Abstract
Spinal cord stimulation (SCS) is a widely used clinical technique to treat ischemic pain in peripheral, cardiac and cerebral vascular diseases. The use of this treatment advanced rapidly during the late 80's and 90's, particularly in Europe. Although the clinical benefits of SCS are clear and the success rate remains high, the mechanisms are not yet completely understood. SCS at lumbar spinal segments (L2-L3) produces vasodilation in the lower limbs and feet which is mediated by antidromic activation of sensory fibers and decreased sympathetic outflow. SCS at thoracic spinal segments (T1-T2) induces several benefits including pain relief, reduction in both frequency and severity of angina attacks, and reduced short-acting nitrate intake. The benefits to the heart are not likely due to an increase, or redistribution of local blood flow, rather, they are associated with SCS-induced myocardial protection and normalization of the intrinsic cardiac nervous system. At somewhat lower cervical levels (C3-C6), SCS induces increased blood flow in the upper extremities. SCS at the upper cervical spinal segments (C1-C2) increased cerebral blood flow, which is associated with a decrease in sympathetic activity, an increase in vasomotor center activity and a release of neurohumoral factors. This review will summarize the basic science studies that have contributed to our understanding about mechanisms through which SCS produces beneficial effects when used in the treatment of vascular diseases. Furthermore, this review will particularly focus on the antidromic mechanisms of SCS-induced vasodilation in the lower limbs and feet.
1. Introduction
Spinal cord stimulation (SCS) delivers electrical impulses to different spinal segments via implanted electrodes. SCS has been used to treat various pain related diseases including ischemic pain for almost half century (Stojanovic and Abdi, 2006). Shealy was the first to use SCS to treat pain patients (Shealy et al., 1967). Currently SCS is the most common neuromodulatory treatment for ischemic pain and the overall beneficial effects last for at least 1 year in 80% of patients, and last for up to 5 years in 60% of patients (Deer and Raso, 2006). It is estimated that each year more than 14,000 SCS implantations are performed worldwide (Linderoth and Foreman, 2006). The beneficial effects of SCS when used to treat ischemic pain include pain relief, decreased infarction or ulcer size, decreased oxygen requirement and increased claudication distance. Clinical and basic studies indicate that these beneficial effects are mainly associated with increased blood flow or redistribution of blood flow to the ischemic area, and/or, normalization of the activity in the nervous system (Linderoth and Foreman, 1999; Erdek and Staats, 2003).
SCS is based on the gate-control theory published by Melzack and Wall in 1965 (Melzack and Wall, 1965), which partially explains the mechanism of SCS-induced pain relief. However, new theories have emerged from recent research that enables a more complete understanding of the mechanisms that produce the benefits of SCS for various organ systems. This review summarizes the multiple mechanisms of SCS employed to create beneficial effects on the vascular system in the lower limbs, feet, heart and brain. Moreover, when SCS is applied on C1-C2, T1-T2 and L2-L3, different mechanisms are engaged to produce the beneficial effects of SCS.
2. Effects of SCS on peripheral, cardio and cerebral vascular system
Ischemia typically results from obliteration of blood vessels due to the pathological changes of the tissues. An imbalance between oxygen supply and demand caused by an ischemic condition produces major metabolic changes in the tissue, and also ischemic pain in the different organs, including brain, heart and lower limbs and feet. Thus, SCS is applied at different spinal segments in order to provide benefit to a specific ischemic organ. SCS produces various benefits to the vascular system in the affected organs. SCS at lumbar spinal segments (L2-L3) produces vasodilation in the vasculature of the lower limbs and feet which is mediated by antidromic activation of sensory fibers and decreased sympathetic outflow. SCS at thoracic spinal segments (T1-T2) provides benefits including pain relief, reduction in frequency and severity of angina attacks, as well as reduced use of short-acting nitrate in patients with angina. There is at present no solid evidence that these benefits are due to an increase, or redistribution of local blood flow in heart, rather, these benefits are most likely the result of mechanisms that produce myocardial protection and normalize the intrinsic cardiac nervous system. SCS at cervical spinal segments (C1-C2) can result in an increase of cerebral blood flow, which is a result of a decrease in sympathetic activity, an increase in activation of vasomotor centers and a release of neurohumoral factors.
2.1 Effects of SCS on peripheral vascular system in the lower limbs and feet
2.1.1 Background
Many ischemic conditions in the limbs are due to peripheral arterial occlusive diseases (PAOD), which is caused by obstruction of blood flow into an arterial tree excluding the intracranial or coronary circulations (Garcia, 2006). PAOD is a major cause of disability, loss of work, and lifestyle changes in the United States (Garcia, 2006). The incidence of PAOD is 2 % in men under 50 years old and 5 % in men over 70 years old. However, the incidence of PAOD in women is in average delayed by ten years (Chochola and Linhart, 2006). It is estimated that PAOD affects and reduces the quality of life in about 2 million Americans. Furthermore, millions of Americans also suffer from PAOD-associated impairment of activity of daily living (ADL) and quality of life (QOL) (Marcoux et al., 1996; Hirsch et al., 2001; Golomb et al., 2006). The morbidity and mortality are relatively high because effective treatments are unavailable. SCS at the lower thoracic (T12) and higher lumbar spinal segments (L2-L3) is used to treat patients with PAOD in the lower limbs and feet. This treatment results in increased peripheral circulation in the limbs and feet. This outcome of SCS treatment in PAOD patients was first reported by Cook (Cook, 1976). Currently, SCS is usually considered only after vascular surgery and medications fail to prevent the progress of PAOD. Surprisingly the success rate of SCS-treated PAOD is greater than 70% (Cameron, 2004). The mechanisms of SCS-induced vasodilation in the lower limbs and feet are not yet completely understood. Two theories explaining the mechanisms of SCS-induced vasodilation have been proposed based on the results of three decades of clinical and basic science studies. One theory is that SCS decreases sympathetic outflow and attenuates constriction of arterial vessels; the alternative theory is that SCS antidromically activates sensory fibers and causes the releases of vasodilators.
2.1.2 Animal models and experimental designs
Sprague-Dawley (SD) rats have been used as animal models under normal physiological conditions to investigate the mechanisms of SCS-induced vasodilation in the lower limbs and feet (Linderoth et al., 1991 a, b, 1994, 1995; Croom et al., 1996, 1997 a, b, 1998; Tanaka et al., 2001, 2003 a, b, 2004; Wu et al., 2006 a, c, d, 2007). Details about the experimental methods are described in these articles. A small ball electrode is placed on the ipsilateral dorsal column near the spinal artery. The electrical stimulation parameters are 50 Hz with 0.2 ms duration with monophasic rectangular pulse. In earlier studies, constant voltage (Linderoth et al., 1991 a) and constant current (Croom et al., 1997) techniques are used for setting SCS stimulation parameters. In recent studies, the motor threshold (MT) is used to calculate stimulus intensity. MT is initially determined in each animal by slowly increasing the SCS current from zero until muscle contraction is observed (Tanaka et al., 2001). The contraction is a reflex response to stimulation of dorsal column fibers, i.e., primary afferents. Activation of these afferent fibers potentially could excite the motoneuronal pools that innervate muscles in the lower limbs (Gerasimenko et al., 2006). The experimental SCS (50 Hz; 0.2 ms, monophasic rectangular pulses), is performed at 30%, 60%, 90%, 300% or 10 times of MT (Tanaka et al., 2003 a; Wu et al., 2006). The lowest level of stimulation at 30% of MT is used because it was closest to the threshold of SCS that produced vasodilation. A stimulation level 60% of MT level is also used because it approximates the parameters of clinical applications of SCS (Linderoth et al., 1991 a, b, 1994). A stimulation level 90% of MT intensity is used because it is close to, but below MT. Stimulation levels at 3 and 10 times of MT are used to observe how SCS at high intensity stimulation affects the peripheral blood flow by mimicking traditional antidromic vasodilation (Bayliss, 1901). The effectiveness of SCS depends on the segments being stimulated and synaptic interaction. SCS at the lower cervical spinal segments such as C5-C6 is used to treat ischemic diseases, such as Raynaud's disease in the upper limbs (Robaina et al, 1989; Sciacca et al, 1998; Neuhauser et al, 2001). However, in patients with lower limb diseases, SCS at the upper lumbar spinal segments such as L2-L3 produced the largest increase in cutaneous blood flow in the lower limbs. SCS at the higher levels of spinal segments, such as T10, has been observed to decrease cutaneous blood flow in the hindpaws of rats (our observation). This observation is also consistent with the clinical findings of a reduction of peripheral blood flow when the stimulation is at segments above T10 in patients with PAOD (Ghajar and Miles, 1998). In animal studies, transection of T13, but not T10 spinal segment, abolishes vasodilation produced by SCS at L2 spinal segment (Barron et al, 1999). Also, when muscimol, an inhibitor of synaptic activity, is topically applied on the dorsal surface of the spinal cord, SCS effects on peripheral circulation are attenuated (Barron et al, 1999). These results support the theory that SCS-induced vasodilation is dependent on synaptic integration, but is independent of input from rostral spinal sites or supraspinal areas.
2.1.3 Antidromic mechanism
The idea of an antidromic mechanism for vasodilation was initially proposed by Bayliss (Bayliss, 1901). He observed that dorsal root stimulation at high intensity induced peripheral vasodilation mediated by thin fibers. This finding was also confirmed by the observation that a high intensity stimulation of dorsal roots provoked an increase in muscle blood flow (Hilton and Marshall, 1980; Groth, 1985). Foreman's group has investigated this antidromic mechanism over the past ten years, the results of which will be discussed below (Croom et al., 1996, 1997 a, b, 1998; Tanaka et al., 2001, 2003 a, b, 2004; Wu et al., 2006 a, c, d 2007 a, b).
The types of sensory fibers involved in SCS-induced vasodilation
Several studies have been performed to determine the types of sensory fibers involved in SCS effects on peripheral vasodilation. Application of high concentration of capsaicin (1%) on the tibial nerves in vivo blocked in C-fiber conduction and resulted in a reduced duration and amount of vasodilation induced by SCS at 90% and 10 times of MT, but not at 30 and 60% of MT (Tanaka et al., 2003 a). Antidromic compound action potentials (CAPs) of C-fibers in dorsal roots were only evoked by SCS ≥90% of MT, which indicates that SCS-induced vasodilation ≤60% of MT may be mediated via myelinated fibers, whereas vasodilation at ≥90% of MT may also involve unmyelinated C-fibers. Resiniferatoxin (RTX), an ultrapotent analog of capsaicin and transient receptor potential vanilloid receptor-1 (TRPV1) agonist (Zahner et al, 2003; Pan et al, 2003; Wu et al., 2006 b) applied intravenously as well as on the spinal cord, peripheral nerves and in the paw abolished vasodilation produced by SCS (Wu et al., 2006 a, c). Thus, these data indicate that SCS-induced vasodilation is predominantly mediated via TRPV1 containing sensory fibers in unmyelinated C-fibers or myelinated Aδ sensory fibers.
The role of vasodilators
Several vasodilators are contained in sensory endings and are released during SCS. CGRP and other vasodilators are co-localized in TRPV1 sensory containing terminals (Eguchi et al, 2004). The activation of TRPV1 and the subsequent depolarization of the sensory terminals caused the release of these vasodilators which enter the vascular smooth muscle layer (Caterina et al., 1997; Bernardini et al., 2004; Funakoshi et al., 2006). CGRP is one of the most powerful microvascular vasodilators (Brain et al., 1985). It is approximately 10 fold more powerful than prostaglandins and 100−1000 times more effective than other typical vasodilators such as, acetylcholine, adenosine and Substance P (SP) (Brain and Grant, 2004). CGRP plays a crucial role in producing vasodilation and maintaining low vascular resistance by binding to the CGRP-1 receptor (Jansen-Olesen et al., 2001; Brain and Grant, 2004,). CGRP 8−37, a classic antagonist of CGRP-1 receptor, has been used to block CGRP-induced vasodilation (Chiba et al., 1989). Intravenous or paw injection of CGRP 8−37 attenuates SCS-induced vasodilation at all intensities (Tanaka et al., 2001; Wu et al., 2006 c). This result suggests that CGRP binding CGRP-1 receptor plays a pivotal role in mediating SCS effects. However, adrenomedullin (ADM), another peptide from the calcitonin family, co-localizes with CGRP in some tissues, including perivascular nerves and dorsal root ganglia (Hobara et al., 2004). ADM is invloved in angiogenesis (Ribatti et al., 2005) and endothelial protection (Kato et al., 2005). ADM also can produce vasodilation by binding CGRP-1 receptor, although its vasodilation effect is relatively weaker when compared to CGRP (Aiyar et al., 1996; McLatchie et al., 1998; Hagner et al., 2002). Thus, ADM may at least be partially involved in SCS-induced vasodilation. Further studies are needed to determine the exact roles of ADM in SCS effects on ischemic diseases. Nitric oxide (NO) is considered to be another important component involved in SCS-induced vasodilation (Croom et al., 1997 b). Intravenous injection of non selected nitric oxide synthase inhibitors, N (omega)-nitro-L-arginine methyl ester (L-NAME) and N(G)-monomethyl-l-arginine (L-NMMA), decreased SCS-induced vasodilation (Croom et al., 1997 b). Local paw injection of L-NAME has also been shown to decrease vasodilation by SCS (Wu et al., 2007b). NO can be released either from TRPV1 containing nerve endings, or from endothelial cells after CGRP activates their intracellular pathways. In order to differentiate the sources of NO during SCS, a neuronal NOS (nNOS) inhibitor 4S)-N-(4-Amino-5[aminoethyl]aminopentyl)-N’-nitroguanidine, TFA, was directly injected into the paw. However, even a high dose of this neuronal NOS (nNOS) inhibitor did not inhibit SCS-induced vasodilation (Wu et al., 2007b). This indicates that NO is not released from nerve terminals, but instead may be released from endothelial cells during SCS. The peripheral pathways are summarized in Fig 1. Intravenous injection of this specific neuronal NOS (nNOS) inhibitor partially attenuated vasodilation produced by SCS (our unpublished data), suggesting that neuronal NOS may play a role in other tissues such as spinal cord. A previous study in dogs (Sanchez-Ledesma et al., 1990) also showed that, after 60 min of SCS, local arterial vasoactive intestinal peptide increases by 33%, and local arterial and venous histamine increase by 26 and 29%, respectively, but no difference was found in levels of SP.
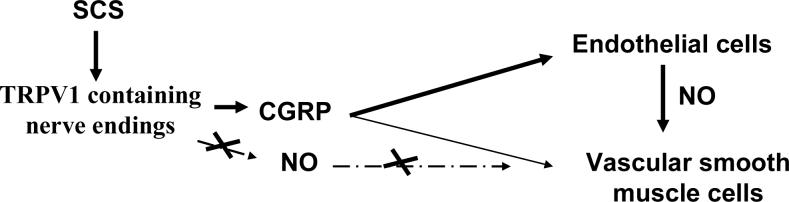
Figure 1. The Summary of Antidormic Mechanisms of SCS-Induced Vasodilation in Limbs and Feet.
The diagram summarizes the antidromic mechanisms that SCS activates TRPV1 containing sensory neurons. The neural information is transmitted from the site of stimulation in the spinal segments to the nerve endings in the peripheral tissues and results in the production and release of vasodilators, including CGRP. CGRP, the most powerful vasodilator, then binds its receptors in endothelial cells and their activation leads to the production and subsequent release of NO to vascular smooth muscle cells, which results in relaxation.
The roles of ERK and AKT
The central spinal terminals of TRPV1 containing sensory fibers in L 3−5 are necessary to produce SCS-induced vasodilation (Wu et al., 2006 a). However, it is unclear how SCS activates these terminals at the spinal level. Anatomically, these sensory central terminals are localized near lamina I and II spinal neurons of the dorsal horn (Sugiura et al., 1993). Activation of extracellular signal-regulated kinase (ERK) and protein kinase B (AKT) in lamina I and II of the dorsal horn are significantly increased after SCS at 90% of MT in the rats (Wu et al., 2006 d). Moreover, local administration of U0126, an inhibitor of ERK kinase, and LY294002, an inhibitor of PI3K upstream of AKT onto the spinal cord significantly decreased SCS-induced vasodilation at 60% and 90% of MT (Wu et al., 2006 d). These results indicate that ERK and AKT play important roles in SCS-induced vasodilation. Other experiments have shown that during SCS the amount of GABA in the spinal cord is increased, while the amount of excitatory amino acids (EAA), glutamate and aspartate are decreased during SCS (Cui et al., 1997 a, b, 1998). It has been shown that substance P, N-methyl-D-aspartate (NMDA) receptors and non-NMDA receptors, are associated with antidromic activity in acute neurogenic inflammation evoked by intradermal injection of capsaicin (Lin et al., 1999, 2004). More research at the biochemical level of the spinal cord is necessary to identify how SCS activates TRPV1 containing sensory fibers.
Section summary
Current knowledge supports the antidromic hypothesis that, at the spinal L2−5 segments SCS activates interneurons containing ERK, AKT and possibly other intracellular signaling molecules, which subsequently stimulate spinal terminals of TRPV1 containing sensory fibers. The neural information is transmitted from the site of stimulation in the spinal segments to the nerve endings in the peripheral tissues and results in the production and release of vasodilators, including CGRP. CGRP, the most powerful vasodilator, then binds its receptors in endothelial cells and their activation leads to the production and subsequent release of NO to vascular smooth muscle cells, which results in relaxation. CGRP also binds to its receptors in vascular smooth muscle cells and activates intracellular signaling pathways, which also produce relaxation of vascular smooth muscle cells. The result is that relaxation of vascular smooth muscle cells results in a decrease in vascular resistance and an increase in local blood flow (Fig 1).
2.1.4 Sympathetic mechanism
It has been proposed that SCS-induced vasodilation may also occur via sympathetic mechanisms. Specifically, SCS suppresses sympathetic activity and subsequently produces marked vasodilation. This theory was proposed based on the results from the clinical observations that a sympathetic block or sympathectomy produced pain relief and vasodilation mimicking the effects of treatment with SCS (Augustinsson et al., 1985; Linderoth et al., 1991 a; Horsch et al., 2004). It is known that the activity of the sympathetic system regulates vascular tone in skin and muscle tissue via autonomic ganglia (Linderoth et al., 1994). Acetylcholine binds to postsynaptic nicotinic receptors and subsequently activates adrenergic receptors in the peripheral tissue, such as vascular smooth muscles. Stimulation of α1 and α2 – adrenoreceptors induces vasoconstriction via the contraction of vascular smooth muscle. In contrast activation of ß2- adrenoreceptors produces vasodilation through the relaxation of vascular smooth cells (Parkinson, 1990 a, b).
SCS–induced cutaneous vasodilation in the rat hindpaw at 66% of MT was abolished by complete surgical sympathectomy (Linderoth et al., 1991 a). Furthermore, the roles of cholinergic and adrenergic receptors in SCS-induced vasodilation were examined (Linderoth et al., 1994). Administration of ganglionic blocker, hexamethonium, or neuronal nicotinic ganglionic blocker, chlorisondamine, abolished the vasodilation produced by SCS. The adrenergic receptor blockers, phentolamine and prazosine in high doses also suppressed SCS-induced vasodilation. However, muscarinic receptor antagonists, pirenzepine and atropine, and the α2 – adrenoreceptor blocker, yohimbine did not alter the SCS effect (Linderoth et al., 1994). These results indicate that the vasodilatory effect of SCS is mediated by an inhibitory effect on peripheral vasoconstriction that is maintained by efferent sympathetic activity including nicotinic transmission in the ganglia and the postganglionic α1- adrenergic receptors. However, conflicting information exists regarding the importance of this mechanism in the peripheral actions of SCS. For example, a α2 agonist, clonidine in high doses suppressed SCS-induced vasodilation. Also some patients demonstrated vasodilation with SCS even after chemical or surgical sympathectomy (Meglio et al., 1986; Broseta et al., 1986; Jacobs et al., 1988). It should be noted that, in humans, sympathectomy is rarely total. Therefore some autonomic responsiveness to SCS may have remained intact.
2.1.5 The relative importance of antidromic and sympathetic mechanism
This section will discuss how the antidromic mechanism and a sympathetic mechanism may interact to produce vasodilation. An early study suggested that the antidromic mechanism did not contribute to vasodilation resulting from SCS. Dorsal root transection did not affect the increased blood flow following SCS at a low intensity in Albino rats (Linderoth et al., 1989). This result is incompatible with an antidromic mechanism. The importance of sympathetic mechanisms in SCS-induced vasodilation were generally supported by experiments conducted using SD rats living in the cold environment of northern Europe where the rats maintain high sympathetic nervous system activity (Linderoth et al., 1991 a, b, 1994, 1995). This relationship has been further investigated by using SD rats in the United States under controlled environmental conditions (Tanaka et al., 2003 b). In a 2003 study by Tanaka, the hindpaw was placed a cooling copper coil to produce local cooling (<25 degrees °C) or moderate local cooling (25−28 degrees °C), resulting in increased sympathetic activities (Freedman et al., 1992). Vasodilation produced by SCS was divided into early and late phases. SCS at all intensities (30%, 60%, and 90% of MT) produced vasodilation at the early phase of vasodilation in the cooled and the moderately cooled hindpaw. The late phase of vasodilation produced by SCS at 90% of MT in the cooled hindpaw was blocked by hexamethonium, a ganglionic blocker. However hexamethonium did not affect the early phase of vasodilation produced by SCS (Tanaka et al., 2003 b). In contrast, CGRP 8−37, a CGRP-1 receptor antagonist, abolished both the early and the late phase responses to SCS. The late phase of vasodilation produced by SCS at 90% of MT in the cooled hindpaw still existed after dorsal rhizotomy (Tanaka et al., 2003 b). The results indicate that SCS-induced vasodilation in the cooled hindpaw (<25 degrees °C) is mediated via both antidromic and sympathetic mechanisms. In contrast, the antidromic mechanism may predominate with moderate skin temperatures (25−28 degrees °C). Therefore, two complementary mechanisms may exist for SCS-induced vasodilatation. The balance of the dual mechanisms depends on the sympathetic activity level, SCS intensity, and individual patients or animal strains.
In addition to peripheral occlusive diseases, vasospastic diseases (mainly Raynaud's disease) also cause ischemic symptoms. Raynaud's phenomenon usually is triggered by cold temperatures, or by emotions such as stress and anxiety (Cooke and Marshall, 2005). The pathological mechanisms are related to the high level of sympathetic activity (Stallworth et al., 1981; Sakakibara et al., 2002; Cooke and Marshall, 2005) and low level of CGRP expression in the local sensory fibers (Mourad and Priollet, 1997; Bunker et al., 1996). The island skin flap animal has been used to mimic the vasospastic condition (Linderoth et al., 1995 b; Qi et al., 2002). In brief, a groin neurovascular flap that exposed the epigastric vessels was proposed in the rat in the supine position. To produce vasospasm, the isolated superficial epigastric artery was repetitively compressed to complete closure with two microforceps for 10 seconds (Linderoth et al., 1995 b). Preemptive SCS showed strong protective effects on the vasospasm (Linderoth et al., 1995 b). Furthermore, another study showed that preemptive SCS increased the survival rate of skin flaps rendered ischemic by occluding the blood supply to the tissue for as long as 12 hours (Gherardini et al., 1999). This cytoprotective effect was markedly reduced by concomitant administration of the CGRP-1 receptor antagonist CGRP 8−37 (Gherardini et al., 1999). Experimental flap survival was increased by the preoperative administration of antiadrenergic drugs such as guanethidine, reserpine, and 6-hydroxydopamine (Jurell and Jonsson, 1976). Therefore, in this pathological ischemic condition of vasospasm and critical ischemia, the dual mechanisms of the release of vasodilators by the antidromic activation of sensory fibers and the suppression of sympathetic activity are probably all involved in the prevention of vasospasm and in cytoprotection.
2.1.6 Other comments
PAOD is a multifactorial disease. In addition to atherosclerosis, multiple risk factors contribute to the complex pathological process of PAOD including genetics, obesity, diabetes mellitus, high blood cholesterol, hypertension and living environment (Garcia, 2006). Therefore, SCS treatment is likely to induce vasodilation in PAOD patients through multiple pathways (Fig 2). Besides these two classic hypotheses, other mechanisms are also potentially involved in SCS effects. First, the release of vasodilators and other peptides may improve endothelial function (Yu et al., 2007) and supress the formation of new atherosclerotic plaques (Kato et al., 2005). The role of endothelial cells and their contribution to the vasodilation effect of SCS are still unclear. New surgical techniques for denuding endothelial cells in peripheral arteries need to be established in order for further studies to be conducted. Second, the release of CGRP and possibly ADM during SCS may also have the ability to stimulate angiogenesis and thus contribute to the long-term effects of SCS (Jang et al., 2000; Nikitenko et al., 2000; Zudaire et al., 2003). Therefore, SCS may not only increase local blood flow in the ischemic area in the short term, but also rebuild new vessel systems to provide increased blood flow to the ischemic area over a long period. Third, opening, or/and rebuilding the arterial collaterals is proposed as an important treatment of PAOD, which compensates for the insufficient blood flow resulting from occluded arteries (Baklanov and Simons, 2003). Through this proposed mechanism, SCS is likely to stimulate and open arterial collaterals and provide extra blood flow to the ischemic area (our preliminary data & discussion with Dr. Li Linggen in the first affiliated hospital of Heilongjiang University of Chinese Medicine, P.R.China). Fourth, accumulating data from clinical studies also show that SCS has similar effects on vascular diseases in patients with, or without diabetes mellitus. Transcutaneous oxygen tension (TcPO2) changes were used as a predictive index of SCS therapy success (Petrakis and Sciacca, 1999, 2000). Thus, SCS has the potential benefit of repairing the damaged nerves and vessels caused by hyperglycemia (Wu et al., 2007a). Fifth, SCS is well known to reduce pain transmission (Linderoth and Foreman, 1999), which contributes to the relief of ischemic pain. Results from a clinical study show that opioid peptides are released during SCS. In that study, plasma Met-enkephalin increased during SCS, indicating an involvement of opioids in the regulation and improvement of the microcirculation by SCS (Fontana et al, 2004). However, clinically there is little or no evidence that opioids are involved in the SCS effects (Eliasson et al., 1998; Meyerson and Linderoth, 2000)
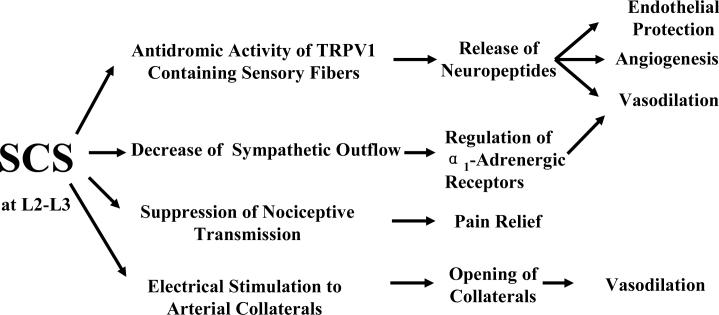
Figure 2. Potential Mechanisms of SCS-induced Vasodilation in the Limbs.
The diagram illustrates that SCS –induced vasodilation may be mediated via 1) antidromic activity of TRPVR1 containing sensory fibers; 2) decrease of sympathetic outflow; and 3) electrical stimulation and opening of collaterals. In addition to vasodilation, the potential mechanisms for other SCS benefits including pain relief, endothelial protection and angiogenesis are also described.
2.2 Effects of SCS on cardiovascular system
2.2.1 Background
Refractory angina pectoris is one of the most common results of heart diseases, and is clinically characterized by discomfort in the chest, jaw, shoulder, back, or arm, which is typically aggravated by exertion or emotional stress (Gibbons et al., 1999). Vasospasm and vessel occlusion are two common mechanisms resulting in the decrease in blood supply to the heart. The imbalance between the supply and the demand of oxygen to the heart is crucial for the development of angina pectoris. More and more patients with chronic angina pectoris resist conventional treatments (Dejongste et al., 2004). SCS has been used to therapy-resistant angina pectoris since the 1980's (Murphy and Giles, 1987; Mannheimer et al., 1988; Augustinsson. 1989). Application of SCS at T1-T2 or higher spinal segments provides promising benefits in patients with angina pectoris. These benefits includes pain relief, reduction in both frequency and to some extent severity of angina attacks, and a reduced short-acting nitrate intake (Jessurun et al., 1996; Stojanovic and Abdi, 2002; Yu, et al, 2004; Chua and Keogh, 2005; Buchser et al., 2006;). Thus, SCS improves the quality of life in patients with angina pectoris by alleviating the symptoms (Yu, et al, 2004). Animal studies have been performed to explain the underlying mechanisms that contribute to pain relief and improved cardiac functions in human.
The mechanisms of SCS effects in heart are more complex and conflicting compared to SCS effects in the limbs and brain. Several hypotheses have been proposed based on animal models to interpret the SCS effects. SCS is suggested to have anti-anginal effects resulting from inhibition of nociceptive transmission (Chandler et al., 1993). Another theory is that SCS may cause a redistribution of the local blood flow and a decrease in the coronary oxygen demand (Eliasson et al., 1993; Jessurun et al., 1996; Linderoth and Foreman, 1999). Recently, it was proposed that SCS stabilizes the intrinsic cardiac nervous system and improves cardiac function (Foreman et al., 2000; Armour et al., 2002; Cardinal et al., 2006). Pre-emptive SCS is likely to protect myocardium from effects of critical ischemia (Southerland et al., 2006). Importantly, there is no solid evidence that SCS has the capability to augment local blood flow in the heart, which is different from the SCS effects on the limbs and brain (Norrsell et al., 1998; Kingma et al 2001).
2.2.2 Animal models and experimental designs
Several different coronary ischemic models have been established in order to study SCS effects on the heart (Kingma et al 2001; Cardinal et al, 2006; Southerland et al., 2006). Acute complete occlusion of the left anterior descending coronary artery (LAD) in dogs (Kingma et al 2001), rabbits (Southerland et al., 2006) or rats (Pirat et al., 2007) have been used as an acute ischemic model. A chronic ischemic model has also been established by implanting an ameroid constrictor around proximal left circumflex coronary artery (Cardinal, et al., 2004). Since the material of the constrictor absorbs fluid and swells slowly in 3−4 weeks (Tomoike et al., 1983), the obstruction to blood flow was produced very slowly and created a relatively chronic ischemic condition in the heart. Also different stressors, such as transient bouts of rapid ventricular pacing and angiotensin II administration, are used to activate the intrinsic cardiac nervous system and mimic clinical heart diseases (Cardinal, et al., 2004). In addition to these models, mediastinal nerve stimulation has been reported to induce bradycardias and atrial tachyarrhythmias in dogs (Cardinal, et al., 2006). 90% of MT (50 Hz, 0.2-ms duration) is the acceptable intensity for SCS studies in the heart (Kingma et al 2001; Cardinal, et al., 2006), and the quadrupolar electrode catheter is commonly used (Cardinal, et al., 2006). T1-T2 spinal segments are the most common and optimal places to be stimulated and the electrode is placed slightly to the left of midline (Cardinal, et al., 2006). However, SCS applied at segments higher than the T1 level also provides some cardioprotection (Gonzalez-Darder et al., 1998; Southerland et al., 2006).
2.2.3 Mechanisms for changes in blood flow
The effect of SCS on cardiac blood flow is still controversial. Unlike the obvious increase in blood flow elicited by SCS in the limbs and brain, few studies report that SCS augments coronary or cardiac blood flow, or redistributes myocardial blood flow (MBF). Effects of SCS on changes of MBF in patients with angina pectoris has been investigated by using positron emission tomography (PET) (Mobilia et al., 1998). The results showed that SCS increased mean MBF and redistributed MBF between the regions with low or normal basal flow and the regions with high basal flow. Also, SCS was reported to improve angina pectoris with a concomitant improvement of myocardial perfusion in cardiac syndrome X (Jessurun et al., 2003). However, another clinical study obtained the opposite results (Norrsell et al., 1998) and found that the anti-ischaemic effect of SCS is independent of an increase in coronary flow velocity. In an experimental animal study, Kingma et al., (2001) investigated this hypothesis by establishing acute complete occlusion of LAD in the dogs. The results showed that SCS did not increase local flow in the myocardium or redistribute blood flow in the non-ischemic or ischemic myocardium. However, a limitation of this study is that occlusions were performed on normal hearts. To make the study more accurate, the experiment should be performed in heart with an ischemia and/or infarction. A clinical study also showed that in the long term, SCS resulted in a decrease of myocardial ischemia. The effect might be due to the formation of better coronary collateralisation because of enhanced physical activity of the patients, instead of a direct effect of SCS (Diedrichs et al, 2005).
Overall, in contrast to the effects of SCS on the peripheral circulation in the limbs, SCS does not produce vasodilation in the cardiovascular system of animals with a normal heart (Kingma et al, 2001). However, neuropeptides including CGRP and NO, as well as TRPV1 containing sensory fibers exist and play important roles in the regulation of cardiovascular system (Ferdinardy et al., 1997; Chottova Dvorakova et al., 2005; Funakoshi et al., 2006; Zyara et al., 2006; Ye et al., 2006). One explanation is that SCS does not activate TRPV1 containing sensory fibers resulting in vasodilation, but stimulates other types of fibers that regulate the cardiac nervous system (see section 2.2.4). Another theory is that SCS may produce release of CGRP strongly affects local circuit neurons and myocardium, but not blood vessels. This may be due to location of nerve endings or to the amount of CGRP released. Therefore, SCS does not produce vasodilation, but is able to adjust the intrinsic cardiac nervous system and protect the heart. Further studies are necessary to elucidate this question.
2.2.4 Intrinsic cardiac nervous system related mechanism
The intrinsic cardiac nervous system is located in the cardiac ganglion plexi of epicardial fat pads adjacent to and within the myocardium (Ardell, 2004). This system includes parasympathetic efferent, sensory afferent, sympathetic efferent and interconnecting local circuit neurons. Interconnecting local circuit neurons have the abilities not only to interact locally, but also to connect with neurons in higher centers resulting in the coordination of regional cardiac function. This population of neurons provides rapid and timely reflex coordination of autonomic neuronal outflow to the heart (Armour, 1999). Clinical observations show that SCS reduces the magnitude of ST segment changes in the electrocardiogram (ECG) induced by exercise and rapid cardiac pacing (Sanderson et al., 1992). SCS is considered to improve cardiac function to a considerable degree by regulating the intrinsic cardiac nervous system (Linderoth and Foreman et al., 2006). Several studies, especially from International Working Group on Neurocardiology (IWGN) support this theory. Bradycardias and atrial arrhythmias can be neuronally induced by mediastinal nerve stimulation in dogs (Cardinal et al., 2006). SCS significantly suppresses these arrhythmias provoked by ischemia (Issa et al., 2005; Cardinal et al., 2006). This effect is blocked after bilateral stellectomy (Cardinal et al., 2006). These data indicate that SCS prevents the induction of atrial arrhythmias induced by excessive activation of intrinsic cardiac neurons (mediastinal nerve stimulation), which is dependent on the integrity of nerves coursing through the subclasvian ansae and stellate ganglia (Cardinal et al., 2006). In another study, SCS reduced the ST segment elevations in an ischemic challenged heart. Ameroid constrictors were implanted around the left circumflex coronary artery (LCx) in canines to mimic the chronic ischemic heart (Cardinal et al., 2004). ST segment displacements were triggered by collateral-dependent myocardium in response to two stressors, transient bouts of rapid ventricular pacing (240/min for 1 min) and angiotensin II administration (100 μg/ml. 0.4 ml/min) to the intrinsic neurons of the right atrial ganglionated plexus (Cardinal et al., 2004). SCS significantly attenuated the increased ST elevation induced by the chronically activated intrinsic interneurons. However SCS did not reduce the increased ST elevation induced by the metabolically determined effects of ventricular rapid process (Cardinal et al., 2004). Importantly, SCS blocked the chemical activation of the intrinsic cardiac nervous system, supporting the theory that SCS improves cardiac function resulting from the normalization of intrinsic cardiac nervous system. In the other words, SCS stabilizes the activity of the intrinsic cardiac nervous system that is typically activated by ischemia. Modulation of sympathetic activity and sensory fibers by SCS are likely to be involved in this mechanism (Linderoth and Foreman, 2006), although the details are still unclear.
2.2.5 Pain suppression mechanisms
The gate-control theory (Melzack and Wall, 1965) supports the general concept that SCS may have inhibitory effects on nociception, which can relieve different kinds of pain, including ischemic pain in the heart. A previous study showed that in anaesthetized monkeys, SCS reduced the number of action potentials of spinothalamic tract neurons evoked by electrical stimulation of cardiopulmonary sympathetic afferent fibers. Furthermore SCS also decreased neuronal activities evoked by intracardiac injection of bradykinin, a mediator of inflammation (Chandler et al., 1993). This study indicates that SCS may also reduce pain transmission via a decrease in the firing of spinothalamic tract cells. However, it is unclear if the suppression of spinothalamic tract activities is a direct effect or results from a reduction in nociceptive information transmitted by afferent fibers.
2.2.6 Protective mechanisms in the myocardium
The results of animal studies lead to the idea that SCS may produce protective changes in the myocardium that allow the heart to resist damage under circumstances producing critical ischemia. SCS has been reported to produce the local release of catecholamine in the myocardium (Armour et al., 2002; Ardell et al., 2005). Catecholamines have also been reported to protect heart from transient myocardial ischemia-induced apoptosis mediated by α1 –PKC pathway and ß-PKA pathway (Broadley and Penson, 2004;). A recent study also reported that this cardioprotection was dependent on cardiac adrenergic efferent neurons (Southerland et al., 2006). Other neuropeptides, such as NO (Sanada and Kitakaze, 2004) and beta-endorphin (Eliasson et al., 1998) are potentially released during SCS and also may prevent myocardial ischemia. The overall potential mechanisms of SCS effects on heart are summarized in Fig 3.
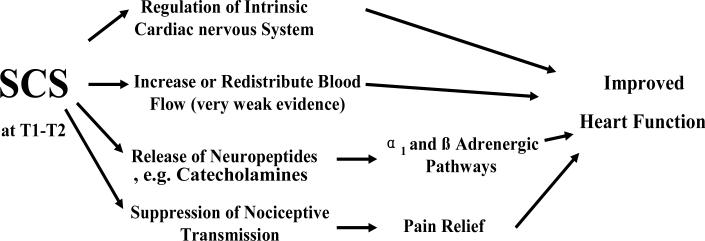
Figure 3. Potential Mechanisms of SCS-improved Heart Function.
The diagram illustrates that SCS-improved Heart Function may be due to 1) an increase or redistribution of blood flow; 2) regulation of the intrinsic cardiac nervous system; 3) release of neuropeptides; and 4) suppression of nociceptive transmission.
2.3 Effects of SCS on cerebral vascular system
2.3.1 Background
Hosobuchi was the first to report that SCS at cervical level, or cervical spinal cord stimulation (cSCS), produced a significant increase in cerebral blood flow (CBF) (Hosobuchi, 1985). Since then, the multiple clinical and basic studies have confirmed this effect. Effects of cSCS on the cerebral vascular system include an increase in CBF (Meglio et al., 1991 a; Ebel et al., 2001; Robaina et al., 2004; Karadag et al., 2005) combined with a decrease in cerebral vascular resistance and an increase in blood flow velocity (Meglio et al., 1991 b), leading to an enhancement of the local-regional delivery of oxygen (Clavo et al., 2002, 2003, 2004), Further effects of cSCS on the cerebral vascular system also include a decrease in induced vasospasms (Visocchi et al., 2001; Gurelik et al., 2005) and an improvement of neurological dysfunction (Gurelik et al., 2005), a modification of glucose metabolism (Momose et al., 1989; Clavo et al., 2006), and the prevention of early spasm due to subarachnoid hemorrhage (SAH) (Visocchi et al., 2001). cSCS effects on cerebral vasodilation depend on a decrease of sympathetic tone (Sagher et al., 2000; Patel et al., 2003), activation of vasomotor centers (Sagher et al., 2000; Patel et al., 2004) and release of neurohumoral factors (Hosobuchi, 1985; Goksel et al., 2001).
2.3.2 Animal models and experimental designs
The animals used for experimental models for the experimental studies include rats, goats, dogs, cats and rabbits. Rats are used to study the cSCS effects under the normal physiological conditions (Sagher et al., 2000, 2003; Patel et al., 2003). The effects of cSCS in pathological conditions are studied with several vasospasm models that have been established in the larger animals such as rabbits (Visocchi et al., 1994; Goksel et al., 2001) and cats (Matsui et al., 1989; Isono et al., 1994). Cerebral ischemia can be induced by cerebral vasospasm after SAH as described by Karadag (Karadag et al., 2005) and Goksel (Goksel et al., 2001). In brief, fresh autologous blood (1 ml/Kg) from each animal's femoral artery was injected into cisterna magna (CM). The locations of cSCS can range from C1 to C6 and the optimal placements of the stimulating electrode is on segments C1-C3 (Patel et al., 2003; Goksel et al., 2001; Sagher et al., 2000). It showed that CBF was increased by more than 80% above baseline with the amplitude of 1.5 mA (Zhong et al., 2004). The optimal pulse width and frequency of cSCS was found to be 0.25 ms, and 50 Hz. A persistent increase in CBF can be produced when applying cSCS for up to 20 minutes. In another study in cats, cSCS at a 4V-stimulus amplitude (25 Hz and 0.1 msec) provided the appropriate microcirculatory enhancement without any harmful effects, and this was sustained 30 min after SCS (Inoue et al., 2000).
2.3.3 Sympathetic mechanisms
Sagher was the first to assess the role of sympathetic tone in cSCS-induced cerebral vasodilation (Sagher et al., 2000), although the earlier studies suggested this mechanism (Broseta et al., 1994; Isono et al., 1994; Visocchi et al., 1994). Intravenous administration of hexamethonium (10 mg/Kg) prior to the initiation of cSCS in the rats abolished the cSCS-induced cerebral vasodilation (Sagher et al., 2000). Another study also showed that cSCS-induced cerebral vasodilation was abolished by both a high-dose of hexamethonium (20 mg/Kg) and prazosin (a selective α-1 receptor blocker, 1 mg/Kg) (Patel et al., 2003). Idazoxan (a selective α-2 receptor blocker) and propranolol (a non-selective ß blocker) did not attenuate the cSCS effect (Patel et al., 2003). However a controversial study showed that cSCS-induced cerebral vasodilation is partially attenuated by propranolol (Garcia-March et al., 1989). Overall sympathetic tone is likely to play an important role in cSCS-induced cerebral vasodilation and this mechanism is mainly mediated by α-1 adrenergic receptors (Patel et al., 2003).
2.3.4 Vasomotor mechanisms
Transection at the spinal cervicomedullary junction significantly attenuated the cSCS-induced cerebral vasodilation, indicating that the cSCS effect may involve supraspinal mechanisms (Sagher et al., 2000). In another study, spinalization, but not resection of the superior cervical ganglion (SCG), abolished cerebral vasodilation produced by cSCS (Patel et al., 2004). The data support the idea that a vasomotor mechanism in the brainstem is crucial in the production of cSCS-induced cerebral vasodilation. Another study indicated that cSCS may activate supraspinal vasomotor centers including the ascending reticular activating system, hypothalamus, thalamus, cingulate gyrus and frontal cortex (Yamaguchi et al., 1995). It is known that the vasomotor centers and their nuclei are implicated in the regulation of CBF. Electrical stimulation of the cerebellar fastigial nucleus can lead to a significant increase in CBF (Mraovitch et al., 1986; Takahashi et al., 1995), which is mainly mediated by rostral ventrolateral nucleus in the medulla (Chida et al., 1990). Therefore, cSCS-induced cerebral vasodilation may initially activate vasomotor centers that include the cerebellar fastigial nucleus and rostral ventrolateral medulla nucleus via ascending spinocerebellar pathways (Patel et al., 2004. Another related theory is that functional activation of the frontal lobe by the ascending reticular pathways mediated via the thalamo-cortical projections during cSCS, is also associated with cSCS-induced cerebral vasodilation (Mazzone et al., 1995, 1996).
2.3.5 Neurohumoral mechanisms
The release of neurohumoral factors is another potential mechanism of cSCS-induced cerebral vasodilation (Hosobuchi, 1985). Indomethacin, a cyclooxygenase inhibitor, partially blocks the effect of cSCS (Hosobuchi, 1985). The role of NO synthase in cSCS-induced cerebral vasodilation during a cerebral vasospasm was investigated in the albino rabbits (Goksel et al., 2001). After the intracisternal injection of L-NAME, cSCS still produced cerebral vasodilation. However, this vasodilation was smaller when compared to the vasodilation produced by cSCS without L-NAME administration (Goksel et al., 2001). Therefore, NO, a basic mediator of vasodilation, is likely to be involved in cSCS-induced cerebral vasodilation. However this study did not determine whether NO synthase was from endothelial NOS, or the neuronal NOS that likely comes from nonadrenergic-noncholinergic nerve fibers (Nozaki et al., 1993). CGRP is another vasodilator that potentially contributes to cerebral vasodilation produced by cSCS in the same way that it contributes to SCS-induced vasodilation in the limbs (Croom et al., 1997). Immunochemical studies show that CGRP containing nerves innervate the cerebrovascular tree (Edvinsson et al., 1987, 1995; Suzuki et al., 1989). Furthermore, stimulation of trigeminal ganglion induced vasodilation mediated by these CGRP containing nerves (Escott et al., 1995). TRPV1 is widely expressed in several brain nuclei and human brain endothelial cells (Mezey et al., 2000; Golech et al., 2004). The studies of immunofluorescence show a high degree of colocalization of TRPV1 with CGRP and SP in the brain neurons, such as those in trigeminal ganglionic neurons (Bae et al., 2004) and corneal neurons (Murata, and Masuko, 2006). Recently, TRPV1 has been shown to be associated with cSCS-induced cerebral vasodilation (Yang et al., 2007). Thus, it is very likely that activation of TRPV1 and release of CGRP during cSCS, is partially involved in cSCS-induced vasodilation. Other vasodilators, such as histamine (Garcia-March et al., 1989) and vasopressin, are possibly involved in this process.
In addition, as we discussed for SCS-induced vasodilation in the lower limbs and feet (2.1.6), cSCS could potentially stimulate and open arterial collaterals of cerebral vascular systems, which could subsequently produce vasodilation. The overall potential mechanisms of the effects of SCS on the cerebral vascular system are summarized in Fig 4. The experimental findings of increased cerebral blood flow and prevention of vasospasm have hitherto, surprisingly, resulted in very few clinical studies mostly in comatose or vegetative patients after vascular catastrophes. (Momose et al., 1989; Funahashi et al., 1989; Kuwata 1993; Simpson 1997).
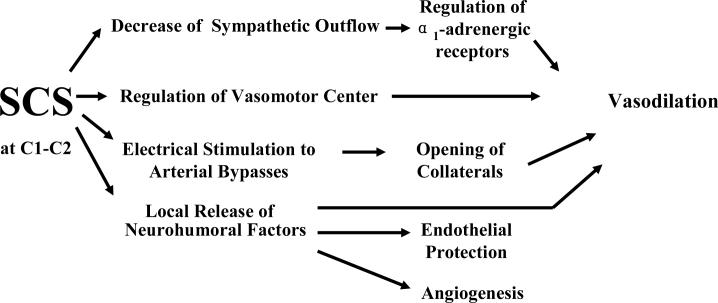
Figure 4. Potential Mechanisms of SCS-induced Vasodilation in Brain.
The diagram illustrates that SCS –induced vasodilation may be related to 1) decrease of sympathetic outflow; 2) regulation of vasomotor center; 3) electrical stimulation and opening of collaterals; and 4) local release of neurohumoral factors. Additionally the release of neurohumoral factors may contribute to endothelial protection and angiogenesis.
3. Summary
In Summary, the effects of SCS on the vascular system are complex and involve multiple mechanisms (Table 1). Based on current knowledge, antidromic activation of sensory fibers and the subsequent release of vasodilators are the most important mechanisms for producing the benefits of SCS at L2-L3 in treatment of PAOD. The benefits to the heart from SCS at T1-T2 to heart are mostly likely due to a normalization of the intrinsic cardiac nervous system. The vasodilation of the cerebral vascular system produced by SCS at C1-C2 may largely be mediated by a suppression of sympathetic activity. Further studies are necessary in order to elucidate the profound mechanisms of SCS effects on the vascular system.
Table 1. The Summary of Different Mechanisms of SCS Effects on Peripheral, Cardiovascular and Cerebral vascular systems.
The table illustrates the different mechanisms regarding SCS effects on different organs: 1) effects of SCS at C1-C2 on cerebrovascular system are associated with the decrease of sympathetic outflow and activation of TRPV1; 2) effects of SCS at T1-T2 on cardiovascular system are largely dependent on adjusting intrinsic cardiac nervous system potentially via sympathetic and sensory neurons; 3) effects of SCS at L2-L3 on peripheral vascular system is due to antidromic activation of TRPV1 containing sensory neurons and a decrease of sympathetic outflow. Symbols explanation: + represents a positive relationship between SCS effects and one of mechanisms; ? represents an unproven relationship.
| SCS | Targeted Tissues | Mechanisms | ||||||
|---|---|---|---|---|---|---|---|---|
| Sensory fibers | Sympat hetic outflow | CGRP | TRPV1 | NO | other | |||
| C1-C2 | Brain | Vasodilation/Ischemia | ? | + | ? | + | + | Brain nucleus (+) |
| C1-C2 T1-T2 | Heart | Improved heart function/Angina pectoris | + | + | ? | ? | ? | Intrinsic cardiac nervous system (+) |
| L2-L3 | Lower limbs | Vasodilation/Ischemia | + | + | + | + | + | ERK and AKT phosphorylation in spinal cord (?) |
Other future studies should be focused on the effect of SCS on endothelial cells. Endothelial dysfunction is a critical pathologic process that can reduce vasodilation and form a proinflammatory and prothrombic condition. Endothelial dysfunction is closely related to the pathological process in most vascular diseases. It is thought that the release of neuropeptides during SCS may have beneficial effects on endothelial cells. However the effects of SCS on the prevention and recovery from endothelial dysfunction have not been studied.
Current experiments have concentrated on the mechanisms related to relative short duration (1−30 min) of SCS treatment in vascular diseases. However, mechanisms involved in producing effects in the vascular system accompanying chronic SCS treatment are still completely unknown. Thus, future experiments should include the development of pathological ischemic animal models with chronically implanted electrodes on the spinal cord for use in the long-term investigation of SCS and its effects on angiogenesis and the vascular system.
With the exception of the heart, the SCS effects on the vascular system of visceral organs, such as gastrointestinal tract, still remain unknown. Therefore, a significant amount of research is necessary in order to elucidate on the effects of SCS on the vascular system of visceral organs.
We believe that the clinical efficacy of SCS combined with a better understanding of the basic mechanisms of SCS effects on the vascular system will lead to increased and effective use of SCS in the treatment of properly screened patients who suffer from cerebral, peripheral, and cardiovascular diseases.
Acknowledgement
We are grateful to Dr. Xiaoli yang (Xi'an Jiaotong University), Chao Qin and Jay P. Farber (The University of Oklahoma Health Sciences Center) for their excellent advice during the preparation of this manuscript. We also thank Brooke Stephenson (The University of Oklahoma Health Sciences Center) for the comments to this manuscript. This review study is supported by NIH grant HL075524 & NS35471 (R.D.F), 2005 University of Oklahoma Health Sciences Center Graduate Student Association Research Grant (M.W) and American Heart Association (AHA) Predoctoral Fellowship 0615642Z (M.W).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiyar N, Rand K, Elshourbagy NA, Zeng Z, Adamou JE, Bergsma DJ, Li Y. A cDNA encoding the calcitonin gene-related peptide type 1 receptor. J Biol Chem. 1996;271:11325–11329. doi: 10.1074/jbc.271.19.11325. [DOI] [PubMed] [Google Scholar]
- Ardell JL, Southerland EM, Milhorn D, et al. Pre-emptive spinal cord stimulation mitigates myocardial ischemia-induced infarction via alpha adrenergic receptors. Experimental Biology, Abstract#. 2005:356.10. [Google Scholar]
- Ardell JL. Intrathoracic neuronal regulation of cardiac function. In: Armour JA, Ardell, editors. Basic and Clinical Neurocardiology. Oxford University Press; 2004. pp. 118–152. [Google Scholar]
- Armour JA. Myocardial ischaemia and the cardiac nervous system. Cardiovasc Res. 1999;41:41–54. doi: 10.1016/s0008-6363(98)00252-1. [DOI] [PubMed] [Google Scholar]
- Armour JA, Linderoth B, Arora RC, DeJongste MJ, Ardell JL, Kingma JG, Jr., Hill M, Foreman RD. Long-term modulation of the intrinsic cardiac nervous system by spinal cord neurons in normal and ischaemic hearts. Auton Neurosci. 2002;95:71–79. doi: 10.1016/s1566-0702(01)00377-0. [DOI] [PubMed] [Google Scholar]
- Augustinsson LE. Spinal cord electrical stimulation in severe angina pectoris: surgical technique, intraoperative physiology, complications, and side effects. Pacing Clin Electrophysiol. 1989;12:693–694. doi: 10.1111/j.1540-8159.1989.tb02716.x. [DOI] [PubMed] [Google Scholar]
- Augustinsson LE, Carlsson CA, Holm J, Jivegard L. Epidural electrical stimulation in severe limb ischemia. Pain relief, increased blood flow, and a possible limb-saving effect. Ann Surg. 1985;202:104–110. doi: 10.1097/00000658-198507000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baklanov D, Simons M. Arteriogenesis: lessons learned from clinical trials. Endothelium. 2003;10:217–223. doi: 10.1080/10623320390246397. [DOI] [PubMed] [Google Scholar]
- Bae YC, Oh JM, Hwang SJ, Shigenaga Y, Valtschanoff JG. Expression of vanilloid receptor TRPV1 in the rat trigeminal sensory nuclei. J Comp Neurol. 2004;478:62–71. doi: 10.1002/cne.20272. [DOI] [PubMed] [Google Scholar]
- Barron KW, Croom JE, Ray CA, Chandler MJ, Foreman RD. Spinal integration of antidromic mediated cutaneous vasodilation during dorsal spinal cord stimulation in the rat. Neurosci Lett. 1999;260:173–176. doi: 10.1016/s0304-3940(98)00972-0. [DOI] [PubMed] [Google Scholar]
- Bayliss WM. On the origin from the spinal cord of the vasodilator fibers of the hind-limb and on the nature of these fibers. J Physiol. 1901;26:173–209. doi: 10.1113/jphysiol.1901.sp000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardini N, Neuhuber W, Reeh PW, Sauer SK. Morphological evidence for functional capsaicin receptor expression and calcitonin gene-related peptide exocytosis in isolated peripheral nerve axons of the mouse. Neuroscience. 2004;126:585–590. doi: 10.1016/j.neuroscience.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev. 2004;84:903–934. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- Broadley KJ, Penson PE. The roles of alpha- and beta-adrenoceptor stimulation in myocardial ischaemia. Auton Autacoid Pharmacol. 2004;24:87–93. doi: 10.1111/j.1474-8673.2004.00324.x. [DOI] [PubMed] [Google Scholar]
- Broseta J, Barbera J, De Vera JA, Barcia-Solario JL, Garcia-March G, Gonzales-Darder J, Rovaina F, Joanes V. Spinal cord stimulation in peripheral arterial disease. J. Neurosurg. 1986;64:71–80. doi: 10.3171/jns.1986.64.1.0071. [DOI] [PubMed] [Google Scholar]
- Broseta J, Garcia-March G, Sanchez-Ledesma MJ, Goncalves J, Silva I, Barcia JA, Llacer JL, Barcia-Salorio JL. High-cervical spinal cord electrical stimulation in brain low perfusion syndromes: experimental basis and preliminary clinical report. Stereotact Funct Neurosurg. 1994;62:171–178. doi: 10.1159/000098614. [DOI] [PubMed] [Google Scholar]
- Buchser E, Durrer A, Albrecht E. Spinal cord stimulation for the management of refractory angina pectoris. J Pain Symptom Manage. 2006;4(Suppl):S36–42. doi: 10.1016/j.jpainsymman.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Bunker CB, Goldsmith PC, Leslie TA, Hayes N, Foreman JC, Dowd PM. Calcitonin gene-related peptide, endothelin-1, the cutaneous microvasculature and Raynaud's phenomenon. Br J Dermatol. 1996;134:399–406. [PubMed] [Google Scholar]
- Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J Neurosurg. 2004;100:254–267. doi: 10.3171/spi.2004.100.3.0254. [DOI] [PubMed] [Google Scholar]
- Cardinal R, Ardell JL, Linderoth B, Vermeulen M, Foreman RD, Armour JA. Spinal cord activation differentially modulates ischaemic electrical responses to different stressors in canine ventricles. Auton Neurosci. 2004;111:37–47. doi: 10.1016/j.autneu.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Cardinal R, Page P, Vermeulen M, Bouchard C, Ardell JL, Foreman RD, Armour JA. Spinal cord stimulation suppresses bradycardias and atrial tachyarrhythmias induced by mediastinal nerve stimulation in dogs. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1369–1375. doi: 10.1152/ajpregu.00056.2006. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chandler MJ, Brennan TJ, Garrison DW, Kim KS, Schwartz PJ, Foreman RD. A mechanism of cardiac pain suppression by spinal cord stimulation: implications for patients with angina pectoris. Eur Heart J. 1993;14:96–105. doi: 10.1093/eurheartj/14.1.96. [DOI] [PubMed] [Google Scholar]
- Chiba T, Yamaguchi A, Yamatani T, Nakamura A, Morishita T, Inui T, Fukase M, Noda T, Fujita T. Calcitonin gene-related peptide receptor antagonist human CGRP-(8−37). Am J Physiol. 1989;256:E331–335. doi: 10.1152/ajpendo.1989.256.2.E331. [DOI] [PubMed] [Google Scholar]
- Chida K, Iadecola C, Reis DJ. Lesions of rostral ventrolateral medulla abolish some cardio- and cerebrovascular components of the cerebellar fastigial pressor and depressor responses. Brain Res. 1990;508:93–104. doi: 10.1016/0006-8993(90)91122-w. [DOI] [PubMed] [Google Scholar]
- Chochola M, Linhart A. Epidemiology of ischemic diseases of the lower extremities. Cas Lek Cesk. 2006;145:368–70. [PubMed] [Google Scholar]
- Chottova Dvorakova M, Kuncova J, Pfeil U, McGregor GP, Sviglerova J, Slavikova J, Kummer W. Cardiomyopathy in streptozotocin-induced diabetes involves intra-axonal accumulation of calcitonin gene-related peptide and altered expression of its receptor in rats. Neuroscience. 2005;134:51–58. doi: 10.1016/j.neuroscience.2005.03.058. [DOI] [PubMed] [Google Scholar]
- Chua R, Keogh A. Spinal cord stimulation significantly improves refractory angina pectoris-a local experience spinal cord stimulation in refractory angina. Heart Lung Circ. 2005;14:3–7. doi: 10.1016/j.hlc.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Clavo B, Robaina F, Catala L, Perez JL, Lloret M, Carames MA, Morera J, Lopez L, Suarez G, Macias D, Rivero J, Hernandez MA. Effect of cervical spinal cord stimulation on regional blood flow and oxygenation in advanced head and neck tumours. Ann Oncol. 2004;15:802–807. doi: 10.1093/annonc/mdh189. [DOI] [PubMed] [Google Scholar]
- Clavo B, Robaina F, Catala L, Valcarcel B, Morera J, Carames MA, Ruiz-Egea E, Panero F, Lloret M, Hernandez MA. Increased locoregional blood flow in brain tumors after cervical spinal cord stimulation. J Neurosurg. 2003;98:1263–1270. doi: 10.3171/jns.2003.98.6.1263. [DOI] [PubMed] [Google Scholar]
- Clavo B, Robaina F, Montz R, Domper M, Carames MA, Morera J, Pinar B, Hernandez MA, Santullano V, Carreras JL. Modification of glucose metabolism in brain tumors by using cervical spinal cord stimulation. J Neurosurg. 2006;104:537–541. doi: 10.3171/jns.2006.104.4.537. [DOI] [PubMed] [Google Scholar]
- Clavo B, Robaina F, Morera J, Ruiz-Egea E, Perez JL, Macias D, Carames MA, Catala L, Hernandez MA, Gunderoth M. Increase of brain tumor oxygenation during cervical spinal cord stimulation. Report of three cases. J Neurosurg. 2002;96:94–100. doi: 10.3171/spi.2002.96.1.0094. [DOI] [PubMed] [Google Scholar]
- Cook AW, Oygar A, Baggenstos P, Pacheco S, Kleriga E. Vascular disease of extremities: Electrical stimulation of spinal cord and posterior roots. New York State J. of Med. 1976;76:366–368. [PubMed] [Google Scholar]
- Cooke JP, Marshall JM. Mechanisms of Raynaud's disease. Vasc Med. 2005;10:293–307. doi: 10.1191/1358863x05vm639ra. [DOI] [PubMed] [Google Scholar]
- Croom JE, Barron KW, Chandler MJ, Foreman RD. Cutaneous blood flow increases in the rat hindpaw during dorsal column stimulation. 1996;728:281–286. doi: 10.1016/0006-8993(96)00554-9. [DOI] [PubMed] [Google Scholar]
- Croom JE, Foreman RD, Chandler MJ, Barron KW. Cutaneous vasodilation during dorsal column stimulation is mediated by dorsal roots and CGRP. Am J Physiol. 1997 a;272:950–957. doi: 10.1152/ajpheart.1997.272.2.H950. [DOI] [PubMed] [Google Scholar]
- Croom JE, Foreman RD, Chandler MJ, Barron KW. Reevaluation of the role of sympathetic nervous system in cutaneous vasodilation during dorsal spinal cord stimulation: Are multiple mechanisms active? Neuromodulation. 1998;1:91–101. doi: 10.1111/j.1525-1403.1998.tb00022.x. [DOI] [PubMed] [Google Scholar]
- Croom JE, Foreman RD, Chandler MJ, Koss MC, Barron KW. Role of nitric oxide in cutaneous blood flow increases in the rat hindpaw during dorsal column stimulation. Neurosurgery. 1997 b discussion 571;40:565–70. doi: 10.1097/00006123-199703000-00027. [DOI] [PubMed] [Google Scholar]
- Cui JC, Linderoth B, Meyerson BA. Incidence of mononeuropathy in rats is influenced by pre-emptive alteration of spinal excitability. Eur J Pain. 1997;1:53–59. doi: 10.1016/s1090-3801(97)90053-7. [DOI] [PubMed] [Google Scholar]
- Cui JG, Meyerson BA, Sollevi A, Linderoth B. Effect of spinal cord stimulation on tactile hypersensitivity in mononeuropathic rats is potentiated by simultaneous GABA(B) and adenosine receptor activation. Neurosci Lett. 1998 a;247:183–186. doi: 10.1016/s0304-3940(98)00324-3. [DOI] [PubMed] [Google Scholar]
- Cui JG, O'Connor WT, Ungerstedt U, Linderoth B, Meyerson BA. Spinal cord stimulation attenuates augmented dorsal horn release of excitatory amino acids in mononeuropathy via a GABAergic mechanism. Pain. 1998 b;73:87–95. doi: 10.1016/s0304-3959(97)00077-8. [DOI] [PubMed] [Google Scholar]
- Deer TR, Raso LJ. Spinal cord stimulation for refractory angina pectoris and peripheral vascular disease. Pain Physician. 2006;9:347–52. [PubMed] [Google Scholar]
- DeJongste MJ, Tio RA, Foreman RD. Chronic therapeutically refractory angina pectoris. Heart. 2004;90:225–230. doi: 10.1136/hrt.2003.025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichs H, Zobel C, Theissen P, Weber M, Koulousakis A, Schicha H, Schwinger RH. Symptomatic relief precedes improvement of myocardial blood flow in patients under spinal cord stimulation. Curr Control Trials Cardiovasc Med. 2005;6:7. doi: 10.1186/1468-6708-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel H, Schomacker K, Balogh A, Volz M, Funke J, Schicha H, Klug N. High cervical spinal cord stimulation (CSCS) increases regional cerebral blood flow after induced subarachnoid haemorrhage in rats. Minim Invasive Neurosurg. 2001;44:167–171. doi: 10.1055/s-2001-18149. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Ekman R, Jansen I, McCulloch J, Uddman R. Calcitonin gene-related peptide and cerebral blood vessels: distribution and vasomotor effects. J Cereb Blood Flow Metab. 1987;7:720–728. doi: 10.1038/jcbfm.1987.126. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Jansen Olesen I, Kingman TA, McCulloch J, Uddman R. Modification of vasoconstrictor responses in cerebral blood vessels by lesioning of the trigeminal nerve: possible involvement of CGRP. Cephalalgia. 1995;15:373–383. doi: 10.1046/j.1468-2982.1995.1505373.x. [DOI] [PubMed] [Google Scholar]
- Eguchi S, Tezuka S, Hobara N, Akiyama S, Kurosaki Y, Kawasaki H. Vanilloid receptors mediate adrenergic nerve- and CGRP-containing nerve-dependent vasodilation induced by nicotine in rat mesenteric resistance arteries. Br J Pharmacol. 2004;142:1137–1146. doi: 10.1038/sj.bjp.0705773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson T, Albertsson P, Hardhammar P, Emanuelsson H, Augustinsson LE, Mannheimer C. Spinal cord stimulation in angina pectoris with normal coronary arteriograms. Coron Artery Dis. 1993;4:819–827. doi: 10.1097/00019501-199309000-00009. [DOI] [PubMed] [Google Scholar]
- Eliasson T, Mannheimer C, Waagstein F, Andersson B, Bergh CH, Augustinsson LE, Hedner T, Larson G. Myocardial turnover of endogenous opioids and calcitonin-gene-related peptide in the human heart and the effects of spinal cord stimulation on pacing-induced angina pectoris. Cardiology. 1998;89:170–177. doi: 10.1159/000006783. [DOI] [PubMed] [Google Scholar]
- Erdek MA, Staats PS. Spinal cord stimulation for angina pectoris and peripheral vascular disease. Anesthesiol Clin North America. 2003;21:797–804. doi: 10.1016/s0889-8537(03)00090-7. [DOI] [PubMed] [Google Scholar]
- Escott KJ, Beattie DT, Connor HE, Brain SD. Trigeminal ganglion stimulation increases facial skin blood flow in the rat: a major role for calcitonin gene-related peptide. Brain Res. 1995;669:93–99. doi: 10.1016/0006-8993(94)01247-f. [DOI] [PubMed] [Google Scholar]
- Ferdinandy P, Csont T, Csonka C, Torok M, Dux M, Nemeth J, Horvath LI, Dux L, Szilvassy Z, Jancso G. Capsaicin-sensitive local sensory innervation is involved in pacing-induced preconditioning in rat hearts: role of nitric oxide and CGRP? Naunyn Schmiedebergs Arch Pharmacol. 1997;356:356–363. doi: 10.1007/pl00005062. [DOI] [PubMed] [Google Scholar]
- Fontana F, Bernardi P, Lanfranchi G, Spampinato S, Di Toro R, Conti E, Bonafe F, Coccheri S. Opioid peptide response to spinal cord stimulation in chronic critical limb ischemia. Peptides. 2004;25:571–575. doi: 10.1016/j.peptides.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Foreman RD, Linderoth B, Ardell JL, Barron KW, Chandler MJ, Hull SS, Jr., TerHorst GJ, DeJongste MJ, Armour JA. Modulation of intrinsic cardiac neurons by spinal cord stimulation: implications for its therapeutic use in angina pectoris. Cardiovasc Res. 2000;47:367–375. doi: 10.1016/s0008-6363(00)00095-x. [DOI] [PubMed] [Google Scholar]
- Freedman RR, Sabharwal SC, Moten M, Migaly P. Local temperature modulates alpha 1- and alpha 2-adrenergic vasoconstriction in men. Am J Physiol. 1992;263:H1197–1200. doi: 10.1152/ajpheart.1992.263.4.H1197. [DOI] [PubMed] [Google Scholar]
- Funahashi K, Komai N, Ogura M, Kuwata T, Nakai M, Tsuji N. Effects and indications of spinal cord stimulation on the vegetative syndrome. No Shinkei Geka. 1989;17:917–923. [PubMed] [Google Scholar]
- Funakoshi K, Nakano M, Atobe Y, Goris RC, Kadota T, Yazama F. Differential development of TRPV1-expressing sensory nerves in peripheral organs. Cell Tissue Res. 2006;323:27–41. doi: 10.1007/s00441-005-0013-3. [DOI] [PubMed] [Google Scholar]
- Garcia LA. Epidemiology and pathophysiology of lower extremity peripheral arterial disease. J Endovasc Ther. 2006;13:II3–9. doi: 10.1177/15266028060130S204. [DOI] [PubMed] [Google Scholar]
- Garcia-March G, Sanchez-Ledesma MJ, Anaya J, Broseta J. Cerebral and carotid haemodynamic changes following cervical spinal cord stimulation. An experimental study. Acta Neurochir Suppl (Wien) 1989;46:102–104. doi: 10.1007/978-3-7091-9029-6_25. [DOI] [PubMed] [Google Scholar]
- Gerasimenko YP, Lavrov IA, Courtine G, Ichiyama RM, Dy CJ, Zhong H, Roy RR, Edgerton VR. Spinal cord reflexes induced by epidural spinal cord stimulation in normal awake rats. J Neurosci Methods. 2006;157:253–263. doi: 10.1016/j.jneumeth.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Ghajar AW, Miles JB. The differential effect of the level of spinal cord stimulation on patients with advanced peripheral vascular disease in the lower limbs. 1998;12:402–408. doi: 10.1080/02688699844583. [DOI] [PubMed] [Google Scholar]
- Gherardini G, Lundeberg T, Cui JG, Eriksson SV, Trubek S, Linderoth B. Spinal cord stimulation improves survival in ischemic skin flaps: an experimental study of the possible mediation by calcitonin gene-related peptide. Plast Reconstr Surg. 1999;103:1221–1228. doi: 10.1097/00006534-199904040-00018. [DOI] [PubMed] [Google Scholar]
- Gibbons RJ, Chatterjee K, Daley J, Douglas JS, Fihn SD, Gardin JM, Grunwald MA, Levy D, Lytle BW, O'Rourke RA, Schafer WP, Williams SV, Ritchie JL, Cheitlin MD, Eagle KA, Gardner TJ, Garson A, Jr., Russell RO, Ryan TJ, Smith SC., Jr. ACC/AHA/ACP-ASIM guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients With Chronic Stable Angina). J Am Coll Cardiol. 1999;33:2092–2197. doi: 10.1016/s0735-1097(99)00150-3. [DOI] [PubMed] [Google Scholar]
- Goksel HM, Karadag O, Turaclar U, Tas F, Oztoprak I. Nitric oxide synthase inhibition attenuates vasoactive response to spinal cord stimulation in an experimental cerebral vasospasm model. Acta Neurochir (Wien) 2001;143:383–390. doi: 10.1007/s007010170094. discussion 390−391. [DOI] [PubMed] [Google Scholar]
- Golech SA, McCarron RM, Chen Y, Bembry J, Lenz F, Mechoulam R, Shohami E, Spatz M. Human brain endothelium: coexpression and function of vanilloid and endocannabinoid receptors. Brain Res Mol Brain Res. 2004;132:87–92. doi: 10.1016/j.molbrainres.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation. 2006;114:688–699. doi: 10.1161/CIRCULATIONAHA.105.593442. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Darder JM, Gonzalez-Martinez V, Canela-Moya P. Cervical spinal cord stimulation in the treatment of severe angina pectoris. Neurosurgery. 1998;8:16–23. [Google Scholar]
- Groth KE. Spinal cord stimulation for the treatment of peripheral vascular disease. In: Fields HL, et al., editors. Advances in pain research and therapy. Vol. 9. Taven Press; New York: 1985. pp. 861–870. [Google Scholar]
- Gurelik M, Kayabas M, Karadag O, Goksel HM, Akyuz A, Topaktas S. Cervical spinal cord stimulation improves neurological dysfunction induced by cerebral vasospasm. Neuroscience. 2005;134:827–832. doi: 10.1016/j.neuroscience.2005.04.062. [DOI] [PubMed] [Google Scholar]
- Hagner S, Stahl U, Knoblauch B, McGregor GP, Lang RE. Calcitonin receptor-like receptor: identification and distribution in human peripheral tissues. Cell Tissue Res. 2002;310:1–50. doi: 10.1007/s00441-002-0616-x. [DOI] [PubMed] [Google Scholar]
- Hilton SM, Marshall JM. Dorsal root vasodilatation in cat skeletal muscle. J Physiol. 1980;299:277–288. doi: 10.1113/jphysiol.1980.sp013124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin J, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- Hobara N, Nakamura A, Ohtsuka A, Narasaki M, Shibata K, Gomoita Y, Kawasaki H. Distribution of adrenomedullin-containing perivascular nerves in the rat mesenteric artery. Peptides. 2004;25:589–599. doi: 10.1016/j.peptides.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Horsch S, Schulte S, Hess S. Spinal cord stimulation in the treatment of peripheral vascular disease: results of a single-center study of 258 patients. Angiology. 2004;55:111–118. doi: 10.1177/000331970405500201. [DOI] [PubMed] [Google Scholar]
- Hosobuchi Y. Electrical stimulation of the cervical spinal cord increases cerebral blood flow in humans. Appl Neurophysiol. 1985;48:372–376. doi: 10.1159/000101161. [DOI] [PubMed] [Google Scholar]
- Inoue M, Nakase H, Hirabayashi H, Hoshida T, Sakaki T. Effect of stimulation of the dorsal aspect of the cervical spinal cord on local cerebral blood flow and EEG in the cat. Neurol Res. 2000;22:386–392. doi: 10.1080/01616412.2000.11740688. [DOI] [PubMed] [Google Scholar]
- Isono M, Kaga A, Fujiki M, Mori T, Hori S. Effect of spinal cord stimulation on cerebral blood flow in cats. Stereotact Funct Neurosurg. 1995;64:40–46. doi: 10.1159/000098732. [DOI] [PubMed] [Google Scholar]
- Issa ZF, Zhou X, Ujhelyi MR, Rosenberger J, Bhakta D, Groh WJ, Miller JM, Zipes DP. Thoracic spinal cord stimulation reduces the risk of ischemic ventricular arrhythmias in a postinfarction heart failure canine model. Circulation. 2005;111:3217–3220. doi: 10.1161/CIRCULATIONAHA.104.507897. [DOI] [PubMed] [Google Scholar]
- Jacobs MJHM, Jorning JG, Joshi SR, Kitslaar PJEHM, Slaaf DW, Reneman RS. Epidural spinal cord electrical stimulation improves microvascular blood flow in severe limb ischemia. Ann. Surg. 1988;207:179–183. doi: 10.1097/00000658-198802000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang YC, Isik FF, Gibran NS. Nerve distribution in hemangiomas depends on the proliferative state of the microvasculature. J Surg Res. 2000;93:144–148. doi: 10.1006/jsre.2000.5973. [DOI] [PubMed] [Google Scholar]
- Jansen-Olesen I, Kaarill L, Edvinsson L. Characterization of CGRP(1) receptors in the guinea pig basilar artery. Eur J Pharmacol. 2001;414:249–258. doi: 10.1016/s0014-2999(01)00760-9. [DOI] [PubMed] [Google Scholar]
- Jessurun GA, DeJongste MJ, Blanksma PK. Current views on neurostimulation in the treatment of cardiac ischemic syndromes. Pain. 1996;66:109–116. doi: 10.1016/0304-3959(96)03001-1. [DOI] [PubMed] [Google Scholar]
- Jessurun GA, Hautvast RW, Tio RA, DeJongste MJ. Electrical neuromodulation improves myocardial perfusion and ameliorates refractory angina pectoris in patients with syndrome X: fad or future? Eur J Pain. 2003;7:507–512. doi: 10.1016/S1090-3801(03)00022-3. [DOI] [PubMed] [Google Scholar]
- Jurell G, Jonsson CE. Increased survival of experimental skin flaps in rats following treatment with antiadrenergic drugs. Scand J Plast Reconstr Surg. 1976;10:169–172. doi: 10.3109/02844317609012964. [DOI] [PubMed] [Google Scholar]
- Karadag O, Eroglu E, Gurelik M, Goksel HM, Kilic E, Gulturk S. Cervical spinal cord stimulation increases cerebral cortical blood flow in an experimental cerebral vasospasm model. Acta Neurochir (Wien) 2005;147:79–84. doi: 10.1007/s00701-004-0410-5. discussion 84. [DOI] [PubMed] [Google Scholar]
- Kato J, Tsuruda T, Kita T, Kitamura K, Eto T. Adrenomedullin: a protective factor for blood vessels. Arterioscler Thromb Vasc Biol. 2005;25:2480–2487. doi: 10.1161/01.ATV.0000184759.91369.f8. [DOI] [PubMed] [Google Scholar]
- Kingma JG, Jr., Linderoth B, Ardell JL, Armour JA, DeJongste MJ, Foreman RD. Neuromodulation therapy does not influence blood flow distribution or left-ventricular dynamics during acute myocardial ischemia. Auton Neurosci. 2001;91:47–54. doi: 10.1016/S1566-0702(01)00285-5. [DOI] [PubMed] [Google Scholar]
- Kuwata T. Effects of the cervical spinal cord stimulation on persistent vegetative syndrome: experimental and clinical study. No Shinkei Geka. 1993;21:325–331. [PubMed] [Google Scholar]
- Lin Q, Wu J, Willis WD. Dorsal root reflexes and cutaneous neurogenic inflammation after intradermal injection of capsaicin in rats. J Neurophysiol. 1999;82:2602–2611. doi: 10.1152/jn.1999.82.5.2602. [DOI] [PubMed] [Google Scholar]
- Lin Q, Zou X, Ren Y, Wang J, Fang L, Willis WD. Involvement of peripheral neuropeptide Y receptors in sympathetic modulation of acute cutaneous flare induced by intradermal capsaicin. Neuroscience. 2004;123:337–347. doi: 10.1016/j.neuroscience.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Linderoth B, Fedorcsak I, Meyerson BA. Is vasodilatation following dorsal column stimulation mediated by antidromic activation of small diameter afferents? Acta Neurochir Suppl (Wien) 1989;46:99–101. doi: 10.1007/978-3-7091-9029-6_24. [DOI] [PubMed] [Google Scholar]
- Linderoth B, Fedorcsak I, Meyerson BA. Peripheral vasodilatation after spinal cord stimulation: animal studies of putative effector mechanisms. Neurosurgery. 1991 a;28:187–195. [PubMed] [Google Scholar]
- Linderoth B, Gunasekera L, Meyerson BA. Effects of sympathectomy on skin and musclemicroculation during dorsal column stimulation: animal studies. Neurosurgery. 1991 b;29:874–879. doi: 10.1097/00006123-199112000-00012. [DOI] [PubMed] [Google Scholar]
- Linderoth B, Herregodts P, Meyerson BA. Sympathetic mediation of peripheral mediation of peripheral vasodilation induced by spinal cord stimulation: animal studies of the role of cholinergic and adrenergic receptor subtypes. Neurosurgery. 1994;35:711–719. doi: 10.1227/00006123-199410000-00018. [DOI] [PubMed] [Google Scholar]
- Linderoth B. Spinal cord stimulation in ischemia and ischemic pain. In: Horsch S, Claeys L, editors. Spinal Cord Stimulation: An innovative Method in the Treatment of PVD and Angina. Steinkopff Verlag; Darmstadt: 1995 a. pp. 19–35. [Google Scholar]
- Linderoth B, Gherardini G, Ren B, Lundeberg T. Preemptive spinal cord stimulation reduces ischemia in an animal model of vasospasm. Neurosurgery. 1995 b;37:266–271. doi: 10.1227/00006123-199508000-00011. discussion 271−272. [DOI] [PubMed] [Google Scholar]
- Linderoth B, Foreman RD. Physiology of spinal cord stimulation: review and update. Neuromodulation. 1999;2:150–164. doi: 10.1046/j.1525-1403.1999.00150.x. [DOI] [PubMed] [Google Scholar]
- Linderoth B, Foreman RD. Mechanisms of spinal cord stimulation in painful syndromes: role of animal models. Pain Med. 2006;7:S14–26. [Google Scholar]
- Mannheimer C, Augustinsson LE, Carlsson CA, Manhem K, Wilhelmsson C. Epidural spinal electrical stimulation in severe angina pectoris. Br Heart J. 1988;59:56–61. doi: 10.1136/hrt.59.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcoux RM, Larrat EP, Taubman AH, Wilson J. Screening for peripheral arterial disease. J Am Pharm Assoc (Wash) 1996;36:370–373. doi: 10.1016/s1086-5802(16)30076-6. [DOI] [PubMed] [Google Scholar]
- Matsui T, Hosobuchi Y. The effects of cervical spinal cord stimulation (cSCS) on experimental stroke. Pacing Clin Electrophysiol. 1989;12:726–732. doi: 10.1111/j.1540-8159.1989.tb02723.x. [DOI] [PubMed] [Google Scholar]
- Mazzone P, Pisani R, Nobili F, Arrigo A, Gambaro M, Rodriguez G. Assessment of regional cerebral blood flow during spinal cord stimulation in humans. Stereotact Funct Neurosurg. 1995;64:197–201. doi: 10.1159/000098748. [DOI] [PubMed] [Google Scholar]
- Mazzone P, Rodriguez G, Arrigo A, Nobili F, Pisani R, Rosadini G. Cerebral haemodynamic changes induced by spinal cord stimulation in man. Ital J Neurol Sci. 1996;17:55–57. doi: 10.1007/BF01995709. [DOI] [PubMed] [Google Scholar]
- McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- Meglio M, Cioni B, Rossi GF, Sandric S, Santarelli P. Spinal cord stimulation affects the central mechanisms of regulation of heart rate. Appl. Neurophysiol. 1986;49:139–146. doi: 10.1159/000100138. [DOI] [PubMed] [Google Scholar]
- Meglio M, Cioni B, Visocchi M, Nobili F, Rodriguez G, Rosadini G, Chiappini F, Sandric S. Spinal cord stimulation and cerebral haemodynamics. Acta Neurochir (Wien) 1991 a;111:43–48. doi: 10.1007/BF01402512. [DOI] [PubMed] [Google Scholar]
- Meglio M, Cioni B, Visocchi M. Cerebral hemodynamics during spinal cord stimulation. Pacing Clin Electrophysiol. 1991 b;14:127–130. doi: 10.1111/j.1540-8159.1991.tb04057.x. [DOI] [PubMed] [Google Scholar]
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–9. doi: 10.1126/science.150.3699.971. 1965. [DOI] [PubMed] [Google Scholar]
- Meyerson BA, Linderoth B. Mechanisms of spinal cord stimulation in neuropathic pain. Neurol Res. 2000;22:285–292. doi: 10.1080/01616412.2000.11740672. [DOI] [PubMed] [Google Scholar]
- Mezey E, Toth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, Guo A, Blumberg PM, Szallasi A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci. 2000;97:3655–3660. doi: 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobilia G, Zuin G, Zanco P, Di Pede F, Pinato G, Neri G, Cargnel S, Raviele A, Ferlin G, Buchberger R. Effects of spinal cord stimulation on regional myocardial blood flow in patients with refractory angina. A positron emission tomography study. G Ital Cardiol. 1998;28:1113–1119. [PubMed] [Google Scholar]
- Momose T, Matsui T, Kosaka N, Ohtake T, Watanabe T, Nishikawa J, Abe K, Tanaka J, Takakura K, Iio M. Effect of cervical spinal cord stimulation (cSCS) on cerebral glucose metabolism and blood flow in a vegetative patient assessed by positron emission tomography (PET) and single photon emission computed tomography (SPECT). Radiat Med. 1989;7:243–246. [PubMed] [Google Scholar]
- Mourad JJ, Priollet P. Physiopathology of Raynaud phenomenon: current data. Rev Med Interne. 1997;18:611–617. doi: 10.1016/s0248-8663(97)82462-9. [DOI] [PubMed] [Google Scholar]
- Mraovitch S, Pinard E, Seylaz J. Two neural mechanisms in rat fastigial nucleus regulating systemic and cerebral circulation. Am J Physiol. 1986;25:1H153–63. doi: 10.1152/ajpheart.1986.251.1.H153. [DOI] [PubMed] [Google Scholar]
- Murata Y, Masuko S. Peripheral and central distribution of TRPV1, substance P and CGRP of rat corneal neurons. Brain Res. 2006;1085:87–94. doi: 10.1016/j.brainres.2006.02.035. [DOI] [PubMed] [Google Scholar]
- Murphy DF, Giles KE. Dorsal column stimulation for pain relief from intractable angina pectoris. Pain. 1987;28:365–368. doi: 10.1016/0304-3959(87)90070-4. [DOI] [PubMed] [Google Scholar]
- Neuhauser B, Perkmann R, Klingler PJ, Giacomuzzi S, Kofler A, Fraedrich G. Clinical and objective data on spinal cord stimulation for the treatment of severe Raynaud's phenomenon. Am Surg. 2001;67:1096–1097. [PubMed] [Google Scholar]
- Nikitenko LL, MacKenzie IZ, Rees MC, Bicknell R. Adrenomedullin is an autocrine regulator of endothelial growth in human endometrium. Mol Hum Reprod. 2000;6:811–819. doi: 10.1093/molehr/6.9.811. [DOI] [PubMed] [Google Scholar]
- Norrsell H, Eliasson T, Albertsson P, Augustinsson LE, Emanuelsson H, Eriksson P, Mannheimer C. Effects of spinal cord stimulation on coronary blood flow velocity. Coron Artery Dis. 1998;9:273–278. doi: 10.1097/00019501-199809050-00005. [DOI] [PubMed] [Google Scholar]
- Nozaki K, Moskowitz MA, Maynard KI, Koketsu N, Dawson TM, Bredt DS, Snyder SH. Possible origins and distribution of immunoreactive nitric oxide synthase-containing nerve fibers in cerebral arteries. J Cereb Blood Flow Metab. 1993;13:70–79. doi: 10.1038/jcbfm.1993.9. [DOI] [PubMed] [Google Scholar]
- Pan HL, Khan GM, Alloway KD, Chen SR. Resiniferatoxin induces paradoxical changes in thermal and mechanical sensitivities in rats: mechanism of action. J Neurosci. 2003;23:2911–2919. doi: 10.1523/JNEUROSCI.23-07-02911.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson D. Adrenergic receptors in the autonomic nervous system. In: Loewy AD, Spyer KM, editors. Central regulation of autonomic functions. Oxford University Press; New York: 1990 a. pp. 17–27. [Google Scholar]
- Parkinson D. Cholinergic receptors. In: Loewy AD, Spyer KM, editors. Central regulation of autonomic functions. Oxford University Press; New York: 1990 b. pp. 28–43. [Google Scholar]
- Patel S, Huang DL, Sagher O. Sympathetic mechanisms in cerebral blood flow alterations induced by spinal cord stimulation. J Neurosurg. 2003;99:754–761. doi: 10.3171/jns.2003.99.4.0754. [DOI] [PubMed] [Google Scholar]
- Patel S, Huang DL, Sagher O. Evidence for a central pathway in the cerebrovascular effects of spinal cord stimulation. Neurosurgery. 2004;55:201–206. doi: 10.1227/01.neu.0000126949.28912.71. discussion 206. [DOI] [PubMed] [Google Scholar]
- Petrakis IE, Sciacca V. Epidural spinal cord electrical stimulation in diabetic critical lower limb ischemia. J Diabetes Complications. 1999;13:293–299. doi: 10.1016/s1056-8727(99)00061-6. [DOI] [PubMed] [Google Scholar]
- Petrakis IE, Sciacca V. Prospective study of transcutaneous oxygen tension (TcPO2) measurement in the testing period of spinal cord stimulation in diabetic patients with critical lower limb ischaemia. Int Angiol. 2000;19:18–25. [PubMed] [Google Scholar]
- Pirat B, Muderrisoglu H, Unal MT, Ozdemir H, Yildirir A, Yucel M, Turkoglu S. Recombinant human-activated protein C inhibits cardiomyocyte apoptosis in a rat model of myocardial ischemia-reperfusion. Coron Artery Dis. 2007;18:61–66. doi: 10.1097/MCA.0b013e328010a44a. [DOI] [PubMed] [Google Scholar]
- Qi Y, Gazelius B, Linderoth B, Lundeberg T. Arterial blood flow and microcirculatory changes in a rat groin flap after thrombosis induced by electrical stimulation of the artery. Microvasc Res. 2002;63:179–185. doi: 10.1006/mvre.2001.2382. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Nico B, Spinazzi R, Vacca A, Nussdorfer GG. The role of adrenomedullin in angiogenesis. Peptides. 2005;26:1670–1675. doi: 10.1016/j.peptides.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Robaina FJ, Dominguez M, Diaz M, Rodriguez JL, de Vera JA. Spinal cord stimulation for relief of chronic pain in vasospastic disorders of the upper limbs. Neurosurgery. 1989;24:63–67. doi: 10.1227/00006123-198901000-00010. [DOI] [PubMed] [Google Scholar]
- Robaina F, Clavo B, Catala F, Carames MA, Morera J. Blood flow increase by cervical spinal cord stimulation in middle cerebral and common carotid arteries. Neuromodulation. 2004;7:26–31. doi: 10.1111/j.1525-1403.2004.04003.x. [DOI] [PubMed] [Google Scholar]
- Sagher O, Huang DL. Effects of cervical spinal cord stimulation on cerebral blood flow in the rat. J Neurosurg. 2000;93:71–76. doi: 10.3171/spi.2000.93.1.0071. [DOI] [PubMed] [Google Scholar]
- Sagher O, Huang DL, Keep RF. Spinal cord stimulation reducing infarct volume in a model of focal cerebral ischemia in rats. J Neurosurg. 2003;99:131–137. doi: 10.3171/jns.2003.99.1.0131. [DOI] [PubMed] [Google Scholar]
- Sakakibara H, Luo J, Zhu SK, Hirata M, Abe M. Autonomic nervous activity during hand immersion in cold water in patients with vibration-induced white finger. Ind Health. 2002;40:254–259. doi: 10.2486/indhealth.40.254. [DOI] [PubMed] [Google Scholar]
- Sanada S, Kitakaze M. Ischemic preconditioning: emerging evidence, controversy, and translational trials. Int J Cardiol. 2004;97:263–276. doi: 10.1016/j.ijcard.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ledesma MJ, Garcia-March G, Goncalves J, Anaya J, Silva I, Gonzalez-Buitrago JM, Navajo JA, Broseta J. Role of vasoactive substances in the segmentary vasomotor response following spinal cord stimulation. An experimental study. Stereotact Funct Neurosurg. 1990;54−55:224–231. doi: 10.1159/000100216. [DOI] [PubMed] [Google Scholar]
- Sanderson JE, Brooksby P, Waterhouse D, Palmer RB, Neubauer K. Epidural spinal electrical stimulation for severe angina: a study of its effects on symptoms, exercise tolerance and degree of ischaemia. Eur Heart J. 1992;13:628–633. doi: 10.1093/oxfordjournals.eurheartj.a060226. [DOI] [PubMed] [Google Scholar]
- Sciacca V, Petrakis I, Borzomati V. Spinal cord stimulation in vibration white finger. Vasa. 1998;27:247–249. [PubMed] [Google Scholar]
- Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg. 1967;46:489–491. [PubMed] [Google Scholar]
- Simpson BA. Spinal cord stimulation. Br J Neurosurg. 1997;11:5–11. doi: 10.1080/02688699746627. [DOI] [PubMed] [Google Scholar]
- Southerland EM, Milhorn DM, Foreman RD, Linderoth B, Dejongste MJ, Armour JA, Subramanian V, Singh K, Singh M, Ardell JL. Pre-emptive, but not reactive, spinal cord stimulation mitigates transient ischemia induced myocardial infarction via cardiac adrenergic neurons. Am J Physiol Heart Circ Physiol. 2006;292:H311–317. doi: 10.1152/ajpheart.00087.2006. [DOI] [PubMed] [Google Scholar]
- Stallworth JM, Horne JB, Plonk GW., Jr. A noninvasive method to assess sympathetic activity. Am Surg. 1981;47:333–337. [PubMed] [Google Scholar]
- Stojanovic MP, Abdi S. Spinal cord stimulation. Pain Physician. 2002;5:156–66. [PubMed] [Google Scholar]
- Sugiura Y, Terui N, Hosoya Y, Tonosaki Y, Nishiyama K, Honda T. Quantitative analysis of central terminal projections of visceral and somatic unmyelinated (C) primary afferent fibers in the guinea pig. J Comp Neurol. 1993;332:315–325. doi: 10.1002/cne.903320305. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Hardebo JE, Owman C. Origins and pathways of cerebrovascular nerves storing substance P and calcitonin gene-related peptide in rat. Neuroscience. 1989;31:427–438. doi: 10.1016/0306-4522(89)90385-0. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Crane AM, Jehle J, Cook M, Kennedy C, Sokoloff L. Role of the cerebellar fastigial nucleus in the physiological regulation of cerebral blood flow. J Cereb Blood Flow Metab. 1995;15:128–142. doi: 10.1038/jcbfm.1995.15. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Barron KW, Chandler MJ, Linderoth B, Foreman RD. Low intensity spinal cord stimulation may induce cutaneous vasodilation via CGRP release. Brain Res. 2001;896:183–187. doi: 10.1016/s0006-8993(01)02144-8. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Barron KW, Chandler MJ, Linderoth B, Foreman RD. Role of primary afferents in spinal cord stimulation-induced vasodilation: characterization of fiber types. Brain Res. 2003 a;959:191–198. doi: 10.1016/s0006-8993(02)03740-x. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Barron KW, Chandler MJ, Linderoth B, Foreman RD. Local cooling alters neural mechanisms producing changes in peripheral blood flow by spinal cord stimulation. Auton Neurosci. 2003 b;28:117–127. doi: 10.1016/S1566-0702(03)00017-1. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Komori N, Barron KW, Chandler MJ, Linderoth B, Foreman RD. Mechanisms of sustained cutaneous vasodilation induced by spinal cord stimulation. Auton Neurosci. 2004;114:55–60. doi: 10.1016/j.autneu.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Tomoike H, Inou T, Watanabe K, Mizukami M, Kikuchi Y, Nakamura M. Functional significance of collaterals during ameroid-induced coronary stenosis in conscious dogs. Interrelationships among regional shortening, regional flow and grade of coronary stenosis. Circulation. 1983;67:1001–1008. doi: 10.1161/01.cir.67.5.1001. [DOI] [PubMed] [Google Scholar]
- Visocchi M, Argiolas L, Meglio M, Cioni B, Basso PD, Rollo M, Cabezas D. Spinal cord stimulation and early experimental cerebral spasm: the “functional monitoring” and the “preventing effect”. Acta Neurochir (Wien) 2001;143(2):177–185. doi: 10.1007/s007010170126. [DOI] [PubMed] [Google Scholar]
- Visocchi M, Cioni B, Vergari S, Marano G, Pentimalli L, Meglio M. Spinal cord stimulation and cerebral blood flow: an experimental study. Stereotact Funct Neurosurg. 1994;62:186–190. doi: 10.1159/000098616. [DOI] [PubMed] [Google Scholar]
- Visocchi M, Di-Rocco F, Meglio M. Protective effect of spinal cord stimulation on experimental early cerebral vasospasm. Conclusive results. Stereotact Funct Neurosurg. 2001;76:269–275. doi: 10.1159/000066730. [DOI] [PubMed] [Google Scholar]
- Wu M, Komori N, Qin C, Farber JP, Linderoth B, Foreman RD. Sensory fibers containing vanilloid receptor-1(VR-1) mediate spinal cord stimulation-induced vasodilation. Brain Res. 2006 a;1107:177–184. doi: 10.1016/j.brainres.2006.05.087. [DOI] [PubMed] [Google Scholar]
- Wu M, Qin C, Foreman RD, Farber JP. Transient receptor potential vanilloid receptor-1 does not contribute to slowly adapting airway receptor activation by ammonia. Auton Neurosci. 2006 b;133:121–127. doi: 10.1016/j.autneu.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Wu M, Komori N, Qin C, Farber JP, Linderoth B, Foreman RD. Extracellular signal-regulated kinase (ERK) and protein kinase B (AKT) pathways involved in spinal cord stimulation (SCS)-induced vasodilation. FASEB J. 2006 d;20:A1402. doi: 10.1016/j.brainres.2007.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Qin C, Farber JP, Linderoth B, Foreman RD. Role of peripheral terminals of vanilloid receptor-1 containing sensory fibers in spinal cord stimulation-induced vasodilation. 36th Society of Neuroscience Meeting. Abstract #. 2006 c;454:5/220. [Google Scholar]
- Wu M, Thorkilsen M, Qin C, Farber JP, Linderoth B, Foreman RD. Effects of spinal cord stimulation on peripheral circulation in stretozotocin-induced diabetic rats. Neuromodulation. 2007a;10:217–223. doi: 10.1111/j.1525-1403.2007.00111.x. [DOI] [PubMed] [Google Scholar]
- Wu M, Komori N, Qin C, Farber JP, Linderoth B, Foreman RD. Roles of peripheral terminals of transient receptor potential vanilloid-1 containing sensory fibers in spinal cord stimulation-induced peripheral vasodilation. Brain Res. 2007b;1156:80–92. doi: 10.1016/j.brainres.2007.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Farber JP, Wu M, Foreman RD, Qin C. Role of TRPV1 in augmentation of cerebral blood flow by cervical spinal cord stimulation in rats. FASEB J. 2007;21:1b563. doi: 10.1016/j.neuroscience.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N, Seki H, Ikeda K, Yamashita J. Effects of cervical spinal cord stimulation on glucose consumption in patients with posttraumatic prolonged unconsciousness. Neurol Med Chir (Tokyo) 1995;35:797–803. doi: 10.2176/nmc.35.797. [DOI] [PubMed] [Google Scholar]
- Ye F, Deng PY, Li D, Luo D, Li NS, Deng S, Deng HW, Li YJ. Involvement of endothelial cell-derived CGRP in heat stress-induced protection of endothelial function. Vascul Pharmacol. 2006;46:238–246. doi: 10.1016/j.vph.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Yu W, Maru F, Edner M, Hellstrom K, Kahan T, Persson H. Spinal cord stimulation for refractory angina pectoris: a retrospective analysis of efficacy and cost-benefit. Coron Artery Dis. 2004;15:31–37. doi: 10.1097/00019501-200402000-00005. [DOI] [PubMed] [Google Scholar]
- Yu XJ, Li CY, Wang KY, Dai HY. Calcitonin gene-related peptide regulates the expression of vascular endothelial growth factor in human HaCaT keratinocytes by activation of ERK1/2 MAPK. Regul Pept. 2007;137:134–139. doi: 10.1016/j.regpep.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Zahner MR, Li DP, Chen SR, Pan HL. Cardiac vanilloid receptor 1-expressing afferent nerves and their role in the cardiogenic sympathetic reflex in rats. J Physiol. 2003;551:515–523. doi: 10.1113/jphysiol.2003.048207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Huang DL, Sagher O. Parameters influencing augmentation of cerebral blood flow by cervical spinal cord stimulation. Acta Neurochir (Wien) 2004;146:1227–1234. doi: 10.1007/s00701-004-0364-7. [DOI] [PubMed] [Google Scholar]
- Zudaire E, Martinez A, Cuttitta F. Adrenomedullin and cancer. Regul Pept. 2003;112:175–183. doi: 10.1016/s0167-0115(03)00037-5. [DOI] [PubMed] [Google Scholar]
- Zvara A, Bencsik P, Fodor G, Csont T, Hackler L, Jr., Dux M, Furst S, Jancso G, Puskas LG, Ferdinandy P. Capsaicin-sensitive sensory neurons regulate myocardial function and gene expression pattern of rat hearts: a DNA microarray study. FASEB J. 2006;20:160–162. doi: 10.1096/fj.05-4060fje. [DOI] [PubMed] [Google Scholar]