Abstract
Advanced atherogenesis is characterized by the presence of markers of enhanced prothrombotic capacity, attenuated fibrinolysis, and by clinical conditions associated with defective coagulation. Diabetes may be associated with enhanced lesion instability and atherosclerotic plaque rupture. Plaques obtained from 206 patients undergoing carotid endarterectomy were divided into diabetic (type 2) and nondiabetic and analyzed by Western blotting and immunohistochemistry to detect tissue factor (TF), metalloproteinases (MMP)-2, -8, -9, and fibrin/fibrinogen related antigens, and in situ zymography to detect MMP activity. Plasma samples were quantified for TF procoagulant activity, C-reactive protein, fibrinogen and D-dimer. Diabetic and symptomatic patients with hypoechogenic plaques had increased plasma TF activity and D-dimer, compared with those with hyperechogenic plaques (p = 0.03, p = 0.007, respectively). Diabetic, symptomatic patients had higher plasma D-dimer levels than asymptomatic patients (p = 0.03). There was a significant correlation between intramural TF levels and D-dimer in diabetic patients with symptomatic disease (p = 0.001, r2 = 0.4). In diabetic patients, plasma fibrinogen levels were higher in patients with hypoechogenic plaques (p = 0.007). Diabetic patients with ulcerated plaques had higher plasma D-dimer and MMP-8 levels than those with fibrous plaques (p = 0.02, p = 0.01, respectively). This data suggests that currently available circulating markers may be clinically useful to select diabetic patients at higher risk of atherothrombosis. Increased procoagulant activity in diabetic patients may be linked to increased mural remodeling.
Keywords: Diabetes, atherosclerosis, carotid artery, tissue factor, D-dimer, matrix metalloproteinase
Introduction
Diabetes mellitus is associated with severe atherosclerosis in humans (Paoletti et al 2006). Patients with metabolic syndrome are at increased risk for both progressive carotid atherosclerosis and coronary heart disease (Diamant et al 2002). Chronic perturbation of diabetic vasculature leads to increased complexity of atherosclerotic plaques (King et al 1998). Furthermore, diabetics have enhanced lesion instability (Death et al 2003).
Several studies have suggested an association between circulating tissue factor (TF) and progression of atherothrombosis. Diabetes, hypercholesterolemia, and smoking, are associated with a higher incidence of thrombotic complications. Poorly controlled diabetic patients have increased circulating TF activity associated with increased blood thrombogenicity (Viles-Gonzalez et al 2006). These findings suggest that high levels of circulating TF may be the mechanism of action responsible for increased thrombotic complications associated with the presence of diabetes (Sambola et al 2003; Steffel et al 2006; Meerarani et al 2007). Increased expression of TF in high-grade stenosis of the ICA is associated with plaque destabilization evidenced clinically both by a history of previous ischemic symptoms and the detection of microemboli in long-term transcraneal Doppler ultrasonography monitoring of the ipsilateral middle cerebral artery (Jander et al 2001). Diabetics with vascular disease have raised plasma fibrinogen (Lipinski 2001; Corrado et al 2006), which is considered to be an important risk factor in atherosclerosis (Tkac et al 2003; Krupinski et al 2007). Fibrinogen remains an independent risk factor for both cardiac and extracardiac atherothrombotic complications. Fibrinogen and its effector, thrombin, substantially determine the extent and outcome of atherothrombotic complications, because they are the molecules linking the mutually dependent events in atherogenesis, coagulation/fibrinolysis, rheology, and inflammation (Shah 2006; Krupinski et al 2007a, 2007b).
The mechanisms that mediate vascular complications in diabetic patients are not yet fully understood. Patients with an increased risk of symptomatic vascular disease and development of type 2 diabetes have an atherogenic ‘prediabetic’ state. This can be measured by increased triglyceride levels, increased systolic blood pressure, and decreased levels of HDL cholesterol occurring before the onset of type 2 diabetes (Haffner 2002). Elevated glucose induces discordant metalloprotease (MMP) expression from endothelial cells and macrophages (Ho et al 2007). High glucose induces endothelial cell expression and activity of MMP-1, -2, but reduces expression of MMP-3. In monocytes MMP-9 activity and expression is induced by high glucose (Death et al 2003). Dermal fibroblasts from diabetic patients were also found to have elevated MMP-2,-3 production (Wall et al 2003). The increased MMP-1, -2, -9 activities induced by high glucose exposure could promote matrix degradation thereby accelerating atherogenesis and potentially reducing plaque stability in diabetes (Derosa et al 2007; Turu et al 2007).
This study was designed to identify links between the prothrombotic state, intramural remodeling, and inflammatory markers in diabetic patients with vulnerable carotid disease (Rubio et al 2005).
Materials and methods
Patients
Patients (n = 206) undergoing symptomatic and asymptomatic carotid endarterectomy were used in this study. Symptomatic patients presented with stroke or transient ischemic attack (TIA) in the territory of the affected carotid artery within 6 months prior to endarterectomy according to North American Symptomatic Carotid Endarterectomy Trial criteria (NASCET 1991). Patients were divided into diabetics (n = 53) and nondiabetics (n = 73) according to clinical evidence of type II diabetes (Table 1). All patients had CT-scan and MR imaging, followed by MR angiography or conventional arteriography and the degree of carotid stenosis was calculated according to Bladin and colleagues (1995). Patients were screened for the presence of bilateral pathology (>50% contralateral stenosis). Duplex colour ultrasound analysis was used to classify plaque stability in terms of hypoechogenic or hyperechogenic according to the Gray-Weale criteria (1988). The presence of vascular risk factors (VRF) was recorded and previous statin or antiplatelet treatment was noted. If a patient was anticoagulated he/she was excluded from further analysis. For quantitative assay of D-dimer expression and high sensitivity C-reactive protein (hsCRP) patients with history of acute arterial or venous thromboembolism, active infections or inflammatory conditions, renal failure, hepatic disease, neoplasms, recent trauma, or surgery were excluded. The study was approved by the local ethical committee.
Table 1.
Characteristics of study patients
| Variable | Diabetics (n = 87) |
Nondiabetics (n = 119) |
|---|---|---|
| Age, y | 68 | 69 |
| Male/Female | 72/20 | 105/19 |
| Hypertensión | 69 | 84 |
| Hypercholesterolemia | 66 | 66 |
| Cigarette smoking | 70 | 87 |
| Bilateral carotid disease* | 35 | 47 |
| Symptomatic carotid dis | 61 | 83 |
| Stenosis severity | 83 | 85 |
| CAD | 30 | 31 |
| PVD | 25 | 34 |
| Antiplatelets | 58 | 83 |
| Statins | 33 | 37 |
| hypoechogenic plaque on ultrasound | 36 | 52 |
| Ulcerated plaque on histology | 47 | 54 |
Notes: more than 50% contralateral stenosis on ultrasound
Abbreviations: CAD, coronary artery disease; PVD, peripheral vascular disease.
Blood sampling and tests
Plasma samples were collected after overnight fasting and prior to surgery, frozen in liquid nitrogen and stored at −80 °C for further processing. D-dimer was measured in plasma by automated latex enhanced immunoassay on IL Coagulation Systems (IL Test D-Dimer, 20008500, Biokit, SA). HsCRP was tested in EDTA-plasma samples by particle enhanced immunonephelometry using BN Systems (Dade Behring, Marburg, Germany).
MMP-2, MMP-8, and MMP-9 levels were determined by commercially available enzyme-linked immunosorbent assay (ELISA, Biotrak Amersham Pharmacia, UK). Our laboratory reference ranges for healthy controls were: 41 ± 27.8 ng/ml for MMP-9 (n = 62, 58% males, mean age 43 years, normal range 25–97 ng/ml), 3.10 ± 1.47 ng/ml for MMP-8 (n = 57, normal range 0.16–6 ng/ml) and 630.8 ± 101.8 ng/ml for MMP-2 (n = 40, 47% males, mean age 43 years, normal range 427–835 ng/ml). The mean intra-assay coefficients of variation were <10% for all MMPs.
Circulating TF activity was measured by Actichrome TF, a chromogenic assay (American Diagnostica, Inc., Stamford, CT, USA). This method measures the peptidyl activity of human TF in lysed cells and human plasma. Samples were mixed with human factor VIIa and human factor X. The reagents were incubated at 37 °C, allowing for the formation of the TF/factor VIIa complex (TF/VIIa), and the activation of the human factor X to factor Xa. The amount of factor Xa generated was measured by its ability to cleave Septrozyme® Xa, a highly specific chromogenic substrate for factor Xa, which was added to the reaction solution. The cleaved substrate released a para-nitroaniline chromophore into the reaction solution. The absorbance was measured at 405 nm and compared with values obtained from a standard curve generated using known amounts of active human TF.
All other standard hematological and biochemistry analysis were routinely performed at the hospital laboratory.
Carotid specimens
Carotid specimens were excised by the vascular surgeon without damage to the plaque surface. They were immediately rinsed in 0.9% saline and cut longitudinally into two specimens. One was snap frozen in liquid nitrogen and stored at −80 °C and the other was fixed for 24 hours in buffered formalin and later cryoprotected in 30% sucrose and frozen in OCT for histology. Plaque morphology was assessed immediately after surgery and later by histology of H&E stained sections. Plaques were classified according to AHA classification as unstable (ulcerated or ulcerated with hemorrhage) or stable (fibrous or fibrous with old hemorrhage) (Stary et al 1995).
Western blotting
For gel electrophoresis, samples (n = 30, 15 diabetics and nondiabetics) were homogenized with 300 μl of ice-cold homogenisation buffer containing 1% sodium deoxycholate, 1% v/v Triton X-100, 100 μM EDTA, 1 μM leupeptin, 1 μM pepstatin, 1 μM aprotinin, and 200 μM PMSF. Samples were centrifuged at 5000 rpm for 2 min and stored in aliquots at −20 °C. The protein concentration of each sample was determined using the Bradford assay. SDS-PAGE (10%) was carried out as previously described (Slevin et al 2002). Membranes were incubated overnight at 4 °C with antihuman MMP-9 and MMP-8 antibodies (Chemicon MAB13415; MAB19045, respectively) (Turu et al 2006) and anti-human antibody to TF (American Diagnostica Inc, ID4501, 1:100). The remaining procedure was performed according to standard protocols.
Statistical analysis
Normality of continuous variables was checked by Kolmogorov-Smirnov normality test. Normally distributed clinical, histological and Western blotting variables were compared between groups by analysis of variance (ANOVA). When samples were not normally distributed, Kruskal-Wallis was used. Differences in frequencies of categorical variables were checked by chi-square test.
Results
Table 1 summarizes clinical characteristics of the studied population. There was no difference in age, sex, vascular risks factor distribution, presence of bilateral carotid disease, carotid stenosis severity, plaque stability on ultrasound, coexisting coronary artery disease (CAD), or peripheral vascular disease (PVD) between diabetic and nondiabetic patients. Furthermore, as summarized in Table 2, there were no differences in biochemical parameters studied between diabetics and nondiabetics. However, when diabetes was associated with symptomatic carotid disease or vulnerable carotid plaques, differences were significant as presented below.
Table 2.
Biochemical parameters of study patients
| Variable | Diabetics | Nondiabetics | P |
|---|---|---|---|
| TF plasma activity | 13.9 | 13.3 | 0.7 |
| TF plaque expression (ng/ml) | 0.75 | 0.7 | 0.8 |
| D-dimer in plasma (ng/ml) | 793 | 731 | 0.9 |
| D-dimer in plaque | 6.2 | 5.1 | 0.5 |
| MMP-2 (ng/ml) | 804 | 764 | 0.6 |
| MMP-8 (ng/ml) | 6.2 | 9.1 | 0.2 |
| MMP-9 (ng/ml) | 80.7 | 111 | 0.2 |
| hsCRP (mg/l) | 8.8 | 6.8 | 0.6 |
| Fibrinogen (g/l) | 4.3 | 4.1 | 0.4 |
| WB counts | 7700 | 7271 | 0.6 |
| Cholesterol | 4.8 | 5.2 | 0.1 |
| LDL-C (mmol/l) | 2.8 | 3.0 | 0.4 |
| TG (mmol/l) | 1.4 | 1.5 | 0.4 |
Abbreviations: hsCRP, high sensitivity C-reactive protein; LDL-C, low density lipoprotein-cholesterol; MMP, metalloproteinases; TF, tissue factor; TG, triglycerin; WB, white blood cells.
Hemostatic markers in diabetic patients
Diabetic and symptomatic patients with hypoechogenic plaques identified on ultrasound had increased plasma TF activity compared with nondiabetics (16.34 PM vs 10.4 PM, p = 0.03; Figure 1a). Regression analysis in the same group of patients showed a strong correlation between TF activity in plasma and intramural levels of MMP-8 (p = 0.03, r2 = 0.2, Figure 1b). There was also a correlation between intramural TF levels and both circulating active MMP-8 and intramural D-dimer in symptomatic, diabetic patients with hypoechogenic plaque on ultrasound (p = 0.02, r2 = 0.6, Figure 2a; p = 0.001, r2 = 0.4, Figure 2b, respectively).
Figure 1.
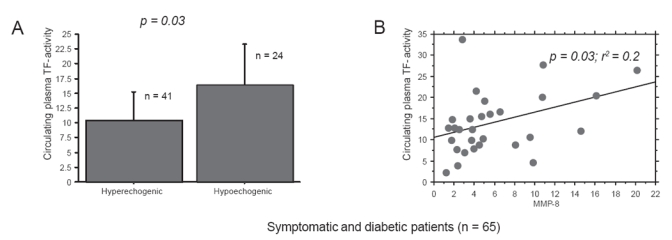
(A) TF activity in plasma samples taken prior to endartrectomy in diabetics and symptomatic patients measured was higher in patients presenting hypoechogenic plaque on ultrasound as compared with patients with hyperechogenic plaque (p = 0.03). (B) On regression plot in the same group of patients there was correlation between TF activity in plasma and intramural levels of MMP-8 (p = 0.03, r2 = 0.2).
Abbreviations: MMP, metalloproteinases; TF, tissue factor.
Figure 2.

(A) Regression plot for symptomatic, diabetic patients with hypoechogenic plaque on ultrasound with correlation between intramural TF levels and circulating active MMP-8 (p = 0.02, r2 = 0.6) (B) Regression plot for symptomatic, diabetic patients with hypoechogenic plaque on ultrasound with correlation between intramural TF levels and intramural D-dimer levels (p = 0.001, r2 = 0.4).
Abbreviations: MMP, metalloproteinases; TF, tissue factor.
Markers of fibrinolysis and inflammation
Diabetics with symptomatic carotid disease had higher levels of plasma D-dimer if the plaque was hypoechogenic on ultrasound as compared with patients with hyperechogenic plaques (p = 0.007, Figure 3a). Diabetics with histologically identified ulcerated plaques had higher plasma D-dimer than diabetics with fibrous plaques (p = 0.02; Figure 3b). Diabetic patients with symptomatic carotid disease had higher plasma D-dimer than nondiabetics (p = 0,03; Figure 3c). Plasma fibrinogen, but not CRP levels were higher in diabetics with hypoechogenic plaques, identified by ultrasound, as compared with diabetics with hyperechogenic plaques (4.6 mg/l vs 3.6 mg/l, p = 0.007).
Figure 3.

(A) Diabetics with symptomatic carotid disease had higher levels of plasma D-dimer if the plaque was hypoechogenic on ultrasound as compared with patients with hyperechogenic plaque (p = 0.007). (B) Diabetics with ulcerated plaque had more plasma D-dimer than diabetics with fibrose plaque (p = 0.02). (C) Diabetics with symptomatic carotid disease had higher levels of plasma D-dimer as compared with diabetic asymptomatic patients (p = 0.03).
Markers of plaque remodeling
Diabetic patients with histologically identified ulcerated carotid plaques had significantly higher plasma levels of MMP-8 than diabetics with stable, fibrous plaques (p = 0.01, Figure 4a). In symptomatic, diabetic patients with hypoechogenic plaques, identified by ultrasound, there was a correlation between total cholesterol levels and intramural MMP-8 levels (p = 0.02, r2 = 0.5, Figure 4b). On western blot diabetic patients had higher plaque TF, active MMP-8, but not MMP-9 expression. The later was more expressed in diabetics with ulcerated, hemorrhagic plaques. Representative western blots are presented in Figure 5.
Figure 4.

(A) Diabetic patients with ulcerated carotid plaque on morphology had higher plasma levels MMP-8 than diabetics with stable, fibrose plaque (p = 0.01). (B) In symptomatic, diabetic patients with hypoechogenic plaque on ultrasound on regression plot there was correlation between total cholesterol levels and intramural MMP-8 levels (p = 0.02, r2 = 0.5).
Abbreviations: MMP, metalloproteinases.
Figure 5.

Representative Western blot showing TF protein levels (47 kDa), pro- and active MMP-8 (85 and 64 kDa, respectively) and pro- and active MMP-9 (92 and 78 kDa, respectively) in CEA plaques from diabetic (Pt1*, Pt2*, Pt4*, and Pt6*) and nondiabetic (Pt3, Pt5, and Pt7) patients. Ponceau staining of the membranes (not shown) and beta-smooth muscle actin (β-SMA) were used as loading controls. C (−) is a negative control, blots were incubated without primary antibodies to TF, MMP-8, and MMP-9.
Abbreviations: CEA; MMP, metalloproteinases; TF, tissue factor.
Discussion
In this study, diabetic patients with unstable carotid disease measured by the presence of symptomatic carotid disease, plaque echogenecity on ultrasound, or plaque vulnerability as evidenced by ulceration on histology, had significantly higher levels of markers of hemostasis, fibrinolysis and plaque remodeling compared with diabetics with stable disease.
TF is thought to be responsible for the prothrombotic state in patients with diabetes, and development of significant carotid artery stenosis with associated plaque remodeling (Sambola et al 2003; Vaidyula et al 2006). Mononuclear cells and monocytes from patients with type 2 diabetes have increased procoagulant activity as measured by TF expression (Konieczkowski and Skrinska 2001). Increased synthesis of TXA2 and TF may potentiate thrombosis and increase fibrin deposition, events that play primary roles in the development of vascular disease. During hyperglycemia-hyperinsulinemia, which is a characteristic of type 2 diabetes, elevation of plasma coagulation factors may constitute a potential for enhanced thrombin generation and thrombosis when triggered by exposure of TF, such as during arterial plaque rupture (Corti et al 2004; Vaidyula et al 2006). Changes of the TF pathway of blood coagulation have been described in diabetes and could be involved in its vascular complications.
Samad and colleagues (2001) found that hyperinsulinemia associated with insulin-resistant states, such as obesity and noninsulin-dependent diabetes mellitus, induced local TF gene expression in multiple tissues, thus contributing via the TF pathway to the increased risk of atherothrombotic disease. There was a strong correlation between expression of the receptor for advanced glycation end products (RAGE) and TF, in long-standing uncontrolled type 2 diabetics (Buchs et al 2004). In our study, diabetics with symptomatic carotid disease had increased circulating tissue factor. Numerous hemostatic factors like vWF, t-PA, PAI-1 are increased in patients with type 2 diabetes or impaired glucose tolerance. Recently, TF was shown to be associated with existing microvascular and neurogenical complications in patients with type 2 diabetes, and also correlated with tPA and vWF expression. Patients with diabetes and TF concentrations of >3,000 pg/ml were 15 times more likely to present with microvascular disease and 10 times more likely to have neurogenical complications (Sommeijer et al 2006). In elderly patients with IGT, intermediates of the TF pathway, ie, TFPI are already increased as compared with normal subjects. Interestingly, endothelium-dependent hemostatic markers do not appear to correlate with lipid metabolism (Leurs et al 2002)
Cell-derived microparticles support coagulation and inflammation and they may be involved in accelerated atherosclerosis in diabetic patients (Morel et al 2006). TF, possibly of granulocytic origin, was exposed on microparticle subpopulations in asymptomatic patients with well-regulated type 2 diabetes. TF-positive microparticles were asociated with components of the metabolic syndrome but not with coagulation. Thus, the presence of TF on microparticles may be involved in processes other than coagulation, including transcellular signaling or angiogenesis (Diamant et al 2002).
In this study population no differences in markers of thrombosis or plaque stability were found between diabetics and nondiabetics. However, diabetic and symptomatic patients with hypoechogenic plaques, identified on ultrasound, had increased expression of circulating and intramural markers of thrombosis, fibrinolysis, and plaque remodeling as compared with patients with stable disease. Other factors and not only diabetes itself may affect changes within the arterial wall and subsequent remodelling leading to symptomatic vascular disease. In noncomplicated patients, the increase in FVII and TFPI was highly dependent on obesity index and age rather than on diabetes itself (Vambergue et al 2001). In the study described here, diabetic patients with hypercholesterolemia had higher intramural MMP-8 levels. Tissue factor activity correlated with intramural MMP-8 levels in diabetics with vulnerable carotid disease, suggesting that plaque remodeling may induce prothrombotic activity. Furthermore, in this vulnerable group of patients there was a significant correlation between total cholesterol levels and intramural MMP-8 and the presence of intramural TF and circulating active MMP-8. Independent of plaque stability, in diabetic patients, there was correlation between intramural MMP-8 and circulating TF activity. MMP-8 levels are of special interest since it is known to be released from neutrophils at sites of inflammation and vascular disease and can cleave TF pathway inhibitor, thereby decreasing its anticoagulant activity (Cunningham et al 2002; Krupinski et al 2007). Baugh and colleagues (2003) however, could not demonstrate abnormal differences in MMP-1, -3, -9 productions in blood moncytes in type 2 diabetic patients. MMP expression and activity therefore remains controversial (Turu et al 2006). Further it is well established that RAGE plays a central role in the process of plaque destabilization in the diabetic patients. It has been demonstrated that enhanced expression of COX-2/mPGES-1 in human symptomatic plaques is associated with MMP-induced plaque rupture (Cipollone et al 2001, 2003). We found the correlation between the circulating TF and intramural MMP-8 expression. This may have further patophysiological implications in a view of recent findings suggesting that statins may inhibit plaque RAGE expression by inhibiting the biosynthesis of PGE2-dependent MMPs (Cuccurullo et al 2006). Further studies should address to answer if by control of plaque stability we also reduce prothrombotic state in diabetic patients.
Prothrombotic markers were independent from markers of inflammation like CRP, fibrinogen or leucocyte count. Our results, demonstrate a link between the processes of plaque remodelling and increased risk of thrombosis. Our study suggests, that diabetics may be a choice group for identification of key molecules participating in atherosclerosis and related risks of vascular complications. Identification of circulating risk molecules may directly reflect local advances in atherosclerosis and may help to identify diabetic patients with high risk of vascular complications.
Disclosure
This work was supported by grants: Marie Curie Reintegration Grant ERG-011214 to J.K., SAF2006-07681
References
- Baugh MD, Gavrivolic J, Davies IR, et al. Monocyte matrix metalloproteinase production in type 2 diabetes and controls—a cross sectional study. Cardiovasc Diabetol. 2003;2:3. doi: 10.1186/1475-2840-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladin CF, Alexandrof AV, Murphy J, et al. Carotid stenosis index. A new method of measuring internal carotid artery stenosis. Stroke. 1995;26:230–4. doi: 10.1161/01.str.26.2.230. [DOI] [PubMed] [Google Scholar]
- Buchs AE, Kornberg A, Zahavi M, et al. Increased expression of tissue factor and receptor for advanced Glycation end products in peripheral blood mononuclear cells of patients with type 2 diabetes mellitus with vascular complications. Exp Diabesity Res. 2004;5:163–9. doi: 10.1080/15438600490424325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipollone F, Iezzi A, Fazia M, et al. The receptor RAGE as a progression factor amplifying arachidonate-dependent inflammatory and proteolytic response in human therosclerotic plaques: role of glycemic control. Circulation. 2003;108:1070–7. doi: 10.1161/01.CIR.0000086014.80477.0D. [DOI] [PubMed] [Google Scholar]
- Cipollone F, Prontera C, Pini B, et al. Overexpression of functionally coupled cyclooxygenase-2 and prostaglandin E synthase in symptomatic atherosclerotic plaques as a basis of prostaglandin E(2)-dependent plaque instability. Circulation. 2001;104:921–7. doi: 10.1161/hc3401.093152. [DOI] [PubMed] [Google Scholar]
- Cuccurullo C, Iezzi A, Fazia ML, et al. Suppression of RAGE as a basis of simvastatin-dependent plaque stabilization in type 2 diabetes. Arterioscler Thromb Vasc Biol. 2006;26:2716–23. doi: 10.1161/01.ATV.0000249630.02085.12. [DOI] [PubMed] [Google Scholar]
- Corrado E, Rizzo M, Muratori I, et al. Association of elevated fibrinogen and C-reactive protein levels with carotid lesions in patients with newly diagonosed hypertension or type II diabetes. Arch Med Res. 2006;37:1004–9. doi: 10.1016/j.arcmed.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Corti R, Hutter R, Badimon JJ, et al. Evolving concepts in the triad of atherosclerosis, inflammation and thrombosis. Thromb Thrombolysis. 2004;17:35–44. doi: 10.1023/B:THRO.0000036027.39353.70. [DOI] [PubMed] [Google Scholar]
- Cunningham AC, Hasty KA, Enghild JJ, et al. Structural and functional characterization of tissue factor pathway inhibitor following degradation by matrix metalloproteinase-8. Biochem J. 2002;367:451–8. doi: 10.1042/BJ20020696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Death AK, Fisher EJ, McGrath KC, et al. High glucose alters matrix metalloproteinase expression in two key vascular cells: potential impact on atherosclerosis in diabetes. Atherosclerosis. 2003;168:263–269. doi: 10.1016/s0021-9150(03)00140-0. [DOI] [PubMed] [Google Scholar]
- Derosa G, D’Angelo A, Tinelli C, et al. Evaluation of metalloproteinase 2 and 9 levels and their inhibitors in diabetic and healthy subjects. Diabetes Metab. 2007 doi: 10.1016/j.diabet.2006.11.008. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Diamant M, Nieuwland R, Pablo RF, et al. Elevated numbers of tissue-factor exposing microparticles correlate with components of the metabolic syndrome in uncomplicated type 2 diabetes mellitus. Circulation. 2002;106:2442–7. doi: 10.1161/01.cir.0000036596.59665.c6. [DOI] [PubMed] [Google Scholar]
- Gray-Weale AC, Graham JC, Burnett JR, et al. Carotid artery atheroma: comparison of preoperative B-mode ultrasound appearance with carotid endarterectomy specimen pathology. Cardiovasc Surg. 1988;29:676–81. [PubMed] [Google Scholar]
- Haffner SM. Lipoprotein disorders associated with type 2 diabetes mellitus and insulin resistance. Am J Cardiol. 2002;90:55i–61i. doi: 10.1016/s0002-9149(02)02634-6. [DOI] [PubMed] [Google Scholar]
- Ho FM, Liu SH, Lin WW, et al. Opposite effects of high glucose on MMP-2 and TIMP-2 in human endothelial cells. J Cell Biochem. 2007 doi: 10.1002/jcb.21192. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Jander S, Sitzer M, Wendt A, et al. Expression of tissue factor in high-grade carotid artery stenosis: association with plaque destabilization. Stroke. 2001;32:850–4. doi: 10.1161/01.str.32.4.850. [DOI] [PubMed] [Google Scholar]
- King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025, prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–31. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- Konieczkowski M, Skrinska VA. Increased synthesis of thromboxane A(2) and expression of procoagulant activity by monocytes in response to arachidonic acid in diabetes mellitus. Prostaglandins Leukot Essent Fatty Acids. 2001;65:133–8. doi: 10.1054/plef.2001.0301. [DOI] [PubMed] [Google Scholar]
- Krupinski J, Miguel Turu M, et al. Endogenous expression of C-reactive protein is increased in active (ulcerated non-complicated) human carotid artery plaques. Stroke. 2006;37:1200–4. doi: 10.1161/01.STR.0000217386.37107.be. [DOI] [PubMed] [Google Scholar]
- Krupinski J, Catena E, Miguel M, et al. D-dimer local expression is increased in symptomatic patients undergoing carotid endarterectomy. Int J Cardiol. 2007;116:174–9. doi: 10.1016/j.ijcard.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Krupinski J, Turu M, Slevin M, et al. Carotid plaque, pathogenesis of stroke, and CRP: Implications for treatment of ischemic stroke, hemorrhagic transformation. Curr Treat Opt Cardiovasc Med. 2007;9:229–35. doi: 10.1007/s11936-007-0017-2. [DOI] [PubMed] [Google Scholar]
- Leurs PB, Stolk RP, Hamulyak K, et al. Tissue factor pathway inhibitor and other endothelium-dependent hemostatic factors in elderly individuals with normal or impaired glucose tolerance and type 2 diabetes. Diabetes Care. 2002;25:1340–5. doi: 10.2337/diacare.25.8.1340. [DOI] [PubMed] [Google Scholar]
- Lipinski B. Pathophysiology of oxidative stress in diabetes mellitus. Diabetes Complicat. 2001;15:203–10. doi: 10.1016/s1056-8727(01)00143-x. [DOI] [PubMed] [Google Scholar]
- Meerarani P, Moreno PR, Cimmino G, et al. Atherothrombosis: role of tissue factor; link between diabetes, obesity and inflammation. Indian J Exp Biol. 2007;45:103–10. [PubMed] [Google Scholar]
- Morel O, Toti F, Hugel B, et al. Procoagulant microparticles: disrupting the vascular homeostasis equation? Arterioscler Thromb Vasc Biol. 2006;26:2594–604. doi: 10.1161/01.ATV.0000246775.14471.26. [DOI] [PubMed] [Google Scholar]
- [NASCET] North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high grade carotid stenosis. N Eng J Med. 1991;325:445–53. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- Paoletti R, Bolego C, Poli A, et al. Metabolic syndrome, inflammation and atherosclerosis. Vasc Health Risk Manag. 2006;2:145–52. doi: 10.2147/vhrm.2006.2.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio F, Martinez-Yelamos S, Cadona P, et al. Carotid endarterectomy: Is it still the gold standard? Cerebrovasc Dis. 2005;20:119–22. doi: 10.1159/000089364. [DOI] [PubMed] [Google Scholar]
- Sambola A, Osende J, Hathcock J, et al. Role of risk factors in the modulation of tissue factor activity and blood thrombogenicity. Circulation. 2003;107:973–7. doi: 10.1161/01.cir.0000050621.67499.7d. [DOI] [PubMed] [Google Scholar]
- Shah PK. Thrombogenic risk factors for atherothrombosis. Rev Cardiovasc Med. 2006;7:10–16. [PubMed] [Google Scholar]
- Slevin M, Gaffney J, Kumar S. Angiogenic oligosaccharides of hyaluronan induce multiple signaling pathways impacting vascular endothelial cell mitogenesis and wound healing. J Biol Chem. 2002;277:41046–59. doi: 10.1074/jbc.M109443200. [DOI] [PubMed] [Google Scholar]
- Sommeijer DW, Hansen HR, Van Oerle R, et al. Soluble tissue factor is a candidate marker for progression of microvascular disease in patients with type 2 diabetes. J Thromb Haemost. 2006;4:574–80. doi: 10.1111/j.1538-7836.2005.01763.x. [DOI] [PubMed] [Google Scholar]
- Stary HC, Bleakley-Chandler A, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. Circulation. 1995;92:1355–74. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- Steffel J, Luscher TF, Tanner FC. Tissue factor in cardiovascular diseases: molecular mechanisms and clinical applications. Circulation. 2006;113:722–31. doi: 10.1161/CIRCULATIONAHA.105.567297. [DOI] [PubMed] [Google Scholar]
- Tkac I, Salagovic J, Kozarova M, et al. Angiotensin-converting enzyme genotype, albuminuria and plasma fibrinogen in type 2 diabetes mellitus. Wien Klin Wochenschr. 2003;115:835–9. doi: 10.1007/BF03041044. [DOI] [PubMed] [Google Scholar]
- Turu MM, Krupinski J, Montaner J, et al. Matrix metalloproteinases in plaque and plasma from patients with advanced carotid atherosclerosis. Atherosclerosis. 2006;187:161–9. doi: 10.1016/j.atherosclerosis.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Vambergue A, Rugeri L, Gaveriaux V, et al. Factor VII, tissue factor pathway inhibitor, and monocyte tissue factor in diabetes mellitus: Influence of type of diabetes, obesity index, and age. Thromb Res. 2001;101:367–75. doi: 10.1016/s0049-3848(00)00424-2. [DOI] [PubMed] [Google Scholar]
- Vaidyula VR, Rao AK, Mozzoli M, et al. Effects of hyperglycaemia and hyperinsulinaemia on circulating tissue factor procoagulant activity and platelet CD40 ligand. Diabetes. 2006;55:202–8. [PubMed] [Google Scholar]
- Viles-Gonzalez JF, Fuster V, Badimon JJ. Links between inflammation and thrombogenicity in atherosclerosis. Curr Mol Med. 2006;6:489–99. doi: 10.2174/156652406778018707. [DOI] [PubMed] [Google Scholar]
- Wall SJ, Sampson MJ, Levell N, et al. Elevated matrix metalloproteinase-2 and-3 production from human diabetic dermal fibroblasts. Br J Dermatol. 2003;149:13–16. doi: 10.1046/j.1365-2133.2003.05262.x. [DOI] [PubMed] [Google Scholar]


