Abstract
Aims
The aims of this study were to visualize in vivo binding of donepezil to acetylcholinesterase (AChE) in the brain and to establish a method for measuring the amount of binding of orally administered donepezil.
Methods
[5-11C-methoxy]-donepezil ([11C]-donepezil) was radiolabelled as a positron emission tomography (PET) tracer. The biodistribution of [11C]-donepezil was measured by PET in 10 AD patients and six elderly normal subjects. Two AD patients underwent additional PET measurements after oral administration of donepezil for 6 months.
Results
[11C]-donepezil-PET images demonstrated high densities of tracer distribution in AChE-rich brain regions such as the striatum, thalamus, and cerebellum. Compared with elderly normal subjects, patients with mild AD exhibited about 18–20% reduction of donepezil binding in the neocortex and hippocampus, while patients with moderate AD exhibited about 24–30% reduction of donepezil binding throughout the brain. Orally administered donepezil (5 mg day−1) induced 61.6–63.3% reduction of donepezil binding in AD brains. The distribution volume of [11C]-donepezil in the hippocampus was significantly correlated with MMSE scores in AD patients.
Conclusions
[11C]-donepezil-PET enables quantitative measurement of donepezil binding in the brain. AD patients exhibited reduction of donepezil binding in the brain, even in the early stage of disease. Longitudinal evaluation by this technique enables determination of AChE binding occupancy of orally administered donepezil.
What is already known about this subject
Deficit in central cholinergic neurotransmission is a consistent change associated with Alzheimer's disease (AD).
Donepezil hydrochloride exhibits selective inhibition of acetylcholinesterase (AChE) and is widely used for the treatment of AD.
The biodistribution of donepezil in the brain after administration is not precisely understood in vivo.
There is no method to measure the amount of binding of orally administered donepezil to AChE.
What this study adds
This study clearly visualizes the distribution of donepezil in human brain using [11C]-donepezil and positron emission tomography.
This study demonstrates prominent reduction of the donepezil binding site in the AD brain.
This study provides methodology to measure the AChE binding occupancy of orally administered donepezil and provides a new surrogate marker for evaluation and prediction of response to donepezil treatment.
Keywords: acetylcholinesterase, Alzheimer's disease, donepezil, positron emission tomography (PET)
Introduction
Cholinergic deficit is consistently found in the brain of patients with Alzheimer's disease (AD). Reduction in the activity of choline acetyltransferase (ChAT) and acetylcholinesterase (AChE) is evident in AD brains and correlates with cognitive decline [1, 2]. For this reason, cholinergic enhancement is a major approach to the treatment of AD. Currently, several AChE inhibitors (AChEIs) are widely prescribed to improve cognitive function in patients with AD [3]. However, not all patients respond to these treatments. It is thus important to identify factors that determine individual responses to treatment with AChEIs.
Functional imaging of cholinergic function is a useful strategy for determination of the treatment protocol of demented patients. Use of AChEIs themselves as radiotracers enables direct investigation of the pharmacokinetics of AChEIs using positron emission tomography (PET). Donepezil hydrochloride is currently the AChEI most widely used for the treatment of AD. It exhibits selective binding of AChE compared with butyrylcholinesterase (BuChE) [4]. Radiolabelled donepezil can thus be used as a tracer to measure brain concentrations of AChE. If the distribution of donepezil in the brain can be measured quantitatively by PET, this will be useful for pharmacological evaluation of AChEIs and for prediction of efficacy of treatment with donepezil. In this study, we performed PET examinations using [5-11C-methoxy]-donepezil ([11C]-donepezil) and determined the in vivo binding characteristics of donepezil in AD patients.
Methods
Subjects and patients
Six elderly normal subjects and 10 patients with probable AD were studied to examine the distribution of [11C]-donepezil in the brain. The AD patients were recruited through The Tohoku University Hospital Dementia Patients Registry. The diagnosis of AD was made according to the National Institute of Neurologic Disorders and Stroke/Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) criteria. AD patients were further divided into two groups by severity: a mild AD group (n = 5; MMSE score ≥ 23 points) and a moderate AD group (n = 5; MMSE score < 20 points). The normal control group was comprised of volunteers without impairment of cognitive function who had no cerebrovascular lesions on magnetic resonance (MR) images. After complete description of the study to the patients and subjects, written informed consent was obtained from them. PET study was performed within 3 months after the completion of a medical and neuropsychological examination. Although no significant difference in age was observed between the mild AD group and elderly normal group, the moderate AD group was older than the elderly normal group (Table 1). The MMSE score of the elderly normal subjects (mean ± SD 29.7 ± 0.8) was significantly higher than that of the mild AD (25.0 ± 1.6) and moderate AD (15.4 ± 2.7) subjects.
Table 1.
Subjects and patients demographics
| Gender | Age | MMSE | ||
|---|---|---|---|---|
| Elderly normal | AN1 | M | 64 | 30 |
| AN2 | M | 61 | 30 | |
| AN3 | F | 59 | 30 | |
| AN4 | F | 60 | 30 | |
| AN5 | M | 74 | 28 | |
| AN6 | F | 75 | 30 | |
| Mean | 65.5 | 29.7 | ||
| SD | 7.2 | 0.8 | ||
| Mild AD | AD1 | F | 77 | 24 |
| AD2 | F | 72 | 23 | |
| AD3 | M | 71 | 26 | |
| AD4 | F | 66 | 25 | |
| AD5 | M | 69 | 27 | |
| Mean | 71.0 | 25.0 | ||
| SD | 4.1 | 1.6 | ||
| Moderate AD | AD6 | F | 77 | 14 |
| AD7 | F | 78 | 12 | |
| AD8 | F | 79 | 19 | |
| AD9 | M | 84 | 17 | |
| AD10 | F | 81 | 15 | |
| Mean | 79.8 | 15.4 | ||
| SD | 2.8 | 2.7 |
Radiosynthesis of [5-11C-methoxy]-donepezil
Synthesis of [11C]-donepezil was performed (Figure 1) as described previously [5]. Briefly, 5′-O-desmethylprecursor (M2) was dissolved in methylethylketone and then tetrabutylammonium hydroxide was added. [11C]-Methyl iodide was prepared from [11C]-CO2 and converted to [11C]-methyl triflate ([11C]-MeOTf). [11C]-Donepezil was produced on the loop from [11C]-MeOTf and purified by high performance liquid chromatography (HPLC). The radioactivity obtained was 155.4–814 MBq (4.2–22 mCi), and the radiochemical yield was 25–30% based on [11C]-MeOTf after decay-correction. Specific activity was 111–354 GBq μmol−1 at the end of synthesis (30–40 min from the end of 11C production). Radiochemical purity was greater than 99%.
Figure 1.
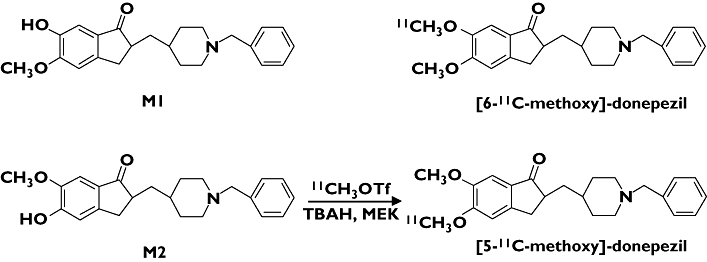
Chemical structures and radiosynthesis of donepezil and its metabolites
PET acquisition protocols
The protocol of the PET study was approved by the Committee on Clinical Investigation at The Tohoku University School of Medicine and the Advisory Committee on Radioactive Substances at Tohoku University. The [11C]-donepezil PET study was performed using a SET-2400 W PET scanner (Shimadzu Inc., Japan) under resting condition with eyes closed. Following a 68Ge/Ga transmission scan of 7 min duration, an emission scan was started soon after intravenous injection of 7.1–9.5 mCi of [11C]-donepezil. Emission data were acquired for 60 min. Standardized uptake value (SUV) images were obtained by normalizing tissue concentration by injected dose and body mass. Arterialized venous blood samples were obtained from a hand vein, heated in a far-infrared mat, and radioactivity was measured in a well-type scintillation counter. Sampled plasma (2 ml) was denaturated with 1 m HClO4 : MeCN (7 : 3) and centrifuged at 3000 × g for 3 min. The supernatant solution was injected into a column (YMC ODS A-324, YMC Co., Ltd, Kyoto, Japan; 10 mm i.d. × 30 cm long) with a solvent system of 0.1 m ammonium formate : acetonitrile (60 : 40) at a flow rate of 5.0 ml min−1. The eluates were collected at time intervals of 0.5 min and were counted for radioactivity with a gamma counter.
Image analysis
Region of interest (ROI) analysis was performed to evaluate the regional distribution of [11C]-donepezil. Circular ROIs (1.0 cm in diameter) were placed on individual axial PET images in the cerebellar hemisphere, striatum, thalamus, lateral frontal cortex (Brodmann's areas (BA) 44, 45, 46, and 47), lateral temporal cortex (BA 20, 21, and 22), parietal cortex (BA 39 and 40), occipital cortex (BA 17), anterior cingulate cortex (BA 24 and 32), posterior cingulate cortex (BA 23 and 31), and medial temporal cortex (BA 27, 28, 34, and 35), referring to the individual MR images. To measure donepezil -binding AChE density in the brain, the distribution volume (DV), the ratio of [11C]-donepezil concentration in tissue to that in plasma at equilibrium, was calculated by Logan's graphical analysis [6], since donepezil reversibly binds to AChE. Using this method, the DV in each ROI was determined from the slopes obtained from the values of each ROI and input function from metabolite-corrected plasma radioactivity. The slopes were determined from the last 15 points of the respective regions. Details of the quantitative analysis will be described elsewhere.
Statistical analysis
Differences in age, MMSE score, and DV among the three groups were evaluated by one-way analysis of variance (anova) followed by Bonferroni's multiple comparison test (GraphPad Prism Software). For each analysis, findings were considered significant at P < 0.05.
Results
Tissue time activity curves (TAC) of [11C]-donepezil in the brain indicated initial rapid uptake of radioactivity followed by gradual clearance from the brain in both elderly normal (Figure 2A) and AD subjects (Figure 2B). Relatively high concentrations of radioactivity of [11C]-donepezil were observed in AChE-rich brain regions such as the striatum, thalamus, and cerebellum, whereas radioactivity uptake in the neocortex including frontal, temporal, and parietal cortices was moderate. Plasma radioactivity of [11C]-donepezil peaked at 30–60 s postinjection, followed by a rapid decline (Figure 2C). Proportions of unchanged [11C]-donepezil in plasma were 91.0 ± 7.0%, 88.1 ± 12.5%, and 82.5 ± 5.1% at 5, 15, and 30 min postinjection, respectively. The metabolite-corrected plasma time–activity curve was used to calculate specific DVs from the region-of-interest-derived regional time–activity curve. [11C]-donepezil exhibited linear regression curves on Logan plot analysis in all brain regions examined (Figure 3). Since the slopes of the regression lines represent the DV of the tracer, these findings indicate a higher DV of donepezil in the striatum than in the frontal cortex. Parametric images of [11C]-donepezil DV clearly revealed higher concentrations of tracer distribution in the striatum and cerebellum than in the neocortex. Patients with mild AD exhibited reduction of DV in the hippocampus and neocortex, compared with elderly normal subjects. The magnitude of DV reduction in the mild AD group was about 20% in the hippocampus and 18% in temporal and parietal cortices. In patients with moderate AD, DV reduction was evident throughout the brain (Figure 4, Figure 5, Table 2). The magnitude of DV reduction was about 30% in the hippocampus and 24% in frontal, temporal, and parietal cortices. Two AD patients (AD3 and AD10) underwent another PET scan after treatment with 5 mg donepezil for 6 months. Orally administered donepezil induced substantial reduction of DV in all regions of brain examined (Figure 6). Mean DV reduction in patient 1 (AD3) was 61.6% (55.5% in the cerebellum, 65.2% in the striatum, 63.6% in the thalamus, 62.5% in the frontal cortex, 61.6% in the temporal cortex, 59.6% in the parietal cortex, 62.6% in the occipital cortex, 60.3% in the anterior cingulate cortex, 59.5% in the posterior cingulate cortex and 65.5% in the medial temporal cortex). Mean DV reduction in patient 2 (AD10) was 63.3% (59.9% in the cerebellum, 72.0% in the striatum, 60.6% in the thalamus, 61.6% in the frontal cortex, 62.9% in the temporal cortex, 62.4% in the parietal cortex, 54.2% in the occipital cortex, 62.4% in the anterior cingulate cortex, 57.9% in the posterior cingulate cortex and 78.8% in medial temporal cortex). Finally, the correlation of donepezil binding with severity of dementia was examined within the AD patient group. As shown in Figure 7, the DV value in the hippocampus was significantly correlated with MMSE scores of AD patients.
Figure 2.
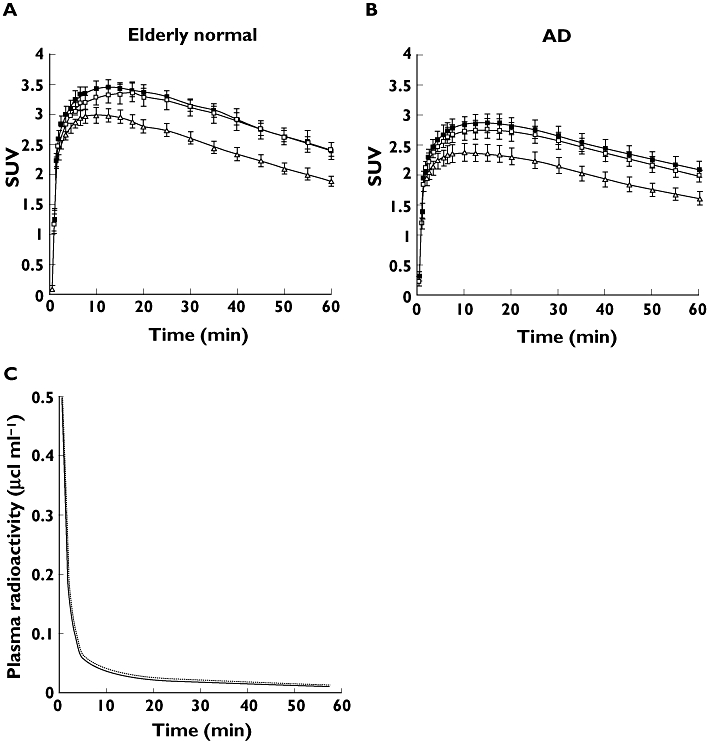
Time activity data for [11C]-donepezil PET in humans. Brain SUV time activity curves for elderly normal subjects (A) and AD patients (B), and plasma time activity curve (C) are shown. The dotted line indicates total time activity curve and the solid line indicates metabolite-corrected time activity curve
Figure 3.

Logan plots for the striatum (□) and frontal cortex (○) for elderly normal subjects (A) and AD patients (B). Cp: plasma concentration of tracer, ROI: region of interest, T: time after injection
Figure 4.

Distribution volume images of [11C]-donepezil in elderly normal subjects (left), patients with mild AD (middle), and patients with moderate AD (right)
Figure 5.

Regional distribution volume data in elderly normal subjects ( ), mild AD (
), mild AD ( ), and moderate AD patients (▪)
), and moderate AD patients (▪)
Table 2.
Regional distribution volume of [11C]-donepezil in elderly normal subjects and AD patients (mean ± SEM)
| Elderly normal | Mild AD | Moderate AD | |
|---|---|---|---|
| Cerebellum | 23.4 ± 3.5 | 19.6 ± 1.4 | 17.6 ± 1.4* |
| Striatum | 24.0 ± 4.1 | 20.0 ± 3.1 | 18.5 ± 1.6* |
| Thalamus | 23.4 ± 4.1 | 19.0 ± 2.1 | 17.1 ± 1.0* |
| Frontal | 18.5 ± 2.5 | 15.8 ± 1.8 | 14.0 ± 1.5* |
| Temporal | 19.7 ± 2.6 | 16.2 ± 1.3* | 15.1 ± 0.9* |
| Parietal | 19.5 ± 2.7 | 16.1 ± 1.7* | 15.0 ± 1.1* |
| Occipital | 19.4 ± 3.2 | 16.1 ± 2.3 | 14.6 ± 1.1* |
| Anterior cingulate | 19.6 ± 2.6 | 16.2 ± 1.7 | 14.7 ± 1.9* |
| Posterior cingulate | 21.1 ± 3.0 | 17.2 ± 2.3 | 16.3 ± 1.7* |
| Hippocampus | 21.4 ± 2.1 | 17.3 ± 2.1* | 14.8 ± 1.2* |
P < 0.05, significantly different from aged normal group.
Figure 6.

Distribution volume images before and after oral administration of donepezil in AD patients
Figure 7.

Correlation between MMSE scores and distribution volume in the hippocampus of AD patients. Pearson r = 0.659 and p = 0.038
Discussion
Currently, in vivo monitoring of brain AChE activity using positron emission tomography (PET) is beneficial in developing strategies for dementia therapy. [11C]-MP4A and [11C]-PMP, which are metabolically trapped acetylcholine analogues, have been successfully applied to the evaluation of AChE activity in the brain [7, 8]. PET studies in AD patients have demonstrated reduction of AChE activity in the early stage of disease, with the degree of reduction correlating with cognitive dysfunction [9, 10]. Another strategy involves use of AChEIs themselves as radiotracers. This method enables direct investigation of the pharmacokinetics of AChEIs. [11C]-physostigmine [11], [11C]-methyl-tacrine [12], and [11C]-CP-126 998 [13] have been designed as radiotracers for clinical PET study. In vivo imaging techniques using such radiotracers can measure the concentrations of tracer-binding AChE. If these radiotracers and therapeutic drugs competitively bind to AChE, the occupancy of binding sites on AChE by therapeutic drugs could be measured by subtraction of post-treatment from pretreatment PET scans.
This PET study demonstrated that intravenously administered [11C]-donepezil rapidly enters the brain and is mainly distributed in the striatum, thalamus, and cerebellum, which are known to contain high densities of AChE compared with the cerebral cortex and hippocampus. This finding is consistent with the findings of our previous study in rats [5]. The regional distribution of [11C]-donepezil was also consistent with regional AChE activity determined in a human postmortem study [14], suggesting selective binding of donepezil to AChE.
Post-treatment evaluation following administration of 5 mg donepezil day−1 revealed a remarkable reduction (61.6–63.3% compared with pretreatment scan) of [11C]-donepezil binding throughout the brain. This indicates that the AChE occupancy by donepezil, when administered in daily doses of 5 mg, was about 35–40% in these two patients. A previous PET study using [11C]-MP4A revealed a mean 39% reduction in AChE activity after oral administration of 3–5 mg donepezil [15]. Intravenous administration of donepezil in monkeys also resulted in a mean 27% reduction of AChE activity at a dose of 100 μg kg−1[16]. These findings together suggest that inhibition of AChE activity matches occupancy of AChEI binding sites. Moreover, orally administered donepezil (5 mg) induced substantial inhibition (43–62%) of the binding of another radiotracer, [11C]-CP-126 998, to AChE [13]. This finding is roughly consistent with our observations. The amount of binding of orally administered donepezil to AChE is considered a key factor in determining therapeutic response. AChE binding occupancy by orally administered donepezil could be modulated by blood–brain barrier permeability, tissue distribution, metabolism, and also by AChE density in the brain. In vivo evaluation of AChE occupancy could thus be a powerful strategy for determining the optimal dose of donepezil. In the future, quantitative evaluation of donepezil binding sites might be used to optimize regimens of treatment with donepezil and to predict the response to treatment. To this purpose, red blood cell AChE inhibition has been explored as a peripheral surrogate marker for the activity of AChEIs [17]. In a future study, we plan to examine the relationship between red blood cell AChE inhibition and [11C]-donepezil binding in the brain.
Patients with moderate AD exhibited significant reduction of [11C]-donepezil DV in all brain regions examined, in comparison with elderly normal subjects. Furthermore, temporo-parietal and hippocampal DVs were significantly reduced even in patients with mild AD, compared with elderly normal group. These reductions suggest early involvement of the cholinergic system in AD, since the AChE in brain is predominantly located in presynaptic cholinergic neurones [18]. A previous [11C]-MP4A PET study demonstrated 21% reduction of hippocampal AChE activity in patients with early onset AD [19]. We observed an approximately 20% reduction in hippocampal DV in the mild AD group and 30% reduction in the moderate AD group. These findings suggest that the concentration of donepezil-binding AChE is matched by regional AChE activity. In a postmortem study, AD patients exhibited reduction of AChE activity, and this reduction was correlated with the severity of dementia [20, 21]. We observed that DV in the hippocampus was correlated with the cognitive status in AD patients, a finding in accord with postmortem data. However, it is important to note that the partial volume effect due to structural atrophy might cause the underestimation of DV in the hippocampus. Analysis after partial volume correction is therefore needed to establish further the relationship between the regional DV of [11C]-donepezil and the severity of dementia in AD.
Compared with previously reported findings of PET imaging with [11C]-MP4A [9] and [11C]-PMP [10], the present [11C]-donepezil-PET study demonstrated a relatively higher cortical retention of radiotracer, suggesting the existence of alternative binding sites for donepezil other than AChE. Donepezil is reported to have high binding affinity for σ1-receptors, which are widely distributed in the brain including the cerebral cortex, hippocampus, and cerebellum [22–24]. A recent human PET study using a σ1-receptor-specific radioligand demonstrated prominent reduction of σ1-receptor density in the cerebral cortex and cerebellum of AD patients [25]. Thus, concomitant binding of donepezil to σ1-receptors might have contributed to the distinctive distribution of [11C]-donepezil we observed in the brain.
Previously, the tracer kinetics of [11C]-donepezil with labelling of the methoxy group at position 6 ([6-11C-methoxy]-donepezil) were examined in mice and rabbits, to test this agent as a candidate for a PET radioligand [26]. However, the regional brain distribution of this radiotracer did not reflect the distribution of AChE in the brain. In contrast, our previous study yielded successful in vivo visualization of AChE by donepezil labelled with 11C at the methoxy group at position 5 ([5-11C-methoxy]-donepezil) [5]. The differences between these findings might be attributable to the affinity of unlabelled metabolites to AChE. Indeed, the unlabelled metabolite of [6-11C-methoxy]-donepezil (M1 in Figure 1) has high binding affinity for AChE (IC50 = 6.4 nm), resulting in competition for binding between 11C-labelled tracer and unlabelled metabolite, while the metabolite of [5-11C-methoxy]-donepezil (M2 in Figure 1) exhibits lower affinity of binding to AChE (IC50 = 1.1 μm) than M1. [5-11C-methoxy]-donepezil is thus suitable for detection of AChE in vivo. In addition, the specific radioactivity of [5-11C-methoxy]-donepezil in this study (111–354 GBq μmol−1) was higher than that of [6-11C-methoxy]-donepezil in a previous study [26]. High specific activity of [11C]-donepezil might therefore be another contributing factor of successful visualization of AChE.
In this study, the distribution of donepezil in human brain was successfully visualized using [11C]-donepezil and PET. Graphical analysis by Logan plots can be used to obtain quantitative estimates of specific donepezil binding. AD patients exhibited significant reduction of donepezil distribution, even in the early stages of disease. This imaging technique will be useful as a new surrogate marker for evaluation of treatment with donepezil.
This work was in part supported by Grants-in-Aid for scientific research (No. 17390156 for K.Yanai and 18019004 for N. Okamura) from the Japan Society of Promotion of Science (JSPS) and the Ministry of Education, Culture, Sports, Science and Technology in Japan, as well as by a grant from the Japan Society of Technology (JST) on research and education in ‘molecular imaging’. The authors thank the volunteers, Dr Syoichi Watanuki for PET operation and Mrs Kazuko Takeda for taking care of the volunteers.
References
- 1.Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet. 1976;2:1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- 2.Coyle JT, Price DL, DeLong MR. Alzheimer's disease: a disorder of cortical cholinergic innervation. Science. 1983;219:1184–90. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- 3.Giacobini E. Cholinesterase inhibitors: from the Calabar bean to Alzheimer therapy. In: Giacobini E, editor. Cholinesterases and Cholinesterase Inhibitors. London: 2000. [Google Scholar]
- 4.Sugimoto H, Ogura H, Arai Y, Iimura Y, Yamanishi Y. Research and development of donepezil hydrochloride, a new type of acetylcholinesterase inhibitor. Jpn J Pharmacol. 2002;89:7–20. doi: 10.1254/jjp.89.7. [DOI] [PubMed] [Google Scholar]
- 5.Funaki Y, Kato M, Iwata R, Sakurai E, Sakurai E, Tashiro M, Ido T, Yanai K. Evaluation of the binding characteristics of [5-(11) C-methoxy]-donepezil in the rat brain for in vivo visualization of acetylcholinesterase. J Pharmacol Sci. 2003;91:105–12. doi: 10.1254/jphs.91.105. [DOI] [PubMed] [Google Scholar]
- 6.Logan J. Graphical analysis of PET data applied to reversible and irreversible tracers. Nucl Med Biol. 2000;27:661–70. doi: 10.1016/s0969-8051(00)00137-2. [DOI] [PubMed] [Google Scholar]
- 7.Kilbourn MR, Snyder SE, Sherman PS, Kuhl DE. In vivo studies of acetylcholinesterase activity using a labeled substrate, N-[11C]methylpiperdin-4-yl propionate ([11C]PMP) Synapse. 1996;22:123–31. doi: 10.1002/(SICI)1098-2396(199602)22:2<123::AID-SYN5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 8.Irie T, Fukushi K, Namba H, Iyo M, Tamagami H, Nagatsuka S, Ikota N. Brain acetylcholinesterase activity: validation of a PET tracer in a rat model of Alzheimer's disease. J Nucl Med. 1996;37:649–55. [PubMed] [Google Scholar]
- 9.Iyo M, Namba H, Fukushi K, Shinotoh H, Nagatsuka S, Suhara T, Sudo Y, Suzuki K, Irie T. Measurement of acetylcholinesterase by positron emission tomography in the brains of healthy controls and patients with Alzheimer's disease. Lancet. 1997;349:1805–9. doi: 10.1016/S0140-6736(96)09124-6. [DOI] [PubMed] [Google Scholar]
- 10.Kuhl DE, Koeppe RA, Minoshima S, Snyder SE, Ficaro EP, Foster NL, Frey KA, Kilbourn MR. In vivo mapping of cerebral acetylcholinesterase activity in aging and Alzheimer's disease. Neurology. 1999;52:691–9. doi: 10.1212/wnl.52.4.691. [DOI] [PubMed] [Google Scholar]
- 11.Blomqvist G, Tavitian B, Pappata S, Crouzel C, Jobert A, Doignon I, Di Giamberardino L. Quantitative measurement of cerebral acetylcholinesterase using [11C]physostigmine and positron emission tomography. J Cereb Blood Flow Metab. 2001;21:114–31. doi: 10.1097/00004647-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Tavitian B, Pappata S, Bonnot-Lours S, Prenant C, Jobert A, Crouzel C, Di Giamberardino L. Positron emission tomography study of [11C]methyl-tetrahydroaminoacridine (methyl-tacrine) in baboon brain. Eur J Pharmacol. 1993;236:229–38. doi: 10.1016/0014-2999(93)90593-7. [DOI] [PubMed] [Google Scholar]
- 13.Bencherif B, Endres CJ, Musachio JL, Villalobos A, Hilton J, Scheffel U, Dannals RF, Williams S, Frost JJ. PET imaging of brain acetylcholinesterase using [11C]CP-126 998, a brain selective enzyme inhibitor. Synapse. 2002;45:1–9. doi: 10.1002/syn.10072. [DOI] [PubMed] [Google Scholar]
- 14.Finkelstein Y, Wolff M, Biegon A. Brain acetylcholinesterase after acute parathion poisoning: a comparative quantitative histochemical analysis post mortem. Ann Neurol. 1988;24:252–7. doi: 10.1002/ana.410240212. [DOI] [PubMed] [Google Scholar]
- 15.Shinotoh H, Aotsuka A, Fukushi K, Nagatsuka S, Tanaka N, Ota T, Tanada S, Irie T. Effect of donepezil on brain acetylcholinesterase activity in patients with AD measured by PET. Neurology. 2001;56:408–10. doi: 10.1212/wnl.56.3.408. [DOI] [PubMed] [Google Scholar]
- 16.Shiraishi T, Kikuchi T, Fukushi K, Shinotoh H, Nagatsuka S, Tanaka N, Ota T, Sato K, Hirano S, Tanada S, Iyo M, Irie T. Estimation of plasma IC50 of donepezil hydrochloride for brain acetylcholinesterase inhibition in monkey using N-[11C]methylpiperidin-4-yl acetate ([11C]MP4A) and PET. Neuropsychopharmacology. 2005;30:2154–61. doi: 10.1038/sj.npp.1300759. [DOI] [PubMed] [Google Scholar]
- 17.Sramek JJ, Cutler NR. RBC cholinesterase inhibition: a useful surrogate marker for cholinesterase inhibitor activity in Alzheimer disease therapy? Alzheimer Dis Assoc Disord. 2000;14:216–27. doi: 10.1097/00002093-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Mesulam MM, Geula C. Overlap between acetylcholinesterase-rich and choline acetyltransferase-positive (cholinergic) axons in human cerebral cortex. Brain Res. 1992;577:112–20. doi: 10.1016/0006-8993(92)90543-i. [DOI] [PubMed] [Google Scholar]
- 19.Shinotoh H, Namba H, Fukushi K, Nagatsuka S, Tanaka N, Aotsuka A, Ota T, Tanada S, Irie T. Progressive loss of cortical acetylcholinesterase activity in association with cognitive decline in Alzheimer's disease: a positron emission tomography study. Ann Neurol. 2000;48:194–200. [PubMed] [Google Scholar]
- 20.Zubenko GS, Moossy J, Martinez AJ, Rao GR, Kopp U, Hanin I. A brain regional analysis of morphologic and cholinergic abnormalities in Alzheimer's disease. Arch Neurol. 1989;46:634–8. doi: 10.1001/archneur.1989.00520420054022. [DOI] [PubMed] [Google Scholar]
- 21.Prohovnik I, Perl DP, Davis KL, Libow L, Lesser G, Haroutunian V. Dissociation of neuropathology from severity of dementia in late-onset Alzheimer disease. Neurology. 2006;66:49–55. doi: 10.1212/01.wnl.0000191298.68045.50. [DOI] [PubMed] [Google Scholar]
- 22.Kato K, Hayako H, Ishihara Y, Marui S, Iwane M, Miyamoto M. TAK-147, an acetylcholinesterase inhibitor, increases choline acetyltransferase activity in cultured rat septal cholinergic neurons. Neurosci Lett. 1999;260:5–8. doi: 10.1016/s0304-3940(98)00943-4. [DOI] [PubMed] [Google Scholar]
- 23.Guitart X, Codony X, Monroy X. Sigma receptors: biology and therapeutic potential. Psychopharmacology. 2004;174:301–19. doi: 10.1007/s00213-004-1920-9. [DOI] [PubMed] [Google Scholar]
- 24.Sakata M, Kimura Y, Naganawa M, Oda K, Ishii K, Chihara K, Ishiwata K. Mapping of human cerebral sigma1 receptors using positron emission tomography and [11C]SA4503. Neuroimage. 2007;35:1–8. doi: 10.1016/j.neuroimage.2006.11.055. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto K, Ishiwata K. Sigma receptor ligands: possible application as therapeutic drugs and as radiopharmaceuticals. Curr Pharm Des. 2006;12:3857–76. doi: 10.2174/138161206778559614. [DOI] [PubMed] [Google Scholar]
- 26.De Vos F, Santens P, Vermeirsch H, Dewolf I, Dumont F, Slegers G, Dierckx RA, De Reuck J. Pharmacological evaluation of [11C]donepezil as a tracer for visualization of acetylcholinesterase by PET. Nucl Med Biol. 2000;27:745–7. doi: 10.1016/s0969-8051(00)00166-9. [DOI] [PubMed] [Google Scholar]


