Abstract
AIM
To explore inhaled technetium-99m-labelled hexakis-methoxy-isobutyl isonitrile (99mTc-sestamibi) for quantifying pulmonary P-glycoprotein (P-gp) expression.
METHODS
The elimination rate from the lungs of 99mTc-sestamibi was recorded scintigraphically for 30 min following inhalation as an aerosol in healthy smokers, nonsmokers and patients with lung disease.
RESULTS
99mTc-sestamibi elimination rates [% min−1 (SD; P vs. healthy nonsmokers)] were: healthy nonsmokers, 0.43 (0.083); healthy smokers, 0.19 (0.056; P < 0.001); chronic obstructive pulmonary disease patients, 0.26 (0.077; P < 0.001). Elimination rates in three patients with interstitial lung disease were not accelerated.
CONCLUSION
Cigarette smoke upregulates lung P-gp. 99mTc-sestamibi elimination in normal smokers could be used to test new P-gp modulators. The findings also have implications for inhaled drug delivery.
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Very little is known about the physiology of P-glycoprotein (P-gp) expression in the lungs.
Ex vivo evidence based on resected lung tissue suggests that pulmonary P-gp is upregulated by cigarette smoke, but there are no in vivo studies to date.
WHAT THIS STUDY ADDS
The novel observation that healthy cigarette smokers have a delayed pulmonary elimination rate of inhaled 99mTc-sestamibi, a P-gp substrate, provides for the first time a potential method for quantifying functional pulmonary P-gp expression that may inform about drug therapy by inhalation as well as provide a non-invasive, quantitative, human biomarker for assessing P-gp modulators.
Keywords: 99mTc-sestamibi, aerosol, chronic obstructive pulmonary disease, lungs, P-glycoprotein, smoking
Introduction
Multi-drug resistance (MDR) proteins, principally P-glycoprotein (P-gp), are widely expressed physiologically. They minimize the transfer of xenobiotic, MDR substrates across critical biological membranes [1, 2], including the blood–brain barrier, the hepatobiliary, gastrointestinal and renal tubular epithelia, and pulmonary blood–gas barrier, and therefore impact significantly on drug therapy in general.
Technetium-99m-labelled hexakis-methoxy-isobutyl isonitrile (99mTc-sestamibi) is an MDR substrate that has been used to image P-gp expression in several settings, especially cancer, in which it has been shown that tracer accumulation correlates inversely with P-gp expression and response to drug treatment [3].
Little is known about the physiology of P-gp in the lungs. Ex vivo data from human lung suggests that pulmonary P-gp is upregulated by cigarette smoke [4] and that multidrug resistance-associated protein (MRP) 1 is downregulated in chronic obstructive pulmonary disease (COPD) [5], but there are no in vivo studies on pulmonary P-gp function. Drug administration via inhalation for both local [6] and systemic [7] therapy may be influenced by lung P-gp expression, but this aspect of P-gp has received little attention. The purpose of our study was to explore a role for inhaled 99mTc-sestamibi for quantifying pulmonary P-gp expression.
Materials and methods
Subjects
Twenty-six healthy White adults [11 smokers of >15 cigarettes per day (age range 25–58, seven men) and 15 nonsmokers (26–60, 11 men)], receiving no medication, were recruited, plus seven patients with COPD (all ex-smokers) and three with interstitial lung disease (ILD; all nonsmokers) (Table 1). Subjects lived in the Cambridge area. Cigarettes were not withheld. The study was approved by the Local Research Ethics Committee and Administration of Radioactive Substances Advisory Committee of the United Kingdom.
Table 1.
ClInIcal detaIls of the patIents wIth chronIc obstructIVe pulmonary dIsease (COPD; n = 7) and InterstItIal lung dIsease (ILD; n = 3)
| Age | Gender | Smoking/gas transfer | Spirometry (l) (FEV1/FVC) | Medication |
|---|---|---|---|---|
| 68 | Male | ex 20 pack years | 0.67/3.17 ratio 21% | Lansoprazole bendrofluazide salbutamol seretide tiotropium |
| 69 | Female | ex 30 pack years | 0.75/1.74 ratio 44% | Serevent salbutamol fluticasone ipratropium |
| 57 | Male | ex 20 pack years | 0.4/1.4 ratio 28% | Theophylline Tiotropium eformoterol salbutamol budesonide Lisinopril Frusemide |
| 72 | Female | ex 21 pack years | 1.07/1.87 ratio 57% | Losartan salbutamol ipratropium Salmeterol Fluticasone Fosamax thyroxine |
| 74 | Male | ex 40 pack years | 0.95/2.37 ratio 34% | Madopar salbutamol beclomethasone Ipratropium |
| 55 | Female | ex 30 pack years | 0.43/1.65 ratio 21% | Tiotropium salbutamol Paroxeine serevent budesonide |
| 67 | Male | ex 40 pack years | 0.49/1.11 ratio 21% | Beclomethasone ipratropium salbutamol |
| 73 | Male | TLCO 53% KCO 87% | 2.65/3.23 ratio 82% | Prednisolone Azathioprine |
| 54 | Female | TLCO 56% KCO 86% | 2.39/2.47 ratio 97% | Aspirin |
| 60 | Male | TLCO 75% KCO 83% | 3.02/4.37 ratio 66% | None |
Smoking history is given for COPD patients and gas transfer for ILD patients. Spirometry is represented as FEV1/FVC, where FEV1 is the forced expiratory volume in 1 s and FVC is the forced vital capacity. The ratio represents FEV1 as a percentage of FVC, which gives an indication of the degree of airflow obstruction. TLCO represents the carbon monoxide transfer factor indicated as a percentage of the predicted value, and KCO is the gas transfer coefficient as a percentage of the predicted value.
Data acquisition
99mTc-sestamibi aerosol (Bristol Myers Squibb, Pharma, Brussels, Belgium) was generated by a lead-shielded system using a disposable Q-Vent circuit (Diagnostic Imaging Ltd, Wellingborough, UK) in a Q-Vent-shielded canister and inhaled via a SealFlex Mask (AMICI,Inc., PA, USA) during relaxed tidal breathing over 5 min in the sitting position. Although 80 MBq 99mTc was added to the circuit, only a small fraction was deposited in the lungs (ed < 0.2 mSv), which were monitored for 30 min by dynamic scintigraphy (Sophy camera DS & ISSophamedical, Buc, France) from the posterior projection with the subject supine.
Data analysis
Regions of interest were placed around the periphery of both lungs, excluding the central airways. Time–activity curves were generated for each lung, summed and corrected for physical decay of 99mTc. No background correction was attempted. Curves were normalized to the maximum count rate.
Statistics
As data in each group were normally distributed, healthy smokers and COPD patients were compared with normal nonsmokers using parametric statistics (nonpaired Student t-testing). A P-value of <0.05 was taken to indicate a significant difference.
Results
Normal subjects
Elimination curves of healthy smokers and nonsmokers tended to form a plateau over the initial 5 min of monitoring (Figure 1) so linear least square fits were applied from 6 min (Figure 1). The mean elimination rate in healthy smokers was 0.19 (SD 0.056) % min−1, significantly reduced compared with healthy nonsmoking subjects, in whom it was 0.43 (0.083) % min−1 (P < 0.001).
Figure 1.
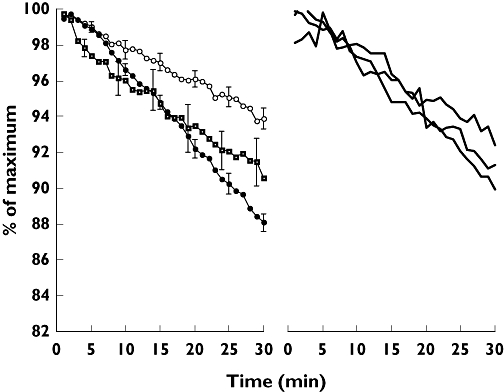
Left panel: composite 99mTc-sestamibi elimination curves in smokers and nonsmokers with healthy lungs and patients with chronic obstructive pulmonary disease (COPD). Error bars are the standard errors. Note that over the initial 5 min of 99mTc-sestamibi elimination, the mean curves for smokers ( ) and nonsmokers (•) are superimposed and less steep than that for COPD patients (
) and nonsmokers (•) are superimposed and less steep than that for COPD patients ( ). Right panel: 99mTc-sestamibi elimination rates in the three individual patients with interstitial lung disease (
). Right panel: 99mTc-sestamibi elimination rates in the three individual patients with interstitial lung disease ( )
)
Patients with lung disease
In contrast to healthy nonsmokers and smokers, the mean linear elimination rate over the initial 5 min of monitoring in COPD patients was relatively rapid compared with the subsequent rate, so linear fits were again applied from 6 min (Figure 1). The mean 99mTc-sestamibi elimination rate in COPD patients was 0.26 (0.077) % min−1, significantly reduced compared with healthy nonsmokers (P < 0.001). 99mTc-sestamibi elimination rates in ILD patients from 6 to 30 min were not accelerated, and were 0.23, 0.32 and 0.35% min−1 (Figure 1).
Over the initial 5 min, the mean elimination rate in COPD patients was 0.62 (0.40) % min−1, significantly faster (P < 0.02) than healthy nonsmokers [0.18 (0.34) % min−1] and healthy smokers [0.15 (0.21) % min−1] over the same period.
Discussion
99mTc forms a stable, positively charged lipophilic complex with sestamibi that is cleared rapidly from blood. It is used clinically for imaging myocardial perfusion and tumours, especially parathyroid tumours. 99mTc-sestamibi is retained within mitochondria after passive diffusion into cells [8]. It is a transport substrate for P-gp, so many P-gp modulators increase the cell-associated concentration of 99mTc-sestamibi [9].
Considering its lipid solubility, 99mTc-sestamibi displayed a surprisingly slow elimination rate from normal lungs with a half-time about twice that of 99mTc-diethyltriaminepentaacetic acid (DTPA) [10]. We suggest this is the result of P-gp-mediated pumping from alveolar epithelial cells back into the airway. In contrast, Nilsson and Wolmer [11] recorded an elimination rate in rabbits faster than 99mTc-DTPA, even though rabbits also express P-gp in their lungs [12], a discrepancy we are unable to explain.
Since 99mTc-sestamibi enters cells, there is less opportunity for it to diffuse through the paracellular route taken by 99mTc-DTPA, explaining why ILD patients, in whom 99mTc-DTPA elimination rates are fast [10], did not have rapid elimination. Because MDR transporters are present in bronchial epithelium, we predicted that disease-induced depletion of P-gp in COPD would result in accelerated 99mTc-sestamibi elimination, but it was delayed, even though MRP1 expression is diminished in ex-smoking COPD patients [13]. Chronic pulmonary injury, however, may lead to sustained upregulation of MDR. Thus, acute inflammatory cytokines generally inhibit P-gp [14], but chronic inflammation may have the opposite effect [15]. It is also not clear why COPD patients displayed a relatively fast elimination over the initial 5 min of monitoring or why the healthy subjects tended to display a plateau.
We speculate that the decreased elimination rate of 99mTc-sestamibi in smokers is the result of upregulation of mdr-1 by cigarette smoke. This is supported by immunohistochemistry of P-gp in surgically resected lung tissue of smokers [8]. The slow elimination in normal smokers could therefore be used as a non-invasive, convenient and quantitative biomarker for the assessment of new P-gp modulators, given either systemically or by inhalation. Pulmonary MDR expression may also be relevant to inhaled drug therapy for treating both local [6] and systemic disease [7]. By preventing the transfer of drugs into the pulmonary or bronchial circulations, local therapy might be facilitated if MDR was expressed or, conversely, systemic therapy facilitated if MDR was downregulated.
In conclusion, 99mTc-sestamibi displays a relatively slow elimination rate from the lungs following inhalation as an aerosol. It is further delayed in healthy cigarette smokers, probably because of P-gp upregulation. Suppression or expression of pulmonary P-gp has important implications for drug delivery via inhalation. Moreover, acceleration of elimination in normal smoking volunteers may represent a useful, non-invasive, simple and quantitative measure of the effectiveness of novel P-gp modulators.
REFERENCES
- 1.Girardin E. Membrane transporter proteins: a challenge for CNS drug development. Dialogues Clin Neurosci. 2006;8:311–21. doi: 10.31887/DCNS.2006.8.3/fgirardin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang XB. A molecular understanding of ATP-dependent solute transport by multidrug resistance associated protein MRP1. Cancer Metastasis Rev. 2007;26:15–37. doi: 10.1007/s10555-007-9041-7. [DOI] [PubMed] [Google Scholar]
- 3.Burak Z, Moretti JL, Ersoy O, Sanli U, Kantar M, Tamgac F, Basdemir G. 99mTc-MIBI imaging as a predictor of therapy response in osteosarcoma compared with multidrug resistance-associated protein and P-glycoprotein expression. J Nucl Med. 2003;44:1394–401. [PubMed] [Google Scholar]
- 4.Lechapt-Zalcman E, Hurbain I, Lacave R, Commo F, Urban T, Antoine M, Milleron B, Bernaudin JF. MDR1-Pgp 170 expression in human bronchus. Eur Respir J. 1997;10:1837–43. doi: 10.1183/09031936.97.10081837. [DOI] [PubMed] [Google Scholar]
- 5.van der Deen M, Marks H, Willemse BW, Postma DS, Muller M, Smit EF, Scheffer GL, Scheper RJ, de Vries EG, Timens W. Diminished expression of multidrug resistance-associated protein 1 (MRP1) in bronchial epithelium of COPD patients. Virchows Arch. 2006;449:682–8. doi: 10.1007/s00428-006-0240-3. [DOI] [PubMed] [Google Scholar]
- 6.Sharma S, White D, Imondi AR. Development of inhalational agents for oncologic use. J Clin Oncol. 2001;19:1839–47. doi: 10.1200/JCO.2001.19.6.1839. [DOI] [PubMed] [Google Scholar]
- 7.Laube BL. The expanding role of aerosols in systemic drug delivery, gene therapy, and vaccination. Respir Care. 2005;50:1161–76. [PubMed] [Google Scholar]
- 8.Piwnica-Worms D, Kronauge JF, Chiu ML. Uptake and retention of hexakis (2-methoxyisobutyl isonitrile) technetium (I) in cultured chick myocardial cells: mitochondrial and plasma membrane potential dependence. Circulation. 1990;82:1826–38. doi: 10.1161/01.cir.82.5.1826. [DOI] [PubMed] [Google Scholar]
- 9.Marian T, Balkay L, Szabo G, Krasznai ZT, Hernadi Z, Galuska L, Szabo-Peli J, Esik O, Tron L, Krasznai Z. Biphasic accumulation kinetics of [99mTc]-hexakis-2-methoxyisobutyl isonitrile in tumour cells and its modulation by lipophilic P-glycoprotein ligands. Eur J Pharm Sci. 2005;25:201–9. doi: 10.1016/j.ejps.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 10.O'Doherty MJ, Peters AM. Pulmonary Tc-99m-DTPA aerosol clearance as an index of lung injury. Eur J Nucl Med. 1997;24:81–7. doi: 10.1007/BF01728316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nilsson K, Wollmer P. Pulmonary clearance of tracers with different lipid and water solubility in experimental surfactant dysfunction. Eur Respir J. 1993;6:505–8. [PubMed] [Google Scholar]
- 12.Roerig DL, Audi AH, Ahlf SB. Kinetic characterization of P-glycoprotein-mediated efflux of rhodamine 6G in the intact rabbit lung. Drug Metab Dispos. 2004;32:953–8. doi: 10.1124/dmd.104.000042. [DOI] [PubMed] [Google Scholar]
- 13.van der Deen M, de Vries EG, Timens W, Scheper RJ, Timmer-Bosscha H, Postma DS. ATP-binding cassette (ABC) transporters in normal and pathological lung. Respir Res. 2005;6:59. doi: 10.1186/1465-9921-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez C, Buyse M, German-Fattal M, Gimenez F. Influence of the pro-inflammatory cytokines on P-glycoprotein expression and functionality. J Pharm Pharm Sci. 2004;7:359–71. [PubMed] [Google Scholar]
- 15.Bauer B, Hartz AM, Miller DS. Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood–brain barrier. Mol Pharmacol. 2007;71:667–75. doi: 10.1124/mol.106.029512. [DOI] [PubMed] [Google Scholar]


