Abstract
AIMS
The risk of adverse events due to chronic benzodiazepine use is high in the elderly. Clinicians need to be able to identify those persons who are at risk of chronic benzodiazepine use, but little is known about the determinants. This study determined social and health related factors that predict new-onset chronic benzodiazepine use in community-dwelling elderly.
METHODS
This study was embedded in an ongoing cohort study among 5364 persons aged ≥57 years. Drug-dispensing medication records were available for the period between 1991 and 2003. We defined chronic benzodiazepine use as use during at least 180 days in a period of 365 consecutive days. The association of various social, psychiatric and somatic variables with new-onset chronic benzodiazepine use was studied with a Cox proportional hazards analysis.
RESULTS
Symptoms of depression, hypertension, pain related joint complaints and the perception of poor physical health predicted new-onset chronic use. In the subsample of participants who had filled at least one prescription in the follow-up period, of these variables only pain related joint complaints increased the risk of new-onset chronic use. Living alone protected against chronic benzodiazepine use.
CONCLUSIONS
The elderly with poor mental and physical health are at an increased risk of chronic benzodiazepine use. Living alone was found to decrease the risk of chronic use, which suggests that social factors may determine drug usage patterns. Very few characteristics predicted chronic benzodiazepine use once patients had received their first prescription. For clinicians, identification of patients at high risk is therefore not straightforward.
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
The risk of adverse events due to chronic benzodiazepine use is high in the elderly.
Cross-sectional studies have shown that increasing age, female gender and poor physical and mental health are associated with benzodiazepine use.
When users were re-examined some years later, chronic somatic disease, pain and stress seemed to contribute to the continuation of benzodiazepine use.
WHAT THIS STUDY ADDS
This is the first longitudinal study that analyzed the determinants of new-onset chronic benzodiazepine use in community-dwelling elderly.
Symptoms of depression, hypertension, pain related joint complaints and the perception of poor physical health predicted new-onset chronic use. Living alone was found to decrease the risk of chronic use.
Keywords: benzodiazepine use, cohort study, elderly
Introduction
Benzodiazepines are among the most frequently prescribed drugs and 10–30% of people aged 65 years or older use them [1, 2]. In older people chronic use of benzodiazepines is particularly problematic because they are at risk of adverse events such as falls, hip fractures and cognitive decline [3, 4]. Chronic benzodiazepine use has been associated with increased use of medical care and suffering from a chronic somatic disease, pain or stress [5–9]. However, these findings are derived from repeated assessments of users and thus focus on continuous chronic use; cohort studies that prospectively determine new-onset chronic use are lacking. Moreover, the role of social factors has not been studied before in a prospective study.
We examined the association between social support and new-onset chronic benzodiazepine use in older people. Our hypothesis was that living with a partner or other housemate, having children, or living in a residence with day-to-day care would have a protective effect. The objective of this study was to analyze social and health related determinants of new-onset chronic benzodiazepine use in a cohort of older, community-dwelling people.
Methods
Study population
This study was part of the Rotterdam Study, a prospective study in which determinants of cardiovascular, neurological, locomotor, ophthalmologic, and psychiatric diseases are investigated on a continuous basis [10]. All inhabitants of Ommoord, a suburb of Rotterdam, aged 55 years or older were invited to participate, and 7983 (78%) agreed to take part. The Medical Ethics Committee of the Erasmus Medical Center approved the study. Written informed consent was obtained from all participants.
In the first examination round from July 1989 to June 1993, the participants were interviewed at home by a trained research assistant and received a physical examination at the research centre. The second round was conducted in July 1993 until December 1995, and served as baseline for this study. The participants completed a questionnaire themselves and were invited to the centre for a physical examination.
Outcome definition
Ninety percent of the participants of the Rotterdam Study fill their prescriptions at one of seven pharmacies in the Ommoord region. These pharmacies are linked to one computer network and they delivered online data about filled benzodiazepine prescriptions between January 1, 1991 and January 1, 2003. In this way, virtually complete information of all dispensed benzodiazepines is collected, because in the Netherlands these are prescription-only drugs. Drugs were coded according to the Anatomical Therapeutic and Chemical (ATC) classification system. We included the ATC-codes N05BA (anxiolytics) and N05CD (hypnotics and sedatives). Non-benzodiazepine compounds such as zopiclone and zolpidem were hardly used in the Rotterdam Study during the study period and were not included in the analyses. We classified each benzodiazepine according to its half-life in the following way: short half-life: alprazolam, bromazepam, brotizolam, flunitrazepam, loprazolam, lorazepam, lormetazepam, midazolam, temazepam, triazolam, and oxazepam; medium half-life: nitrazepam; and long half-life: diazepam, chlordiazepoxide, clorazepinezuur, flurazepam, nitrazepam, and prazepam. For each prescription, the pharmacies registered ATC-code, dosage, prescribed daily dosage and number of tablets.
We defined chronic benzodiazepine use as at least 180 days of continuous or intermittent use during a consecutive period of 365 days. Zandstra et al. have recommended this definition after reviewing the literature [2]. At the first day on which an individual reached the 180th day, he was considered to be a ‘chronic user’. At baseline, 6312 persons participated. After excluding 948 participants who had become chronic users in the 2 years before baseline, a study population of 5364 participants remained at risk of chronic use (Figure 1). Of these, 2490 participants filled at least one prescription for a benzodiazepine during the follow-up period.
Figure 1.
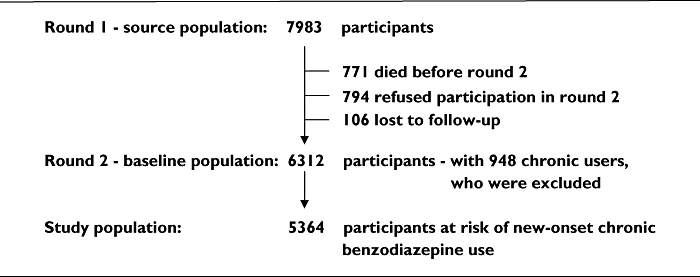
Flow diagram of study population
Determinants
Three domains of potential determinants were studied. The first domain concerned social support. We focused on the availability of direct social support: living situation with or without a partner or other housemate, number of children, and place of residence with or without day-to-day care. This information was retrieved from the participants during the home interview in the first round. At time of the second round, we determined who had become widowed or had moved to another place of residence in the period between the two rounds.
The second domain dealt with psychiatric and somatic health. Symptoms of depression and anxiety were established in the home interview. Subjects with a score of 16 or more on the Center for Epidemiologic Studies Depression Scale (range 0–60) or a score of 8 or more on the depression subscale of the Hospital Anxiety and Depression Scale (range 0–24) were considered as positive for a depressive disorder [11, 12]. Those with a score of 8 or more on the anxiety subscale of the Hospital Anxiety and Depression Scale (range 0–24) were considered as positive for an anxiety disorder [12]. The CES-D was available in 1629 participants and the HADS in 2580. The Mini Mental State Examination was used to screen for cognitive disorders [13]. The number of hours of rest or sleep per night was established by self-report. We used history of myocardial infarction, cerebrovascular accidents, hypertension, and current pain related joint complaints as determined by self-report.
The third domain involved participants' health perception and behaviour. Firstly, we used a measure of Activities of Daily Living based on the Modified Stanford Health Assessment Questionnaire [14]. Secondly, we determined subjective health by asking participants to rate their health as worse, same or better than that of peers. Self-reported alcohol use and smoking were categorized into none, former, and current.
In addition to these three domains, participants' demographic characteristics, i.e. age, sex, highest education attained, and health insurance were taken into account.
Data analysis
We performed two Cox proportional hazards analyses. In these analyses each participant contributed person-years from the date of the baseline interview until follow-up ended either with the event of chronic use or when a participant was censored due to death, loss-to-follow, or the end of study on January 1, 2003. First, we compared the social and health related characteristics of new-onset chronic benzodiazepine users with those of all other participants. We thus aimed to clarify which characteristics increase the risk of chronic use in the general population. We performed the second analysis in the subsample of participants who had filled at least one prescription for a benzodiazepine during the follow-up period. In this way, we analyzed which characteristics increase the risk of new-onset chronic use in the group of benzodiazepine users, i.e. which users go on to become chronic users.
For each analysis, we determined whether the social and health related factors were associated with new-onset chronic benzodiazepine use by calculating the hazard rates adjusted for age and sex. The factors with a P < 0.10 in the age and gender adjusted model were included in the final model. The factors with a P value < 0.05 in the final multivariate analysis were considered to be determinants of chronic use. P values are two-sided and were considered statistically significant if less than 0.05. We used SPSS 11.0 to perform the analyses.
Results
We identified 440 new cases of chronic benzodiazepine use in 39 164 person-years. Thus, of the 2490 participants who had filled at least one prescription for a benzodiazepine, 17.7% became chronic users during the follow-up period, which was on average 7.3 years. Table 1 displays the baseline characteristics of the study population. Table 2 describes the type, half-life and daily dosages in terms of defined daily dosage (DDD) of the benzodiazepines first prescribed and of the benzodiazepines that the chronic users used at the index date. Note that 21 chronic users used two and one chronic user used three different benzodiazepines concurrently.
Table 1.
Baseline characteristics of the study population
| Characteristic | n = 5364 |
|---|---|
| Patient characteristics | |
| Socio demographic variables | |
| Age (years, mean (range)) | 70.2 (56.5–101.8) |
| Gender (% female) | 56.6 |
| Primary school only (%) | 20.6 |
| Public health insurance (%) | 52.5 |
| Social support | |
| Living situation | |
| With partner or other person (%) | 72.0 |
| Alone (%) | 28.0 |
| Number of children (mean (range)) | 2.1 (0–16) |
| Place of residence | |
| Independently (%) | 87.8 |
| Service flat (%) | 8.3 |
| Home for the elderly (%) | 4.0 |
| Psychiatric and physical health | |
| Anxiety (% HADS-A ≥ 8)* | 11.3 |
| Depression (% HADS-D ≥ 8 or CES-D ≥ 16)† | 10.9 |
| MMSE (% < 26) | 10.0 |
| Sleep (% < 6 h) | 6.7 |
| Myocardial infarction (%) | 8.0 |
| Stroke (%) | 1.6 |
| Hypertension (%) | 35.0 |
| Joint or back complaints (%) | 18.6 |
| Other patient characteristics | |
| Activities of daily living (mean score (range))‡ | 1.29 (1.00–4.00) |
| Subjective health (%) | |
| Better | 54.1 |
| Same | 38.3 |
| Worse | 7.7 |
| Alcohol use (%) | |
| Nonuser | 24.7 |
| Former user | 0.9 |
| Current user | 74.4 |
| Smoking (%) | |
| Nonsmoker | 30.9 |
| Former smoker | 49.9 |
| Current smoker | 19.2 |
Hospital Anxiety and Depression Scale (HADS) known for 2580 participants.
Center for Epidemiologic Studies Depression Scale (CES-D) known for 1629.
A score of 1.5 or lower indicates independent functioning.
Table 2.
Characteristics of benzodiazepine prescriptions
| All users (n = 2490) | Chronic users (n = 440) | |
|---|---|---|
| First benzodiazepine prescription | ||
| Type of benzodiazepine | ||
| Anxiolytic (%) | 58.8 | 50.0 |
| Hypnotic/sedative (%) | 41.2 | 50.0 |
| Daily dosage | ||
| Mean | 0.30 | |
| ≤0.5 DDD* (%) | 67.4 | 63.4 |
| 0.5–1.0 DDD (%) | 28.8 | 32.7 |
| >1.0 DDD (%) | 4.0 | 3.9 |
| Half-life | ||
| Short (%) | 67.6 | 69.6 |
| Long (%) | 32.4 | 30.4 |
| Benzodiazepine prescription at index date | ||
| Type of benzodiazepine† | ||
| Anxiolytic (%) | – | 48.4 |
| Hypnotic/sedative (%) | – | 55.9 |
| Daily dosage† | ||
| Mean | – | 0.61 |
| ≤0.5 DDD‡ (%) | – | 69.9 |
| 0.5–1.0 DDD (%) | – | 24.0 |
| >1.0 DDD (%) | – | 6.1 |
| Half-life† | ||
| Short (%) | – | 80.7 |
| Long (%) | – | 20.9 |
DDD stands for defined daily dosage (this is the recommended daily dosage for an adult of 70 kg).
Percentages do not add up to 100% because 22 chronic users used more than one benzodiazepine at the index date.
In chronic users who used more than one benzodiazepine DDDs were added.
Table 3 shows the independent determinants of new-onset chronic benzodiazepine use in bold. Note that the hazard rates (HR) in Table 3 were not adjusted for anxiety and hours of sleep because missing values for these variables exceeded 20%. The hazard rates for anxiety and sleep, as shown in Table 3, were derived from separate Cox proportional hazard analyses for the groups of participants for which these variables were known (complete case analysis). Increasing age, female gender, public health insurance, depressive complaints, less than 6 h of sleep per night, hypertension, pain related joint complaints, poor subjective health, and current smoking predicted new-onset chronic benzodiazepine use. Living alone was a protective factor against new-onset chronic benzodiazepine use. An analysis stratified for gender showed that this protective effect was quite similar in men (HR 0.55; 95% CI 0.29, 1.02) and women (HR 0.71; 95% CI 0.55, 0.93).
Table 3.
Determinants of chronic benzodiazepine use in older people (n = 5364)
| Variable | Hazard rate* (95% CI) |
|---|---|
| Socio-demographic variables | |
| Age (years) | 1.04 (1.03, 1.06) |
| Female | 1.67 (1.31, 2.14) |
| Public health insurance | 1.23 (1.01, 1.51) |
| Social support | |
| Living situation | |
| With partner or other person | 1.00 (reference) |
| Alone | 0.66 (0.52, 0.84) |
| Place of residence | |
| Independently | 1.00 (reference) |
| Service flat | 1.34 (0.98, 1.83) |
| Home for the elderly | 0.72 (0.36, 1.42) |
| Psychiatric and physical health | |
| Anxiety (n = 2580) HADS-A ≥ 8 | 1.46 (0.98, 2.19) |
| Depression HADS-D ≥ 8 or CES-D ≥ 16 | 1.53 (1.14, 2.07) |
| Sleep (n = 2104), <6 h | 1.66 (1.08, 2.54) |
| Myocardial infarction | 1.18 (0.83, 1.67) |
| Hypertension | 1.29 (1.05, 1.57) |
| Pain related joint complaints | 1.45 (1.16, 1.81) |
| Other patient characteristics | |
| Activities of daily living per score | 1.00 (0.81, 1.24) |
| Subjective health | |
| Better | 1.00 (reference) |
| Same | 1.09 (0.88, 1.34) |
| Worse | 1.50 (1.06, 2.13) |
| Alcohol | |
| None | 1.00 (reference) |
| Former | 0.94 (0.30, 2.97) |
| Current | 0.86 (0.68–1.07) |
| Smoking | |
| None | 1.00 (reference) |
| Former | 0.92 (0.71, 1.19) |
| Current | 1.36 (1.01, 1.82) |
Cox proportional hazards analysis. CESD, Center for Epidemiologic Studies Depression Scale; HADS, Hospital Anxiety and Depression Scale. Numbers in bold indicate independent determinants of new-onset chronic benzodiazepine use.
The results of the second analysis, in the subsample of participants who had filled at least one benzodiazepine prescription, are provided in Table 4. Female gender, depressive complaints, hypertension and current smoking were no longer associated with new-onset chronic use. Only, increasing age, public health insurance and pain related joint complaints predicted new-onset chronic benzodiazepine use in those who had filled at least one prescription. Again, living alone was associated with a decreased risk of benzodiazepine use. Post-hoc we analyzed whether a hypnotic or anxiolytic as the first benzodiazepine is associated with the risk of chronic use and found that, while adjusting for age and sex, a hypnotic first benzodiazepine increased the risk of chronic use compared with an anxiolytic first benzodiazepine (HR 1.39; 95% CI 1.15, 1.68).
Table 4.
Determinants of chronic benzodiazepine use in older users* (n = 2490)
| Variable | Hazard rate† (95% CI) |
|---|---|
| Socio-demographic variables | |
| Age (years) | 1.04 (1.03, 1.06) |
| Female | 1.15 (0.90, 1.47) |
| Public health insurance | 1.24 (1.01, 1.51) |
| Social support | |
| Living situation | |
| With partner or other person | 1.00 (reference) |
| Alone | 0.73 (0.58, 0.93) |
| Place of residence | |
| Independently | 1.00 (reference) |
| Service flat | 1.41 (1.04, 1.92) |
| Home for the elderly | 1.50 (0.78, 2.89) |
| Psychiatric and physical health | |
| Depression, HADS-D ≥ 8 or CES-D ≥ 16 | 1.22 (0.91, 1.64) |
| Hypertension | 1.11 (0.91, 1.35) |
| Pain related joint complaints | 1.32 (1.06, 1.64) |
| Other patient characteristics | |
| Subjective health | |
| Better | 1.00 (reference) |
| Same | 1.05 (0.86, 1.29) |
| Worse | 1.34 (0.96, 1.87) |
| Alcohol | |
| None | 1.00 (reference) |
| Former | 0.84 (0.26, 2.65) |
| Current | 0.82 (0.66, 1.03) |
| Smoking | |
| None | 1.00 (reference) |
| Former | 0.88 (0.68, 1.15) |
| Current | 1.21 (0.90, 1.62) |
The subsample of participants who filled at least one prescription.
Cox proportional hazards analysis. Numbers in bold indicate independent determinants of new-onset chronic benzodiazepine use.
Discussion
In this prospective study, we analyzed social and health related risk factors of new-onset chronic benzodiazepine use in an older community-dwelling population. Symptoms of depression, hypertension, pain related joint complaints, and the perception of poor physical health were associated with an increased risk of chronic benzodiazepine use. However, in the subsample of participants who had filled at least one prescription, having pain related joint complaints was the only health related risk factor associated with new-onset chronic benzodiazepine use. In both population samples, increasing age, and public health insurance increased the risk of chronic benzodiazepine use, and living alone reduced the risk of new-onset chronic use.
To our knowledge, we present the first prospective study which has established that both social and health related patient characteristics determine new-onset chronic benzodiazepine use in elderly people. There are two other longitudinal studies that investigated risk factors of new-onset chronic benzodiazepine use in an older, general population, but these were limited by their design [1, 15]. In the study by Egan et al. the number of cases was small (n = 18), so that lack of power may explain that the risk of chronic use was not associated with health status, cognitive status, and anxiety, but with increasing age only [1]. The other study, by Simon et al. did not take somatic or psychological health status into account [15].
Several follow-up studies of elderly users have been published. They analyzed the risk factors for chronic benzodiazepine use in older people who were users already; in other words, risk factors for continuous, not new-onset, chronic use were studied [5–9, 16]. In these studies, neither anxiety, depressive symptoms, cognitive impairment, nor chronic somatic diseases were related to an increased risk of continuous chronic use [6, 7, 9], although having a chronic somatic disease or pain did show a positive association [5, 8]. We also found a relationship with pain related joint complaints in the participants who had filled at least one prescription. This is a disturbing finding, not only because pain related joint complaints, like benzodiazepine use, increase the risk of falls, but also because pain is managed more effectively with analgesics. Moreover, not only are pain related joint complaints related to chronic use, they are often the primary reason for initiating the treatment, as shown by the chart review of Simon et al.[15].
Contrary to our hypothesis that living with a partner or other person would be associated with a reduced risk of new-onset chronic benzodiazepine use, living alone protected against chronic use. Several explanations are conceivable, if this is not a chance finding. First, a person who lives alone does not adjust his sleeping pattern to that of someone else. Responding to the stimuli of one's own circadian rhythm is probably beneficial for one's night's rest. Secondly, living alone may be an indicator of good health. Although we adjusted for various psychiatric and somatic factors the possibility of residual confounding cannot be ruled out. Thirdly, one could argue that persons who live alone pay less attention to a sleeping disorder. However, even in those who filled at least one benzodiazepine prescription, and therefore must have discussed this problem with a physician, living alone was protective against chronic use as well. Previous studies that analyzed the role of living alone or marital status found no association with continuous benzodiazepine use [6–9].
With respect to demographic risk factors, two issues need to be discussed. First, a common perception, based on findings from cross-sectional studies, is that the risk of using benzodiazepines increases with age [17–19]. Our study and the other prospective study adjusting for health status also found that age increased the risk of new-onset chronic use in the general elderly population [1]. This also applied to the subsample of participants who filled at least one prescription. On the other hand, the follow-up studies among users did not find an increasing risk with age, even if they adjusted for health status as well [5, 7–9]. Age seems to be an independent risk factor for new-onset chronic benzodiazepine use, but not for continuing chronic use. This inconsistency in results could be due to different prescription patterns in the different samples. Follow-up studies of benzodiazepine users cannot rule out that the different age groups already have different usage patterns at baseline. For example, younger persons may be more likely than elderly persons to be either nonusers or chronic users.
Another common perception derived from cross-sectional studies is that female sex increases the risk of chronic use [17–19]. We found that female gender was associated with an increased risk in the general population, but this relationship no longer existed in the subsample of participants who filled at least one prescription. This is consistent with many other studies among users [5, 6, 9, 16]. Thus, neither male nor female gender increases the risk to continue benzodiazepine use once a person has begun. The explanation for the predominance of females among chronic users must therefore lie in their high rate to start benzodiazepine use. This suggests that the threshold to begin, not difficulty to stop, is gender related.
Our study has several strengths. A prospective design was combined with a rigorous selection of the study population. Participants who were already chronic users at baseline were carefully identified, using a prebaseline selection period of 2 years. In addition, benzodiazepine use was registered on a continuous basis and the follow-up of 10 years was relatively long. Both contributed to an accurate identification of new-onset chronic use. Although utilization data are more precise than prescription data, we consider day-to-day prescription data more precise than retrospective self-reported use on which the follow-up studies among users have been based.
Following the recommendations of Zandstra et al.[2], we chose a quantitative instead of clinical, DSM-IV based definition of chronic benzodiazepine use. Most elderly benzodiazepine users do not show signs of dependence such as escalation of dose, withdrawal symptoms, or detrimental effects on health and daily life [20]. It is the chronic use of benzodiazepines, not just the misuse, which may result in adverse events in the elderly, such as fractures.
A limitation of this study was that anxiety and depressive complaints could not be classified according to the DSM-IV. Moreover, we only had information on complaints of anxiety in a subsample of participants. This limited our power to detect an association between anxiety and new-onset chronic benzodiazepine use. Also, we could not take into account perceived social support, personality disorders, or attitudes towards benzodiazepine use [21].
Given that 17.7% of the users in our cohort proceeded to chronic benzodiazepine use after the first prescription, this first prescription implies a substantial risk to become a chronic user. A number of demographic, social and health related patient characteristics predicted new-onset chronic benzodiazepine use in the general elderly population, but very few characteristics predicted chronic use once patients had received their first prescription. As it is difficult to identify the patients who are at risk of chronic use, this group cannot be targeted with specific prevention programs. This further supports the already existing recommendations to be very prudent with initiating benzodiazepine therapy in older people. Finally, our finding that living alone protects against chronic benzodiazepine use indicates that more awareness is needed for the role of social patient characteristics in the development of chronic benzodiazepine use.
Financial disclosure: no funding source or affiliation of any of the authors is relevant to the subject of this article.
REFERENCES
- 1.Egan M, Moride Y, Wolfson C, Monette J. Long-term continuous use of benzodiazepines by older adults in Quebec: prevalence, incidence and risk factors. J Am Geriatr Soc. 2000;48:811–6. doi: 10.1111/j.1532-5415.2000.tb04758.x. [DOI] [PubMed] [Google Scholar]
- 2.Zandstra SM, Furer JW, van de Lisdonk EH, van't HM, Bor JH, van Weel C, Zitman FG. Different study criteria affect the prevalence of benzodiazepine use. Soc Psychiatry Psychiatr Epidemiol. 2002;37:139–44. doi: 10.1007/s001270200006. [DOI] [PubMed] [Google Scholar]
- 3.Herings RM, Stricker BH, de Boer A, Bakker A, Sturmans F. Benzodiazepines and the risk of falling leading to femur fractures. Dosage more important than elimination half-life. Arch Intern Med. 1995;155:1801–7. [PubMed] [Google Scholar]
- 4.Barker MJ, Greenwood KM, Jackson M, Crowe SF. Cognitive effects of long-term benzodiazepine use: a meta-analysis. CNS Drugs. 2004;18:37–48. doi: 10.2165/00023210-200418010-00004. [DOI] [PubMed] [Google Scholar]
- 5.Barnas C, Whitworth AB, Fleischhacker WW. Are patterns of benzodiazepine use predictable? A follow-up study of benzodiazepine users. Psychopharmacology (Berl) 1993;111:301–5. doi: 10.1007/BF02244945. [DOI] [PubMed] [Google Scholar]
- 6.Fourrier A, Letenneur L, Dartigues JF, Moore N, Begaud B. Benzodiazepine use in an elderly community-dwelling population. Characteristics of users and factors associated with subsequent use. Eur J Clin Pharmacol. 2001;57:419–25. doi: 10.1007/s002280100326. [DOI] [PubMed] [Google Scholar]
- 7.Jorm AF, Grayson D, Creasey H, Waite L, Broe GA. Long-term benzodiazepine use by elderly people living in the community. Aust N Z J Public Health. 2000;24:7–10. doi: 10.1111/j.1467-842x.2000.tb00715.x. [DOI] [PubMed] [Google Scholar]
- 8.Gray SL, Eggen AE, Blough D, Buchner D, LaCroix AZ. Benzodiazepine use in older adults enrolled in a health maintenance organization. Am J Geriatr Psychiatry. 2003;11:568–76. [PubMed] [Google Scholar]
- 9.Neutel CI, Walop W, Patten SB. Can continuing benzodiazepine use be predicted? Can J Clin Pharmacol. 2003;10:202–6. [PubMed] [Google Scholar]
- 10.Hofman A, Grobbee DE, de Jong PT, van den Ouweland FA. Determinants of disease and disability in the elderly: the Rotterdam Elderly Study. Eur J Epidemiol. 1991;7:403–22. doi: 10.1007/BF00145007. [DOI] [PubMed] [Google Scholar]
- 11.Beekman AT, Deeg DJ, Van Limbeek J, Braam AW, De Vries MZ, Van Tilburg W. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): results from a community-based sample of older subjects in The Netherlands. Psychol Med. 1997;27:231–5. doi: 10.1017/s0033291796003510. [DOI] [PubMed] [Google Scholar]
- 12.Spinhoven P, Ormel J, Sloekers PP, Kempen GI, Speckens AE, Van Hemert AM. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol Med. 1997;27:363–70. doi: 10.1017/s0033291796004382. [DOI] [PubMed] [Google Scholar]
- 13.Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40:922–35. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 14.Pincus T, Summey JA, Soraci SA, Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26:1346–53. doi: 10.1002/art.1780261107. [DOI] [PubMed] [Google Scholar]
- 15.Simon GE, VonKorff M, Barlow W, Pabiniak C, Wagner E. Predictors of chronic benzodiazepine use in a health maintenance organization sample. J Clin Epidemiol. 1996;49:1067–73. doi: 10.1016/0895-4356(96)00139-4. [DOI] [PubMed] [Google Scholar]
- 16.Isacson D. Long-term benzodiazepine use: factors of importance and the development of individual use patterns over time – a 13-year follow-up in a Swedish community. Soc Sci Med. 1997;44:1871–80. doi: 10.1016/s0277-9536(96)00296-1. [DOI] [PubMed] [Google Scholar]
- 17.Magrini N, Vaccheri A, Parma E, D'Alessandro R, Bottoni A, Occhionero M, Montanaro N. Use of benzodiazepines in the Italian general population: prevalence, pattern of use and risk factors for use. Eur J Clin Pharmacol. 1996;50:19–25. doi: 10.1007/s002280050063. [DOI] [PubMed] [Google Scholar]
- 18.van der Waals FW, Mohrs J, Foets M. Sex differences among recipients of benzodiazepines in Dutch general practice. BMJ. 1993;307:363–6. doi: 10.1136/bmj.307.6900.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tu K, Mamdani MM, Hux JE, Tu JB. Progressive trends in the prevalence of benzodiazepine prescribing in older people in Ontario, Canada. J Am Geriatr Soc. 2001;49:1341–5. doi: 10.1046/j.1532-5415.2001.49262.x. [DOI] [PubMed] [Google Scholar]
- 20.Piper A., Jr Addiction to benzodiazepines – how common? Arch Fam Med. 1995;4:964–70. doi: 10.1001/archfami.4.11.964. [DOI] [PubMed] [Google Scholar]
- 21.van Hulten R, Bakker AB, Lodder AC, Teeuw KB, Bakker A, Leufkens HG. The impact of attitudes and beliefs on length of benzodiazepine use: a study among inexperienced and experienced benzodiazepine users. Soc Sci Med. 2003;56:1345–54. doi: 10.1016/s0277-9536(02)00133-8. [DOI] [PubMed] [Google Scholar]


