Abstract
Background
Four electronic devices for self-measurement of brachial blood pressure (BP): the Omron M1 Plus, the Omron M6 Comfort, the Spengler KP7500 D, and the Microlife BP A100 Plus, were evaluated in four separate studies according to the International Protocol of the European Society of Hypertension (ESH).
Design
The International Validation Protocol is divided into 2 phases: the first phase is performed on 15 selected subjects (45 pairs of BP measurements); if the device passes this phase, 18 supplementary subjects are included (54 pairs of BP measurements) making a total number of 33 subjects (99 pairs of BP measurements) on which the final validation is performed.
Methods
The same methodology recommended by the ESH protocol was applied for the 4 studies. In each study and for each subject, 4 BP measurements were performed simultaneously by 2 trained observers using mercury sphygmomanometers alternately with 3 measurements by the tested device. The difference between the BP value given by the device and that obtained by the two observers (mean of the two observers) was calculated for each measure. The 99 pairs of BP differences were classified into 3 categories (≤5, ≤10, ≤15 mmHg). The number of differences in each category was compared with the number required by the International Protocol. An individual analysis was then done to determine for each subject the number of comparisons ≤5 mmHg. At least 22 of the 33 subjects should have 2 of their 3 comparisons ≤5 mmHg.
Results
All 4 tested devices passed the first and the second phase of the validation process. The average differences between the device and mercury sphygmomanometer readings were −1.4 ± 5.5 and −0.4 ± 4.8 mmHg for SBP and DBP respectively for the Omron M1 Plus device, −2.1 ± 7.4 and 0.1 ± 4.9 mmHg for SBP and DBP respectively for the Omron M6 Comfort device, −1.4 ± 8.6 and −0.1 ± 3.5 mmHg for SBP and DBP respectively for the Spengler KP7500 D device, and 1.6 ± 4.2 mmHg and 0.54 ± 2.8 mmHg for SBP and DBP respectively for the Microlife BP A100 Plus device. For all devices, readings differing by less than 5, 10, and 15 mmHg for SBP and DBP values fulfill the recommendation criteria of the International Protocol as well as the individual analysis.
Conclusions
Omron M1 Plus (HEM-4011C-E), Omron M6 Comfort (HEM 7000-E), Spengler KP7500 D, and Microlife BP A100 Plus devices fulfilled the validation recommendations of the International Protocol.
Keywords: Omron M1 Plus (HEM-4011C-E), Omron M6 Comfort (HEM-7000-E), Spengler KP7500 D and Microlife BP A100 Plus, validation, International Protocol, self-blood pressure measurement
Introduction
Advantages of blood pressure (BP) self-measurement have been well documented (Pickering et al 1996; White 1998; O’Brien et al 2005). Indeed, self-BP measurement (sBPm) not only provides valuable information for hypertension diagnosis but also on BP control of the treated patient, and it improves patient’s compliance with antihypertensive therapy (Haynes et al 1976). Therefore, it is appropriate to encourage the widespread use of self-recorded BP as an important adjunct to the clinical care of some patients with hypertension (Thijs et al 1998; Asmar et al 2000). Clinical indications of sBPm have been recently highlighted in several international scientific society recommendations (Asmar et al 2000; O’Brien et al 2005). Obviously, BP self-measurement is only practicable if the devices are accurate, user-friendly, and relatively inexpensive. Particular attention must be paid to ensure the accuracy of the used devices (O’Brien et al 1993a). Ideally, recommended devices should have been submitted to independent clinical validation procedures. During recent years, various automated devices for self-measurement of BP have been fabricated, but only some have been validated (O’Brien et al 1993a, 1996; Foster et al 1994; Modesti et al 1996; Cordoba et al 1997; Bortolotto et al 1999; Naschitz et al 2000; Mattu et al 2001; White et al 2001; El Assaad et al 2002; Cuckson et al 2002; Golara et al 2002; Ploin et al 2002; El Assaad et al 2003; Alpert et al 2004; Nolly et al 2004; Verdecchia et al 2004; Stergiou et al 2006; Topouchian et al 2006) according to recognized protocols specifically designed for this purpose, such as the British Hypertension Society (BHS) protocol (O’Brien et al 1990, 1993b), the Association for the Advancement of Medical Instrumentation (AAMI) protocol (AAMI 1987, 1993) and the most recent International Protocol (O’Brien et al 2002) published by the European Society of Hypertension (ESH). In this study, 4 devices for self-measurement of BP were validated according to the ESH protocol in 4 separate studies (O’Brien et al 2002).
Methods
Devices
Omron M1 plus (HEM-4011C-E)
The Omron M1 Plus device records brachial BP oscillometrically with a BP measurement range of 0–299 mmHg and heart rate range of 40–180 beats/min. Systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate are displayed on a liquid crystal digital (LCD) read-out. Inflation is manual by pumping the inflation bulb. Measurement starts automatically after having stopped inflating the arm cuff. Deflation is made by pressing the air release button to release the air in the arm cuff; this is an automatic pressure release valve. Standard cuff type adult for an arm circumferences ranging from 220 to 320 mm is provided.
Omron M6 comfort (HEM-7000-E)
The Omron M6 Comfort (HEM 7000-E) device records brachial BP oscillometrically with a BP measurement range of 0–299 mmHg and heart rate range of 40–180 beats/min. SBP, DBP, and heart rate are displayed on an LCD read-out. The inflation is performed using a fuzzy-logic electric pumping system and the deflation by an automatic pressure release valve. Standard cuff type adult for an arm circumference ranging from 220 to 420 mm is provided, which fits both standard and large arm circumferences (cuff dimensions: 152 × 600 mm; weight: 240 g).
Spengler KP7500D
The Spengler KP7500D device records brachial BP oscillometrically with a BP measurement range of 20–300 mmHg and heart rate range of 40–200 beats/min. SBP, DBP, and heart rate are displayed on an LCD read-out. Inflation is automatic by a micro rolling pump. Measurement starts automatically after having pressed and released the power button. Deflation is automatic by an automatic pressure release valve to release the air in the arm cuff. Standard adult cuff type for an arm circumference ranging from 220 to 320 mm is provided.
Microlife BP A100 plus
The Microlife BP A100 Plus device records brachial BP oscillometrically with a BP measurement range of 30–280 mmHg and heart rate range of 40–200 beats/min. SBP, DBP, and heart rate are displayed on an LCD read-out. Measurement starts automatically; deflation is also automatic by a constant air release solenoid valve. Standard adult cuff type for an arm circumference ranging from 220 to 320 mm is provided. Two other cuffs, small (arm circumference 170–220 mm), and extra large cuffs (320–420 mm) are optional.
Device validation
Each device was validated separately in specific populations and at different times. Therefore, each patient participated in only one study device validation. The devices were validated according to the ESH protocol. For each study, the manufacturer was asked to loan 2 or 3 devices with 2 different size cuffs (medium and large).
Factors affecting accuracy of measurements were described by the manufacturers according to the requirements of the International Protocol and were taken into consideration during the validation procedure.
The validation team consisted of 3 persons experienced in BP measurement who also had received training based on a CD-ROM (Groupe d’Evaluation et de Mesure de la Société Française d’Hypertension Artérielle 1998) specifically developed by the French Society of Hypertension for the certification of observers involved in clinical studies. Two of the three observers simultaneously measured BP using a standard mercury sphygmomanometer, the components of which had been carefully checked before the study, and the third observer was the supervisor who checked the values obtained by the two observers and measured the BP by the tested device.
Analysis according to the International Protocol consisted of 2 phases. In the first phase, 15 subjects (45 BP measurements) were recruited; devices passing this primary phase proceeded to the second phase, for which a further 18 subjects (54 BP measurements) were recruited. All the devices were validated at the same center, by the same observers but at different times and in different populations.
Subject selection
For each study, selection of subjects was done according to the recommendations of the International Protocol (Table 1). Four different populations were used in these 4 validation procedures. Arm circumferences were measured in each patient to ensure the use of an adequate cuff size; arm circumferences were distributed by chance according to the ESH protocol. In order to fulfill the BP criteria ranges and to optimize recruitment, it is recommended that subjects for the high diastolic and low systolic groups should be recruited first, then those with high systolic and low diastolic, and finally the remaining gaps should be filled. Thirty-three subjects with both SBP and DBP measurements corresponding to the requested BP ranges were selected to validate each of the four devices.
Table 1.
BP ranges for entry BP
| SBP (mmHg) | DBP (mmHg) | |
|---|---|---|
| Low | 90–129 | 40–79 |
| Medium | 130–160 | 80–100 |
| High | 161–180 | 101–130 |
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure.
For the primary phase, 5 of the 15 subjects should have an SBP in each of the ranges. Similarly, 5 of the 15 subjects should have a DBP in each of the ranges. For the second phase, 11 of the 33 subjects (including the first 15 subjects) should have SBP and DBP in each of the ranges. There are 3 ranges for SBP and 3 for DBP, with 11 subjects in each range to provide 99 pairs of measurements. Final analysis is performed on the 99 paired measurements.
Procedure
BP measurements by the observers
The subjects were seated in a quiet room and BP measurements started after a 10-minute rest period. Arm circumference was measured and brachial BP cuff size was adapted to the circumference. All measurements were made on the left arm at the heart level. BP was measured simultaneously (Y tube) with 2 calibrated mercury sphygmomanometers by the two observers alternately with the device undergoing validation. The observers were blinded to each other’s readings.
Measurements were carried out in the following sequence for all 4 validation procedures:
BPA Entry BP, observers 1 and 2 each with independent mercury standard sphygmomanometer. The mean values were used to categorize the subject into a low, medium, or high range separately for SBP and DBP (Table 1).
BPB Device detection BP, observer 3. This BP was measured to allow the tested device to determine the BP characteristics of the subject and was not included in the analysis.
BP1 Observers 1 and 2 with the mercury standard.
BP2 Supervisor with the tested device.
BP3 Observers 1 and 2 with the mercury standard.
BP4 Supervisor with the tested device.
BP5 Observers 1 and 2 with the mercury standard.
BP6 Supervisor with the tested device.
BP7 Observers 1 and 2 with the mercury standard.
Accuracy criteria
The concept of the International Protocol is to classify the differences between the device tested and control measurements according to whether these differences lie within 5, 10, or 15 mmHg. Differences are always calculated by subtracting the tested observer measurement from the device measurement. Differences were classified separately in this way for both SBP and DBP.
Subject measurements
For assessment of accuracy, only measurements BP1 to BP7 were used. The mean of each pair of observer measurements was calculated; this was denoted as observer measurement BP1, BP3, BP5, or BP7. Each device measurement was flanked by two of these observer measurements, and one of these was selected as the comparative measurement as follows:
1- The differences between BP2-BP1, BP2-BP3, BP4-BP3, BP4-BP5, BP6-BP5, and BP6-BP7 were calculated.
2- The absolute values of the differences were calculated.
3- These were paired according to the device reading.
4- If the values in a pair were unequal, the observer measurement corresponding to the smaller difference was used.
5- If the values in a pair were equal, the first of the 2 observer measurements was used.
When this had been completed, there were 3 device readings for SBP and 3 for DBP for each subject. Each of these 6 readings had a single corresponding observer measurement, a difference between the two and a band for that difference categorized as follows: (0–5 mmHg, 6–10 mmHg, 11–15 mmHg, >15 mmHg).
Assessment
After all BP ranges have been filled (Table 1), there were 45 sets of measurements for both SBP and DBP for the first phase (15 subjects) and 99 sets for the second phase (33 subjects).
The number of differences in each zone was calculated and compared to the number required by the International Protocol and a continue/fail grade for first phase and pass/fail grade for second phase (phase 2.1) was determined. Also for the second phase, the number of measurements falling within 5 mmHg was determined for each of the 33 subjects and a pass/fail recommendation was determined according to the protocol (phase 2.2). For this phase at least 22 of the 33 subjects should have at least 2 of their 3 comparisons lying within 5 mmHg. And at most 3 of the 33 subjects can have all three of their comparison over 5 mmHg apart. To pass the validation and to be recommended for clinical use, a device must pass both phase 2.1 and phase 2.2. If it does not, it fails and is not recommended for clinical use.
Results
Different populations were used in each of the validation procedures. The number of subjects screened for each device’s study was: 43 pre-included subjects for the Omron M1 Plus, 52 for the Omron M6 Comfort, 46 for the Spengler KP7500 D, and 47 for the Microlife BP A100 Plus.
Omron M1 plus (HEM-4011C-E)
In the Omron M1 Plus study, mean age of the 33 subjects included was 58 ± 13 (21 men and 12 women), the arm circumference was 28 ± 4 (range: 19–38), and 26 standard cuffs, 6 large cuffs, and 1 small cuff were used (Table 2). The BP difference between the two observers was 0.95 ± 1.4 mmHg and 0.08 ± 0.84 mmHg for SBP and DBP respectively. The mean values of 99 measurements for SBP and DBP were respectively 136.5 ± 20.9/89.6 ± 16.4 mmHg with the Omron M1 Plus (HEM-4011C-E) device and 137.9 ± 22.1/90 ± 16 mmHg with the standard mercury sphygmomanometer. The mean and standard deviation of the difference between the device and the observers were −1.4 ± 5.5 and −0.4 ± 4.8 mmHg for SBP and DBP respectively.
Table 2.
Age, arm circumference distribution, and BP values for all four devices populations
| Omron M1 Plus | Omron M6 Comfort | Spengler KP7500D | Microlife BP A100 Plus | |
|---|---|---|---|---|
| Age (years) | 58 ± 13 | 52 ± 15 | 59 ± 13 | 57 ± 13 |
| Arm circ. distribution (cm) | 28 ± 4 | 29 ± 4 | 30 ± 4 | 29 ± 4 |
| Arm circ. range (cm) | 19–38 | 19–34 | 23–40 | 24–38 |
| BP (SBP/DBP) (mmHg) | 136.5 ± 20.9/89.6 ± 16.4 | 133.8 ± 24.2/85.2 ± 13.9 | 138 ± 20.2/83.6 ± 17.0 | 141.9 ± 24.5/84.5 ± 16.4 |
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure.
In total, 45 measurements (3 measurements × 15 subjects) were available for analysis in the first phase of the validation process, and 99 (3 measurements × 33 subjects) in the second phase. The number of measurements differing from the mercury sphygmomanometer standard by 5, 10, and 15 mmHg or less are shown in Table 3. These results are in accordance with the requested criteria of the International Protocol for the primary and secondary phases. Thus the Omron M1 Plus device fulfills the validation criteria of the International Protocol.
Table 3.
Results of the OMRON M1 Plus (HEM-4011C-E) device
| Phase 1 | ≤5 mmHg | ≤10 mmHg | ≤15 mmHg | Recomm. | |||
|---|---|---|---|---|---|---|---|
| Required | One of | 25 | 35 | 40 | |||
| Achieved | SBP | 41 | 44 | 45 | Continue | ||
| DBP | 39 | 44 | 45 | Continue | |||
| Phase 2.1 | ≤5 mmHg | ≤10 mmHg | ≤15 mmHg | Recomm. | Mean diff. | SD | |
| Required | Two of | 65 | 80 | 95 | |||
| All of | 60 | 75 | 90 | ||||
| Achieved | SBP | 83 | 97 | 99 | Pass | −1.4 | 5.5 |
| DBP | 80 | 93 | 98 | Pass | −0.4 | 4.8 | |
| Phase 2.2 | 2/3 ≤ 5 mmHg | 0/3 ≤ 5 mmHg | Recomm. | ||||
| Required | ≥22 | ≤3 | |||||
| Achieved | SBP | 29 | 0 | Pass | |||
| DBP | 27 | 1 | Pass | ||||
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; Recomm., recommendation; Mean diff., mean difference (mmHg); SD, standard deviation (mmHg).
The difference between the device readings and the mean BP of the device and the two observers for all 99 points for SBP and DBP are shown in Figure 1a and Figure 2a respectively.
Figure 1.
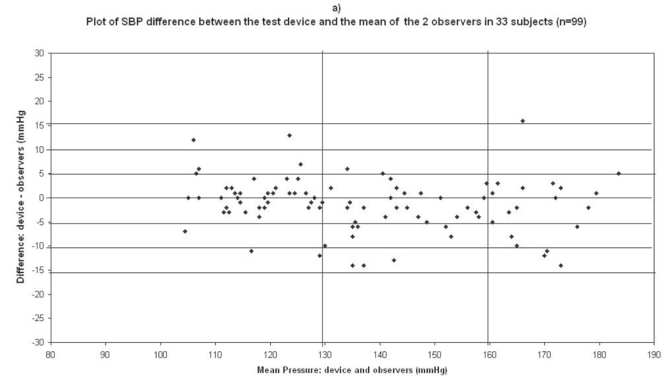
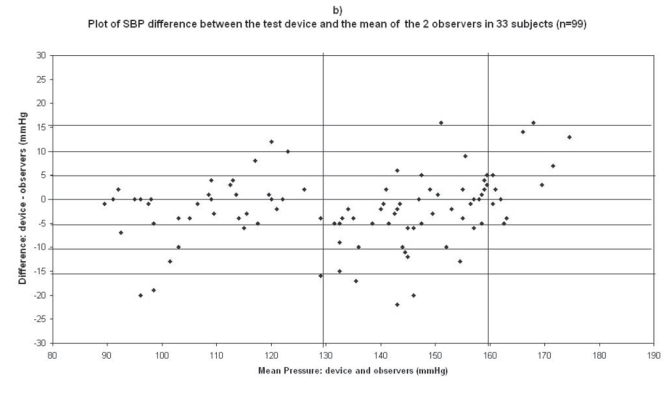
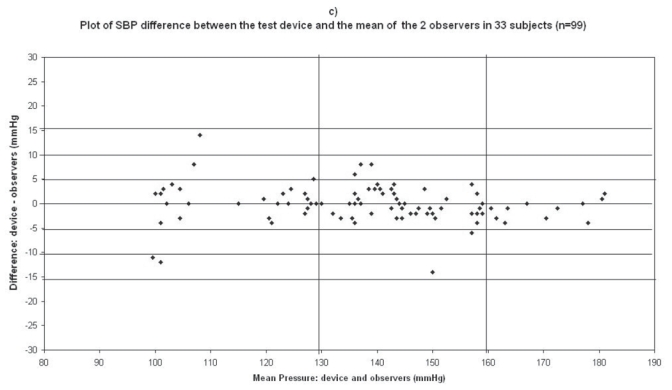
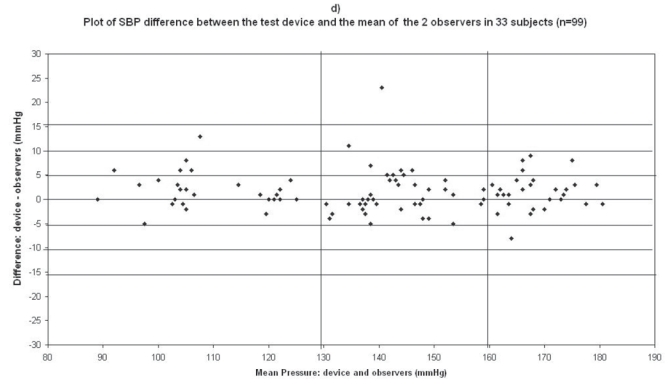
Plots for systolic blood pressure (SBP) difference between the test device readings and the mean of the two observer readings in 33 participants (n = 99) versus the mean of the devices and the mercury sphygmomanometer readings: (a) Omron M1 Plus (HEM 4011C-E), (b) Omron M6 Comfort (HEM-7000-E), (c) Spengler KP7500D, (d) Microlife BP A100 Plus.
Figure 2.
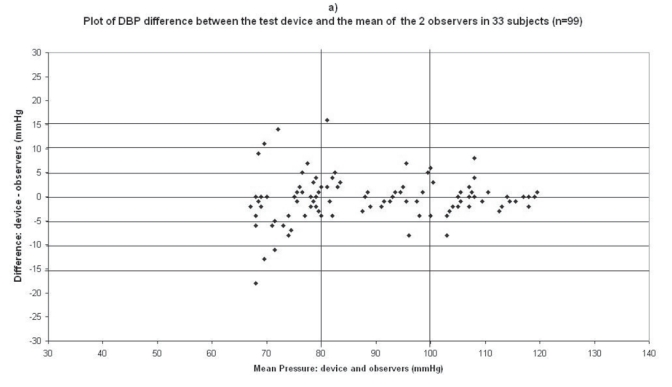
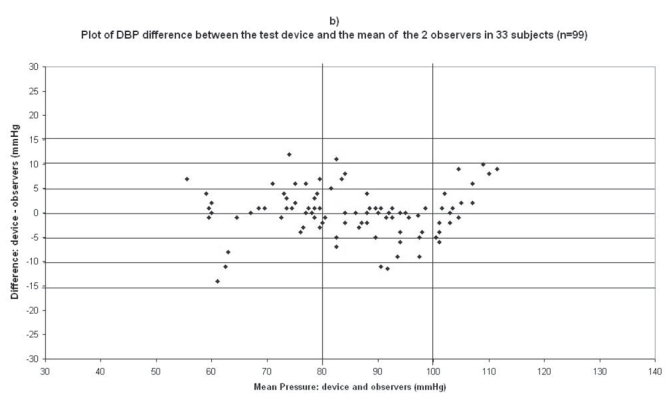
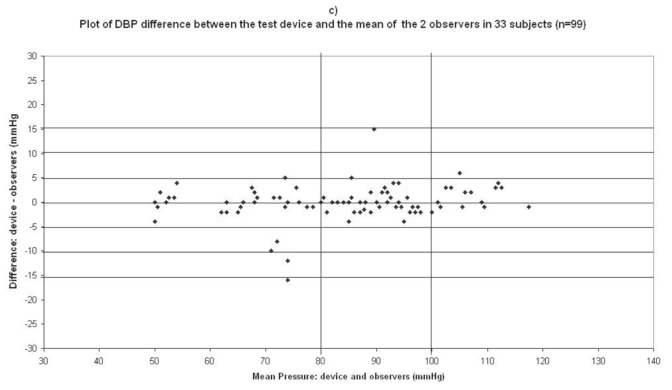
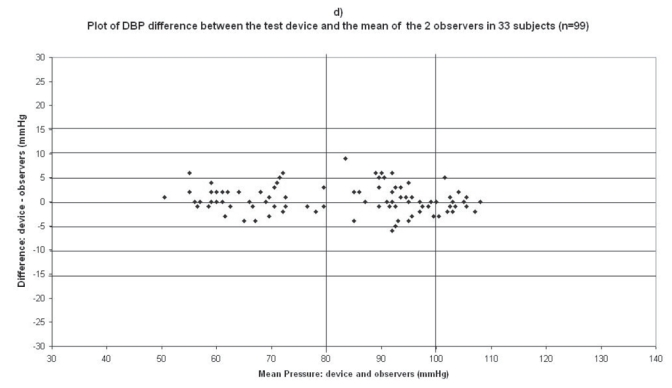
Plots for diastolic blood pressure (DBP) difference between the test device readings and the mean of the two observer readings in 33 participants (n = 99) versus the mean of the devices and the mercury sphygmomanometer readings: (a) Omron M1 Plus (HEM 4011C-E), (b) Omron M6 Comfort (HEM-7000-E), (c) Spengler KP7500D, (d) Microlife BP A100 Plus.
Omron M6 comfort (HEM-7000-E)
In the Omron M6 Comfort (HEM-7000-E) study, mean age of the 33 subjects included was 52 ± 15 (19 men and 14 women), the arm circumference was 29 ± 4 (range: 19–34), so the standard cuff was used for all subjects (Table 2). The difference between the two observers was 0.36 ± 1.88 mmHg and 0.34 ± 1.14 mmHg for SBP and DBP respectively. The mean values of 99 measurements for SBP and DBP were respectively 133.8 ± 24.2/85.2 ± 13.9 mmHg with the Omron M6 Comfort device and 135.9 ± 22.7/85.2 ± 13.8 mmHg with the standard mercury sphygmomanometer. The mean and standard deviation of the difference between the device and the observers were −2.13 ± 7.37 and 0.09 ± 4.91 mmHg for SBP and DBP respectively.
In total, 45 measurements (3 measurements × 15 subjects) were available for analysis in the first phase of the validation process, and 99 (3 measurements × 33 subjects) in the second phase. The number of measurements differing from the mercury standard by 5, 10, and 15 mmHg or less are shown in Table 4. These results are in accordance with the requested criteria of the International Protocol for the primary and secondary phases. Thus the Omron M6 Comfort device fulfills the validation criteria of the International Protocol.
Table 4.
Results of the OMRON M6 Comfort (HEM 7000-E) device
| Phase 1 | ≤5 mmHg | ≤10 mmHg | ≤15 mmHg | Recomm. | |||
|---|---|---|---|---|---|---|---|
| Required | One of | 25 | 35 | 40 | |||
| Achieved | SBP | 32 | 37 | 40 | Continue | ||
| DBP | 38 | 43 | 45 | Continue | |||
| Phase 2.1 | ≤5 mmHg | ≤10 mmHg | ≤15 mmHg | Recomm. | Mean diff. | SD | |
| Required | Two of | 65 | 80 | 95 | |||
| All of | 60 | 75 | 90 | ||||
| Achieved | SBP | 67 | 81 | 90 | Pass | −2.1 | 7.4 |
| DBP | 73 | 93 | 99 | Pass | 0.1 | 4.9 | |
| Phase 2.2 | 2/3 ≤ 5 mmHg | 0/3 ≤ 5 mmHg | Recomm. | ||||
| Required | ≥22 | ≤3 | |||||
| Achieved | SBP | 25 | 3 | Pass | |||
| DBP | 25 | 3 | Pass | ||||
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; Recomm., recommendation; Mean diff., mean difference (mmHg); SD, standard deviation (mmHg).
The difference between the device readings and the mean BP of the device and the two observers for all 99 points for SBP and DBP are shown in Figure 1b and Figure 2b respectively.
Spengler KP7500D
In the Spengler KP7500D study, mean age of the 33 subjects included was 59 ± 13 (21 men and 12 women), the arm circumference was 30 ± 4 (range: 23–40), and 29 standard cuffs and 4 large cuffs were used (Table 2). The difference between the two observers was 0.01 ± 2.01 mmHg and 0.1 ± 1.6 mmHg for SBP and DBP respectively. The mean values of 99 measurements for SBP and DBP were respectively 138 ± 20.2/83.6 ± 17.0 mmHg with the Spengler KP7500D device and 139.4 ± 21.9/83.7 ± 16.3 mmHg with the standard mercury sphygmomanometer. The mean and standard deviation of the difference between the device and the observers were −1.4 ± 8.6 and −0.1 ± 3.5 mmHg for SBP and DBP respectively.
In total, 45 measurements (3 measurements × 15 subjects) were available for analysis in the first phase of the validation process, and 99 (3 measurements × 33 subjects) in the second phase. The number of measurements differing from the mercury standard by 5, 10, and 15 mmHg or less are shown in Table 5. These results are in accordance with the requested criteria of the International Protocol for the primary and secondary phases. Thus the Spengler KP7500D device fulfills the validation criteria of the International Protocol.
Table 5.
Results of the Spengler KP7500D device
| Phase 1 | ≤5 mmHg | ≤10 mmHg | ≤15 mmHg | Recomm. | |||
|---|---|---|---|---|---|---|---|
| Required | One of | 25 | 35 | 40 | |||
| Achieved | SBP | 40 | 40 | 42 | Continue | ||
| DBP | 42 | 43 | 44 | Continue | |||
| Phase 2.1 | ≤5 mmHg | ≤10 mmHg | ≤15 mmHg | Recomm. | Mean diff. | SD | |
| Required | Two of | 65 | 80 | 95 | |||
| All of | 60 | 75 | 90 | ||||
| Achieved | SBP | 86 | 92 | 96 | Pass | −1.4 | 8.6 |
| DBP | 93 | 96 | 98 | Pass | −0.1 | 3.5 | |
| Phase 2.2 | 2/3 ≤ 5 mmHg | 0/3 ≤ 5 mmHg | Recomm. | ||||
| Required | ≥22 | ≤3 | |||||
| Achieved | SBP | 29 | 1 | Pass | |||
| DBP | 31 | 1 | Pass | ||||
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; Recomm., recommendation; Mean diff., mean difference (mmHg); SD, standard deviation (mmHg).
The difference between the device readings and the mean BP of the device and the two observers for all 99 points for SBP and DBP are shown in Figure 1c and Figure 2c respectively.
Microlife BP A100 plus
In the Microlife BP A100 Plus study, mean age of the 33 subjects included was 57 ± 13 (23 men and 10 women), the arm circumference was 29 ± 4 (range: 24–38), and 26 standard cuffs and 7 large cuffs were used (Table 2). The difference between the two observers was 0.067 ± 1.7 mmHg and 0.12 ± 0.98 mmHg for SBP and DBP respectively. The mean values of 99 measurements for SBP and DBP were respectively 141.9 ± 24.5/84.5 ± 16.4 mmHg with the Microlife BP A100 Plus device and 140.3 ± 24.7/83.9 ± 17.35 mmHg with the standard mercury sphygmomanometer. The mean and standard deviation of the difference between the device and the observers were 1.6 ± 4.2 and 0.54 ± 2.8 mmHg for SBP and DBP respectively.
In total, 45 measurements (3 measurements × 15 subjects) were available for analysis in the first phase of the validation process, and 99 (3 measurements × 33 subjects) in the second phase. The number of measurements differing from the mercury standard by 5, 10, and 15 mmHg or less, are shown in Table 6. These results are in accordance with the requested criteria of the International Protocol for the primary and secondary phases. Thus the Microlife BP A100 Plus device fulfills the validation criteria of the International Protocol.
Table 6.
Results of the Microlife BP A100 Plus device
| Phase 1 | ≤5 mmHg | ≤10 mmHg | ≤15 mmHg | Recomm. | |||
|---|---|---|---|---|---|---|---|
| Required | One of | 25 | 35 | 40 | |||
| Achieved | SBP | 40 | 45 | 45 | Continue | ||
| DBP | 42 | 45 | 45 | Continue | |||
| Phase 2.1 | ≤5 mmHg | ≤10 mmHg | ≤15 mmHg | Recomm. | Mean diff. | SD | |
| Required | Two of | 65 | 80 | 95 | |||
| All of | 60 | 75 | 90 | ||||
| Achieved | SBP | 84 | 96 | 98 | Pass | 1.6 | 4.2 |
| DBP | 91 | 99 | 99 | Pass | 0.54 | 2.8 | |
| Phase 2.2 | 2/3 ≤ 5 mmHg | 0/3 > 5 mmHg | Recomm. | ||||
| Required | ≥22 | ≤3 | |||||
| Achieved | SBP | 30 | 2 | Pass | |||
| DBP | 33 | 0 | Pass | ||||
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; Recomm., recommendation; Mean diff., mean difference (mmHg); SD, standard deviation (mmHg).
The difference between the device readings and the mean BP of the device and the two observers for all 99 points for SBP and DBP are shown in Figure 1d and Figure 2d respectively.
Discussion
The tested devices, Omron M1 Plus (HEM-4011C-E), Omron M6 Comfort (HEM-7000-E), Spengler KP7500D, and Microlife BP A100 Plus, fulfilled the validation criteria of the International Protocol for SBP and for DBP. The International Protocol recommendations (O’Brien et al 2002) have been published by the Working Group on Blood Pressure Monitoring of the ESH with the aim of simplifying the two main available guidelines, the BHS (O’Brien et al 1990, 1993b) and AAMI (AAMI 1987, 1993) protocols without sacrificing their integrity. These two validation protocols have many similarities but experience has demonstrated that the conditions they recommend are sometimes extremely difficult to fulfill especially because of the large number of subjects who have to be recruited and the ranges of BP required. It has been demonstrated by the ESH Working Group that validation studies can be performed in such a way as to satisfy the criteria of the much more complicated earlier protocols (O’Brien et al 2002). The main advantage of the International Protocol is that it requires fewer subjects, 33 instead of 85 with the two other protocols.
Our experience with the validation of these devices shows that the recruitment of subjects having low SBP (90–129 mmHg) and especially high DBP (101–130 mmHg) is the major factor that extends the time required for the validation, although the International Protocol recommends that recruitment of subjects should commence by targeting those likely to have pressures in the low-systolic and high-diastolic ranges so that it will be easy to complete the recruitment with the remaining ranges.
Another point that remains a limit of the present study is that the results are based on only one device and the validation was done in only one center; however the International Protocol (O’Brien et al 2002) does not specify the number of devices to be tested or the number of study sites recommended to enhance the heterogeneity of the study population. The AAMI protocol (AAMI 1987, 1993) recommends more than one study site without specifying the number and without noting the number of devices used for the validation. On the other hand, the BHS protocol (O’Brien et al 1990, 1993b) does not specify performing the validation in more than one site but recommends assessing the capability of a number of devices of the tested model to give consistent measurements. If substantial differences between instruments of the same device occur, further device validation is not appropriate. In this regard, Stergiou et al (2006) reported recently similar results for the Microlife BP A100 Plus device.
It is important to mention here that these validation studies were performed in the general population and that the observed results cannot be extrapolated to specific populations such as the elderly, the obese, and children. Specific validation studies are needed in specific populations.
This study shows that increased error at extremes of BP occurs in virtually all non-invasive devices, but the degree of error varies (White et al 2001; El Assaad et al 2002; Geddes et al 1982). It is, however, also important to recognize that this usually bears little clinical relevance since therapeutic decisions would not significantly differ (White et al 2001).
In conclusion, the tested devices, Omron M1 Plus (HEM-4011C-E), Omron M6 Comfort (HEM-7000-E), Spengler KP7500D, and Microlife BP A100 Plus have passed the validation criteria of the International Protocol for validation of BP measuring devices.
References
- Alpert BS. Validation of the Pharma-Smart PS-2000 public use blood pressure monitor. Blood Press Monit. 2004;9:19–23. doi: 10.1097/00126097-200402000-00005. [DOI] [PubMed] [Google Scholar]
- Asmar R, Zanchetti A. Guidelines for the use of self-blood pressure monitoring: a summary report of the first international consensus conference. J Hypertens. 2000;18:493–508. doi: 10.1097/00004872-200018050-00001. [DOI] [PubMed] [Google Scholar]
- [AAMI] Association for the Advancement of Medical Instrumentation. The national standard of electronic or automated sphygmomanometers. AAMI: Arlington VA; 1987. [Google Scholar]
- [AAMI] Association for the Advancement of Medical Instrumentation. American national standard: electronic or automated sphygmomanometers. AAMI: Arlington VA; 1993. [Google Scholar]
- Bortolotto L, Henry O, Hanon O, et al. Validation of the two devices for self-measurement of blood pressure by elderly patients according to the revised British Hypertension Society protocol: the Omron HEM-722C and HEM-735C. Blood Press Monit. 1999;4:21–5. doi: 10.1097/00126097-199904010-00004. [DOI] [PubMed] [Google Scholar]
- Cordoba R, Fuertes MI, Alvarez A, et al. Validation of a self-measuring blood pressure monitor: the Omron-HM 722C. Aten Primaria. 1997;20:247–50. [PubMed] [Google Scholar]
- Cuckson AC, Reinders A, Shabeeh H, et al. British Hypertension Society Validation of the Microlife BP 3BTO-A oscillometric blood pressure monitoring device according to a modified British Hypertension Society protocol. Blood Press Monit. 2002;7:319–24. doi: 10.1097/00126097-200212000-00005. [DOI] [PubMed] [Google Scholar]
- El Assaad M, Topouchian J, Darné B, et al. Validation of the Omron HEM-907 device for blood pressure measurement. Blood Press Monit. 2002;7:237–41. doi: 10.1097/00126097-200208000-00006. [DOI] [PubMed] [Google Scholar]
- El Assaad MA, Topouchian JA, Asmar RG. Evaluation of two devices for self-measurement of blood pressure according to the international protocol: the Omron M5-I and the Omron 705IT. Blood Press Monit. 2003;8:127–33. doi: 10.1097/00126097-200306000-00006. [DOI] [PubMed] [Google Scholar]
- Foster c, McKinlay JM, Cruickshank JM, Coats AJS. Accuracy of the Omron HEM 706 portable monitor for home measurement of blood pressure. J Hum Hypertens. 1994;8:661–4. [PubMed] [Google Scholar]
- Geddes LA, Voellz M, Combs C, et al. Characterization of the oscillometric method for measuring indirect blood pressure. Ann Biomed Eng. 1982;10:271–80. doi: 10.1007/BF02367308. [DOI] [PubMed] [Google Scholar]
- Golara M, Jones C, Randhawa M, et al. Inflationary oscillometric blood pressure monitoring: validation of the OMRON-MIT. Blood Press Monit. 2002;7:325–8. doi: 10.1097/00126097-200212000-00006. [DOI] [PubMed] [Google Scholar]
- Groupe d’Evaluation et de Mesure de la Société Française d’Hypertension Artérielle. La prise de la tension artérielle au cabinet médical. In: Ran D, editor. CD ROM. France: Meudon; 1998. [Google Scholar]
- Haynes R, Sackett D, Gibson E, et al. Improvement of medication compliance in uncontrolled hypertension. Lancet. 1976;1:1265–8. doi: 10.1016/s0140-6736(76)91737-2. [DOI] [PubMed] [Google Scholar]
- Mattu G, Perry T, Wright J. Comparison of the oscillometric blood pressure monitor (BPM-100beta) with the auscultatory mercury sphygmomanometer. Blood Press Monit. 2001;6:153–9. doi: 10.1097/00126097-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Modesti PA, Costoli A, Cecioni I, et al. Clinical evaluation of the QuietTrak blood pressure recorder according to the protocol of the British Hypertension Society. Blood Press Monit. 1996;1:63–8. [PubMed] [Google Scholar]
- Naschitz JE, Loewenstein L, Lewis R, et al. Accuracy of the Omron M4 automatic blood pressure measuring device. J Hum Hypertens. 2000;14:423–7. doi: 10.1038/sj.jhh.1001040. [DOI] [PubMed] [Google Scholar]
- Nolly H, Romero M, Nolly A, et al. Home blood pressure measurement: validation of the Braun BP 2550 (UG) monitor according to the ESH International Protocol. Blood Press Monit. 2004;9:53–8. doi: 10.1097/00126097-200402000-00010. [DOI] [PubMed] [Google Scholar]
- O’Brien E, Asmar R, Beilin L, et al. European Society of Hypertension Working Group on Blood Pressure Monitoring. Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self blood pressure measurement. J Hypertens. 2005;23:697–701. doi: 10.1097/01.hjh.0000163132.84890.c4. [DOI] [PubMed] [Google Scholar]
- O’Brien E, Atkins N, Mee F, et al. Comparative accuracy of six ambulatory devices according to blood pressure levels. J Hypertens. 1993a;11:673–5. doi: 10.1097/00004872-199306000-00012. [DOI] [PubMed] [Google Scholar]
- O’Brien E, Mee F, Atkins N, et al. Evaluation of three devices for sel-measurement of blood pressure according to the revised British Hypertension Society Protocol: the Omron HEM-705CP, Philips HP5332, and Neissei DS-175. Blood Press Monit. 1996;1:55–61. [PubMed] [Google Scholar]
- O’Brien E, Petrie J, Littler WA, et al. The British Hypertension Society Protocol for the evaluation of automated and semi-automated blood pressure measuring devices with special reference to ambulatory systems. J Hypertens. 1990;8:607–19. doi: 10.1097/00004872-199007000-00004. [DOI] [PubMed] [Google Scholar]
- O’Brien E, Petrie J, Littler WA, et al. The British Hypertension Society Protocol for the evaluation of blood pressure measuring devices. J Hypertens. 1993b;11(Suppl 2):S43–S62. doi: 10.1097/00004872-199306000-00013. [DOI] [PubMed] [Google Scholar]
- O’Brien E, Pickering T, Asmar P, et al. Working Group on Blood Pressure Monitoring of the European Society of Hypertension International Protocol for validation of blood pressure measuring devices in adults. Blood Press Monit. 2002;7:3–17. doi: 10.1097/00126097-200202000-00002. [DOI] [PubMed] [Google Scholar]
- Pickering T, Kaplan N, Krakoff L, et al. Recommendations for the use of home (self) and ambulatory blood pressure monitoring. Am J Hypertens. 1996;9:1–11. doi: 10.1016/0895-7061(95)00341-x. [DOI] [PubMed] [Google Scholar]
- Ploin D, Baguet JP, Pierre H, et al. Clinical evaluation of a self blood pressure monitor according to the First International Consensus Conference on Self Blood Pressure Measurement. Blood Press Monit. 2002;7:335–41. doi: 10.1097/00126097-200212000-00008. [DOI] [PubMed] [Google Scholar]
- Stergiou GS, Giovas P, Neofytou MS, et al. Validation of the Microlife BPA100 Plus device for self-home blood pressure measurement according to the International Protocol. Blood Press Monit. 2006;11:157–60. doi: 10.1097/01.mbp.0000209071.84965.bf. [DOI] [PubMed] [Google Scholar]
- Thijs L, Staessen JA, Celis H, et al. Reference values for self-recorded blood pressure. A meta-analysis of summary data. Arch Intern Med. 1998;158:481–8. doi: 10.1001/archinte.158.5.481. [DOI] [PubMed] [Google Scholar]
- Topouchian JA, El Assaad MA, Orobinskaia LY, et al. Validation of two automatic devices for self-measurement of blood pressure according to the International Protocol of the European Society of Hypertension: the Omron M6 (HEM-7001-E) and the Omron R7 (HEM-637-IT) Blood Press Monit. 2006;11:165–71. doi: 10.1097/01.mbp.0000209078.17246.34. [DOI] [PubMed] [Google Scholar]
- Verdecchia P, Angeli F, Poeta F, et al. Validation of the A&D UA-774 (UA-767Plus) device for self-measurement of blood pressure. Blood Press Monit. 2004;9:225–9. doi: 10.1097/00126097-200408000-00008. [DOI] [PubMed] [Google Scholar]
- White W. Out-of-office blood pressure monitoring. In: Izzo J, editor. Hypertension primer. 2. Council for High Blood pressure Research, American Heart Association: Dallas; 1998. pp. 302–6. [Google Scholar]
- White W, Anwar Y. Evaluation of the overall efficacy of the Omron office digital blood pressure HEM-907 monitor in adults. Blood Press Monit. 2001;6:107–10. doi: 10.1097/00126097-200104000-00007. [DOI] [PubMed] [Google Scholar]


