Abstract
AIMS
Co-administration of standard-dose voriconazole and efavirenz results in a substantial decrease in voriconazole levels, while concurrently increasing efavirenz levels. Hence, concomitant use of standard doses of these drugs was initially contraindicated. This study assessed different dose combinations of efavirenz and voriconazole, with the goal of attaining a dose combination that provides systemic exposures similar to standard-dose monotherapy with each drug.
METHODS
This was an open-label, four-treatment, multiple-dose, fixed-sequence study in 16 healthy males. Steady-state pharmacokinetics were assessed following two test treatments (voriconazole 300 mg q12 h + efavirenz 300 mg q24 h and voriconazole 400 mg q12 h + efavirenz 300 mg q24 h) and compared with standard-dose monotherapy (voriconazole 200 mg q12 h or efavirenz 600 mg q24 h).
RESULTS
Dose adjustment to voriconazole 300 mg q12 h with efavirenz 300 mg q24 h decreased voriconazole area under the concentration–time curve (AUCτ) and maximum concentration (Cmax), with changes of −55% [90% confidence interval (CI) −62, −45] and −36% (90% CI −49, −21), respectively, when compared with monotherapy. Voriconazole 400 mg q12 h plus efavirenz 300 mg q24 h decreased voriconazole AUCτ (−7%; 90% CI −23, 13) and increased Cmax (23%; 90% CI −1, 53), while increasing efavirenz AUCτ (17%; 90% CI 6, 29) and not changing Cmax when compared with the respective monotherapy regimens. No serious adverse events were observed with voriconazole plus efavirenz.
CONCLUSIONS
When co-administered, voriconazole dose should be increased to 400 mg q12 h and efavirenz dose decreased to 300 mg q24 h in order to provide systemic exposures similar to standard-dose monotherapy.
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Efavirenz 400 mg q24 h reduces exposure to voriconazole 200 mg q12 h when the two drugs are co-administered.
Furthermore, voriconazole increases the systemic exposure of efavirenz.
Co-administration was therefore initially contraindicated.
WHAT THIS STUDY ADDS
The doses of efavirenz and voriconazole can be adjusted to provide adequate exposure to both drugs when the two are co-administered, without compromising safety.
Appropriate adjustment of doses for both drugs may thus represent an alternative to a mere contraindication.
Keywords: efavirenz, pharmacokinetics, voriconazole
Introduction
Opportunistic fungal infections can occur in human immunodeficiency virus (HIV)–infected subjects due to compromised immune function, and such infections are likely to require antifungal therapy. Voriconazole is an extended-spectrum triazole antifungal agent, available as oral and i.v. formulations, with demonstrated efficacy against invasive aspergillosis and candidaemia [1, 2]. It is at least as effective as fluconazole in treating oesophageal candidiasis in immunocompromised patients, including those with acquired immunodeficiency syndrome [3]. Hence, there is a possibility that voriconazole would be coprescribed with efavirenz, a non-nucleoside reverse transcriptase inhibitor and a component of several highly active antiretroviral regimens.
In vitro, voriconazole undergoes N-oxidation by the cytochrome P450 isozymes CYP2C19, CYP2C9 and CYP3A4 [4], whereas efavirenz is primarily metabolized by the CYP2B6 and CYP3A4 isozymes [5]. Voriconazole also inhibits CYP2C9 and CYP2C19, but has only a mild inhibitory effect on CYP3A4-mediated metabolism [6]. Voriconazole N-oxide is the major circulating metabolite of voriconazole and does not possess antifungal activity. Efavirenz interacts in a complex manner with CYP450 enzymes, both inhibiting [7] and inducing [5, 8–10] CYP3A4, CYP2C19 and CYP2C9.
The potential for pharmacokinetic interactions between co-administered voriconazole and efavirenz has been confirmed in a previous study, in which efavirenz 400 mg q24 h markedly decreased the steady-state Cmax and AUCτ of voriconazole, with mean changes of −66% [90% confidence interval (CI) −57, −73] and −80% (90% CI −75, −84), respectively. In addition, voriconazole 200 mg q12 h increased the steady-state Cmax and AUCτ of efavirenz by 37% (90% CI 29, 46) and 43% (90% CI 36, 51), respectively [11]. It should be noted that the standard therapeutic dose of efavirenz is 600 mg, and that co-administration of standard-dose efavirenz with voriconazole may therefore result in a greater magnitude of change in voriconazole pharmacokinetics. Co-administration of standard doses of voriconazole (200 mg q12 h) and efavirenz (600 mg q24 h) was therefore initially contraindicated [11]. Nevertheless, concomitant administration of both drugs as part of effective therapy has been reported in the literature. In one instance, efavirenz (600 mg q24 h) and voriconazole (200 mg q12 h, later increased to 350 mg q12 h) were co-administered in the successful treatment of pulmonary aspergillosis and breakthrough oesophageal candidiasis in a HIV patient [12].
The goal of this investigation was to explore dose adjustments that could potentially provide systemic exposures similar to those observed with therapeutic maintenance doses of voriconazole (i.e. 200 mg orally, q12 h) or efavirenz (600 mg orally, q24 h) alone.
Methods
Study subjects
The study was conducted in compliance with the ethical principles of the Declaration of Helsinki (revised South Africa, 1996), all International Conference on Harmonisation Good Clinical Practice Guidelines, and local laws and regulations. It was approved by the Ethics Committee of the Hôpital Erasme, Brussels, Belgium. Written informed consent was obtained from all subjects prior to study commencement.
Enrolled subjects were healthy male nonsmokers aged 18–55 years with a body mass index (BMI) of 18–30 kg m−2. Subjects were excluded for: evidence or history of clinically significant disease; a positive test for hepatitis; any condition possibly affecting drug absorption and use of prescription or nonprescription drugs within 7 days of the study, except paracetamol at doses of up to 2 g day−1. Grapefruit or products containing grapefruit were not allowed from 7 days prior to study until termination.
Study design
This was an open-label, multiple-dose, fixed-sequence design study (Figure 1). Subjects received voriconazole (400 mg q12 h on day 1, then 200 mg q12 h on days 2–3) in period 1. After a wash-out of 6 days, subjects received evening doses of efavirenz (600 mg q24 h on days 11–20, then 300 mg q24 h on days 21–34) in period 2. On days 21–27, subjects received voriconazole 300 mg q12 h, which was subsequently increased to 400 mg q12 h on days 28–34. Subjects returned for follow-up within 7 days of the end of treatment. Serial pharmacokinetic samples were collected on day 3 (to determine concentrations of voriconazole and its N-oxide metabolite), day 19 (to determine concentrations of efavirenz), as well as days 26 and 33 (to determine concentrations of all three analytes) at the following time points: predose, and at 0.5, 1, 2, 4, 6, 8, 10, 12, 16, 20 and 24 h postdose. To assess attainment of steady-state levels, trough samples were collected on day 2 and every other day starting on day 12 (except on days of serial pharmacokinetic samples).
Figure 1.
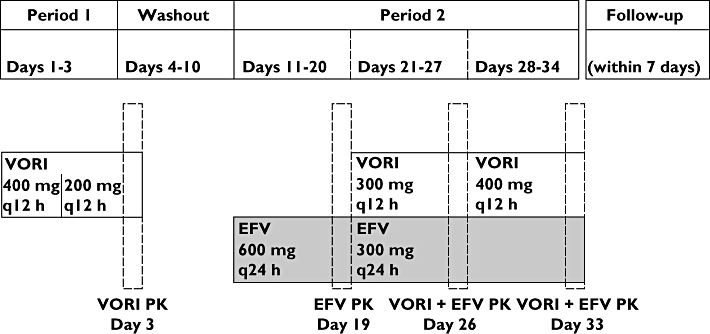
Schematic study design. VORI, voriconazole; EFV, efavirenz; q24 h, once a day; q12 h, twice a day; PK, pharmacokinetics
Sample analyses and pharmacokinetics
Plasma samples were assayed for voriconazole and the N-oxide metabolite using a validated liquid chromatography coupled to tandem-mass spectrometry (LC/MS/MS) method (PPD, Richmond, VA, USA). The plasma samples (0.100 ml) were extracted using a solid-phase extraction procedure, and UK-103 446-01 was employed as an internal standard in the assay for both compounds. For voriconazole, quantification was achieved using peak area ratios of nine calibration standards over a range of 10.0–5000 ng ml−1. For the N-oxide metabolite, quantification was achieved using peak area ratios of eight calibration standards over a range of 20.0–5000 ng ml−1. Calibration curves were constructed using the best-fit line determined by linear regression of the calibration data utilizing a weighting factor of 1/concentration. The accuracy (% difference from nominal) of the quality control samples (QCs) used during sample analysis ranged from −2.68 to 4.91%, with a precision (as measured by percentage relative standard deviation) of ≤16.5% for voriconazole. For the N-oxide metabolite, the accuracy of the QCs ranged from −2.82 to 1.75% with a precision of ≤11.9%.
Plasma samples were assayed for efavirenz using a validated high-performance liquid chromatography with ultraviolet detection method (PPD). Individual 0.100-ml aliquots of plasma containing analyte and internal standard were extracted using a liquid/liquid extraction procedure. Quantification was achieved using peak height ratios of eight calibration standards over a range of 0.100–10.0 μg ml−1. Calibration curves were constructed using the best-fit line determined by linear regression of the calibration data utilizing a weighting factor of 1/ concentration. The accuracy (% difference from nominal) of the QCs used during sample analysis ranged from −1.02 to 0.843% with a precision (as determined by percentage relative standard deviation) of ≤5.79%.
Pharmacokinetic analysis was carried out with WinNonlin™ V.3.2 (Pharsight®, Mountain View, CA, USA) using standard noncompartmental methods. Cmax was estimated directly from experimental data; Tmax was defined as the time of first occurrence of Cmax. The area under the plasma concentration–time curve within the dosing interval (τ), where τ = 12 h for voriconazole and voriconazole N-oxide, and τ = 24 h for efavirenz, was estimated using the log-linear trapezoidal approximation.
Statistical analysis
Assuming a drop-out rate of 25%, 16 subjects were recruited into the study to ensure completion by at least 12 subjects. A sample size of 12 subjects was to provide 90% CIs for the difference between treatments of ± 0.10 and 0.15 on the natural log scale for voriconazole AUC0–τ and Cmax, respectively, with 80% coverage probability. The calculations estimating these 90% CIs were based on intrasubject coefficient for variation estimates for voriconazole (0.11 and 0.17) and efavirenz (0.08 and 0.11) for AUCτ and Cmax, respectively, obtained from previous studies (Pfizer study 150–232 for voriconazole and A1501048 for efavirenz; Pfizer, data on file). The precision of the study was calculated using the methods of Kupper and Hafner extended to crossover designs [13].
Natural log-transformed Cmax and AUCτ were analysed separately for each analyte using a mixed effects model, with treatment as fixed effects and subject as random effect. This model was implemented using the SAS procedure MIXED, with REML method, compound symmetry and Satterthwaite degrees of freedom algorithm (SAS Inc., Cary, NC, USA). Adjusted mean treatment differences in Cmax and AUCτ and corresponding CIs were estimated from the model. These differences and 90% CIs were exponentiated to derive estimates of the ratios of geometric means between treatments and the 90% CI for these ratios. No adjustment was made for multiple comparisons in any analyses. Descriptive statistics were provided for all pharmacokinetic parameters for voriconazole, its N-oxide metabolite and efavirenz.
Results
Study population
All 16 subjects received voriconazole alone and efavirenz alone, 15 received efavirenz plus voriconazole 300 mg and 14 received efavirenz plus voriconazole 400 mg. One subject was discontinued because of an efavirenz-related adverse event (AE) and one subject was no longer willing to participate following the efavirenz plus voriconazole 300-mg phase. Subjects had a mean age of 29.6 years (range 20–42) and a mean BMI of 24.3 kg m−2 (range 19–30); 12 subjects (75%) were White, three (19%) were Black and one (6%) was Asian.
Pharmacokinetics
Co-administration of voriconazole 300 mg q12 h with efavirenz 300 mg q24 h resulted in mean voriconazole concentrations that were considerably lower than with voriconazole 200 mg q12 h alone (Figure 2A). Mean N-oxide metabolite concentrations following co-administration of voriconazole 300 mg and efavirenz were generally lower than those with voriconazole 200 mg alone, except between 2 and 6 h postdose (Figure 2B); mean efavirenz concentrations were lower than with efavirenz 600 mg alone (Figure 2C). These trends in the concentration–time profiles are also reflected in the arithmetic mean (SD) values of Cmax and AUCτ for these analytes (Table 1). Statistical analyses indicated that the ratios of the adjusted geometric mean AUCτ and Cmax of voriconazole decreased by 55% and 36%, respectively (Table 2). On the other hand, voriconazole N-oxide metabolite levels did not change appreciably, as the 90% CIs for both AUCτ and Cmax were contained within the 80–125% range (Table 2). The addition of voriconazole 300 mg to efavirenz 300 mg had little effect on the AUCτ of efavirenz compared with efavirenz 600 mg alone, as the 90% CI was contained within the 80–125% equivalence bounds, but efavirenz Cmax was lowered by 14% (Table 2).
Figure 2.
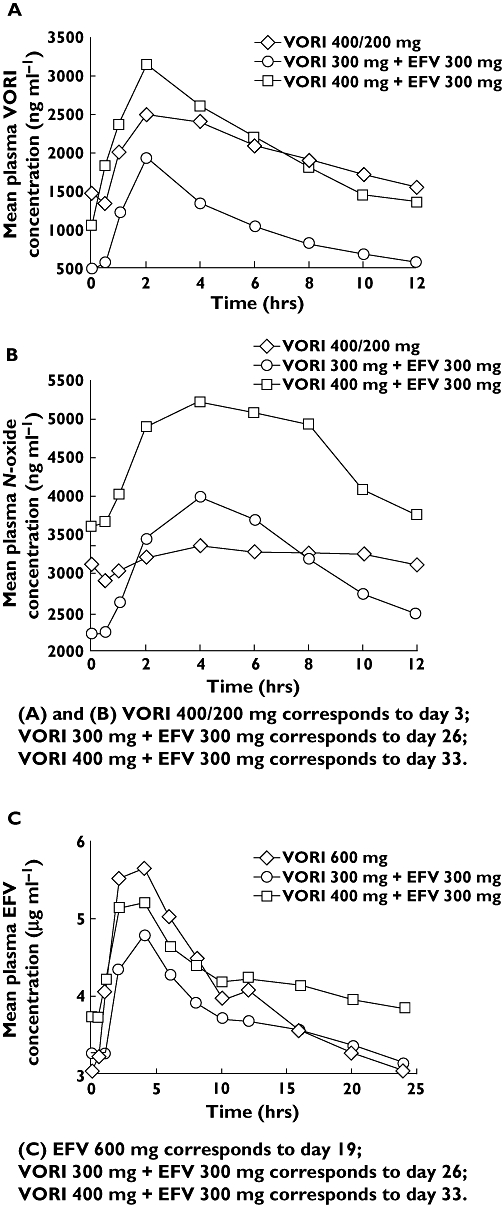
Mean steady-state plasma concentration–time profiles of (A) voriconazole, (B) voriconazole N-oxide metabolite and (C) efavirenz following administration of 200 mg q12 h voriconazole alone or 600 mg q24 h efavirenz alone or co-administration of either 300 or 400 mg q12 h voriconazole with 300 mg q24 h efavirenz. VORI, Voriconazole; EFV, efavirenz
Table 1.
Arithmetic mean (with standard deviation) steady-state pharmacokinetic parameters for voriconazole, voriconazole N-oxide and efavirenz
| Parameter | Reference (VORI 400/200* or EFV 600†) Mean ± SD | Median (range) | Test (VORI 300/EFV 300‡) Mean ± SD | Median (range) | Test (VORI 400/EFV 300§) Mean ± SD | Median (range) |
|---|---|---|---|---|---|---|
| Voriconazole | ||||||
| AUCτ (ng.h.ml−1) | 24 000 ± 14 200 | 23 800 (5 290–60 000) | 12 500 ± 12 400 | 8 080 (2 560–43 500) | 25 100 ± 23 600 | 15 200 (6 640–79 800) |
| Cmax (ng ml−1) | 2 740 ± 1 230 | 2 990 (956–5 310) | 1 990 ± 1 400 | 1 790 (339–4 950) | 3 520 ± 2 120 | 2 650 (1 270–7 910) |
| Tmax (h) | – | 2.0 (1.0–6.0) | – | 2.0 (1.0–4.0) | – | 2.0 (0.5–6.0) |
| Voriconazole N-oxide | ||||||
| AUCτ (ng.h.ml−1) | 39 300 ± 7 420 | 39 800 (24 600–49 200) | 38 900 ± 10 100 | 37 400 (22 000–56 400) | 55 400 ± 13 000 | 53 200 (35 800–80 100) |
| Cmax (ng ml−1) | 3 780 ± 674 | 3 950 (2 710–4 860) | 4 060 ± 763 | 4 050 (2 950–5 270) | 5 470 ± 1 090 | 5 530 (4 040–7 090) |
| Tmax (h) | – | 5.0 (0.0–12.0) | – | 4.0 (2.0–6.0) | – | 6.0 (4.0–8.0) |
| Efavirenz | ||||||
| AUCτ (μg.h.ml−1) | 97.4 ± 45.4 | 85.6 (48.9–209) | 89.4 ± 28.2 | 87.7 (43.4–165) | 103 ± 28.2 | 109 (50.5–165) |
| Cmax (μg ml−1) | 6.09 ± 1.92 | 5.60 (3.81–9.85) | 4.97 ± 1.10 | 5.01 (2.87–7.70) | 5.50 ± 1.12 | 5.78 (3.54–8.06) |
| Tmax (h) | – | 2.0 (2.0–12.0) | – | 4.0 (2.0–6.0) | – | 4.0 (2.0–6.0) |
Day 3.
Day 19.
Day 26.
Day 33. VORI, voriconazole; EFV, efavirenz; SD, standard deviation.
Table 2.
Statistical comparison between reference and test treatments for steady-state pharmacokinetic parameters of voriconazole, voriconazole N-oxide and efavirenz
| Analyte | Parameter | VORI 300/EFV 300 ratio (90% CI)* | VORI 400/EFV 300 ratio (90% CI)† |
|---|---|---|---|
| Voriconazole | AUCτ (ng.h.ml−1) | 45.49% (37.54, 55.11) | 93.37% (77.06, 113.13) |
| Cmax (ng ml−1) | 63.61% (51.08, 79.21) | 122.95% (98.74, 153.11) | |
| Voriconazole N-oxide | AUCτ (ng.h.ml−1) | 99.32% (93.54, 105.46) | 142.66% (134.37, 151.47) |
| Cmax (ng ml−1) | 108.99% (104.80, 113.35) | 146.62% (140.98, 152.48) | |
| Efavirenz | AUCτ (μg.h.ml−1) | 101.01% (91.76, 111.21) | 117.23% (106.48, 129.05) |
| Cmax (μg ml−1) | 86.14% (79.38, 93.48) | 95.67% (88.16, 103.82) |
Ratios are based on the adjusted geometric means and were calculated as day 26/day 3 for voriconazole and N-oxide, and as day 26/day 19 for efavirenz.
Ratios are based on the adjusted geometric means and were calculated as day 33/day 3 for voriconazole and N-oxide, and as day 33/day 19 for efavirenz. VORI, voriconazole; EFV, efavirenz.
Co-administration of voriconazole 400 mg q12 h with efavirenz 300 mg q24 h resulted in mean voriconazole concentrations that were higher until 6 h postdose and lower thereafter, compared with voriconazole 200 mg q12 h alone (Figure 2A). Based on adjusted geometric mean ratios, voriconazole AUCτ was lowered by 7%, whereas Cmax was increased by 23% (Table 2). The AUCτ and Cmax of the N-oxide metabolite were increased by 43% and 47%, respectively (Table 2). Mean efavirenz concentrations were lower than with efavirenz 600 mg q24 h alone until 8 h postdose and higher thereafter (Figure 2C). Based on adjusted geometric mean ratios, efavirenz AUCτ was increased by 17% compared with efavirenz 600 mg alone, whereas the Cmax was similar (i.e. 90% CI contained within 80–125%) (Table 2). Mean trough concentrations of efavirenz are shown in Figure 3. During administration of efavirenz alone (600 mg q24 h), steady-state trough concentrations of efavirenz appeared to be reached by day 19 (mean trough concentration of 3.02 ng ml−1). Similarly, steady-state levels of efavirenz appeared to be achieved by day 26 (mean trough concentration of 3.27 ng ml−1) during co-administration of efavirenz 300 mg q24 h with voriconazole 300 mg q12 h, and by day 33 (mean trough concentration of 3.73 ng ml−1) during co-administration with voriconazole 400 mg q12 h.
Figure 3.
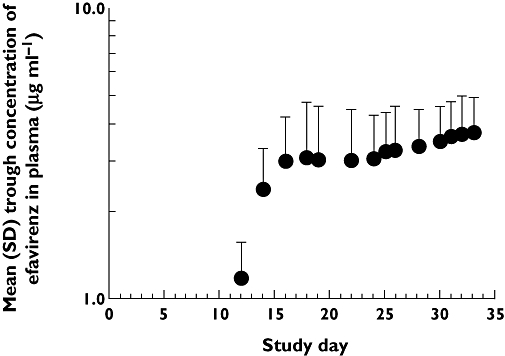
Mean (SD) trough plasma concentrations of efavirenz over the course of the study
The individual subject changes in AUC of voriconazole and efavirenz are presented in Figure 4. As evident from Figure 4 and Table 1, during co-administration the variability in the pharmacokinetics of voriconazole appears to increase, whereas that of efavirenz appears to decrease compared with monotherapy.
Figure 4.
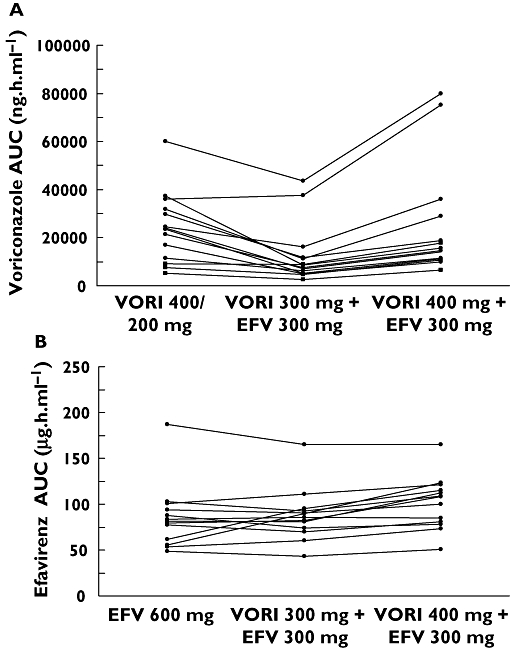
Individual subject change in AUC of voriconazole (A) and efavirenz (B). VORI, voriconazole; EFV, efavirenz
Safety
All subjects in this study reported at least one AE, for a total number of 224; 92% of these were considered treatment related by the investigator. Most AEs were mild (87.5%), but 12.5% were moderate in intensity. The most frequently reported treatment-emergent AEs, regardless of treatment received, were dizziness (n = 27), headache (n = 18) and fatigue (n = 16). During the voriconazole monotherapy phase of the study, visual disturbances (n = 11), nervous system events (n = 9) and psychiatric events (n = 8) were the most common treatment-related AEs (total of 34). The most frequent treatment-related AEs (total of 66) during the efavirenz monotherapy phase were reported to be nervous system (n = 26), gastrointestinal (n = 9) and vascular (n = 7) events. The occurrence of treatment-related AEs during the voriconazole 300 mg plus efavirenz 300 mg (total of 58) and voriconazole 400 mg plus efavirenz 300 mg (total of 49) phases was very similar. Most frequent were visual disturbances (n = 9 and 12, respectively), nervous system events (n = 14 and 12, respectively) and gastrointestinal events (n = 9 and 7, respectively).
No deaths or serious AEs were reported. One subject was permanently discontinued from study medication due to elevated aspartate aminotransferase following 9 days of efavirenz 600 mg. This was first reported 12 h postdose on day 19 and was considered related to the study drug; it resolved after efavirenz discontinuation. There were no other clinically significant changes in laboratory assessments and no clinically significant changes in vital signs or ECGs during the study.
Discussion
The metabolic profiles for voriconazole and efavirenz indicate a potential for pharmacokinetic drug interaction when co-administered. This has been confirmed in a previous study, in which efavirenz (400 mg q24 h) markedly decreased mean steady-state Cmax and AUCτ of voriconazole, and voriconazole in turn increased the systemic exposure of efavirenz [11]. The standard therapeutic dose of efavirenz (600 mg q24 h) is greater than the dose used in that study, and may therefore have an even more significant effect on voriconazole pharmacokinetics. Co-administration of efavirenz with standard doses of voriconazole (200 mg q12 h) was therefore initially contraindicated [11].
The substantial decrease in steady-state voriconazole exposure following co-administration with steady-state efavirenz 400 mg is likely to reflect induction of CYP450 (specifically CYP3A4, CYP2C19 and CYP2C9) metabolic activity by efavirenz [5, 10]. The pharmacologically inactive N-oxide is the initial and most abundant voriconazole metabolite [14]. As evident from Figure 2B, the elimination phase of the voriconazole N-oxide metabolite following co-administration with efavirenz was much steeper than following administration of voriconazole alone, suggesting that degradation of the voriconazole N-oxide metabolite may also be induced by efavirenz. The moderate increases in steady-state efavirenz concentrations are probably due to inhibition of CYP3A4 by voriconazole. Therefore, it appears that the two-way interaction between voriconazole and efavirenz involves complex dynamics between the inductive effect of efavirenz and the inhibitory effect of voriconazole on CYP450 enzymes.
In the present study, the dose of voriconazole was increased to 300 mg or 400 mg q12 h, whereas the dose of efavirenz was reduced to 300 mg q24 h. The objective of these dose adjustments, which were based on the previously obtained data mentioned above [11], was to achieve systemic exposures comparable to those obtained by single agent at a therapeutic dose (i.e. 200 mg q12 h for voriconazole and 600 mg q24 h for efavirenz). The results indicate that 300 mg voriconazole given in combination with 300 mg efavirenz would yield suboptimal exposures to voriconazole. Compared with the respective single-agent treatments, the combination of voriconazole 400 mg q12 h with efavirenz 300 mg q24 h resulted in voriconazole AUCτ decreasing by 7%, whereas voriconazole Cmax increased by 23%; efavirenz AUCτ increased by 17%, whereas efavirenz Cmax was equivalent. These pharmacokinetic parameters are therefore similar to those achieved by voriconazole and efavirenz monotherapy. Notably, during co-administration with phenytoin, which, like efavirenz, is an inducer of CYP450, it is recommended that oral voriconazole doses be similarly increased to 400 mg q12 h [15, 16].
The 23% increase in voriconazole Cmax (when co-administered with efavirenz) is unlikely to be significant from a safety perspective, since higher systemic levels of voriconazole are observed in certain clinical situations that require the voriconazole dose to be increased. For example, higher oral voriconazole maintenance doses of 300 mg q12 h are indicated if the patient response is inadequate with a 200-mg dose, and this results in a 2.5-fold increase in voriconazole exposure (AUCτ) [16]. Similar conclusions can be drawn with respect to the increased plasma levels of the N-oxide (AUCτ and Cmax by 43% and 47%, respectively) observed with the increase in voriconazole dose to 400 mg. The systemic levels of the N-oxide metabolite were not assessed in any previous studies that used higher doses of voriconazole. However, an increase in voriconazole dose from 200 mg to 300 mg q12 h, when patient response is inadequate, would undoubtedly raise the systemic N-oxide levels. Similarly, when phenytoin is co-administered with a 400-mg dose of voriconazole, higher than usual levels of the N-oxide metabolite would be expected due to the inductive effect of phenytoin. It should be noted that higher doses of voriconazole appear to be tolerated in the above-mentioned clinical situations. The increase in voriconazole and its N-oxide metabolite levels when 400 mg voriconazole is co-administered with efavirenz 300 mg is therefore unlikely to be of clinical significance.
Peak efavirenz concentrations following co-administration of 300 mg efavirenz with 400 mg voriconazole were equivalent to those observed with 600 mg efavirenz alone. The 17% increase in efavirenz AUCτ, when co-administered with voriconazole, was mainly due to changes in the terminal phase of the concentration–time curve over the dosing interval. This also resulted in higher trough concentrations of efavirenz, which represent an important predictor of virological success in patients [17], and may presumably result in a somewhat longer duration of exposure to the drug, particularly in cases of poor compliance due to missed doses. Notably, during efavirenz and fluconazole co-administration, drug interaction due to fluconazole 200 mg q24 h increases the AUC of efavirenz 400 mg q24 h by an average of 16% when compared with efavirenz 600 mg q24 h monotherapy [5]. This increase in efavirenz exposure with fluconazole is very similar to the increase observed with voriconazole in this study.
The interpatient variability in voriconazole pharmacokinetics appears to be increased with co-administration of efavirenz. Examination of the individual subject changes in AUC values revealed that this change may have been due to two subjects in whom the AUC of voriconazole increased when the modified doses of voriconazole (400 mg) and efavirenz (300 mg) were co-administered (Figure 4). The most important predictor of voriconazole interpatient variability is the CYP2C19 genotype; poor metabolizers have higher exposure compared with extensive metabolizers [18]. Interestingly, the same two subjects also had higher exposure to voriconazole during the monotherapy phase. Although the CYP2C19 genotype was not determined for subjects enrolled in this study, it is conceivable that the effect of efavirenz may differ based on this genotype. In particular, individuals who are poor metabolizers of voriconazole may be less susceptible to the cytochrome P450 inductive effect of efavirenz, and may thus exhibit higher voriconazole exposure when an increased dose of the drug is co-administered with efavirenz. High concentrations of voriconazole have been associated with an increase in visual AEs and have shown a weak, but statistically significant, relationship with liver function test abnormalities [19]. Therefore, monitoring of visual AEs and liver function may be prudent during co-administration of efavirenz and voriconazole, particularly in a subset of patients with impaired ability to metabolize the latter. In contrast to voriconazole, the variability in efavirenz pharmacokinetics appears to decrease during concomitant administration with voriconazole. This observation, coupled with the apparent increase in the trough concentration of efavirenz during co-administration, is unlikely to influence the activity of efavirenz. On the other hand, it could potentially reduce the variability in efavirenz toxicity associated with variable concentrations of the drug.
In conclusion, this study has demonstrated that the two-way pharmacokinetic interaction resulting from concomitant use of efavirenz and voriconazole can be compensated for by adjusting the doses of both drugs. To achieve exposure levels similar to monotherapy, the voriconazole dose should be increased to 400 mg q12 h and the efavirenz dose decreased to 300 mg q24 h when the two drugs are co-administered.
This study was sponsored by Pfizer Global Research and Development. Clinical research support was provided by the Pfizer Clinical Research Unit in Brussels, Belgium, under the direction of Georges Weissgerber, MD, as principal investigator. Editorial support was provided by D. Wolf, MSc, of PAREXEL and was funded by Pfizer Inc.
REFERENCES
- 1.Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, Kern WV, Marr KA, Ribaud P, Lortholary O, Sylvester R, Rubin RH, Wingard JR, Stark P, Durand C, Caillot D, Thiel E, Chandrasekar PH, Hodges MR, Schlamm HT, Troke PF, de Pauw B Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer and the Global Aspergillus Study Group. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408–15. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 2.Kullberg BJ, Sobel JD, Ruhnke M, Pappas PG, Viscoli C, Rex JH, Cleary JD, Rubinstein E, Church LW, Brown JM, Schlamm HT, Oborska IT, Hilton F, Hodges MR. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomised non-inferiority trial. Lancet. 2005;366:1435–42. doi: 10.1016/S0140-6736(05)67490-9. [DOI] [PubMed] [Google Scholar]
- 3.Ally R, Schürmann D, Kreisel W, Carosi G, Aguirrebengoa K, Dupont B, Hodges M, Troke P, Romero AJ Esophageal Candidiasis Study Group. A randomized, double-blind, double-dummy, multicenter trial of voriconazole and fluconazole in the treatment of esophageal candidiasis in immunocompromised patients. Clin Infect Dis. 2001;33:1447–54. doi: 10.1086/322653. [DOI] [PubMed] [Google Scholar]
- 4.Hyland R, Jones BC, Smith DA. Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab Dispos. 2003;31:540–7. doi: 10.1124/dmd.31.5.540. [DOI] [PubMed] [Google Scholar]
- 5.Bristol-Myers Squibb Company; Sustiva® (efavirenz) prescribing information. [Google Scholar]
- 6.Niwa T, Shiraga T, Takagi A. Effect of antifungal drugs on cytochrome P450 (CYP) 2C9, CYP2C19, and CYP3A4 activities in human liver microsomes. Biol Pharm Bull. 2005;28:1805–8. doi: 10.1248/bpb.28.1805. [DOI] [PubMed] [Google Scholar]
- 7.von Moltke LL, Greenblatt DJ, Granda BW, Giancarlo GM, Duan SX, Daily JP, Harmatz JS, Shader RI. Inhibition of human cytochrome P450 isoforms by nonnucleoside reverse transcriptase inhibitors. J Clin Pharmacol. 2001;41:85–91. doi: 10.1177/00912700122009728. [DOI] [PubMed] [Google Scholar]
- 8.Mouly S, Lown KS, Kornhauser D, Joseph JL, Fiske WD, Benedek IH, Watkins PB. Hepatic but not intestinal CYP3A4 displays dose-dependent induction by efavirenz in humans. Clin Pharmacol Ther. 2002;72:1–9. doi: 10.1067/mcp.2002.124519. [DOI] [PubMed] [Google Scholar]
- 9.Fellay J, Marzolini C, Decosterd L, Golay KP, Baumann P, Buclin T, Telenti A, Eap CB. Variations of CYP3A activity induced by antiretroviral treatment in HIV-1 infected patients. Eur J Clin Pharmacol. 2005;60:865–73. doi: 10.1007/s00228-004-0855-8. [DOI] [PubMed] [Google Scholar]
- 10.Smith PF, DiCenzo R, Morse GD. Clinical pharmacokinetics of non-nucleoside reverse transcriptase inhibitors. Clin Pharmacokinet. 2001;40:893–905. doi: 10.2165/00003088-200140120-00002. [DOI] [PubMed] [Google Scholar]
- 11.Liu P, Foster G, Labadie R, Allison J, Sharma A. Pharmacokinetic interaction between voriconazole and efavirenz at steady state in healthy subjects. Clin Pharmacol Ther. 2005;2:P40. doi: 10.1177/0091270007309703. [DOI] [PubMed] [Google Scholar]
- 12.Gerzenshtein L, Patel SM, Scarsi KK, Postelnick MJ, Flaherty JP. Breakthrough Candida infections in patients receiving voriconazole. Ann Pharmacother. 2005;39:1342–5. doi: 10.1345/aph.1E627. [DOI] [PubMed] [Google Scholar]
- 13.Kupper LL, Hafner KB. How appropriate are popular sample size formulas? Am Stat. 1989;43:101–5. [Google Scholar]
- 14.Roffey SJ, Cole S, Comby P, Gibson D, Jezequel SG, Nedderman AN, Smith DA, Walker DK, Wood N. The disposition of voriconazole in mouse, rat, rabbit, guinea pig, dog, and human. Drug Metab Dispos. 2003;31:731–41. doi: 10.1124/dmd.31.6.731. [DOI] [PubMed] [Google Scholar]
- 15.Purkins L, Wood N, Parviz G, Love ER, Eve MD, Fielding A. Coadministration of voriconazole and phenytoin: pharmacokinetic interaction, safety, and toleration. Br J Clin Pharmacol. 2003;56:37–44. doi: 10.1046/j.1365-2125.2003.01997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfizer Inc.; Vfend® (voriconazole) prescribing information. [Google Scholar]
- 17.Joshi AS, Barrett JS, Fiske WD, Pieniaszek HJ, Ludden TM, Bacheler LT, Ruiz NM. Population pharmacokinetics of efavirenz in Phase II studies and relationship with efficacy. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 1999; San Francisco, Calif. [Poster 1201] [Google Scholar]
- 18.Ikeda Y, Umemura K, Kondo K, Sekiguchi K, Miyoshi S, Nakashima M. Pharmacokinetics of voriconazole and cytochrome P450 2C19 genetic status. Clin Pharmacol Ther. 2004;75:587–8. doi: 10.1016/j.clpt.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Tan K, Brayshaw N, Tomaszewski K, Troke P, Wood N. Investigation of the potential relationships between plasma voriconazole concentrations and visual adverse events or liver function test abnormalities. J Clin Pharm. 2006;46:235–43. doi: 10.1177/0091270005283837. [DOI] [PubMed] [Google Scholar]


