Abstract
Aims
Recent case–control studies suggest a relationship between the use of selective serotonin reuptake inhibitors (SSRIs) and the occurrence of birth defects and other adverse pregnancy outcomes. The aim was to determine the extent of the use of SSRIs before and during pregnancy and its trend over the years 1995–2004 in the Netherlands.
Methods
The study was performed with data from a population-based prescription database. Within this database, women giving birth to a child between 1995 and 2004 were identified. The exposure rate and 95% confidence interval (CI) were calculated as the number of pregnancies per 1000 that were exposed to an SSRI in a defined period (per trimester or in the year preceding delivery). Exposure rates were calculated for 2-year periods: 1995/1996, 1997/1998, 1999/2000, 2001/2002 and 2003/2004. Trends in exposure rates were analysed using the χ2 test for trend.
Results
Included were 14 902 pregnancies for which complete pharmacy records were available from 3 months before pregnancy until delivery. A total of 310 pregnancies were exposed to an SSRI in the year preceding delivery. The exposure rate increased from 12.2 (95% CI 7.0, 19.8) in 1995/1996 to 28.5 (95% CI 23.0, 34.9) in 2003/2004.
Conclusion
There has been a significant increase in the use of SSRIs among pregnant women in the Netherlands over the last 10 years, parallel with the increase in exposure in women of fertile age. In light of the recent warnings about the use of SSRIs in pregnancy, healthcare professionals should be careful in prescribing SSRIs to women planning a pregnancy.
What is already known about this subject
Recently, the use of selective serotonin reuptake inhibitors (SSRIs), particularly paroxetine, in pregnancy has been associated with an increased risk on specific birth defects or other adverse pregnancy outcomes.
However, the extent of SSRI use in pregnancy is largely unknown.
What this study adds
In the last decade the use of SSRIs in the year preceding delivery has increased twofold.
This increase runs parallel with the increase in use of SSRIs among women of fertile age.
Paroxetine is one of the most commonly used SSRIs.
Only recently have sufficient data become available on the use of paroxetine to detect moderate increased risks for specific malformations.
The safety of SSRIs which are less frequently used is not yet established.
Case–control birth defect-monitoring systems may be helpful in providing safety and risk estimates that become more precise as data accumulate for these drugs.
Keywords: drug utilization, exposure rate, pregnancy, selective serotonin reuptake inhibitors
Introduction
Occurrence of significant depressive symptoms and major depressive disorders is not uncommon in pregnant women. Prevalence rates vary between 7% [1] and 20% [2]. Since the introduction of selective serotonin reuptake inhibitors (SSRIs) in the 1980s, the prevalent and incident use of SSRIs has increased over the use of tricyclic antidepressants [3]. Several, mostly prospective, cohort studies on the use of SSRIs in pregnancy have found no increased risk of general major congenital malformations [4–6]. Other cohort studies have found adverse pregnancy outcomes, such as a higher rate of spontaneous abortions [7], lower birth weight and shorter gestation [8] or an increased proportion of children with minor anomalies [9]. The use of SSRIs in pregnancy has also been associated with neonatal withdrawal syndrome [10] and with an increased risk of persistent pulmonary hypertension [11]. The results of recent case–control studies suggest that SSRIs are not major teratogens, but specific SSRIs appear to increase modestly the risk of various specific (cardiac) malformations [12–17].
Since clear data on the prevalence of SSRI use in pregnancy over the last decade are limited, we set out to determine the extent of SSRI use in the trimester before pregnancy and during the different trimesters in pregnancy and to analyse the trend of its use over the years 1995–2004 based on data available for the Netherlands.
Methods
For this study data from the Interaction Database (IADB.nl) were used. The IADB.nl is a population-based prescription database which contains data from prescriptions dispensed from community pharmacies. It covers a population in the northern and eastern parts of the Netherlands. The database comprised data on approximately 220 000 people in 1994, and has gradually expanded to data on approximately 500 000 people in 1999. Registration occurs irrespective of health insurance and is considered representative of the general population. Each prescription record contains information on the name of the drug, the date of dispensing, the quantity dispensed, the dose regimen and the prescribing physician. The indication for prescribing is not known. All the drugs are coded according to the Anatomical Therapeutic Chemical (ATC) classification system [18]. Each patient has a unique identification number, and date of birth, sex and address code are known. Since most patients restrict their visits to a single pharmacy, their medication records are virtually complete. The database does not include information on over-the-counter medication and medications dispensed during hospitalization [19].
Within the IADB.nl a pregnancy database has been generated. To identify mothers, all children born after 1 January 1994 were selected. For each child within the IADB.nl, the female person, 15–50 years older than the child and with the same address code, is considered to be the mother, provided there are no other female persons in that age range with the same address code. Validation of this method has been described in detail by Schirm et al.[20]. Using this method, for 35% of the children the mother can not be identified, because the mother has a separate address code or is registered with another pharmacy. Since the major reason for not identifying the mother is not the lack of pharmacy registration, selection bias towards drug-using families seems to be limited.
Because the actual length of the pregnancy is unknown, the theoretical conception date was determined as the date of birth minus 273 days. The length of the pregnancy is therefore standardized at 39 weeks, which can be divided into three trimesters of 13 weeks.
From this pregnancy database all mothers between 15 and 49 years of age were selected, who gave birth to a child between 1995 and 2004 and for whom complete data were available on the year preceding delivery (13 weeks before the theoretical conception date until delivery). The 13 weeks before conception will be referred to as trimester 0 and the trimesters in pregnancy as trimesters 1, 2 and 3.
Prescriptions for SSRIs were identified by ATC codes starting with N06AB. The theoretical period of use was calculated for each prescription for SSRIs, based on the date of dispensing, the quantity dispensed and the dose regimen. The exposure rate was then calculated as the number of pregnancies per 1000 pregnancies that were in theory exposed to an SSRI in a defined period: women who received a prescription in one trimester which was extended into the next trimester were counted in both trimesters in which they had had access to the drug. Exposure rates were calculated for 2-year periods: 1995/1996, 1997/1998, 1999/2000, 2001/2002 and 2003/2004.
To compare the exposure rates in the pregnant population with those in the general population of women of fertile age (15–49 years), the age-standardized 1-year exposure rates in women of fertile age were also calculated using the 5-year age distribution among the pregnant women. The age-standardized 1-year exposure rates were averaged over the 2-year periods. The rate ratio was calculated as the age-standardized 1-year exposure rate to the pregnancy exposure rate.
The calculation of exposure rates per trimester does not give insight into the patterns of use for individual women. If drugs are prescribed for short-time use, then it is in theory possible that for each trimester the exposed pregnancies occur with new users. To study possible changes in the patterns of use in pregnancy we distinguished between the following groups: (i) women who used SSRIs before pregnancy only, (ii) women who used SSRIs before and continued use in pregnancy, and (iii) women who started use of SSRIs in pregnancy. Patterns of SSRI use were analysed with respect to two time frames: birth years 1995–1999 and 2000–2004.
The defined daily dose (DDD) is the assumed average maintenance dose per day for a drug used for its main indication in adults. Between the birth years 1995–1999 and 2000–2004, we compared the total DDDs prescribed in the year preceding delivery, the total number of days of the prescription(s) and the average DDD calculated as the total DDDs prescribed, divided by the total number of days of the prescription(s) and categorized as ≤ 1 DDD and > 1 DDD.
Trends in exposure rates over 2-year periods were analysed in SPSS 12.0 for Windows (Chicago, IL, USA) using the χ2 test for trend. Continuous data were analysed using the T-test for two groups and one-way ANOVA for more than two groups. The total DDDs prescribed and the total number of days of the prescriptions were analysed using the Mann–Whitney U-test. Proportions were compared using the χ2 test. The 95% confidence interval (CI) for the exposure rates was calculated using the Score method with continuity correction for small proportions [21].
Results
Within our population, 14 902 pregnancies occurring to 10 897 women could be identified between 1995 and 2004. For 7432 women (68%), one pregnancy was identified in the period 1995–2004 and for 3465 women (32%) two or more pregnancies. The maximum number of pregnancies of one woman was six. The mean maternal age at birth was 29.9 years (SD 4.5). The mean maternal age at birth differed significantly between the birth years (P = 0.000), from 29.2 years (SD 4.3) in 1995/1996 to 30.1 years (SD 4.6) in 2001/2002.
In the year preceding delivery, 455 out of 14 902 pregnancies (3.1%) were exposed to an antidepressant (ATC code N06A). The mean age at birth of the women who used an antidepressant in this period was 30.4 (SD 5.0) and 29.9 years (SD 4.5) for women who did not use an antidepressant before or during pregnancy, respectively (P = 0.043).
Exposure to an SSRI (N06AB) in trimester 0–3 occurred in 310 pregnancies (2.1%) with 292 women: 274 women with one exposed pregnancy and 18 women with two exposed pregnancies. In the 10-year period, paroxetine was the most commonly used SSRI (n = 180, 58.1%), followed by fluoxetine (n = 67, 21.6%) and fluvoxamine (n = 39, 12.6%). Citalopram and sertraline were the least used SSRIs, with 8.4% (n = 26) and 3.5% (n = 11). Among the exposed pregnancies, the use of paroxetine increased from 37.5% (6/16 pregnancies) in 1995/1996 to 60.4% in 2003/2004 (55/91 pregnancies), whereas the use of fluoxetine and fluvoxamine decreased from 37.5% (6/16) and 31.3% (5/16), respectively, in 1995/1996 to 19.8% (18/91) and 5.5% (5/91), respectively, in 2003/2004. In 13 pregnancies more than one type of SSRI was used in trimesters 0–3 (subsequently). The two most prevalent combinations were paroxetine and fluoxetine (five pregnancies) and paroxetine and fluvoxamine (four pregnancies).
In Figure 1 exposure rates for SSRIs per trimester are shown per 2-year periods. The pattern of use within periods 0–3 was similar for all 2-year periods. The use of SSRIs was highest in the trimester before conception, decreased in the first trimester and further in the second trimester. The use of SSRIs in the third trimester was comparable to the use in the second trimester. The decrease in use over periods 0–3 was statistically significant for all 2-year periods (1995/1996, χ2 for trend = 11.323, P = 0.001; 1997/1998, χ2 for trend = 25.337, P = 0.000; 1999/2000, χ2 for trend = 33.368, P = 0.000; 2001/2002, χ2 for trend = 29.503, P = 0.000; 1995/1996, χ2 for trend = 21.989, P = 0.000). The use of SSRIs showed a significantly increasing trend over time for each of the trimesters (trimester 0, χ2 for trend = 21.936, P = 0.000; trimester 1, χ2 for trend = 26.038, P = 0.000; trimester 2, χ2 for trend = 30.776, P = 0.000; trimester 3, χ2 for trend = 26.186, P = 0.000).
Figure 1.
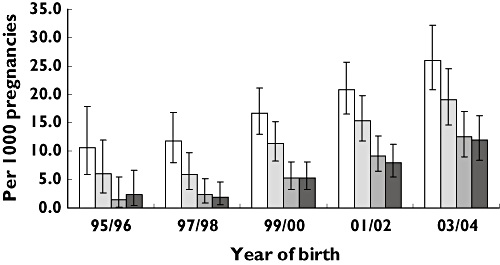
Exposure rate (and 95% confidence interval) for selective serotonin reuptake inhibitors per trimester per 1000 pregnancies per 2-year periods in a cohort of 14 902 pregnancies in the Netherlands (trim 0, (□); trim 1, ( ); trim 2, (
); trim 2, ( ); trim 3, (
); trim 3, ( ))
))
In Figure 2 the exposure rate for any use of an SSRI in the year preceding delivery per 2-year period is presented. The exposure rate increased from 12.2 (95% CI 7.0, 19.8) per 1000 pregnancies in 1995/1996 to 28.5 (95% CI 23.0, 34.9) per 1000 pregnancies in 2003/2004. This increase runs parallel with the increase in use in the general population of women aged 15–49 years. The age-standardized 1-year exposure rate (averaged over 2-year periods) in this population increased from 36.8 per 1000 in 1995/1996 to 75.7 per 1000 in 2003/2004. The rate ratio was 3.0, 4.0, 3.6, 3.2 and 2.7 for the respective 2-year periods. Between 1995 and 2004, the pregnancy rate in women aged 15–49 years was 13.1 per 1000 person years.
Figure 2.
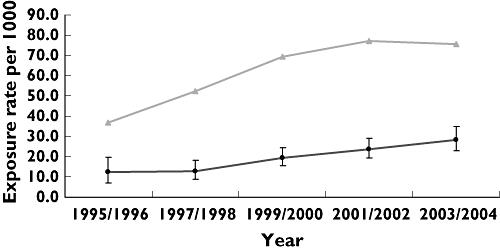
Average exposure rate per 2-year period for any use of selective serotonin reuptake inhibitors in the year preceding delivery (including 95% confidence interval) compared with the average age-standardized 1-year exposure rate per 2-year periods for women of fertile age (15–49 years) (pregnancy, (•); women fertile age, age-standardised, ( ))
))
Among the women who used SSRIs in the year preceding delivery (n = 310), 90.0% of the pregnancies were exposed to SSRIs only (including combinations of SSRIs). In 10.0%, both SSRIs and other types of antidepressants were used. In Table 1 the pattern of SSRI use for pregnancies exposed to SSRIs in the year preceding delivery is presented. Only in 11.6% of the exposed pregnancies did the use of SSRIs start in pregnancy. This percentage did not differ between 1995 and 1999 or between 2000 and 2004. However, when we look at the pregnancies in which an SSRI had already been used before pregnancy, it can be seen that in the more recent time period continuation of SSRI use in pregnancy was more prevalent, with 65.8% vs. 51.9% in the earlier period (P = 0.03). When these analyses were restricted to those pregnancies in which SSRIs had already been used before pregnancy and no other types of antidepressants were used (n = 253), the results were comparable (65.9% vs. 51.9%).
Table 1.
Pattern of use of SSRIs and average DDD in 1995–1999 and 2000–2004 for pregnancies exposed to an SSRI in period from 3 months before conception until delivery
| Total | 1995–1999 | 2000–2004 | |||||
|---|---|---|---|---|---|---|---|
| n = 310 | (100%) | n = 90 | (100%) | n = 220 | <(100%) | P | |
| Use before pregnancy | 274 | 88.4 | 81 | 90.0 | 193 | 87.7 | |
| Use discontinued before theoretical conception date | 105 | 38.3 | 39 | 48.1 | 66 | 34.2 | 0.03 |
| Use continued in pregnancy | 169 | 61.7 | 42 | 51.9 | 127 | 65.8 | |
| Start use in pregnancy | 36 | 11.6 | 9 | 10.0 | 27 | 12.3 | |
| Average DDD prescribed | |||||||
| ≤1 DDD | 229 | 73.9 | 69 | 76.7 | 160 | 72.7 | 0.474 |
| >1 DDD | 81 | 26.1 | 21 | 23.3 | 60 | 27.3 | |
SSRI, Selective serotonin reuptake inhibitor; DDD, defined daily dose. SSRIs include paroxetine (ATC N06AB05; DDD 20 mg), fluoxetine (ATC N06AB03; DDD 20 mg), fluvoxamine (ATC N06AB08; DDD 100 mg), citalopram (ATC N06AB04; DDD 20 mg) and sertraline (ATC N06AB06; DDD 50 mg).
In 2000–2004 the length of use of SSRIs and the total DDDs prescribed were significant higher than in 1995–1999 (median number of days 99.5 vs. 64, P = 0.008; median total DDDs 111.5 vs. 67.5; P = 0.009). The average DDD (average daily dose) in the year preceding delivery varied between 0.3 and 4.0, and 191 women (61.6%) received an average DDD of 1. The proportion of women who received an average DDD ≤ 1 did not differ between 1995 and 1999 or between 2000 and 2004 with 76.7% and 72.7%, respectively (Table 1, P = 0.474).
Discussion
The results of this observational study show that there has been a significant increase in the use of SSRIs during pregnancy in the Netherlands over the last 10 years. The increase in use is present in all trimesters before and during pregnancy and runs parallel with the increase in use of SSRIs in women of fertile age. In addition, in recent years continued use of SSRIs from before pregnancy until the first trimester is more frequent along with an increase in the length of use and the total DDDs prescribed. The average daily dose prescribed did not change. The most commonly prescribed SSRI over the whole study period was paroxetine.
To our knowledge, this study is the first to examine trends in use of SSRIs before and during pregnancy over a 10-years period. The validity of the exposure rates for use of SSRIs in the year preceding delivery is equivalent to the age-standardized 1-year exposure rates in women of fertile age, since both data derive from the same source population. We did not adjust for multiple pregnancies per woman, because we wanted to conduct an observational study in which each pregnancy was considered as an independent event.
The use of a population-based prescription database is an important tool in monitoring the use of drugs among pregnant women. The data are recorded prospectively and cover prescriptions from different prescribers. The IADB.nl was gradually expanded between 1994 and 1999 by including pharmacies from geographical areas not previously covered. The pharmacies that were added to the IADB.nl were representative of the north-eastern region. When the average exposure rates were calculated per 2-year period for any use of SSRIs in the year preceding delivery restricted to those pharmacies that participated in the IADB.nl in the first 2 years (1994 and 1995), the results were comparable to the exposure rates found in Figure 2. It is therefore unlikely that the results of this study have been influenced by the expansion of the database.
There are several limitations in using an administrative prescription database, such as the unknown actual use and the standardized length of pregnancy. Since the actual use is unknown, the exposure rates found in this study are an estimate. The use of SSRIs in pregnancy may be an overestimation if women stop taking their medication when they plan to become pregnant or when they discover they are pregnant. Also, because the length of the pregnancy was standardized at 39 weeks, misclassification of exposure is possible. The use of SSRIs in the first trimester could be an overestimation if use in pregnancy is associated with preterm birth. From the literature it is not clear if there is a relation between the use of SSRIs and preterm birth. Some studies have found such an association [9, 22], whereas others have not [8, 23]. However, definitions of exposure and preterm birth differ between these studies.
Also, the methodology used to identify mothers and pregnancies has its limitations. We were able to identify approximately 65% of the mothers for children included in the IADB.nl. Since the validated method has a sensitivity of 99% [20], it is not to be expected that nonpregnant women were misclassified as being pregnant. Failure to identify a pregnancy when the child is known can mostly be attributed to administrative reasons, and selection towards drug-using families seems therefore limited. The detection rate may be improved using less strict criteria. On the other hand, this would probably lead to an undesirable loss of sensitivity. Furthermore, pregnancies are not identified if they resulted in a spontaneous or induced abortion, a still birth or an early neonatal death or if the child is not (yet) registered with a pharmacy. A Dutch study on drug use in children, using pharmacy dispensing data from the IADB.nl, has shown that approximately 80% of the children had used at least one prescription drug (and therefore were registered with a pharmacy) within the first 2 years of life [24]. Since it may take some time for a new-born child to be registered with a pharmacy, the number of unidentified pregnancies may be larger in more recent than in previous years. This is likely to result in an underestimation of maternal drug use in more recent years.
The prevalence rates per trimester for use of SSRIs followed the same pattern as for antidepressants in general, found in a Dutch cohort of 29 005 women giving birth between January 2000 and July 2003 [25]. In this study, the use of antidepressants decreased from 2.9% before pregnancy to 2.1% in the first trimester and 1.8% in the second and third trimesters. Paroxetine was the most commonly used antidepressant (approximately 47% of all antidepressants used). Reefhuis et al.[26] have found a prevalence of SSRI use of 2.8% among a cohort of 4094 mothers who gave birth to a healthy child between October 1997 and December 2002. The data were obtained from a population-based case–control study of congenital anomalies, conducted in eight states in the USA. Self reported measures were used. That prevalence of use is comparable to that (2.9%) in a matched control group consisting of mothers of a healthy child found in a study by Chambers et al.[11], and estimates from both studies were somewhat higher than in our study (i.e. 2.1%). The higher use can be explained by the fact that, in general, use of antidepressants is higher in the USA.
In the late 1990s a number of prospective cohort studies were published which did not find an increased overall risk of major congenital malformations after the use of SSRIs in pregnancy [4, 6, 9, 27]. However, most of these cohort studies lacked sufficient power to detect increased risk of specific congenital malformations. Case–control studies have more statistical power to detect moderately increased risks for specific birth defects than cohort studies. They are more efficient in terms of sample size and time. Case–control studies are also sensitive to selection and recall bias, which can be minimized by the choice of an appropriate control group and the use of prospectively collected (pharmacy) data on prenatal medication use.
Since paroxetine is one the most commonly used SSRIs among pregnant women, sufficient data are now becoming available to detect these moderately increased risks for specific congenital malformations [11–13]. The safety of SSRIs that are less frequently used has not yet been established. It is very important that more data become available on the safety of the newer antidepressants. Case–control birth defect-monitoring systems that include information on prenatal medication use might be helpful in providing these safety and risk estimates. As data accumulate, the risk estimates for these drugs will become more precise [28].
The decision whether to use antidepressants in pregnancy should be taken after careful consideration of the benefits and risks for both mother and child. In some cases the benefits of treatment may well outweigh the teratogenic risks. Untreated depression in pregnancy appears to carry substantial perinatal risks, such as preterm birth, restricted fetal growth, preeclampsia, spontaneous abortions and delayed cognitive and emotional development. These adverse effects may be caused by psychopathological events which have physiological effects on the fetus. Depression may also lead to unhealthy behaviour that can indirectly affect the outcome of the pregnancy [29]. Also, a recent cohort study has found that women who discontinue antidepressant medication close to conception experience more frequent relapses of major depression during pregnancy than those who maintain their medication [30].
The American College of Obstetricians and Gynaecologists has recently recommended that treatment with all SSRIs during pregnancy should be individualized, and paroxetine use among pregnant women or women planning a pregnancy should be avoided, if possible. Women of fertile age who take SSRIs should be advised to consult a specialist before they become pregnant to develop a treatment plan regarding their condition and the use of SSRIs, in which risks and benefits for mother and child are well considered [31]. In the Netherlands, where 80% of pregnancies are planned, it should then be possible to avoid the unnecessary use of SSRIs in pregnancy as much as possible.
Competing interests
P.K. has been reimbursed by Eli Lilly, the manufacturer of fluoxetine, and Jansen Cilag/Organon, the manufacturer of mirtazepine, for attending several conferences, and has been paid by GlaxoSmithKline, manufacturer of paroxetine, and by Pfizer, the manufacturer of sertraline, for running educational programmes. All other authors declare they have no conflict of interests to declare.
References
- 1.Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR. Prevalence of depression during pregnancy: systematic review. Obstet Gynecol. 2004;103:698–709. doi: 10.1097/01.AOG.0000116689.75396.5f. [DOI] [PubMed] [Google Scholar]
- 2.Marcus SM, Flynn HA, Blow FC, Barry KL. Depressive symptoms among pregnant women screened in obstetrics settings. J Womens Health (Larchmt) 2003;12:373–80. doi: 10.1089/154099903765448880. [DOI] [PubMed] [Google Scholar]
- 3.Meijer WE, Heerdink ER, Leufkens HG, Herings RM, Egberts AC, Nolen WA. Incidence and determinants of long-term use of antidepressants. Eur J Clin Pharmacol. 2004;60:57–61. doi: 10.1007/s00228-004-0726-3. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein DJ, Corbin LA, Sundell KL. Effects of first-trimester fluoxetine exposure on the newborn. Obstet Gynecol. 1997;89(5 Part 1):713–8. doi: 10.1016/s0029-7844(97)00070-7. [DOI] [PubMed] [Google Scholar]
- 5.Hendrick V, Smith LM, Suri R, Hwang S, Haynes D, Altshuler L. Birth outcomes after prenatal exposure to antidepressant medication. Am J Obstet Gynecol. 2003;188:812–5. doi: 10.1067/mob.2003.172. [DOI] [PubMed] [Google Scholar]
- 6.Kulin NA, Pastuszak A, Sage SR, Schick-Boschetto B, Spivey G, Feldkamp M, Ormond K, Matsui D, Stein-Schechman AK, Cook L, Brochu J, Rieder M, Koren G. Pregnancy outcome following maternal use of the new selective serotonin reuptake inhibitors: a prospective controlled multicenter study. JAMA. 1998;279:609–10. doi: 10.1001/jama.279.8.609. [DOI] [PubMed] [Google Scholar]
- 7.Chun-Fai-Chan B, Koren G, Fayez I, Kalra S, Voyer-Lavigne S, Boshier A, Shakir S, Einarson A. Pregnancy outcome of women exposed to bupropion during pregnancy: a prospective comparative study. Am J Obstet Gynecol. 2005;192:932–6. doi: 10.1016/j.ajog.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 8.Simon GE, Cunningham ML, Davis RL. Outcomes of prenatal antidepressant exposure. Am J Psychiatry. 2002;159:2055–61. doi: 10.1176/appi.ajp.159.12.2055. [DOI] [PubMed] [Google Scholar]
- 9.Chambers CD, Johnson KA, Dick LM, Felix RJ, Jones KL. Birth outcomes in pregnant women taking fluoxetine. N Engl J Med. 1996;335:1010–5. doi: 10.1056/NEJM199610033351402. [DOI] [PubMed] [Google Scholar]
- 10.Sanz EJ, las-Cuevas C, Kiuru A, Bate A, Edwards R. Selective serotonin reuptake inhibitors in pregnant women and neonatal withdrawal syndrome: a database analysis. Lancet. 2005;365:482–7. doi: 10.1016/S0140-6736(05)17865-9. [DOI] [PubMed] [Google Scholar]
- 11.Chambers CD, Hernandez-Diaz S, Van Marter LJ, Werler MM, Louik C, Jones KL, Mitchel AA. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354:579–87. doi: 10.1056/NEJMoa052744. [DOI] [PubMed] [Google Scholar]
- 12.GlaxoSmithKline Clinical Trial Register. [15 November 2005]. Epidemiology Study: Preliminary Report on Bupropion in Pregnancy and the Occurrence of Cardiovascular and Major Congenital Malformation. Available at http://ctrgskcouk/Summary/paroxetine/epip083.pdf.
- 13.Kallen B, Otterblad Olausson P. Antidepressant drugs during pregnancy and infant congenital heart defect. Reprod Toxicol. 2006;21:221–2. doi: 10.1016/j.reprotox.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Wogelius P, Norgaard M, Gislum M, Pedersen L, Munk E, Mortensen PB, Lipworth L, Toft Sorensen H. Maternal use of selective serotonin reuptake inhibitors and risk of congenital malformations. Epidemiology. 2006;17:701–4. doi: 10.1097/01.ede.0000239581.76793.ae. [DOI] [PubMed] [Google Scholar]
- 15.Berard A, Ramos E, Rey E, Blais L, St-Andre M, Oraichi D. First trimester exposure to paroxetine and risk of cardiac malformations in infants: the importance of dosage. Birth Defects Res B Dev Reprod Toxicol. 2007;80:18–27. doi: 10.1002/bdrb.20099. [DOI] [PubMed] [Google Scholar]
- 16.Alwan S, Reefhuis J, Rasmussen SA, Olney RS, Friedman JM. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med. 2007;356:2684–92. doi: 10.1056/NEJMoa066584. [DOI] [PubMed] [Google Scholar]
- 17.Louik C, Lin AE, Werler MM, Hernandez-Diaz S, Mitchell AA. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med. 2007;356:2675–83. doi: 10.1056/NEJMoa067407. [DOI] [PubMed] [Google Scholar]
- 18.WHO Collaborating Centre for Drugs Statistics Methodology. [2 December 2004]. Available at http://www.whoccno/atcddd/
- 19.Tobi H, van den Berg PB, De Jong-van den Berg LTW. The Interaction Database: synergy of science and practice in pharmacy. In: Brause RW, Hanisch E, editors. Medical Data Analysis. Berlin: Springerverlag; 2000. pp. 206–11. [Google Scholar]
- 20.Schirm E, Tobi H, de Jong-van den Berg LT. Identifying parents in pharmacy data: a tool for the continuous monitoring of drug exposure to unborn children. J Clin Epidemiol. 2004;57:737–41. doi: 10.1016/j.jclinepi.2002.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Tobi H, van den Berg PB, de Jong-van den Berg LT. Small proportions: what to report for confidence intervals? Pharmacoepidemiol Drug Saf. 2005;14:239–47. doi: 10.1002/pds.1081. [DOI] [PubMed] [Google Scholar]
- 22.Wen SW, Yang Q, Garner P, Fraser W, Olatunbosun O, Nimrod C, Walker M. Selective serotonin reuptake inhibitors and adverse pregnancy outcomes. Am J Obstet Gynecol. 2006;194:961–6. doi: 10.1016/j.ajog.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Malm H, Klaukka T, Neuvonen PJ. Risks associated with selective serotonin reuptake inhibitors in pregnancy. Obstet Gynecol. 2005;106:1289–96. doi: 10.1097/01.AOG.0000187302.61812.53. [DOI] [PubMed] [Google Scholar]
- 24.Schirm EBP, Gebben H, Sauer P, de Jong-van den Berg L. Drug use of children in the community assessed through pharmacy dispensing data. Br J Clin Pharmacol. 2000;50:473–8. doi: 10.1046/j.1365-2125.2000.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ververs T, Kaasenbrood H, Visser G, Schobben F, de Jong-van den Berg Egberts T. Prevalence and patterns of antidepressant drug use during pregnancy. Eur J Clin Pharmacol. 2006;62:863–70. doi: 10.1007/s00228-006-0177-0. [DOI] [PubMed] [Google Scholar]
- 26.Reefhuis J, Rasmussen SA, Friedman JM. Selective serotonin-reuptake inhibitors and persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354:2188–90. doi: 10.1056/NEJMc060602. [DOI] [PubMed] [Google Scholar]
- 27.Pastuszak A, Schick-Boschetto B, Zuber C, Feldkamp M, Pinelli M, Sihn S, Donnenfeld A, McCormack M, Leen-Mitchell M, Woodland C, Gardner A, Hom M, Koren G. Pregnancy outcome following first-trimester exposure to fluoxetine (Prozac) JAMA. 1993;269:2246–8. [PubMed] [Google Scholar]
- 28.Mitchell AA. Systematic identification of drugs that cause birth defects – a new opportunity. N Engl J Med. 2003;349:2556–9. doi: 10.1056/NEJMsb031395. [DOI] [PubMed] [Google Scholar]
- 29.Bonari L, Pinto N, Ahn E, Einarson A, Steiner M, Koren G. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry. 2004;49:726–35. doi: 10.1177/070674370404901103. [DOI] [PubMed] [Google Scholar]
- 30.Cohen LS, Altshuler LL, Harlow BL, Nonacs R, Newport DJ, Viguera AC, Suri R, Burt VK, Hendrik V, Reminick AM, Loughead A, Vitonis AF, Stowe ZN. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295:499–507. doi: 10.1001/jama.295.5.499. [DOI] [PubMed] [Google Scholar]
- 31.ACOG Committee on Obstetric Practice. ACOG Committee Opinion No. 354: treatment with selective serotonin reuptake inhibitors during pregnancy. Obstet Gynecol. 2006;108:1601–3. doi: 10.1097/00006250-200612000-00058. [DOI] [PubMed] [Google Scholar]


