Abstract
AIM
Observational retrospective studies of the association between use of β2 agonists and the risk of acute myocardial infarction (MI) have demonstrated conflicting results, particularly among first-time users. The aim of this study was to examine the association between β2 agonist use and first nonfatal acute MI.
METHODS
We conducted a case–control study (2476 cases) nested in a cohort of antihypertensive drug users in the Dutch PHARMO RLS database. PHARMO RLS consists of drug dispensing linked to the national hospitalizations register. Each case of nonfatal acute MI was matched with up to 12 control patients by gender, age and region. Drug and disease history and the severity of the underlying respiratory disease were adjusted for.
RESULTS
Risk of acute MI was increased in current β2 agonist users [crude odds ratio (OR) 1.36, 95% confidence interval (CI) 1.15, 1.61]. However, this excess risk was reduced after adjustment for severity of asthma and chronic obstructive pulmonary disease (adjusted OR 1.18, 95% CI 0.93, 1.49). The risk was highest in patients with ischaemic heart disease and low cumulative dose of β2 agonists (adjusted OR 2.47, 95% CI 1.60, 3.82).
CONCLUSION
Most users of β2 agonists did not have an increased risk of acute MI. Only patients with ischaemic heart disease with low cumulative exposure to β2 agonists had an increased risk of acute MI. It is likely that this increased risk was related to latent cardiovascular disease rather than to the direct effects of β2 agonists.
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Use of β2 agonists has been associated with tachycardia, an abnormal ECG and atrial fibrillation.
Previous observational studies of the association between use of β2 agonists and the risk of acute myocardial infarction (MI) have demonstrated conflicting results.
Instead of a causal effect, the positive association between β2 agonist use and MI may be explained by latent ischaemic heart disease, which has symptoms that appear similar to respiratory complaints in chronic obstructive pulmonary disease.
WHAT THIS STUDY ADDS
The majority of β2 agonist users in our study population did not have an increased risk of nonfatal acute MI.
Only patients with ischaemic heart disease and who had recently started β2 agonists had an increased risk of acute MI.
It is likely that this increased risk was related to latent cardiovascular disease rather than direct effects of β2 agonists.
Keywords: β-agonists, salbutamol, chest pain, confounding factors (epidemiology), myocardial infarction, myocardial ischaemia
Introduction
Beta-2 agonists are the most frequently used drugs in the treatment of obstructive airway disease (OAD), which is defined as asthma or chronic obstructive pulmonary disease (COPD). Although β2 agonists are usually inhaled with low systemic absorption, there have been reports of increased plasma levels [1]. Beta-2 receptors are present in the myocardium, where they mediate contraction [2]. Through this mechanism, use of β2 agonists has been associated with tachycardia, an abnormal ECG and atrial fibrillation [3–5].
Observational retrospective studies of the association between use of β2 agonists and the risk of acute myocardial infarction (MI) have demonstrated conflicting results, particularly among first-time users [6–8]. Explanations for these discrepancies include a role of the underlying disease (COPD or hypertension) in the aetiology of MI and lack of statistical adjustment for use of β-blockers or nebulized administration forms of respiratory medications. It has also been suggested that β2 agonists may be prescribed to patients with latent ischaemic heart disease, which has symptoms that appear similar to respiratory complaints in OAD [8].
However, none of these hypotheses have yet been tested. Furthermore, previous studies have not quantified β2 agonist exposure in a very detailed fashion [6–8]. Because cardiovascular disease is highly prevalent in patients with COPD [9, 10], our study aimed to examine the association between β2 agonists and first nonfatal acute MI in antihypertensive drug users, who represent a population at an increased risk of MI.
Methods
Base population
The setting of the study was the PHARMO record linkage system (RLS, http://www.pharmo.nl). PHARMO RLS includes the demographic details and complete medication history of more than two million community-dwelling residents in the Netherlands. These pharmacy data are then linked to hospital admission records as well as several other health registries, including pathology, clinical laboratory findings and general practitioner data. Since virtually all patients in the Netherlands are registered with a single community pharmacy, independently of prescriber, pharmacy records are virtually complete with regard to prescription drugs. Patients are included in the database regardless of their health insurance or socioeconomic status, and represent about 13% of the general population. Several independent validation studies have shown that PHARMO RLS has a high level of completeness and validity. For this study, only drug dispensing data and hospitalization data from January 1991 through December 2003 were used [11, 12]. PHARMO RLS has previously been used to study drug-induced cardiovascular outcomes [13, 14].
Cohort definition
Patients registered in the PHARMO RLS for at least 1 year and using antihypertensive drugs (thiazide diuretics, β-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers or centrally acting agents) were included in the study population, as these patients are at increased risk of acute MI.
Study design
A nested case–control analysis was conducted within the cohort. The outcome of interest was the first nonfatal acute MI [International Classification of Diseases 9 (ICD-9) code 410] that occurred within 100 days after the last dispensing of antihypertensive drugs. A period of 100 days was selected as Dutch health insurance policies cover the dispensing of the majority of drugs for 3 months. We did not include patients who suffered from a fatal MI because they may have died before hospitalization and then their MI data may not have been registered in PHARMO RLS. The date of first admission for nonfatal acute MI was defined as the index date. Only cases aged 18 years at the index date were included in the analysis. Each case was matched with up to 12 control patients by year of birth (±2 years), gender and geographical area. Control patients had similar eligibility criteria to cases. Controls were assigned the same index date as the case with whom they were matched.
Exposure
Current users of β2 agonists were defined as patients who received at least one dispensing within 100 days before the index date. Recent users received their last dispensing for β2 agonists in the 100 days up to 1 year before the index date, and past users were patients with their last dispensing at least 1 year before the index date. For current users, we calculated the cumulative dose and the average daily dose (calculated by division of the cumulative dose by the treatment time, expressed as inhaled salbutamol equivalents (eq.) using defined daily dosages [15, 16]: salbutamol is a synonym of albuterol). Exposure to β2 agonists was also examined in patients with a history of ischaemic heart disease (i.e. patients who were exposed to at least one nitrate prescription [17] or who had been hospitalized for ischaemic heart disease, or who had undergone a percutaneous transluminal coronary angioplasty or coronary artery bypass graft).
Covariates
The analysis was adjusted for cardiovascular risk factors, defined as dispensings for antihypertensive drugs 100 days before the index date, nonsteroidal anti-inflammatory drugs or aspirin in the 2 months before, and loop diuretics, digoxin, antiarrhythmics, spironolactone, nitrates, statins, fibrates, anticoagulants and antidiabetics in the 6 months before the index date. In addition, we adjusted for hospitalizations for hypertensive disease, diabetes mellitus, hyperlipidaemia, ischaemic heart disease, rheumatic heart disease, diseases of the pulmonary circulation, other forms of heart disease including heart failure and cardiac dysrhythmias, and cerebrovascular disease at any time prior to the index date. Because of the reported inverse association between lung function and coronary heart disease [18, 19], we also adjusted for indicators of the severity of the underlying respiratory disease, including hospitalizations for OAD in the 1 year before and exposure to inhaled corticosteroids (ICS), inhaled anticholinergics, xanthine derivatives, acetylcystein, nebulized medications and oral corticosteroids (OCs, using the average daily dose) in the 6 months prior to the index date. Furthermore, we adjusted for antibiotics (tetracyclines, penicillins, β-lactam antibacterials, sulphonamides and macrolides) within 3 days of an OC dispensing (a marker for an exacerbation of COPD [20]).
Statistical analysis
Conditional logistic regression (SAS version 9.1.3, PHREG procedure; SAS Inc., Cary, NC, USA) was used to quantify the association between use of β2 agonists and the risk of nonfatal acute MI. We conducted two differently adjusted analyses: first, we adjusted the results for the indicators of the severity of the underlying respiratory disease. Second, we also adjusted for cardiovascular risk factors using backward elimination. In order to visualize the relationship between the risk of acute MI and recency of β2 agonist use (i.e. the time between the index date and the most recent dispensing) and cumulative β2 agonist use, we used smoothing spline regression plots (SAS version 9.1.3). In a sensitivity analysis, analysis was restricted to patients who were likely to have had a previous diagnosis of COPD. We used a COPD definition that has previously been used by Suissa et al.[8]. These patients were 55 years old at the index date and had filled at least three dispensings for bronchodilators at two or more different dates in any 1-year period before the index date.
Results
A total of 2476 subjects was identified with an acute MI during follow-up. The mean age at the index date was 67 years and 59% of the cases were male. Almost all cases were matched with 10 controls. Table 1 shows their baseline characteristics. As expected, cases were more likely to receive antidiabetics and drugs for the treatment of ischaemic heart disease. Indicators of the severity of OAD were associated with an increased risk of acute MI [crude odds ratio (OR) 1.35, 95% confidence interval (CI) 1.10, 1.64 with use of inhaled anticholinergics, OR of 1.37, 95% CI 1.15, 1.63 with use of ICS, and OR of 1.69, 95% CI 0.91, 3.10 with hospitalization for OAD].
Table 1.
Baseline characteristics of cases and controls
| Characteristic | Cases (n = 2476) | (%) | Controls (n = 24 252) | (%) |
|---|---|---|---|---|
| Mean age (years) | 67.3 | 67.2 | ||
| Women | 1008 | 40.7 | 10 014 | 41.3 |
| Antihypertensive drug use in the 100 days before | ||||
| Thiazide diuretics | 668 | 27.0 | 8 275 | 34.1 |
| β-Blockers | 1331 | 53.8 | 11 401 | 47.0 |
| Calcium channel blockers | 869 | 35.1 | 6 232 | 25.7 |
| ACE-inhibitors | 685 | 27.7 | 7 773 | 32.1 |
| Angiotensin II receptor blockers | 233 | 9.4 | 2 507 | 10.3 |
| Other antihypertensive drugs | 20 | 0.8 | 174 | 0.7 |
| Cardiovascular drug use in the 6 months before | ||||
| Lipid-lowering drugs | 526 | 21.2 | 5 028 | 20.7 |
| Statins | 512 | 20.7 | 4 876 | 20.1 |
| Fibrates | 31 | 1.3 | 268 | 1.1 |
| Antidiabetic agents | 406 | 16.4 | 2 937 | 12.1 |
| Loop diuretics | 299 | 12.1 | 2 413 | 9.9 |
| Spironolactone | 37 | 1.5 | 361 | 1.5 |
| Potassium sparing diuretics | 268 | 10.8 | 3 049 | 12.6 |
| Anticoagulants | 283 | 11.4 | 2 838 | 11.7 |
| Digoxin | 105 | 4.2 | 1 243 | 5.1 |
| Antiarrhythmics other than digoxin | 147 | 5.9 | 1 625 | 6.7 |
| Nitrates | 792 | 32.0 | 3 276 | 13.5 |
| Respiratory drug use in the 6 months before | ||||
| Beta-2 agonists | 216 | 8.7 | 1 728 | 7.1 |
| Inhaled corticosteroids | 196 | 7.9 | 1 503 | 6.2 |
| Anticholinergics | 138 | 5.6 | 1 088 | 4.5 |
| Xanthine derivatives | 32 | 1.3 | 217 | 0.9 |
| Acetylcystein | 84 | 3.4 | 721 | 3.0 |
| Oral corticosteroids by daily dose | ||||
| <7.5 mg | 44 | 1.8 | 339 | 1.4 |
| 7.5–15 mg | 15 | 0.6 | 173 | 0.7 |
| ≥15 mg | 10 | 0.4 | 72 | 0.3 |
As shown in Table 2, the risk of acute MI was increased in current users of β2 agonists (crude OR 1.36, 95% CI 1.15, 1.61). After adjustment for severity of OAD, the excess risk was reduced and was statistically comparable to nonusers (adjusted OR 1.18, 95% CI 0.93, 1.49). There was no difference between current exposure to short-acting β2 agonists (adjusted OR 0.98, 95% CI 0.74, 1.30) and to long-acting β2 agonists (adjusted OR 1.28, 95% CI 0.92, 1.78). The results also did not change when restricting the study population to patients with COPD (adjusted OR 1.35, 95% CI 0.52, 3.51 for exposure to <200 µg salbutamol equivalent/day, adjusted OR 0.89, 95% CI 0.48, 1.67 for exposure to 200–500 µg salbutamol equivalent/day, and adjusted OR 0.97, 95% CI 0.62, 1.52 for >500 µg salbutamol equivalent/day). Concomitant use of β-blockers and β2 agonists did not increase risk of acute MI, yielding an adjusted OR of 1.31 (95% CI 0.97, 1.87), whereas current β2 agonist users who had never been exposed to β-blockers had an adjusted of OR 0.87 (95% CI 0.64, 1.19) for risk of acute MI.
Table 2.
Use of β2 agonists and risk of acute myocardial infarction (MI)
| Exposure to β2 agonists before index date | Cases (n = 2476) | Controls (n = 24 252) | Crude OR (95% CI) | Adjusted OR (95% CI)* | Adjusted OR (95% CI)† |
|---|---|---|---|---|---|
| Never use | 2109 | 21 231 | 1.00 referent | 1.00 referent | 1.00 referent |
| Current use | 166 | 1 272 | 1.36 (1.15, 1.61) | 1.18 (0.93, 1.49) | 1.16 (0.91, 1.47) |
| First time use | 15 | 98 | 1.57 (0.91, 2.72) | 1.45 (0.83, 2.53) | 1.29 (0.74, 2.27) |
| Average daily dose, inhaled salbutamol eq. | |||||
| ≤400 µg | 50 | 372 | 1.37 (1.01, 1.85) | 1.19 (0.85, 1.67) | 1.13 (0.80, 1.59) |
| 400–800 µg | 48 | 370 | 1.37 (1.01, 1.86) | 1.17 (0.82, 1.66) | 1.20 (0.84, 1.72) |
| 800–1600 µg | 45 | 355 | 1.33 (0.97, 1.82) | 1.08 (0.74, 1.57) | 1.12 (0.76, 1.65) |
| >1600 µg | 8 | 77 | 1.11 (0.53, 2.30) | 0.88 (0.41, 1.91) | 0.88 (0.41, 1.90) |
| Recent use | 68 | 596 | 1.16 (0.90, 1.50) | 1.09 (0.84, 1.43) | 1.07 (0.82, 1.41) |
| Past use | 133 | 1 153 | 1.17 (0.97, 1.42) | 1.15 (0.95, 1.39) | 1.13 (0.93, 1.36) |
Adjusted for indicators of the severity of obstructive airway disease (OAD) included exacerbations, the use of inhaled glucocorticoids (GCs), anticholinergics, xanthine derivatives, nebulized medication, daily dose of oral GCs (<7.5, 7.5–15, ≥15 mg), and acetylcystein 6 months before index date. Hospitalizations for OAD 1 year before the index date were also considered an indicator of the severity of OAD.
Adjusted for indicators of the severity of OAD (*) and general risk factors of MI including the use of antidiabetics, statins, fibrates, nitrates, digoxin, thiazide diuretics, calcium-channel blockers, β-blockers, ACE inhibitors and angiotensin II receptor blockers 6 months prior to the index date, use of nonsteroidal anti-inflammatory drugs 2 months before index date, and a history of cardiovascular disease, pulmonary disease and rheumatoid arthritis at any time before the index date.
Figure 1 shows that the risk of acute MI was increased particularly in patients who had received their last β2 agonist prescription shortly before the index date. The adjusted OR for patients with a β2 agonist dispensing in the 30 days prior to the index date was 1.45 (95% CI 1.10, 1.92). High cumulative exposure to β2 agonists was not associated with an increased risk of acute MI (Figure 2). Recent starters [i.e. patients with low cumulative exposure (<0.25 g)] had the highest risk of acute MI (adjusted OR 1.38, 95% CI 0.98, 1.94).
Figure 1.
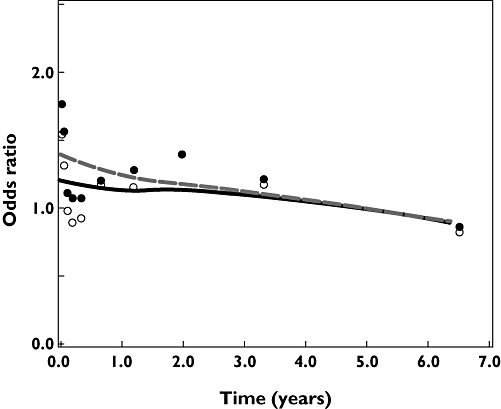
Risk of acute myocardial infarction (MI) and time between index date and last dispensing for β2 agonists. Adjusted for confounders in Table 2 (model under footnote †). Adjusted odds ratio ( ); Crude odds ratio (
); Crude odds ratio ( )
)
Figure 2.
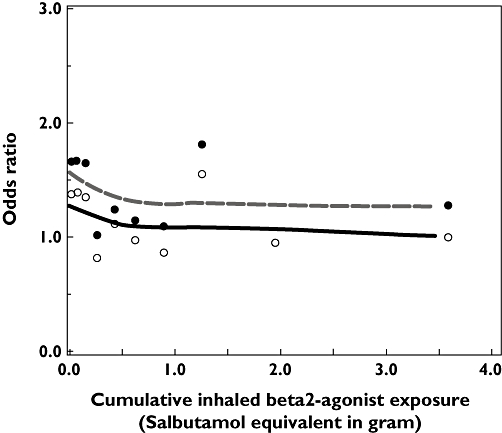
Risk of acute myocardial infarction (MI) and cumulative dose of β2 agonists use among current users. Adjusted for confounders in Table 2 (model under footnote †). Adjusted odds ratio ( ); Crude odds ratio (
); Crude odds ratio ( )
)
In order to explore further the higher risks in patients who received β2 agonists shortly before the index date and who had low cumulative exposure, the study population was stratified by history of ischaemic heart disease. It was found that current users of β2 agonists with a history of ischaemic heart disease had a twofold increased risk of acute MI compared with nonusers (Table 3). This risk was highest in patients with ischaemic heart disease who had a low cumulative dose of β2 agonists (i.e. recent starters, adjusted OR of 2.47, 95% CI 1.60, 3.82).
Table 3.
Use of β2 agonist and risk of acute myocardial infarction stratified by history of ischaemic heart disease
| No history of ischaemic heart disease | History of ischaemic heart disease | |||||
|---|---|---|---|---|---|---|
| Use of β2 agonists | Cases n = 1616 | Controls n = 20 295 | Adjusted OR* (95% CI) | Cases n = 860 | Controls n = 3957 | Adjusted OR* (95% CI) |
| Current use (100 days before) | 88 | 977 | 0.88 (0.67, 1.17) | 78 | 295 | 1.93 (1.41, 2.65) |
| First time use | 9 | 74 | 1.13 (0.56, 2.29) | 6 | 24 | 1.69 (0.67, 4.27) |
| Average daily dose, inhaled salbutamol eq. | ||||||
| ≤400 µg | 19 | 282 | 0.66 (0.41, 1.08) | 31 | 90 | 2.38 (1.50, 3.76) |
| 400–800 µg | 27 | 294 | 0.92 (0.59, 1.43) | 21 | 76 | 2.11 (1.24, 3.59) |
| 800–1600 µg | 30 | 271 | 1.10 (0.71, 1.71) | 15 | 84 | 1.33 (0.73, 2.43) |
| >1600 µg | 3 | 56 | 0.49 (0.15, 1.61) | 5 | 21 | 1.84 (0.66, 5.11) |
| Cumulative dose, inhaled salbutamol eq. | ||||||
| ≤0.25 g | 28 | 329 | 0.83 (0.55, 1.25) | 33 | 94 | 2.47 (1.60, 3.82) |
| 0.25–1.00 g | 23 | 330 | 0.72 (0.45, 1.15) | 28 | 106 | 1.91 (1.19, 3.05) |
| >1.00 g | 37 | 318 | 1.14 (0.76, 1.72) | 17 | 95 | 1.29 (0.72, 2.29) |
Adjusted for the same confounders as in Table 2 (see footnote †), excluding the use of nitrates. Reference: nonusers of β2 agonists.
Figure 3 shows that current β2 agonist users with a history of ischaemic heart disease who had recently started nitrates had a fourfold increased risk of acute MI (adjusted OR 3.80, 95% CI 1.74, 8.30). The risk was reduced in those with long-term nitrate use (adjusted OR 1.53, 95% CI 1.00, 2.33) with 10 prior nitrate dispensings.
Figure 3.
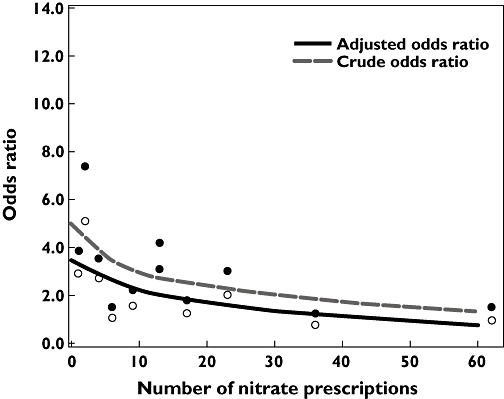
Risk of acute Myocardial infarction (MI) and number of previous nitrate dispensing in current users of both β2 agonists and nitrates. Adjusted for confounders in Table 2 (model under footnote †), excluding nitrates. Adjusted odds ratio ( ); Crude odds ratio (
); Crude odds ratio ( )
)
Discussion
In our study population of patients receiving treatment for hypertension, those using β2 agonists were not associated with an increased risk of acute MI compared with nonusers after adjustment for underlying respiratory disease severity. However, the risk was increased in recent starters of β2 agonists who had a history of ischaemic heart disease.
There are several possible explanations for our finding of an increased risk of acute MI in recent starters of β2 agonist use with a history of ischaemic heart disease. A likely explanation is that the dyspnoea-like symptoms were caused by latent cardiovascular disease rather than OAD. Acute ischaemia or cardiomyopathy can increase the left ventricular end-diastolic volume. This mechanism has been associated with an increase in pulmonary vascular pressure, leading to dyspnoea either by producing hypoxaemia or by stimulation of pulmonary vascular and/or interstitial receptors [21]. Thus, these patients may have been prescribed β2 agonists due to a similarity in clinical presentation.
An alternative explanation for our findings is direct effects of β2 agonists in this subgroup of patients with ischaemic heart disease. It has been described that stimulation of cardiac β2 receptors may result in tachycardia, ECG changes and atrial fibrillation [2–5]. In dose–response studies, it has been demonstrated that higher β2 agonist dosages cause more severe cardiac side-effects [22, 23]. Through this mechanism, we would expect a positive association between daily or cumulative doses of β2 agonists and risk of acute MI. However, none of our dose analyses supported this hypothesis. Higher daily doses of β2 agonists were not associated with higher risks of acute MI, and the cumulative dose was inversely correlated with the risk of acute MI. The lack of direct effect of β2 agonists on the risk of acute MI is also supported by a meta-analysis of randomized controlled clinical trials that compared β2 agonist users with placebo. Although the risk of sinus tachycardia was increased threefold, no significantly increased risk of major cardiovascular outcomes (including acute MI) was found [24, 25].
Although our main finding of a lack of association between use of β2 agonists and risk of acute MI is similar to the results from a Canadian case–control study [8], the differences in the statistical approaches of the two studies must be noted: the Canadian study evaluated three exposure categories by counting the number of dispensed canisters, and its analyses were stratified by history of cardiovascular disease. We chose to quantify the cumulative dispensed dose and the recency of use in a very detailed way using smoothing spline visualizations. We stratified our analysis by ischaemic heart disease and number of nitrate prescriptions in order to identify high-risk patients. Our findings contradict those of a US case–control study that reported increased risk of acute MI in first-time users of β2 agonists. The American study adjusted only for cardiovascular risk factors and not for the severity of OAD and use of respiratory medications and β-blockers. Also, it did not evaluate the possibility that β2 agonists may be prescribed to patients with symptoms of latent ischaemic heart disease rather than asthma/COPD [7]. In our study, increased risk of acute MI was most apparent in patients who had a low cumulative exposure of β2 agonists and a history of ischaemic heart disease. Our finding of the lack of effect of long-term use of β2 agonists and risk of acute MI is consistent with all other published studies [7, 8].
The major limitation of our study is probably the inability to adjust fully for underlying disease, because only hospital records and drug dispensing records were available. Severity of the underlying respiratory disease could only be determined with proxy indicators rather than the lung function parameters [18, 19]. However, the initially elevated crude MI risk dropped towards the null value after adjustment for the proxy indicators of the severity of the underlying respiratory disease; we expect that better adjustment would direct the OR further towards null. Unfortunately, we did not have data on smoking, which may be an important confounder. For this study, data on fatal MIs could not be used because these are not routinely registered in the Dutch national hospitalization registry. However, there is no particular reason to suspect that outcome for the type of acute MI (fatal/nonfatal) is likely to be influenced by β2 agonist exposure. Another limitation is that our findings apply to users of antihypertensive drugs and cannot be extrapolated to all users of β2 agonists. Due to the nested study design, changes of MI risk over calendar time were difficult to track. Lastly, some of our subgroup analyses contain small numbers, and have therefore limited statistical power to detect effects.
Nevertheless, our study is the largest study to have examined the association between use of β2 agonists and risk of acute MI in a high-risk population. Unlike other studies, we were able to analyse small exposure groups (using spline regression) rather than lumping different exposures in a few categories. Therefore, we were also able to stratify patients with a history of ischaemic heart disease in a very detailed fashion. Another strength of our study is the virtually complete drug-dispensing records that gave us the opportunity to calculate dispensed dosages, instead of counting canister numbers. This is important in the light of the wide variety in administration forms (e.g. metered dose inhalers, inhalation powder in capsules and disks) that had been available during the period of data collection. In the Netherlands, drug dispensings were reimbursed regardless of socioeconomic status or employment. Moreover, drug-dispensing data were routinely collected, since 94% of Dutch patients always receive their drug dispensing from the same pharmacy [26].
In conclusion, we have found that the majority of β2 agonist users in our study population did not have an increased risk of nonfatal acute MI. Only patients with ischaemic heart disease with low cumulative exposure to β2 agonists had an increased risk of acute MI. It is likely that this increased risk was related to latent cardiovascular disease rather than direct effects of β2 agonists. Cardiovascular risk assessment should be considered in users of β2 agonists and antihypertensive medication who suffer from latent ischaemic heart disease.
Competing interests
J-W.L. has received an unrestricted grant by GlaxoSmithKline for the support of translational research in the field of asthma and COPD. H.L. has received an unrestricted research grant from GlaxoSmithKline for the support of pharmacoepidemiological research in the field of asthma and COPD.
The authors thank Indira Vishnubhatla for her assistance in editing the English of this manuscript.
REFERENCES
- 1.Anderson PJ, Zhou X, Breen P, Gann L, Logsdon TW, Compadre CM, Hiller FC. Pharmacokinetics of (R,S)-Albuterol after aerosol inhalation in healthy adult volunteers. J Pharm Sci. 1998;87:841–4. doi: 10.1021/js970445u. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman B, Taylor P. Neurotransmission: the autonomic and somatic motor nervous systems. In: Hardman J, Limbird L, Gilman AG, editors. Goodman and Gilman's the Pharmacological Basis of Therapeutics. 10. New York: McGraw-Hill; 2002. p. 141. [Google Scholar]
- 3.Breeden CC, Safirstein BH. Albuterol and spacer-induced atrial fibrillation. Chest. 1990;98:762–3. doi: 10.1378/chest.98.3.762. [DOI] [PubMed] [Google Scholar]
- 4.Vincent HH, Veld AJ, Boomsma F, Schalekamp M. Prevention of epinephrine-induced hypokalemia by nonselective beta blockers. Am J Cardiol. 1985;56:10D–14D. doi: 10.1016/0002-9149(85)91108-7. [DOI] [PubMed] [Google Scholar]
- 5.Kung M, Croley SW, Phillips BA. Systemic cardiovascular and metabolic effects associated with the inhalation of an increased dose of albuterol. Influence of mouth rinsing and gargling. Chest. 1987;91:382–7. doi: 10.1378/chest.91.3.382. [DOI] [PubMed] [Google Scholar]
- 6.Au DH, Curtis JR, Every NR, McDonnell MB, Fihn SD. Association between inhaled beta-agonists and the risk of unstable angina and myocardial infarction. Chest. 2002;121:846–51. doi: 10.1378/chest.121.3.846. [DOI] [PubMed] [Google Scholar]
- 7.Au DH, Lemaitre RN, Curtis JR, Smith NL, Psaty BM. The risk of myocardial infarction associated with inhaled beta-adrenoceptor agonists. Am J Respir Crit Care Med. 2000;161:827–30. doi: 10.1164/ajrccm.161.3.9904006. [DOI] [PubMed] [Google Scholar]
- 8.Suissa S, Assimes T, Ernst P. Inhaled short acting beta agonist use in COPD and the risk of acute myocardial infarction. Thorax. 2003;58:43–6. doi: 10.1136/thorax.58.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutten FH, Cramer MJ, Grobbee DE, Sachs APE, Kirkels JH, Lammers JJ, Hoes AW. Unrecognized heart failure in elderly patients with stable chronic obstructive pulmonary disease. Eur Heart J. 2005;26:1887–94. doi: 10.1093/eurheartj/ehi291. [DOI] [PubMed] [Google Scholar]
- 10.Sidney S, Sorel M, Quesenberry CP, Jr, DeLuise C, Lanes S, Eisner MD. COPD and incident cardiovascular disease hospitalizations and mortality: Kaiser Permanente Medical Care Program. Chest. 2005;128:2068–75. doi: 10.1378/chest.128.4.2068. [DOI] [PubMed] [Google Scholar]
- 11.Heerdink ER, Leufkens HG, Herings RM, Otterranger JP, Stricker BH, Bakker A. NSAIDs associated with increased risk of congestive heart failure in elderly patients taking diuretics. Arch Intern Med. 1998;158:1108–12. doi: 10.1001/archinte.158.10.1108. [DOI] [PubMed] [Google Scholar]
- 12.Herings RM. Utrecht: Universiteit Utrecht; 1993. PHARMO: a Record Linkage System for Postmarketing Surveillance of Prescription Drugs in The Netherlands. [Google Scholar]
- 13.Erkens JA, Klungel OH, Herings RM, Stolk RP, Spoelstra JA, Grobee DE, Leufkens HG. Use of fluorquinolones is associated with a reduced risk of coronary heart disease in diabetes mellitus type 2 patients. Eur Heart J. 2002;23:1575–9. doi: 10.1053/euhj.2002.3173. [DOI] [PubMed] [Google Scholar]
- 14.Bouvy ML, Heerdink ER, De Bruin ML, Herings RM, Leufkens HG, Hoes AW. Use of sympathomimetic drugs leads to increased risk of hospitalization for arrhythmias in patients with congestive heart failure. Arch Intern Med. 2000;160:2477–80. doi: 10.1001/archinte.160.16.2477. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. ATC Classification Index with Ddds 2002. Nydalen: WHO Collaborating Centre for Drug Statistics Methodology Norwegian Institute of Public Health; 2002. [Google Scholar]
- 16.De Vries F, van Staa TP, Bracke MS. Severity of obstructive airway disease and risk of osteoporotic fracture. Eur Respir J. 2005;25:879–84. doi: 10.1183/09031936.05.00058204. [DOI] [PubMed] [Google Scholar]
- 17.Maitland-van der Zee AH, Klungel OH, Stricker BH, van de Kuip DAM, Witteman JCM, Hofman A, van Hoof J. Repeated nitrate prescriptions as a potential marker for angina pectoris. A comparison with medical information from the Rotterdam Study. Pharm World Sci. 2003;25:70–2. doi: 10.1023/a:1023292830670. [DOI] [PubMed] [Google Scholar]
- 18.Schroeder EB, Welch VL, Couper D, Nieto FJ, Liao D, Rosamond WD, Heiss G. Lung function and incident coronary heart disease: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2003;158:1171–81. doi: 10.1093/aje/kwg276. [DOI] [PubMed] [Google Scholar]
- 19.Anthonisen NR, Connett JE, Enright PL, Manfreda J. Hospitalizations and mortality in the Lung Health Study. Am J Respir Crit Care Med. 2002;166:333–9. doi: 10.1164/rccm.2110093. [DOI] [PubMed] [Google Scholar]
- 20.Van Ganse E, van der Linden PD, Leufkens HG, Herings RM, Vincken W, Ernst P. Asthma medications and disease exacerbations: an epidemiological study as a method for asthma surveillance. Eur Respir J. 1995;8:1856–60. doi: 10.1183/09031936.95.08111856. [DOI] [PubMed] [Google Scholar]
- 21.Schwartzstein R. Approach to the Patient with Dyspnea. Waltham, MA: UpToDate Inc.; 2006. [Google Scholar]
- 22.Newhouse MT, Chapman KR, McCallum AL, Abboud RT, Bowie DM, Hodder RV, Paré PD, Mesic-Fuchs H, Molfino NA. Cardiovascular safety of high doses of inhaled fenoterol and albuterol in acute severe asthma. Chest. 1996;110:595–603. doi: 10.1378/chest.110.3.595. [DOI] [PubMed] [Google Scholar]
- 23.Jartti T, Kaila T, Tahvanainen K, Kuusela T, Vanto T, Välimäki I. The acute effects of inhaled salbutamol on the beat-to-beat variability of heart rate and blood pressure assessed by spectral analysis. Br J Clin Pharmacol. 1997;43:421–8. doi: 10.1046/j.1365-2125.1997.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salpeter SR. Cardiovascular safety of beta (2)-adrenoceptor agonist use in patients with obstructive airway disease: a systematic review. Drugs Aging. 2004;21:405–14. doi: 10.2165/00002512-200421060-00005. [DOI] [PubMed] [Google Scholar]
- 25.Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. Chest. 2004;125:2309–21. doi: 10.1378/chest.125.6.2309. [DOI] [PubMed] [Google Scholar]
- 26.Van der Schee E, Van Dijk L, Blom L, et al. Consumer-panel healthcare searches for gaps. Pharm Weekbl. 2004;139:618–22. (in Dutch). [Google Scholar]


