Abstract
AIM
To assess the two-way pharmacokinetic interaction between voriconazole and Ortho-Novum® 1/35, an oral contraceptive containing norethindrone 1 mg and ethinyl oestradiol 35 μg.
METHODS
In this open-label, three-period, fixed-sequence study, 16 healthy females received voriconazole (400 mg q12 h, day 1; 200 mg q12 h, days 2–4) (period 1), oral contraceptive (q24 h, days 12–32) (period 2), and combination voriconazole (400 mg q12 h, day 57; 200 mg q12 h, days 58–60) and oral contraceptive (q24 h, days 40–60) (period 3).
RESULTS
Voriconazole geometric mean AUCτ and Cmax increased 46% (12 682–18 495 ng h ml−1; 90% confidence interval [CI] 32, 61) and 14% (2485–2840 ng ml−1; 90% CI 3, 27), respectively, when co-administered with oral contraceptive vs. voriconazole alone. Ethinyl oestradiol geometric mean AUCτ and Cmax increased 61% (1031–1657 ng h ml−1; 90% CI 50, 72) and 36% (119–161 ng ml−1; 90% CI 28, 45), respectively, and norethindrone geometric mean AUCτ and Cmax increased 53% (116–177 ng h ml−1; 90% CI 44, 64) and 15% (18–20 ng ml−1; 90% CI 3, 28), respectively, during voriconazole co-administration vs. oral contraceptive alone. Neither ethinyl oestradiol nor norethindrone levels were reduced in subjects following voriconazole co-administration. Adverse events (AEs) were generally mild, occurring less in subjects receiving voriconazole alone (36 events) vs. oral contraceptive alone (88 events) or combination treatment (68 events); four subjects experienced a severe AE.
CONCLUSIONS
Co-administration of voriconazole and oral contraceptive increased systemic exposures of all analytes relative to respective monotherapy. Although generally safe and well tolerated, it is recommended that patients receiving co-administered voriconazole and oral contraceptive be monitored for development of AEs commonly associated with these medications.
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Voriconazole, a broad-spectrum antifungal drug, is a substrate and inhibitor of CYP2C19 and CYP3A4 isozymes.
Ethinyl oestradiol and norethindrone, components of the combination oral contraceptive drug Ortho-Novum® 1/35, also are substrates of cytochrome P450 CYP2C19 and CYP3A4 isozymes.
Because co-administration of voriconazole and Ortho-Novum® 1/35 could potentially result in pharmacokinetic interactions that increase systemic exposure of one or both drugs to unsafe levels, clinical studies are needed to define better the two-way pharmacokinetic interaction between these drugs.
WHAT THIS STUDY ADDS
Although co-administered voriconazole and oral contraceptive did result in increased systemic exposures of all three drugs relative to respective monotherapy, co-administered treatment was generally safe and well tolerated.
It is recommended, however, that patients receiving co-administered voriconazole and oral contraceptives be monitored for the development of adverse events commonly associated with these medications.
Keywords: combined, contraceptives, oral, pharmacokinetics, voriconazole
Introduction
Voriconazole (Vfend®; Pfizer Inc., New York, NY, USA) is a broad-spectrum triazole antifungal agent approved for the primary treatment of acute invasive aspergillosis, and as salvage therapy for serious fungal infections caused by Scedosporium apiospermum and Fusarium species [1]. It is also used for the treatment of oesophageal candidiasis and candidaemia [1]. Following oral administration, voriconazole is rapidly absorbed, with a time to maximum plasma concentration (Tmax) achieved 1–2 h after dosing. Voriconazole has an estimated absolute oral bioavailability of 96%. Voriconazole is extensively metabolized, and <1% of the administered dose is excreted unchanged in urine and faeces. In adults, voriconazole has nonlinear pharmacokinetics with regard to dose, presumably due to saturation of its metabolism; there is therefore a greater than proportional increase in exposure when the dose of voriconazole is increased. Voriconazole undergoes N-oxidation by the cytochrome P450 isozymes CYP2C19, CYP2C9 and CYP3A4, with results of in vitro metabolism studies indicating that the affinity of voriconazole is highest for CYP2C19, lower for CYP2C9, and appreciably lower for CYP3A4 [1, 2]. Voriconazole N-oxide is the major circulating metabolite and does not possess antifungal activity.
Ortho-Novum® 1/35 (Ortho-McNeil Pharmaceutical, Inc., Raritan, NJ, USA) is a low-dose combination oral contraceptive containing 1 mg norethindrone (NE) and 35 μg ethinyl oestradiol (EE) [3]. EE is well absorbed, but is subject to an extensive first-pass effect on oral administration and has an average oral availability of approximately 50%. In addition, wide variation occurs in its bioavailability, ranging from 20 to 65% [4]. The site of this presystemic elimination is the gut wall. Sulphate conjugation accounts for approximately 60% of the first-pass metabolism [4]. In addition, EE undergoes glucuronide conjugation and CYP3A-mediated hydroxylation. Once absorbed, the major route of elimination is by hydroxylation. Enterohepatic circulation of EE can occur and approximately 30% of the dose may be recovered in the faeces [5]. The clearance of EE is about 5.4 ml kg min−1; its volume of distribution is approximately 3.5 l kg−1 and its elimination half-life is approximately 10 ± 6 h [4]. NE is rapidly absorbed and is partially metabolized by CYP3A4, primarily through reduction followed by sulphation [6].
Loss of oral contraceptive efficacy due to drug interactions is a significant clinical concern, which is believed to be primarily due to induction of cytochrome P450 enzyme metabolism [7]. In contrast, drugs that inhibit cytochrome P450 enzymes have the potential to increase the concentrations of combination oral contraceptives. While the inductive effect is not of concern with voriconazole, voriconazole is expected to compete with EE and NE for hepatic metabolism via CYP3A4, and perhaps also CYP2C19. Furthermore, the inhibitory activity of voriconazole on the P450 isozymes is also a concern for drug interaction. Since voriconazole is likely to be used for antifungal therapy in women taking oral contraceptives, the current study investigated the two-way pharmacokinetic interaction between these two agents.
Methods
Subjects
Sixteen healthy female subjects between the ages of 18 and 40 years were enrolled into the study. To be eligible, subjects had to be in good health, as determined by a detailed medical history, full physical examination, 12-lead electrocardiogram and clinical laboratory tests. Subjects were required to have a negative serum pregnancy test at screening and prior to commencing all study periods. Subjects who were taking, or had taken any prescription or over-the-counter drug, vitamins or dietary supplement within 7 days or 5 half-lives (whichever was longer) before the first dose of study medication were excluded. The results of a urine drug screen had to be negative for recreational drugs and drugs of abuse (amphetamine, barbiturate, benzodiazepine, cocaine, opiate, marijuana, alcohol). Subjects were not permitted to consume grapefruit juice within 4 days before dosing or use herbal supplements including St John's Wort within 30 days before dosing.
The study was conducted from May 2004 to December 2004, and subjects' written informed consent obtained before participation, in conformity with the ethical principles originating in or derived from the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practices Guidelines. The study protocol was approved by the Institutional Review Board at Bronson Methodist Hospital in Kalamazoo, MI.
Study design
This was an open-label, three-period, fixed-sequence study. Subjects received study treatments relative to their menstrual cycle day and study day as presented in Table 1. Subjects were administered voriconazole in period 1 (400 mg q12 h on day 1; 200 mg q12 h on days 2–4), oral contraceptive (Ortho-Novum® 1/35) in period 2 (q24 h on days 12–32), and combination voriconazole (400 mg q12 h on day 57; 200 mg q12 h on days 58–60) and oral contraceptive (q24 h on days 40–60) in period 3. The morning dose of voriconazole was administered at approximately 07.00 h after an overnight fast, and the evening dose was administered at approximately 19.00 h. When Ortho-Novum 1/35 was given alone, it was administered at the same time each day, preferably between 07.00 and 08.00 h. While confined to the clinical research unit on day 32, Ortho-Novum was to be administered at approximately 07.00 h, prior to breakfast; breakfast was not to be administered until at least 1 h after dosing. When Ortho-Novum 1/35 was administered concomitantly with voriconazole, it was administered with the morning voriconazole dose at approximately 07.00 h.
Table 1.
Study design schematic
| Study period | |||||
|---|---|---|---|---|---|
| Period 1 | Wash-out | Period 2 | Wash-out | Period 3 | |
| Study day* | 1–4 | 5–11 | 12–32 | 33–39 | 40–60 |
| Menstrual cycle day | 18–21 | 22–28 | 1–21 | 22–28 | 1–21 |
| Voriconazole dosing | Day 1: 400 mg q12 h | None | None | None | Day 57: 400 mg q12 h |
| Days 2–3: 200 mg q12 h | Days 58–60: 200 mg q12 h | ||||
| Day 4: 200 mg qam | |||||
| Ortho-Novum® 1/35 dosing | None | None | Days 12–32: q24 h | None | Days 40–60: q24 h |
Serial pharmacokinetic sampling was conducted on study days 4, 32 and 60.
Subjects were confined to the clinical research unit on the following days during each period for pharmacokinetic assessments: period 1, days 0–5; period 2, days 31–33; and period 3, days 56–61. On other days, subjects visited the clinic on an outpatient basis for study evaluations. There was a wash-out interval of ≥7 days between study periods to allow oral contraceptive dosing to begin on the first day of each subject's menstrual cycle (or as close to the first day as possible) in periods 2 and 3. The overall duration of the study was approximately 74 days, including a 10–14-day follow-up period.
Pharmacokinetic assessments
Steady-state pharmacokinetics were determined on day 4 (voriconazole and its N-oxide metabolite), day 32 (EE and NE) and day 60 (all four analytes). Serial blood samples (3–5 ml each) were collected in tubes containing sodium heparin at the following time points: predose (immediately before the morning dose) and at 0.5, 1, 2, 3, 4, 6, 8, 10, 12, 16 h (days 32 and 60) and 24 h (days 32 and 60) postdose. In addition, morning predose trough samples were collected for 2 days prior to the day of serial pharmacokinetic sampling. Blood samples were centrifuged at 1700 g for 10 min at 4°C; the plasma was stored in appropriately labelled screw-capped polypropylene tubes at −20°C within 1 h of collection.
Plasma samples were assayed for voriconazole and the N-oxide metabolite using a validated liquid chromatography coupled to tandem-mass spectrometry (LC/MS/MS) method (PPD, Richmond, VA, USA). Plasma aliquots (100 μl) were acidified with 500 μl of 20 mM ammonium formate solution (pH 3) and fortified with 20 μl of an internal standard solution (UK-103 446-01 at 2500 ng ml−1 concentration). Analytes were isolated through solid phase extraction (SPE) using a Varian Bond Elut Matrix C18 96-well plate automated with a Tomtec Quadra 96 Model 320. The SPE eluate was diluted fourfold to a final extract formation of 1 : 1 methanol/20 mM ammonium acetate, pH 4.0.
A 20-μl aliquot of the final extract was injected into an LC/MS/MS system (HP 1100 Series pump interfaced to a PE-Sciex API 3000 triple quadruple mass spectrometer with an electrospray source) set up with a Luna C18 column (2 × 50 mm, 5 μm; Phenomenex, Torrance, CA, USA). The mobile phases used were methanol/20 mM ammonium acetate (pH 4.0), 70 : 30, v/v (mobile phase A) and methanol (mobile phase B) at a flow rate of 0.200 ml min−1. Separation of the analytes was achieved by running 100% of mobile phase A isocratically for 3.2 min, then from 90% to 10% of mobile phase A over the next 0.3 min, and then running 10% of mobile phase A isocratically for 1.0 min.
The mass spectrometer was operated in the positive ionization mode and monitored the transition ions m/z 350.3→281.2 (voriconazole), m/z 366.2→223.9 (N-oxide metabolite) and m/z 384.3→314.8 (internal standard). Quantification was achieved using peak area ratios of nine calibration standards over a range of 10–5000 ng ml−1 for voriconazole, and eight calibration standards over a range of 20–5000 ng ml−1 for the N-oxide metabolite. Calibration curves were constructed using the best-fit line determined by linear regression of the calibration data, utilizing a weighting factor of 1/concentration. The lower limits of quantification for the assays during sample analysis were 10 ng ml−1 and 20 ng ml−1 for voriconazole and the N-oxide metabolite, respectively. Plasma samples were analysed in seven acceptable analytical runs for both voriconazole and for the N-oxide metabolite. For voriconazole, the percent relative error (%RE) of the quality controls (QCs) used during sample analysis ranged from −1.77 to −1.41, with a percent relative standard deviation (%RSD) of ≤4.09. For the N-oxide metabolite, %RE of the QCs ranged from −1.31 to 0.321, with a %RSD of ≤8.11. The presence of 50 ng ml−1 of NE and 1 ng ml−1 of EE in plasma QCs was not found to interfere with the voriconazole/N-oxide metabolite analysis.
Plasma samples were assayed for NE and EE using a validated liquid chromatography coupled to LC/MS/MS method (PPD). Individual 0.5-ml aliquots of plasma containing analyte and internal standard were extracted using a liquid/liquid extraction procedure. Extracted samples were analysed by LC/MS/MS. Quantification was achieved using peak area ratios of nine calibration standards over a range of 50–25 000 pg ml−1 for NE (lower limit of quantification was 50 pg ml−1) and eight calibration standards over a range of 2–500 pg ml−1 for EE (lower limit of quantification was 2 pg ml−1). Calibration curves were constructed using the best-fit line determined by linear regression of the calibration data utilizing a weighting factor of concentration−1. Plasma samples were analysed in six acceptable analytical runs for NE and in five acceptable runs for EE. For NE, the %RE of the QCs used during sample analysis ranged from −7.69 to 8.04 with a %RSD of ≤3.99; for EE, the %RE of the QCs ranged from −1.66 to 15.7 with a %RSD of ≤8.81. The presence of 10 μg ml−1 of voriconazole and the N-oxide metabolite in plasma QCs did not interfere with the NE/EE analysis.
Pharmacokinetic analyses were carried out with WinNonlin (V.3.2; Pharsight®, Mountain View, CA, USA) using standard noncompartmental methods. Maximum observed plasma concentrations (Cmax) and time of first occurrence of Cmax (Tmax) were estimated directly from experimental data. Area under the curve over the dosing interval (AUCτ; where τ was 12 h for voriconazole and 24 h for oral contraceptive analytes) was estimated using the linear log−1 trapezoidal approximation.
Safety assessments
Safety was evaluated throughout the study based on adverse event (AE) monitoring, clinical laboratory values, vital sign measurements, electrocardiogram results and physical examination findings.
Statistical analysis
Sample size calculations for this study were based on the intrasubject percentage coefficient of variation (%CV) estimate for NE AUC (0.21) determined in a previous Pfizer study (Study AA981-066, data on file), which assessed the effect of atorvastatin on the pharmacokinetics of Ortho-Novum® 1/35. In that study, the observed %CV estimates for AUC were lower with voriconazole (0.11) and EE (0.12–0.17) compared with NE. Based on these results, a sample size of 12 subjects was deemed sufficient in the present study to provide an 80% probability of calculating the 90% confidence intervals (CI) for the relative bioavailability estimates of AUCτ for NE in the presence and absence of voriconazole, and 16 subjects were enrolled to ensure that 12 subjects completed the study.
An analysis of variance (anova) model was used to compare the natural log transformed AUCτ and Cmax for the test treatment to the reference treatment between treatment periods for each analyte separately. This model was implemented using the SAS procedure MIXED, with treatment as fixed effect and subject as random effect utilizing REML method, compound symmetry and Satterthwaite degrees of freedom algorithm. Adjusted mean treatment differences in Cmax and AUCτ and corresponding CIs were estimated from the model. These differences and 90% CIs were exponentiated to derive estimates of the ratios of geometric means between treatments and the 90% CI for these ratios. No adjustment was made for multiple comparisons in any analysis.
Results
Study population
Fifteen of the 16 enrolled subjects completed the study as planned. One subject discontinued the study during period 2 due to personal reasons; this subject was excluded from pharmacokinetic analyses for all study periods and from period 3 safety analyses. Mean age was 25.9 years (range 19–36 years) and mean body mass index (BMI) was 25.9 kg m−2 (range 19.3–31.3 kg m−2). Thirteen subjects (81.3%) were White, one was Black (6.3%), one was Hispanic and one was other/multiracial.
Effect of combination oral contraceptive on voriconazole pharmacokinetics
Assessment of predose trough concentrations indicated that steady-state levels of voriconazole were reached before assessment of pharmacokinetics. The mean steady-state voriconazole and N-oxide concentration–time profiles for voriconazole administered alone (day 4) and with the combination oral contraceptive (day 60) are shown in Figure 1. Administration of the combination oral contraceptive increased the mean steady-state concentration of voriconazole at day 60 relative to voriconazole given alone at day 4, whereas the mean steady-state concentration of the N-oxide metabolite was decreased. The increases in geometric mean voriconazole AUCτ and Cmax following co-administration with the combination oral contraceptive were approximately 46% (12 682–18 495 ng h ml−1) and 14% (2485–2840 ng ml−1), respectively. Arithmetic mean (SD) data and statistical analyses based on geometric mean data are shown in Table 2. The 90% CIs for each pharmacokinetic parameter comparison were not entirely contained within the (80%, 125%) equivalence limits (Table 2). N-oxide metabolite geometric mean steady-state AUCτ and Cmax values decreased by approximately 9% (39 015–35 529 ng h ml−1) and 13% (3880–3364 ng ml−1), respectively, following co-administration of voriconazole and the combination oral contraceptive relative to values for administration of voriconazole alone; however, the boundaries of the 90% CI for each comparison were contained entirely within the (80%, 125%) equivalence limit (Table 2). The change in AUC for voriconazole for each subject is shown in Figure 2; of the 14 patients, only one demonstrated a decrease in AUC.
Figure 1.
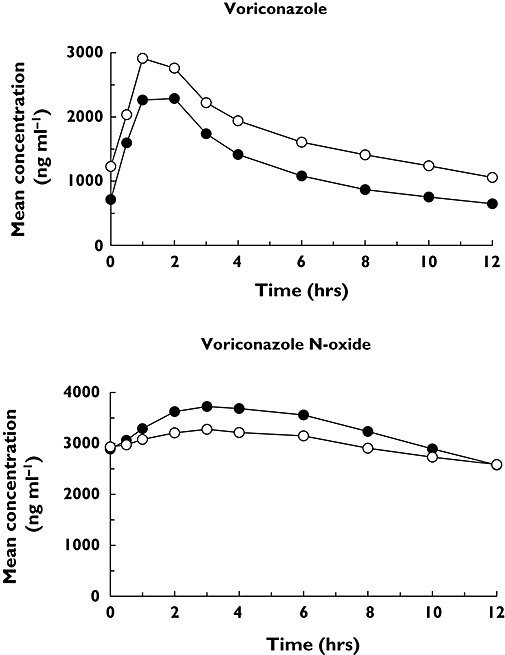
Steady-state mean voriconazole and voriconazole N-oxide plasma concentration vs. time profiles following administration of voriconazole alone (day 4) or in combination with oral contraceptive (day 60). Day 4 (•); Day 60 (○)
Table 2.
Pharmacokinetic parameters of voriconazole and N-oxide metabolite
| Arithmetic mean (SD) | |||
|---|---|---|---|
| Pharmacokinetic parameter | Day 4 | Day 60 | % Ratio (90% CI)* |
| Voriconazole | |||
| AUCτ (ng h ml−1) | 14 893 (8 949) | 20 986 (10 393) | 145.84 (132.13, 160.97) |
| Cmax (ng ml−1) | 2 637 (921) | 3 016 (1 041) | 114.30 (103.05, 126.77) |
| Tmax (h)† | 1 (0.5, 2) | 1 (1, 6) | –‡ |
| N-oxide metabolite | |||
| AUCτ (ng h ml−1) | 39 556 (6 563) | 36 002 (5 779) | 91.07 (88.15, 94.08) |
| Cmax (ng ml−1) | 3 921 (556) | 3 401 (500) | 86.70 (83.57, 89.95) |
| Tmax (h)† | 3 (0.5, 6) | 3 (0, 6) | –‡ |
Represents adjusted geometric mean ratio of day 60/4-values.
Median (range).
Not analysed. SD, Standard deviation; CI, confidence interval.
Figure 2.

Individual subject AUC for voriconazole following administration of voriconazole alone (day 4) or in combination with Ortho-Novum 1/35 (day 60)
Effect of voriconazole on EE and NE pharmacokinetics
Assessment of predose trough concentrations indicated that steady-state levels of EE and NE were reached before assessment of pharmacokinetics. The mean steady-state EE and NE concentration–time profiles when administered alone (day 32) and co-administered with voriconazole (day 60) are shown in Figure 3. Following co-administration with voriconazole, the mean steady-state EE and NE concentrations were higher compared with oral contraceptive administered alone. The increases in geometric mean steady-state EE AUCτ and Cmax following co-administration with voriconazole were approximately 61% (1031–1657 ng h ml−1) and 36% (119–161 ng ml−1), respectively. Arithmetic mean (SD) data and statistical analyses based on geometric mean data are shown in Table 3. The 90% CI for each pharmacokinetic parameter comparison were entirely outside the (80%, 125%) equivalence limits (Table 3). For NE, the geometric mean increases in AUCτ and Cmax following co-administration with voriconazole were approximately 53% (116–177 ng h ml−1) and 15% (18–20 ng ml−1), respectively, and the 90% CI for each pharmacokinetic parameter comparison was not entirely contained within the (80%, 125%) equivalence limits (Table 3). The change in AUC for EE and NE for each subject is shown in Figure 4; no subject had a decrease in the AUC of EE or NE.
Figure 3.
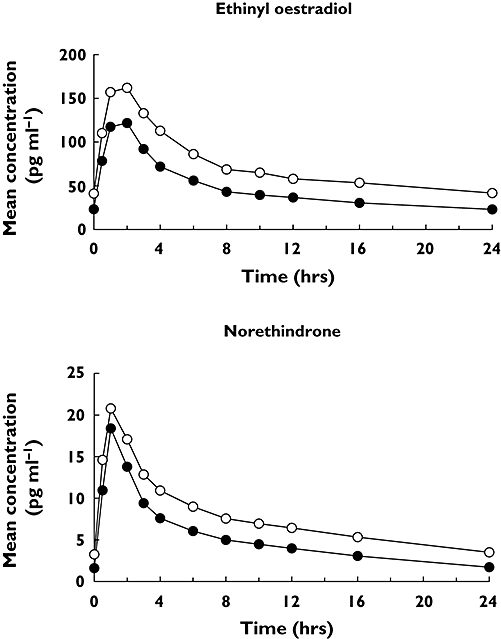
Steady-state mean ethinyl oestradiol and norethindrone plasma concentration vs. time profiles following administration of Ortho-Novum® 1/35 alone (day 32) or in combination with voriconazole (day 60). Day 32 (•); Day 60 (○)
Table 3.
Pharmacokinetic parameters of ethinyl oestradiol and norethindrone
| Arithmetic mean (SD) | |||
|---|---|---|---|
| Pharmacokinetic parameter | Day 32 | Day 60 | % Ratio (90% CI)* |
| Ethinyl oestradiol | |||
| AUCτ (pg h ml−1) | 1116 (510) | 1748 (639) | 160.68 (149.81, 172.35) |
| Cmax (pg ml−1) | 125 (41) | 168 (51) | 135.99 (127.67, 144.85) |
| Tmax (h)† | 2 (0.5, 2) | 2 (1, 2) | –‡ |
| Norethindrone | |||
| AUCτ (ng h ml−1) | 122 (47) | 182 (49) | 153.39 (143.80, 163.62) |
| Cmax (ng ml−1) | 18 (5) | 21 (5) | 114.91 (103.05, 128.14) |
| Tmax (h)† | 1 (1, 2) | 1 (0.5, 1) | –‡ |
Represents adjusted geometric mean ratio of day 60/32 values.
Median (range).
Not analysed. SD, Standard deviation; CI, confidence interval.
Figure 4.
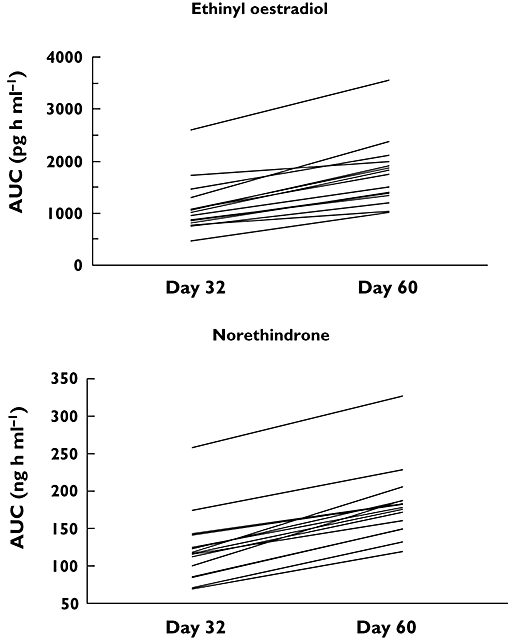
Individual subject AUC for ethinyl oestradiol and norethindrone following administration of Ortho-Novum® 1/35 alone (day 32) or in combination with voriconazole (day 60)
Safety
No subject discontinued the study due to AEs or laboratory abnormalities. There were no serious AEs during the study. The total number of all-causality AEs was lower during treatment with voriconazole alone (36 events) than during treatment with oral contraceptive either alone (88 events) or in combination with voriconazole (68 events). The occurrence of treatment-related AEs showed a similar trend. The most commonly reported all-causality AEs during treatment with voriconazole alone were visual complaints (abnormal vision, chromatopsia and photophobia), nausea and dizziness, whereas headache, metrorrhagia, nausea and abdominal pain were the most commonly reported all-causality AEs during treatment with oral contraceptive alone. The most commonly reported all-causality AEs during combination treatment were headache, abnormal vision, dizziness, nausea and chromatopsia. The most commonly reported treatment-related AEs were similar to those summarized above. Among all-causality AEs and treatment-related AEs, abnormal vision, chromatopsia and photophobia occurred only during treatment with voriconazole (either alone or in combination), and not during treatment with oral contraceptive. Headache and metrorrhagia occurred more frequently during treatment with oral contraceptive (either alone or in combination) than during treatment with voriconazole alone. Metrorrhagia did not occur during treatment with voriconazole alone. Most AEs were mild, with only four subjects experiencing a severe AE. Three subjects experienced severe treatment-related abdominal pain during treatment with oral contraceptive, and one subject experienced severe treatment-related headache during combination treatment.
Discussion
The purpose of this study was to evaluate the two-way pharmacokinetic drug interaction and tolerability of concomitant administration of a combination oral contraceptive (Ortho-Novum® 1/35) and oral voriconazole. The results have shown that exposure to both components of the combination oral contraceptive, EE and NE, were higher when co-administered with voriconazole than when given alone. EE and NE are both partially metabolized by CYP3A4 [4]. Hence, the observed increase in exposure of EE and NE when co-administered with voriconazole is probably due to inhibition of CYP3A4 by voriconazole. Fluconazole also causes an increase in the levels of EE and NE, albeit to a lesser magnitude, presumably because it is a weak inhibitor of CYP3A4 relative to voriconazole [8]. Similarly, inhibitors of CYP3A4 such as erythromycin, clarithromycin, ketoconazole and itraconazole may increase plasma concentrations of oestrogens, which may result in side-effects [9, 10].
Given the increase in plasma concentrations of EE and NE in each subject in the current study, it is unlikely that oral contraceptive efficacy may be compromised because of co-administration with voriconazole. However, considering the 61% and 53% increases in AUCs for EE and NE, respectively, monitoring of AEs related to these treatments is recommended. Frequently occurring AEs associated with administration of oral contraceptives include nausea, vomiting and migraine headache [3]. Serious AEs associated with administration of oral contraceptives include increased risk of venous thromboembolism (VTE), which is linked to the oestrogen component (i.e. EE) of the combination oral contraceptives. A quantitative relationship between increased oestrogen levels and the risk of VTEs has not been elucidated, but it is generally believed that higher levels increase the risk [11]. The risk of VTE also depends on other cofactors including BMI, smoking, age, duration of use and family history [12]. It should be noted that in the current study of healthy volunteers, the 3-week, sustained, increased level of oestrogen resulting from co-administration with voriconazole did not result in any thromboembolic events.
Over the past several decades, the dose of oestrogen in combination oral contraceptives has been progressively reduced from 80 μg to 50 or 35 μg; EE doses <35 μg also are used clinically. We therefore compared the systemic exposure of EE in the current study (mean AUC and Cmax 1750 pg h ml−1 and 168 pg ml−1, respectively) during co-administration of voriconazole with Ortho-Novum® 1/35 with that seen historically with higher doses of a combination oral contraceptive. Ouellet et al. have reported that following administration of a combination oral contraceptive (Demulen®; Pharmacia Corp., Chicago, IL, USA) containing 50 μg of EE in healthy female volunteers, the AUC and Cmax of EE alone were 1670 pg h ml−1 and 104 pg ml−1, respectively [13]. In another study by Fattore et al. administration of a combination oral contraceptive (Evanor-D®; Wyeth, Aprilia, Italy) containing 50 μg of EE in healthy female volunteers resulted in mean AUC and Cmax of EE alone of 1667 pg h ml−1 and 180 pg ml−1, respectively [14]. Although EE levels in the present study were increased because of co-administration of Ortho-Novum® 1/35 and voriconazole, these co-administered levels were still within the range that would be expected with combination oral contraceptives containing 50 μg of EE. It therefore appears that the voriconazole-mediated increase in oestrogen levels, and probably oestrogen-related AEs, would be similar to those seen with a 50-μg dose of EE.
The current study has also demonstrated that co-administration with a combination oral contraceptive resulted in an increase in voriconazole exposure and a reduction in the exposure of the inactive N-oxide metabolite. The observed increase in voriconazole levels when co-administered with combination oral contraceptives is mainly due to inhibition of CYP2C19 by EE, as shown in previous studies [15–18]. It is not anticipated that this increase in voriconazole exposure would have any safety implications. For example, an increase in voriconazole dose from 200 to 300 mg is indicated for patients who do not respond well to therapy [1]. This increase in dose from 200 to 300 mg results in a 2.5-fold increase in voriconazole AUC due to the nonlinear pharmacokinetics, presumably resulting from saturation of its metabolism; the resulting higher systemic exposures are generally well tolerated [1]. Furthermore, in the present study voriconazole was generally well tolerated whether given alone or in combination with oral contraceptive. No new or unexpected AEs were observed. The only AE with a clear increase in reporting frequency during co-administration was abnormal vision, and this is consistent with voriconazole's AE profile [1]. Nonetheless, monitoring of AEs related to voriconazole is recommended when co-administered with combination oral contraceptives.
Multiple cytochrome P450 enzymes (CYP3A4, CYP2C19, CYP2C9) are involved in the metabolism of voriconazole. Of these isozymes, CYP2C19 exhibits significant genetic polymorphism; up to 20% of the Asian population are poor metabolizers, whereas 2–6% of Whites are poor metabolizers [19]. Poor metabolizers and heterozygous extensive metabolizers of CYP2C19 are shown to have higher voriconazole exposures than their homozygous extensive metabolizer counterparts [1]. Mikus et al. have demonstrated that co-administration of voriconazole with the potent CYP3A4 inhibitor, ritonavir, resulted in higher levels of voriconazole in CYP2C19 poor metabolizers compared with extensive metabolizers [20]. Therefore, due to blockade of multiple metabolic pathways, one could expect CYP2C19 poor metabolizers to have higher levels of voriconazole compared with extensive metabolizer when co-administered with oral contraceptives.
Tan et al. have conducted comprehensive analyses of potential relationships between plasma voriconazole levels and visual AEs or liver function test (LFT) abnormalities from 1053 patients across 10 Phase II and III safety and efficacy trials of voriconazole [21]. There was a significant relationship between plasma voriconazole levels and visual AEs, no significant relationship between voriconazole levels and alanine transaminase abnormalities, and a weak but statistically significant association between aspartate transaminase, alkaline phosphatase and bilirubin abnormalities. Furthermore, a receiver–operating characteristic curve analysis indicated that plasma voriconazole levels could not be used to discriminate between true-positive and false-positive LFT abnormalities based on voriconazole concentrations ranging from 0.5 to 10 μg ml−1, suggesting that individual plasma voriconazole concentrations cannot be regarded as a good predictor for LFT abnormalities within that range [21]. Albeit in a smaller cohort of patients, other investigators have explored the potential relationship between voriconazole plasma concentrations and AEs; these reports suggest that most abnormal LFTs occur at voriconazole levels >6 μg ml−1[22, 23]. Although the incidence of AEs may increase with voriconazole levels, there appears to be no clearly discernable threshold for voriconazole levels that would be predictive of LFT abnormalities [24]. Considering this, close monitoring of AEs related to voriconazole, in addition to monitoring of AEs related to oral contraceptives, is recommended.
In summary, this drug–drug interaction study has demonstrated that voriconazole administered concomitantly with a combination oral contraceptive in healthy female subjects was generally safe and well-tolerated. Co-administration resulted in a two-way pharmacokinetic interaction leading to an increase in exposure of voriconazole, EE and NE; this increase may be greater in CYP2C19 poor metabolizers. Therefore, if these drugs are to be co-administered, it is recommended that patients be monitored for AEs and toxicity related to voriconazole and the combination oral contraceptive.
The study was sponsored by Pfizer Global Research and Development. Editorial support was provided by T. Wetter, PhD, at PAREXEL, and was funded by Pfizer Inc. EA, BC, AF, PC, RL and RG are all Pfizer employees. GF is a former Pfizer employee. All authors hold Pfizer Stock.
REFERENCES
- 1.Pfizer Inc. Vfend®. US Prescribing Information. New York, NY: Pfizer Inc.; 2006. [Google Scholar]
- 2.Hyland R, Jones BC, Smith DA. Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab Dispos. 2003;31:540–7. doi: 10.1124/dmd.31.5.540. [DOI] [PubMed] [Google Scholar]
- 3.Ortho-McNeil Pharmaceutical, Inc. Ortho-Novum® 1/35. US Prescribing Information. Raritan, NJ: Ortho-McNeil Pharmaceutical, Inc.; 2006. [Google Scholar]
- 4.Goldzieher JW. Pharmacokinetics and metabolism of ethinyl estrogens. In: Goldzieher JW, Fotherby K, editors. Pharmacology of the Contraceptive Steroids. New York: Raven Press; 1994. pp. 127–51. [Google Scholar]
- 5.Back D, Breckenridge A, MacIver M, Orme M, Purba HS, Rowe PH, Taylor L. The gut wall metabolism of ethinyl estradiol and its contribution to the presystemic metabolism of ethinyl estradiol in humans. Br J Clin Pharmacol. 1982;13:325–30. doi: 10.1111/j.1365-2125.1982.tb01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craig W, Hendrix CW, Jackson KA, Whitmore E, Guidos A, Kretzer R, Liss CM, Shah LP, Khoo KC, McLane J, Trapnell CB. The effect of isotrentoin on the pharmacokinetics and pharmacodynamics of ethinyl estradiol and norethindrone. Clin Pharmacol Ther. 2004;75:464–75. doi: 10.1016/j.clpt.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Doose DR, Wang S-S, Padmanabhan M, Schwabe S, Jacobs D, Bialer M. Effect of topiramate or carbamazepine on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in healthy obese and nonobese female subjects. Epilepsia. 2003;44:540–9. doi: 10.1046/j.1528-1157.2003.55602.x. [DOI] [PubMed] [Google Scholar]
- 8.Hilbert J, Kuye O, Friedman H. Evaluation of interaction between fluconazole and an oral contraceptive in healthy women. Obstet Gynecol. 2001;98:218–23. doi: 10.1016/s0029-7844(01)01443-0. [DOI] [PubMed] [Google Scholar]
- 9.Wyeth Pharmaceuticals Inc. Prempro™. US Prescribing Information. Philadelphia, PA: Wyeth Pharmaceuticals Inc.; 2007. [Google Scholar]
- 10.Ortho-McNeil Pharmaceutical, Inc. Ortho Tri-Cyclen® Lo. US Prescribing Information. Raritan, NJ: Ortho-McNeil Pharmaceutical, Inc.; 2004. [Google Scholar]
- 11.Sherif K. Benefits and risks of oral contraceptives. Am J Obstet Gynecol. 1999;180:343S–348S. doi: 10.1016/s0002-9378(99)70694-0. [DOI] [PubMed] [Google Scholar]
- 12.Castelli WP. Cardiovascular disease: pathogenesis, epidemiology and risk among users of oral contraceptives who smoke. Am J Obstet Gynecol. 1999;180:349S–356S. doi: 10.1016/s0002-9378(99)70695-2. [DOI] [PubMed] [Google Scholar]
- 13.Oeullet D, Hsu A, Qian J, Locke C, Eason C, Cavanaugh J, Leonard J, Granneman R. Effect of ritonavir on the pharmacokinetics of ethinylestradiol in healthy female volunteers. Br J Clin Pharmacol. 1998;46:111–6. doi: 10.1046/j.1365-2125.1998.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fattore C, Cipolla G, Gatti G, Limido GL, Sturm Y, Bernasconi C, Perucca E. Induction of ethinylestradiol and levonorgestrel metabolism by oxcarbazepine in healthy women. Epilepsia. 1999;40:783–7. doi: 10.1111/j.1528-1157.1999.tb00779.x. [DOI] [PubMed] [Google Scholar]
- 15.Laine K, Yasar U, Widen J, Tybring G. A screening study on the liability of eight different female sex steroids to inhibit CYP2C9, 2C19 and 3A4 activities in human liver microsomes. Pharmacol Toxicol. 2003;93:77–81. [PubMed] [Google Scholar]
- 16.Tamminga WJ, Wemer J, Oosterhuis B, Weiling J, Wilffert B, de Leij LF, de Zeeuw RA, Jonkman JH. CYP2D6 and CYP2C19 activity in a large population of Dutch healthy volunteers: indications for oral contraceptive-related gender differences. Eur J Clin Pharmacol. 1999;55:177–84. doi: 10.1007/s002280050615. [DOI] [PubMed] [Google Scholar]
- 17.Laine K, Tybring G, Bertilsson L. No sex-related differences but significant inhibition by oral contraceptives of CYP2C19 activity as measured by the probe drugs mephenytoin and omeprazole in healthy Swedish white subjects. Clin Pharmacol Ther. 2000;68:151–9. doi: 10.1067/mcp.2000.108949. [DOI] [PubMed] [Google Scholar]
- 18.Hagg S, Spigset O, Dahlqvist R. Influence of gender and oral contraceptives on CYP2D6 and CYP2C19 activity in healthy volunteers. Br J Clin Pharmacol. 2001;51:169–73. doi: 10.1111/j.1365-2125.2001.01328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flockhart DA. Drug interactions and the cytochrome P450 system. The role of cytochrome P450 2C19. Clin Pharmacokinet. 1995;29(Suppl. 1):45–52. doi: 10.2165/00003088-199500291-00008. [DOI] [PubMed] [Google Scholar]
- 20.Mikus G, Schöwel V, Drzewinska M, Rengelshausen J, Ding R, Riedel KD, Burhenne J, Weiss J, Thomsen T, Haefeli WE. Potent cytochrome P450 2C19 genotype-related interaction between voriconazole and the cytochrome P450 3A4 inhibitor ritonavir. Clin Pharmacol Ther. 2006;80:126–35. doi: 10.1016/j.clpt.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Tan K, Brayshaw N, Tomaszewski K, Troke P, Wood N. Investigation of the potential relationships between plasma voriconazole concentrations and visual adverse events or liver function test abnormalities. J Clin Pharmacol. 2006;46:235–43. doi: 10.1177/0091270005283837. [DOI] [PubMed] [Google Scholar]
- 22.Denning DW, Ribaud P, Milpied N, Caillot D, Herbrecht R, Thiel E, Haas A, Ruhnke M, Lode HN. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin Infect Dis. 2002;34:563–71. doi: 10.1086/324620. [DOI] [PubMed] [Google Scholar]
- 23.Potoski BA, Brown J. The safety of voriconazole. Clin Infect Dis. 2002;35:1273–5. doi: 10.1086/343746. [DOI] [PubMed] [Google Scholar]
- 24.Lutsar I, Hodges MR, Tomaszewski K, Troke PF, Wood ND. Safety of voriconazole and dose individualization. Clin Infect Dis. 2003;36:1087–8. doi: 10.1086/374248. [DOI] [PubMed] [Google Scholar]


