Abstract
Integrin-linked kinase (ILK) is key for cell survival, migration, and adhesion, but little is known about its role in epidermal development and homeostasis in vivo. We generated mice with conditional inactivation of the Ilk gene in squamous epithelia. These mice die perinatally and exhibit skin blistering and severe defects in hair follicle morphogenesis, including greatly reduced follicle numbers, failure to progress beyond very early developmental stages, and pronounced defects in follicular keratinocyte proliferation. ILK-deficient epidermis shows abnormalities in adhesion to the basement membrane and in differentiation. ILK-deficient cultured keratinocytes fail to attach and spread efficiently and exhibit multiple abnormalities in actin cytoskeletal organization. Ilk gene inactivation in cultured keratinocytes causes impaired ability to form stable lamellipodia, to directionally migrate, and to polarize. These defects are accompanied by abnormal distribution of active Cdc42 to cell protrusions, as well as reduced activation of Rac1 upon induction of cell migration in scraped keratinocyte monolayers. Significantly, alterations in cell spreading and forward movement in single cells can be rescued by expression of constitutively active Rac1 or RhoG. Our studies underscore a central and distinct role for ILK in hair follicle development and in polarized cell movements, two key aspects of epithelial morphogenesis and function.
INTRODUCTION
Integrins are heterodimeric transmembrane proteins composed of an α regulatory and a β signal-transduction subunit (Giancotti and Ruoshlati, 1999; Schoenwaelder and Burridge, 1999). Integrin-linked kinase (ILK) serves as a scaffold between the cytoplasmic domains of certain integrins and the actin cytoskeleton. In silico analysis of ILK indicates the existence of three N-terminal ankyrin repeats, a pleckstrin homology (PH) domain, and a C-terminal domain with substantial homology to Ser/Thr kinases (reviewed in Hannigan et al., 2005; Legate et al., 2006). These domains mediate interactions with a variety of proteins, including β1 and β3 integrins, PINCH, paxillin and parvins. Through these interactions, ILK functions both as an adaptor between integrins and the actin cytoskeleton, and as a hub around which several important signaling pathways are centered (Grashoff et al., 2004; Hannigan et al., 2005; Legate et al., 2006). ILK also plays a key role in cell movement through poorly understood mechanisms (Grashoff et al., 2003; Terpstra et al., 2003) and is essential for embryogenesis, as ILK-null mouse embryos fail to implant, showing alterations in the actin cytoskeleton and impaired polarization (Sakai et al., 2003).
In addition to these generalized roles, ILK may also fulfill cell type-specific functions in tissues such as the epidermis, a stratified epithelium in which integrins mediate many processes central to its morphogenesis and homeostasis. Epidermal integrins are normally expressed only in the lowermost basal layer, which is composed of undifferentiated keratinocytes. As basal keratinocytes undergo terminal differentiation, they cease to express integrins, detach from the laminin 332–containing basement membrane upon which they rest, move upward to form the suprabasal layers, and form cell–cell contacts (Watt, 2003; Morasso and Tomic-Canic, 2005). The most abundant integrins in resting epidermis are α6β4 and α3β1, which bind to laminin 332 and are necessary for keratinocyte adhesion to the basement membrane (reviewed in Burgeson and Christiano, 1997; Watt, 2003), followed by integrins α2β1 and α5β1, which mediate interactions with collagen/laminin, and fibronectin, respectively. Keratinocytes from mice lacking α6β4 integrins fail to form hemidesmosomes, resulting in epidermal detachment from the basement membrane and formation of large blisters, similar to human junctional epidermolysis bullosa (Downer et al., 1993; Georges-Labouesse et al., 1996; van der Neut et al., 1996). On the other hand, α3β1-null mice exhibit a mild skin phenotype, with formation of microblisters in limbs and abnormalities in laminin 332 assembly (Di Persio et al., 1997). In stark contrast, inactivation of all β1 integrins gives rise to multiple, pronounced defects, including substantial loss of basement membrane, reduction in hemidesmosomes, impaired proliferation, and defects in hair follicle invagination into the dermis (Brackebusch et al., 2000; Raghavan et al., 2000), suggesting that α3β1 integrins mediate some, but not all of the functions of αβ1 dimers.
Although all those studies have shown the importance of β1 integrins in the epidermis, they have provided few insights into downstream mechanisms. Whether ILK is involved in any of these integrin-dependent functions is unknown. However, ILK is important in the epidermis, as evidenced by its involvement in the formation and maintenance of cell–cell junctions in cultured differentiating epidermal keratinocytes (Vespa et al., 2005). As integrin expression decreases in differentiated keratinocytes in culture and in vivo, it is possible that ILK fulfills this role in a manner independent of integrin function. To investigate the functions of ILK in the epidermis, we have generated a conditional mouse mutant using Cre-loxP technology. We now demonstrate that ILK plays essential roles in multiple aspects of epidermal function, including tissue integrity and hair follicle morphogenesis, as well as keratinocyte polarization and capacity for directional migration.
MATERIALS AND METHODS
Cell Culture, Transfections, and Adenovirus Infections
Primary epidermal keratinocytes were isolated from 3-d-old mice and cultured as described (D'Souza et al., 2001). To inactivate the Ilk gene in primary cultured keratinocytes, adenoviral infections were conducted by incubating keratinocytes 2 d after isolation in serum-free growth medium with a Cre-encoding adenovirus (AdCre) for 5 h at a multiplicity of infection of 75, followed by culture in normal growth medium for 96 h before experimentation, or for times indicated in individual experiments. Under these conditions, ≥99% of primary mouse keratinocytes were infected, as revealed by immunofluorescence. In all experiments, control cultures that were infected with a β-galactosidase–encoding adenovirus (Ad-βgal) exhibited responses that were indistinguishable from those cells that were not infected. For reconstitution experiments, cells were infected with AdCre as above, followed 96 h later by a second infection with the ILK- and green fluorescent protein (GFP)-encoding adenovirus, used at a multiplicity of infection of 100. Cells infected with the second virus were visualized by GFP fluorescence. In experiments in which exogenous expression of red fluorescent protein (RFP)-wGBD (N-WASP GTPase-binding domain), GFP-Rac1 (wild type or G12V), GFP-RhoG, or C1199 Tiam1 was induced, keratinocytes were first infected with AdCre, followed 20 h later by transfection with the appropriate plasmid. Cells were cultured for 30 additional hours and processed for direct or immunofluorescence microscopy.
Time-Lapse Videomicroscopy and Scrape-Wound Assays
For time-lapse videomicroscopy of sparse cultures, keratinocytes were trypsinized from stock plates, resuspended in serum-free growth medium containing bovine serum albumin (BSA; 2.5% wt/vol) and 0.05 mM CaCl2, and plated onto culture dishes coated with laminin 332. Cells were placed on a stage heated to 37°C and observed on a Leica DMIRBE microscope (Richmond Hill, Canada) equipped with an Orca II digital camera (Hamamatsu, Hamamatsu City, Japan). Images and movies were generated using Openlab 3.5 software (Improvision, Coventry, United Kingdom). For scrape-wound assays, primary mouse keratinocytes grown to confluence on the extracellular matrix proteins indicated in individual experiments were incubated for 8 h in serum-free growth medium supplemented with 2.5% BSA and scrape-wounded using the narrow end of a 200-μl pipette tip. The cultures were rinsed to remove cell debris, cultured in serum-free growth medium containing 2.5% BSA and 0.05 mM Ca2+ for 16 h, and photographed or processed for immunofluorescence. At least 15 fields were analyzed for each scrape wound, and representative photographs are shown.
Measurement of Rac1 Activity
Rac1 activity was measured by pulldown assays using a GST fusion protein containing the GTPase-binding domain from human PAK1 (GST-PBD) as described (Benard et al., 1999; Katoh et al., 2005), with minor modifications. Untreated ILKf/f keratinocytes or cells infected with AdCre were cultured in normal growth medium for 5 d after infection, followed by medium supplemented with 1% BSA for 24 h. To determine Rac1 activity in migrating cells, 40 scratch wounds were made with a micropipette tip in the confluent monolayers. The cells were rinsed and cultured in fresh medium supplemented with 1% BSA. At timed intervals after scraping, cells were lysed for 5 min in ice-cold buffer A (50 mM Tris-HCl, pH 7.4, 100 mM NaCl, 2 mM MgCl2, 1% Igepal, 10% glycerol, 1 mM dithiothreitol, and protease inhibitors) and collected by scraping, and lysates were clarified by centrifugation (10,000 × g, 4°C, 5 min). Two 20-μl aliquots were removed and used as a loading control for pulldown assays and to determine protein concentration in the lysates. Equal amounts of protein in the remaining lysates were incubated on ice for 60 min with purified GST-PBD fusion protein (1 μg for each 50 μg protein in lysate) and with glutathione-Sepharose beads. The beads were washed four times with ice-cold buffer A, and bound proteins were analyzed by denaturing PAGE and immunoblotting. The results shown are representative of four experiments.
RESULTS
Generation of Mice with Epidermis-restricted Deletion of the Ilk Gene
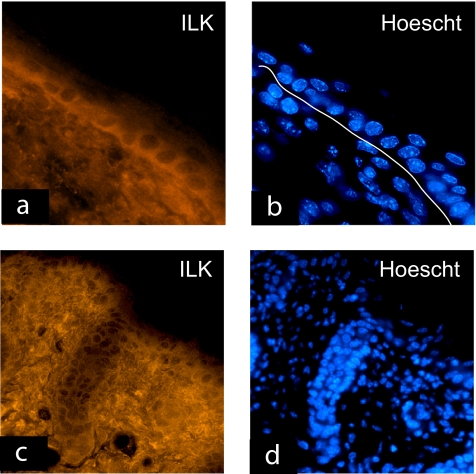
ILK is abundantly expressed in primary cultured keratinocytes, irrespective of their differentiation status (Vespa et al., 2003). In 4-d-old mouse skin, we observed that ILK is present in the basal and all the suprabasal layers of the interfollicular epidermis and is also abundantly expressed in the dermis (Figure 1, a and b). ILK is also readily detected throughout the hair follicles, where it preferentially localizes to the cell periphery in the outer root sheath and to the hair matrix (Figure 1, c and d).
Figure 1.
Expression of ILK in the skin. Frozen sections of 4-d-old mouse skin were used in immunofluorescence analysis with an anti-ILK antibody, and treated with Hoechst 33258 to visualize the nuclei, as indicated in individual micrographs. Expression of ILK is evident in all layers of the interfollicular epidermis and in the dermis (a), with predominance in the basolateral regions of basal keratinocytes. The line traced in panel b is just underneath the dermal/epidermal junction. ILK is also expressed all along the anagen hair follicles (c and d). Bar, 50 μm.
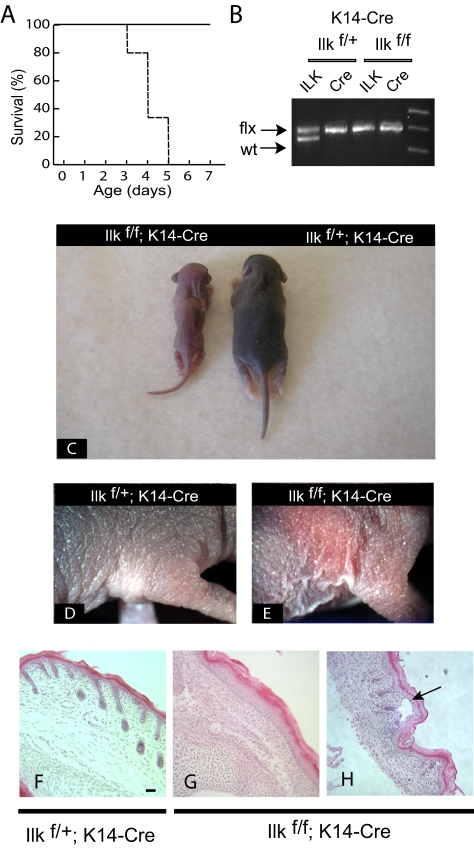
To investigate the role of ILK in keratinocytes and during epidermal morphogenesis, we generated mutant mice with keratinocyte-specific deletion of the Ilk gene. To this end, we first bred Ilkf/f mice (Terpstra et al., 2003) with transgenic mice that specifically express Cre recombinase under the control of the keratin 14 promoter (Dassule et al., 2000) to obtain Ilkf/+;K14-Cre mice. The latter, with only one functional copy of the Ilk gene were indistinguishable from wild-type littermates, indicating a lack of dosage effects. Ilkf/+; K14-Cre mice were subsequently bred with Ilkf/f animals, to generate animals with keratinocyte-specific deletion of the Ilk gene (Ilkf/f; K14-Cre), which were born with the expected 25% Mendelian ratio (34 mice of 150 total), although their median survival was only 4 d (Figure 2A). The homozygous ILKf/f mice carrying the K14-Cre transgene showed no detectable ILK protein in epidermal tissue and had no obvious defects at birth (data not shown). However, reduced pigmentation of the skin and growth delay were evident as early as 1 d after birth and were maintained thereafter, in a manner reminiscent of that observed for mice with epidermal inactivation of the β1 integrin gene (Brackebusch et al., 2000). By 3 d of age, these mice exhibited severe growth delays, failure to thrive, and clear defects in hair growth (Figure 2C). In addition, the skin of these mice appeared thin, scaly, and fragile (Figure 2, D and E).
Figure 2.
Phenotypic abnormalities in mice with targeted inactivation of the Ilk gene in the epidermis. (A) Cumulative survival curve (Kaplan-Meier survival plot) of Ilkf/f;K14Cre mice (- - - -) and control Ilkf/+;K14Cre littermates (——, n = 36). (B) Ilkf/f mice were bred with Ilkf/;K14Cre transgenic mice, and their progeny was genotyped for presence the Cre transgene and the mutant, floxed Ilk allele at 4 d of age, using PCR. (C) Visible abnormalities in Ilkf/f;K14Cre mice. The photograph shows the appearance of the animals genotyped in B at 4 d of age. The mouse with ILK-deficient epidermis shows growth retardation and its skin lacks pigmentation, apparent in the ILK-expressing littermate. (D and E) Presence of dry, scaly and fragile skin in the absence of epidermal ILK expression. (F–H) Histological examination of ILK-deficient epidermis reveals abnormally low hair follicle abundance (G) and presence of blisters, where the epidermis has detached from the dermis (arrow in H). Bar, 100 μm.
Defects in the Epidermis and in Hair Follicle Morphogenesis in ILK-deficient Mice
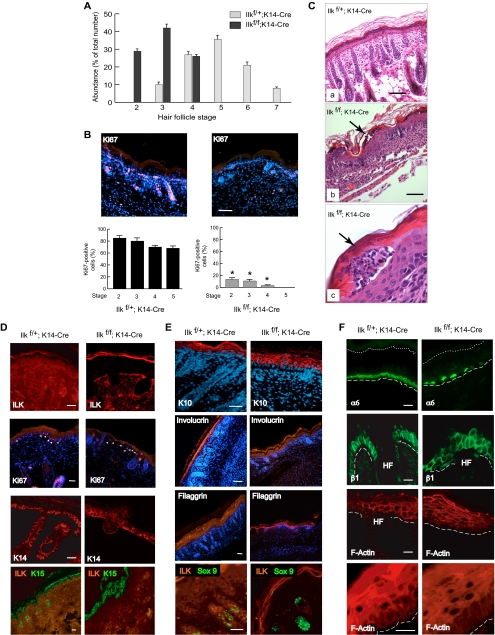
Histological examination of the epidermis in Ilkf/f; K14-Cre mice revealed several abnormalities. Specifically, hair follicle morphogenesis was impaired in ILK-deficient epidermis. Analysis of hair follicle morphology and abundance in 4-d-old skin (Figure 2, F and G) revealed a 55% decrease in the number of follicles in mutant epidermis, with areas in which follicles were not apparent at all. At this age, hair follicles are actively growing in the first anagen phase. We staged hair follicle growth according to well-defined morphological criteria (Paus et al., 1999) and found severe growth retardation in mutant follicles. Thus, although 81% of ILK-expressing follicles had reached stages 4–6, a similar proportion of ILK-deficient follicles had only developed to stages 2 and 3 (Figure 3A). Further, nearly 30% of ILK-expressing follicles had reached stages 6 and 7, whereas no ILK-null follicles were apparent beyond stage 4 (Figure 3A). We next investigated whether mutant keratinocytes exhibited proliferation defects, which might contribute to the reduced formation and development of ILK-deficient follicles. During the growth phase, keratinocytes in the hair follicle proliferate extensively, especially those in the distal and mid-outer root sheath, as well as in the proximal hair matrix (Magerl et al., 2001). We assessed the expression of the proliferation marker Ki67, and observed that ∼80% of cells in wild-type follicles were positive for Ki67 expression (Figure 3B). In stark contrast, only 15% of stage 2 and 5% of stage 4 ILK-deficient follicular keratinocytes expressed detectable Ki67 (Figure 3B), indicating that the defects in hair follicle formation in the absence of ILK are associated with impaired cell proliferation.
Figure 3.
Alterations in the epidermis and hair follicles of Ilkf/f;K14Cre mice. (A) Hair follicle development was histologically assessed in epidermal tissue from 4-d-old Ilkf/+;K14Cre mice or Ilkf/f;K14Cre littermates, as indicated, and the fraction of follicles at a given stage of development was scored according to the criteria outlined by Paus et al. (1999). The results are expressed as the mean + SD of the stages of 600 follicles assessed per mouse (n = 4). (B) Reduced expression of Ki67 in ILK-deficient hair follicles. The presence of the proliferation marker Ki67 was assessed by immunofluorescence microscopy in epidermal sections of Ilkf/+;K14Cre or Ilkf/f;K14Cre mice using an anti-Ki67 antibody. Nuclei were visualized with Hoechst 33258. Nuclei not expressing Ki67 appear dark blue. Quantification of the relative abundance of Ki67-positive nuclei in ILK-expressing and ILK-deficient hair follicles at stages 2–5 is shown under the corresponding tissue micrographs (average + SD), evaluated from 80 follicles in each of five mice. Virtually all ILK-expressing follicles have cells positive for Ki67 staining, in contrast to ILK-deficient follicles (* p < 0.001 relative to stage-matched ILK-expressing follicles). Bar, 200 μm. (C) Histological abnormalities and presence of epidermal blisters in ILK-deficient epidermis. Epidermal tissue sections from mice of the indicated genotype were prepared and stained with hematoxylin and eosin. The arrows in micrographs b and c indicate intraepidermal blisters in the upper suprabasal layers and abnormal underlying epidermis. Bar, (a and b) 100 μm, (c) or 10 μm. (D) Proliferation and expression of basal cell markers in ILK-deficient epidermis. Sections of Ilkf/+;K14Cre or Ilkf/f;K14Cre epidermis from 4-d-old mice were processed for indirect immunofluorescence, using the antibodies indicated in individual panels. Expression of Ki67 is restricted to basal keratinocytes, and positive cells are indicated with arrowheads. Nuclei were visualized with Hoechst 33258. Bar, 50 μm. (E) Expression of differentiation markers in ILK-deficient epidermis. Sections of Ilkf/+;K14Cre or Ilkf/f;K14Cre epidermis from 4-d-old mice were processed for indirect immunofluorescence, using the antibodies indicated in individual panels. Nuclei were visualized with Hoechst 33258. Bar, 100 μm. (F) Integrin expression and F-actin organization in ILK-deficient epidermis. Sections of Ilkf/+;K14Cre or Ilkf/f;K14Cre epidermis from 4-d-old mice were either processed for indirect immunofluorescence, using antibodies against integrin α6 or β1, or were stained with rhodamine-conjugated phalloidin, to visualize the actin cytoskeleton by confocal microscopy, as indicated. Nuclei were detected with Hoechst 33258. Bar, 50 μm
The abnormalities in hair follicle morphogenesis in the absence of ILK expression prompted us to investigate whether the interfollicular epidermis was also affected by Ilk gene inactivation. Histological examination of ILK-deficient epidermis revealed the presence of numerous microblisters at the junction of the basal layer and the basement membrane, which were present on different parts of the body, including dorsal skin, the footpads, and all along the fore- and hind limbs (Figure 2H). We did not observe any blisters in ILK-expressing epidermis. In those animals that were able to survive to 3 or 4 d of age, additional lesions throughout the epidermis were observed, more abundant in areas subjected to high friction. Specifically, there were numerous regions in which intraepidermal separation within the upper layers was evident, as well as extensive erosions on the epithelial surface. Affected regions frequently exhibited acanthosis, increased intracellular epidermal spaces, edema, and inflammation. (Figure 3C and data not shown).
On verification of the uniform loss of ILK protein expression throughout the epidermis and hair follicles in Ilkf/f;K14Cre mice (Figure 3D), we next assessed the presence of abnormalities in proliferation patterns in ILK-deficient basal keratinocytes, as indicated by expression of Ki67. In contrast to follicular keratinocytes, the abundance of Ki67-positive ILK-deficient basal cells (120 cells/mm epidermis) was indistinguishable from that of ILK-expressing keratinocytes (113 cells/mm epidermis), and no suprabasal expression of Ki67 was detected in intact, histologically normal epidermis (Figure 3D).
The differentiation status of keratinocytes in ILK-deficient epidermis was assessed by examining expression of the basal cell markers keratins 14 and 15 (normally restricted to cells in the basal layer and in the hair follicle in 0–4-d-old mice), the spinous markers keratin 10 and involucrin, and filaggrin (granular layer marker). We observed similar expression patterns for keratins 14 and 15 in ILK-expressing and in intact ILK-deficient epidermis. However, these two keratins were also expressed in a few suprabasal layers in some regions of the ILK-deficient epidermis, especially those areas associated with acanthosis, suggesting altered and/or delayed maturation of basal keratinocytes (Figure 3D). In contrast, keratin 10, involucrin, and filaggrin were expressed in the suprabasal, but not basal, layers of ILK-deficient epidermis, similar to normal tissue (Figure 3E). Keratin 10 immunofluorescence also indicated altered morphology in suprabasal, ILK-deficient keratinocytes, but no evidence of altered levels of these proteins was detected.
We next examined the status of follicular keratinocytes in those few hair follicles formed in the absence of ILK. To this end, we assessed expression of the transcription factor Sox9, a well-established marker of the outer root sheath (Vidal et al., 2005). Sox9 is dispensable for hair follicle formation, but is required for the maintenance of outer root sheath identity. Expression of Sox9 is first detected in murine hair placodes at ∼14.5 d of gestation, but it becomes restricted to the presumptive outer root sheath by 18.5 d of gestation. After birth, Sox9 is expressed in the upper regions of the outer root sheath and in the stem cells of the hair follicle bulge. Analysis of Sox9 expression revealed the presence of this transcription factor in ILK-deficient hair placodes from 4-d-old mice (Figure 3E), indicating that ILK is dispensable for specification of at least some keratinocyte lineages, such as the outer root sheath.
Consideration of the role of ILK as a downstream effector of integrin β1, together with the abnormal adhesion of ILK-deficient keratinocytes to the dermo-epidermal junction, prompted us to examine the status of integrins in ILK-deficient epidermis. We first focused on integrin α6, which, together with its partner integrin β4, is an essential component of the hemidesmosomes that contribute to epidermal adhesion to the dermo-epidermal junction, and localizes all along the regions in basal keratinocytes in direct contact with the underlying basement membrane (Figure 3F). Loss of ILK expression markedly perturbed the distribution of integrins α6 and β4 in the basal layer, which exhibited a discontinuous pattern and were undetectable in numerous regions, irrespective of whether visible separations at the dermo-epidermal junction occurred (Figure 3F and data not shown). Thus, loss of ILK expression is associated with abnormalities in hemidesmosomes, which likely impair epidermal interactions with the basement membrane.
Expression of β1 integrin is normally confined to the basal layer, but can extend to suprabasal layers during regeneration after injury and in hyperproliferative conditions, such as psoriasis and papillomavirus infections (Hertle et al., 1992; Cooper et al., 2006). ILK-deficient epidermis shows altered β1 integrin expression, reminiscent of that observed in healing epidermis. Specifically, we readily detected regions with β1 integrin expression within several suprabasal layers, both in intact epidermis and in areas affected by epidermal detachment from the basement membrane (Figure 3F and data not shown).
An important role of ILK is its contribution to maintenance and remodeling of the actin cytoskeleton and cell morphology. In ILK-expressing epidermis, basal keratinocytes are columnar and exhibit actin filaments parallel to the cell membrane, especially in their apical and lateral aspects, whereas suprabasal cells show F-actin filaments around the cell periphery (Figure 3F). In contrast, ILK-deficient keratinocytes appear disorganized. Only a fraction of basal cells maintain a columnar morphology, and suprabasal cells appear flattened. Actin filaments in basal keratinocytes are distributed all around the cell periphery, including regions in contact with the basement membrane (Figure 3F), whereas suprabasal cells exhibited less defined actin bundles along the cell perimeter, as well as actin filaments traversing the cytoplasm (Figure 3F). These observations are consistent with the concept that ILK regulates keratinocyte morphology and actin cytoskeleton in vivo, and its loss causes defects in keratinocyte organization within the epidermis.
ILK Expression Is Necessary for Keratinocyte Spreading and Proliferation in Culture
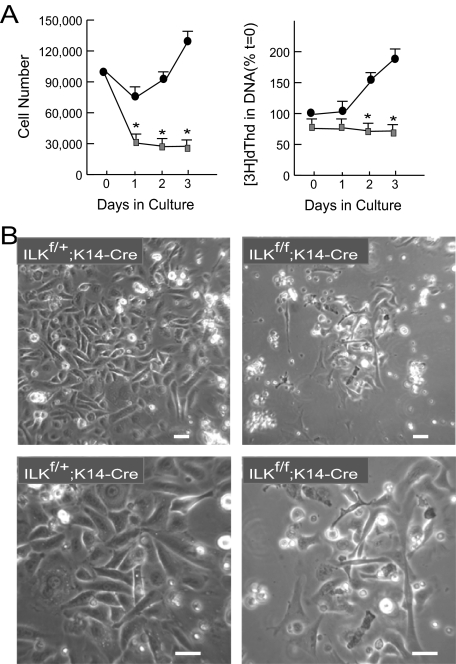
To begin to explore at the cellular level the alterations in keratinocytes due to ILK deficiency, we first isolated and cultured keratinocytes from newborn Ilkf/f;K14-Cre and Ilkf/+;K14-Cre littermates. ILK is necessary for normal attachment and spreading in various cell types, and we observed in ILK-deficient keratinocytes marked differences in spreading, morphology, and proliferation. Specifically, 65–75% of ILK-expressing cells had attached and spread 24 h after plating, compared with 25–30% of ILK-deficient keratinocytes. In addition, the doubling time of ILK-deficient keratinocytes increased to >72 h, whereas that of ILK-expressing cells was 40 h (Figure 4A). Similarly, the DNA synthetic capacity of cultured ILK-deficient keratinocytes was significantly reduced compared with that of ILK-expressing cells (Figure 4A), although no significant differences in the number of apoptotic cells were detected between the two cell populations (data not shown).
Figure 4.
Defects in adhesion and proliferation of cultured ILK-deficient keratinocytes. (A) Primary keratinocytes isolated from 3-d-old Ilkf/+;K14-Cre mice or Ilkf/f;K14-Cre littermates were plated at a density of 100,000 cells/well in 12-well culture dishes at t = 0. The number of cells/well and [3H]dThd incorporated into DNA (expressed as a percentage of [3H]dThd incorporated in Ilkf/+;K14-Cre keratinocytes at t = 0), were determined at the indicated intervals after initial seeding. The results represent the average + SEM of three experiments done in triplicate. * p < 0.05 (ANOVA). (B) Phase-contrast micrographs of live cultured keratinocytes from Ilkf/+;K14Cre mice or Ilkf/f;K14Cre littermates, 3 d after isolation and plating. Bar, 30 μm.
ILK-deficient keratinocytes also exhibited altered morphology, with the majority of the cells being either round and phase-contrast bright, indicating defective cell spreading, or elongated and reminiscent of fibroblasts, rather than showing the characteristic cobblestone-like morphology of epithelial cells (Figure 4B). Thus, ILK is necessary for epidermal keratinocyte spreading and to sustain normal proliferation in culture.
Reduced Spreading and Actin Cytoskeleton Abnormalities in ILK-deficient Keratinocytes
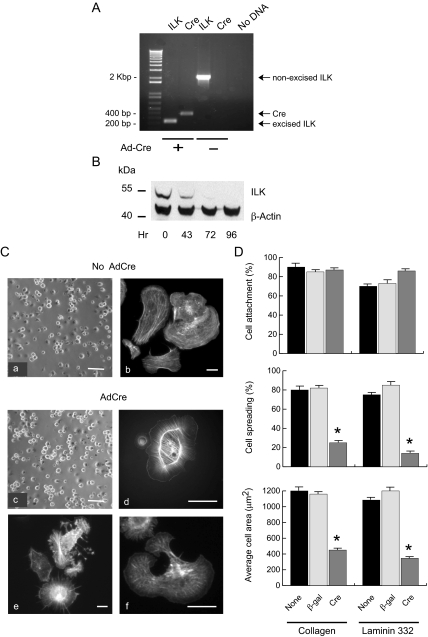
The impaired ability of Ilkf/f; K14-Cre keratinocytes to proliferate in culture precluded the isolation of sufficient cells to more fully elucidate the role that ILK plays in epidermal cells. Thus, we isolated and cultured ILKf/f keratinocytes, which express ILK and are indistinguishable from wild-type cells. After isolating these cells and allowing them to grow, we exogenously expressed Cre recombinase by adenovirus-mediated gene transfer. Twenty-four hours after infection with AdCre we verified that the floxed Ilk gene had been excised and found undetectable levels of nonexcised alleles in these cultures (Figure 5A). ILK protein levels decreased in a time-dependent manner after Ilk gene excision, and we estimated its half-life to be ∼40 h under these conditions (Figure 5B and data not shown). ILK was undetectable by 72–96 h after AdCre infection, and importantly, loss of ILK protein had no significant effect on cellular β-actin levels (Figure 5B).
Figure 5.
Abnormalities in cell spreading and actin cytoskeleton in ILK-deficient keratinocytes. (A) Epidermal keratinocytes from Ilkf/f mice were isolated and plated and 24 h later were infected with Cre-encoding adenoviruses (Ad-Cre) at an moi of 75. Twenty-four hours after AdCre infection, genomic DNA from infected and noninfected cultures was isolated and genotyped by PCR to assess excision of the loxP-containing Ilk alleles. Amplicon sizes corresponding to nonexcised floxed ILK, excised ILK, and Cre DNA are 2.1 kb, 230 base pairs, and 391 base pairs, respectively. (B) Immunoblot analysis of ILK protein levels in keratinocytes treated with AdCre viruses. Cell extracts were isolated at the indicated times after infection with AdCre, resolved by denaturing gel electrophoresis, and transferred to membranes. The membranes were probed with antibodies against ILK or β-actin, as indicated. (C) Four days after infection with adenovirus encoding Cre (AdCre) or β-gal (No AdCre), Ilkf/f keratinocytes were briefly trypsinized and seeded on culture dishes coated with a laminin 332 matrix. Phase-contrast micrographs of live cells were obtained 45 min later (a and c). Parallel cultures were processed for fluorescence microscopy 60 min after plating and treated with rhodamine-labeled phalloidin to visualize the actin cytoskeleton (b and d–f). Bar, 100 μm (a and c) or 15 μm (b and d–f). (D) Ilkf/f keratinocytes were infected with adenovirus encoding the indicated protein, 72 h later they were trypsinized, and 50,000 cells for each condition were seeded on collagen or a laminin 332 substrate. The percentage of cells that attached, or attached and spread, was determined 45 min after plating. Images of cells labeled with rhodamine-labeled phalloidin were acquired and analyzed with Openlab to measure cell surface. * p < 0.01 relative to uninfected cells (ANOVA).
We next determined whether ILK is involved in keratinocyte responses to laminin 332, a major component of the basement membrane at the dermo-epidermal junction. Adhesion to and migration of keratinocytes on laminin 332 occurs through α6β4 and α3β1 integrins (Choma et al., 2004). Normal keratinocytes adhered well to culture surfaces coated with a laminin 332 matrix, and ∼85% of the attached cells had spread within 45 min of plating. Many of these cells polarized, acquiring fan-like morphology with formation of lamellipodia, and exhibited well-organized actin fibers, consistent with previously reported observations (Choma et al., 2004; Figure 5C, micrographs a and b). ILK-deficient keratinocytes attached to laminin 332, but showed significantly reduced spreading (Figure 5, Cc and D). We also observed substantial alterations in the morphology and actin cytoskeleton of spread cells, which consisted of four main variants. Some cells showed a round morphology with few processes, accompanied by formation of thick actin cables surrounding the nucleus and finer perpendicular fibers (Figure 5Cd). Other cells showed abundant fine processes, similar to filopodia, but which branched at various points. In these cells, actin filaments were found along the extensions, as aggregates at the tip of the branches, and in star-like arrangements (Figure 5Ce). A third group of cells exhibited round morphology with circumferential extensions containing actin filaments all along (Figure 5Ce), similar to the phenotype reported for integrin α3-null keratinocytes (Choma et al., 2004). Finally, multiple protrusions formed in some cells, in which actin fibers were visible in perpendicular, rather than parallel, orientation to the protruding cell membrane (Figure 5Cf). We also investigated if attachment to collagen and spreading, mediated by α2β1 integrins in these cells, were affected by loss of ILK expression, and found similar defects to those described above for cells plated on a laminin 332 matrix (Figure 5D and data not shown). Together, our observations demonstrate that ILK is involved in mediating cell spreading and actin cytoskeletal organization in response to laminin 332 and collagen, suggesting that ILK is a downstream effector of integrins α2β1 and α3β1 in keratinocytes.
Alterations in Migration and Organization of the Leading Edge in ILK-deficient Keratinocytes
The interaction of a cell with extracellular matrix substrates is key not only for adhesion and spreading, but also for directional migration. Consequently, the abnormalities in ILK-deficient keratinocytes described above prompted us to investigate the migratory capability of these cells. To this end, we used a scrape-wound model in which the confluent cells at the leading edge of the wound extend processes, migrating to cover the denuded area. This process is critically dependent on cell interactions with extracellular matrix substrates and cytoskeletal reorganization. Confluent monolayers of keratinocytes were scraped, and migration was observed for 16 h. ILK-expressing keratinocytes efficiently migrated and had completely closed the wound by 16 h (Figure 6A). In stark contrast, ILK-deficient cells did not migrate during this time (Figure 6A). Because wound closure requires new deposition of laminin 332 by the migrating keratinocytes (Frank and Carter, 2004), we investigated whether the defective migration in ILK-deficient cells was mainly associated with inability to deposit this substrate. We reasoned that, in this case, exogenously supplying laminin 332 would restore migration to cover the wound. ILK-deficient keratinocytes were unable to migrate when laminin 332 or extracellular matrix components were provided, respectively, by the addition of laminin 332-containing conditioned medium or chelated fetal bovine serum after scraping (data not shown). Thus, ILK is required for sustained migration of keratinocytes, irrespective of the ability of these cells to deposit laminin 332.
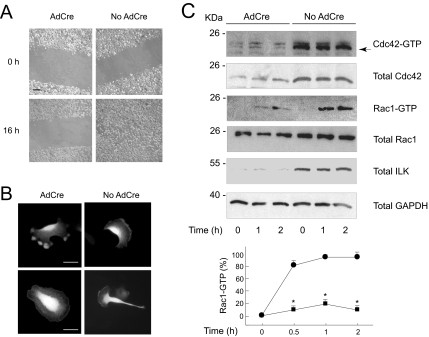
Figure 6.
Inactivation of ILK in keratinocytes prevents directional cell migration and Rac1-GTP formation. (A) Four days after infection with AdCre (AdCre) or an empty adenovirus (No AdCre), confluent monolayers of Ilkf/f keratinocytes were scrape-wounded and cultured in serum-free growth medium and 2.5% BSA. Photographs show representative images all along the wound edges at time = 0, and after allowing the cells to migrate into the denuded areas for 16 h. Bar, 200 μm. (B) Ilkf/f keratinocytes were infected with AdCre or with an empty adenovirus (No AdCre), followed 20 h later by transfection with a vector encoding RFP-wGBD. The cells were cultured for an additional 24-h interval and photographed as they generated extensions, which resulted in directional migration in the control population (No AdCre), but not in the AdCre-infected, ILK-deficient cells. Bar, 50 μm. (C) Ilkf/f keratinocytes were infected with AdCre or an empty adenovirus (No AdCre) and cultured for 4 d in normal growth medium. The confluent monolayers were cultured in serum-free medium containing 0.25% BSA for 16 h, at which time multiple scrape wounds were produced, and cell lysates were obtained at the indicated intervals after wounding. Active, GTP-bound Cdc42 (Cdc42-GTP) and Rac1 (Rac1-GTP) were isolated in GST-PAK pull down assays. The levels of indicated total cellular proteins were also assessed by immunoblot from samples of lysates containing each 20 μg protein. The graph represents the average + SD from densitometric analysis of Rac1GTP levels normalized to abundance of total Rac1 corresponding to the same time point (n = 4, * p < 0.005).
Directional migration requires establishment of cell polarity through formation of front and rear ends. Cell polarization occurs through spatially regulated formation of signaling complexes that regulate actin polymerization and formation of cell protrusions at the leading edge. Key to these events are the Rho GTPases Cdc42 and Rac1, whose active forms localize to the leading edge of the migrating cell and induce formation, respectively, of filopodia and lamellipodia necessary to propel the cell forward (Nobes and Hall, 1995; Ridley et al., 2003). Because of the importance of Rho GTPases in cell movement, together with the impaired migratory capacity of ILK-deficient keratinocytes, we next examined the distribution and extent of Rho GTPase activation in cells that were briefly trypsinized and replated on a laminin 332 matrix. To visualize active Cdc42 (hereafter termed Cdc42-GTP), we took advantage of the ability of a chimeric protein containing monomeric RFP fused to the GBD of N-WASP (RFP-wGBD) to interact with and hence serve as a specific marker for Cdc42-GTP (Kalman et al., 1999; Benink and Bement, 2005). Exogenously expressed RFP-wGBD was observed throughout the cytoplasm in static Ilkf/f keratinocytes and was concentrated as a ribbon along the plasma membrane at the leading edge, but not at the rear end, of migrating cells (Figure 6B and data not shown). Similar patterns of distribution were observed in Ilkf/f cells infected with Ad-βgal, a control recombinant adenovirus that encodes β-galactosidase (data not shown). Excision of the Ilk gene by treatment of Ilkf/f keratinocytes with AdCre markedly altered RFP-wGBD distribution (Figure 6B). Many ILK-deficient cells with a nonpolarized morphology showed RFP-wGBD localization close to the plasma membrane along their entire diameter. Other cells showed several protrusions simultaneously forming in multiple directions, but not a defined lamellipodium. In these cells, intense RFP-wGBD signals were present at the tips of the cell extensions. We also assessed the levels and kinetics of Cdc42 activation after generation of multiple scrape wounds in serum-starved confluent keratinocytes. Cdc42-GTP was readily detected in ILK-expressing monolayers before scratching, and its levels remained essentially unchanged after scrape wounding (Figure 6C). Similarly, Cdc42-GTP abundance in resting ILK-deficient cells did not significantly differ from those observed after scratching. Although the levels of both total Cdc42 and Cdc42-GTP in ILK-deficient cells were substantially lower than those in ILK-expressing cells (Figure 6C), the ratio of Cdc42-GTP to total Cdc42 was similar in both cell types. Thus, ILK is dispensable for Cdc42 activation and localization to cell extensions, but it is necessary for the localization of Cdc42-GTP to a leading lamellipodium.
Keratinocyte spreading and migration on laminin 332 are mediated through α3β1 integrins. These processes also involve Rac1 activation, reorganization of the actin cytoskeleton, stabilization of leading lamellipodia and directional cell movement (Choma et al., 2004; Hamelers et al., 2005). Consequently, we next examined whether the activation of Rac1 induced by migration stimuli on laminin 332 was altered in ILK-deficient cells. We generated multiple scrape wounds in serum-starved confluent keratinocyte monolayers and assessed levels of GTP-bound, active Rac1 (Rac1-GTP) in cell lysates at timed intervals after scratching. Rac1-GTP was undetectable in normal resting cells, but was readily observed shortly after scratching. Maximum levels of Rac1-GTP in ILK-expressing keratinocytes were reached by 30 min after scraping and were maintained for at least 2 h (Figure 6C and data not shown). Rac1-GTP was also detected in ILK-deficient keratinocytes, but the kinetics and extent of Rac1 activation differed from those in normal cells. Specifically, maximum Rac1-GTP levels were sustained for a shorter interval in mutant cells, as Rac1-GTP levels began to fall 1 h after scratching and constituted only ∼10% of those observed in ILK-expressing cells by 2 h (Figure 6C). Our findings support the notion that ILK plays a central role in modulating Rac1 activation and maintenance of Rac1-GTP pools during induction of directional migration stimulated through α3β1 integrins in keratinocytes.
Impaired Directional Migration and Effects of Rac1 Activation in ILK-deficient Keratinocytes
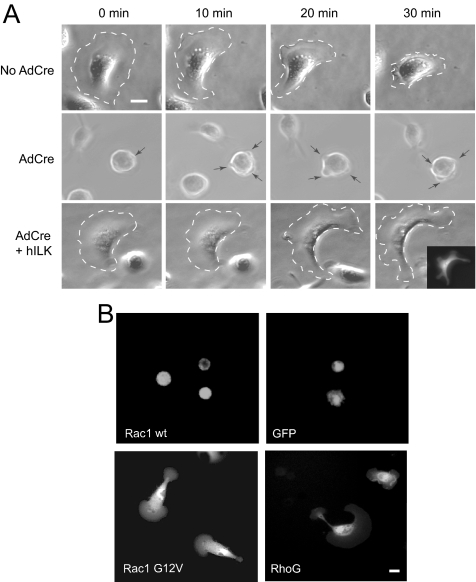
Having established that ILK-deficient keratinocytes exhibit impaired Rac1 activation and migration into the denuded area of an otherwise confluent monolayer, we next examined whether ILK deficiency also causes alterations in spreading and directional, polarized movement in subconfluent cells. To this end, we analyzed by time-lapse videomicroscopy the ability of cells trypsinized and sparsely replated to spread and migrate on a laminin 332-coated surface in the absence of serum, to eliminate any potential contribution of growth factor stimulation on cell migration. ILK-expressing keratinocytes effectively spread, and 30 min after plating ∼30% of the cells displayed large, stable lamellipodia at the cell front, typical of cells migrating on this substrate (Frank and Carter, 2004). Formation of a stable lamellipodium preceded retraction of the rear end of the cell and forward movement (Figure 7A). Continuous cell migration in the direction of the lamellipodium ensued for up to 2 h, in agreement with previous reports (Choma et al., 2004). Similar results were obtained with ILKf/f cells infected with the Ad-βgal adenovirus, or with heterozygous ILKf/+ cells infected with AdCre (data not shown). In stark contrast, <1% of ILK-deficient cells showed these phenotypic changes characteristic of directional migration. The majority of these cells attached efficiently to the laminin 332 matrix, but failed to spread, maintaining a round, phase-contrast, bright appearance. Significantly, numerous short-lived, small pseudopodia, which failed to progress into stable lamellipodia, constantly formed in these cells (Figure 7A). As a result, ILK-deficient cells did not move forward, but rather displayed frequent and random changes in position, indicating that, although formation of cell extensions does not absolutely require ILK, production of a stable lamellipodium for directional migration is critically dependent on this protein. In agreement with this concept, exogenous expression of human ILK in ILK-deficient keratinocytes resulted in the generation of cells that formed stable polarized lamellipodia and exhibited directional migration in a manner and frequency similar to that observed in normal keratinocytes (Figure 7A). Notably, these characteristics of ILK-deficient cells resemble those reported in keratinocytes devoid of α3β1 integrins (Choma et al., 2004), again consistent with the concept that ILK is a likely downstream target of α3β1 integrin stimulation in these cells. We next began to investigate whether the above abnormalities in cell spreading and migration were associated with insufficient Rac1 pools or with a disruption of the link between integrin activation by laminin 332 and Rac1 activation. Thus, we first determined the effect of exogenously expressing wild-type, GFP-tagged Rac1 on keratinocytes sparsely replated on laminin 332 matrix. The impairment in spreading and migrating previously observed in ILK-deficient cells (Figure 7A) was maintained in keratinocytes overexpressing wild-type Rac1, whose phenotype was, in turn, indistinguishable from that of cells expressing GFP as a control (Figure 7B). Thus, keratinocytes transfected with wild-type GFP-Rac1 showed a rounded morphology, production of short-lived cell extensions and no evidence of spreading, lamellipodia formation or directional migration. We also investigated the effect of expressing a constitutively active GFP-Rac1 G12V mutant in ILK-deficient cells, which is able to constitutively localize to focal adhesions and to the leading edge of migrating cells (Chang et al., 2007). We found that cells expressing GFP-Rac1G12V were able to spread after plating and to acquire a polarized fan morphology forming a leading lamellipodium, which allowed them to migrate (Figure 7B). Together, our observations suggest that ILK may be a crucial link between signals from laminin 332-stimulated integrins to downstream factors that recruit and/or activate Rac1.
Figure 7.
Rescue of defects in ILK-deficient keratinocyte spreading and forward cell movement by Rac1 G12V and RhoG. (A) Ilkf/f keratinocytes were infected with empty adenovirus (No AdCre), infected with AdCre (AdCre), or infected with AdCre followed 4 d later by infection with an adenovirus encoding both human ILK and GFP (AdCre + hILK). All cells were cultured in serum-free medium for 16 h. The cells were detached from the culture dish and seeded sparsely onto a laminin 332 matrix–coated surface in serum-free medium and observed by time-lapse videomicroscopy. Dashed borders indicate the outer edge of spread and migrating cells, and arrows denote the position of transient protrusions in an ILK-deficient cell. The inset is a direct fluorescence image of the cell indicated in the (AdCre + hILK) panels, demonstrating expression of GFP. Bar, 25 μm. (B) Ilkf/f keratinocytes were infected with AdCre, followed 20 h later by transfection with vectors encoding the indicated proteins. The cells were cultured for additional 24 h, trypsinized, and reseeded on a surface coated with laminin 332-matrix. Forty-five minutes after seeding, images of live cells were obtained by videomicroscopy. Bar, 25 μm.
We also explored the potential involvement of two Rac1 guanine-exchange factors that are activated by integrin stimulation: RhoG/Dock/Elmo complexes and Tiam1 (Katoh and Negishi, 2003; Hamelers et al., 2005; Katoh et al., 2005). RhoG forms complexes with Dock and Elmo proteins, which localize to the plasma membrane and function to recruit and activate Rac1 to promote integrin-dependent cell spreading and migration. Exogenous expression of GFP-tagged, wild-type RhoG in ILK-deficient keratinocytes resulted in cell spreading and migration similar to those observed with GFP-Rac1 G12V in cells plated on the laminin 332 matrix (Figure 7B). Whether the mechanism of rescue directly involves Rac1 activation or other pathways remains to be determined. Similarly, when we expressed a constitutively active mutant of Tiam 1 (C1199 Tiam1), another direct Rac1 activator which mimics Tiam 1-induced activation of Rac1 stimulated by the interaction of laminin 332 with α3β1 integrins in keratinocytes (Michiels et al., 1997; Hamelers et al., 2005), spreading of ILK-deficient cells was noted (data not shown). Together, these observations suggest that ILK is an essential component of the signaling scaffold that links integrin stimulation with Rac1 recruitment to the plasma membrane and activation in keratinocytes to produce cell spreading and forward movement. Further, it would appear that sufficient endogenous Rac1 is present in ILK-deficient cells to sustain spreading and migration, provided that at least some signaling pathways between integrin stimulation and Rac1 activation are functional and/or amplified, although this idea awaits experimental confirmation.
DISCUSSION
Integrin-linked kinase is central in the formation of cell–cell junctions in cultured epidermal keratinocytes, a fundamental aspect of epidermal function (Vespa et al., 2003, 2005). Given that inactivation of the Ilk gene results in preimplantation lethality, we generated a conditional mouse model to investigate the role of ILK in formation and maintenance of the epidermis and its appendages. Our studies have uncovered a novel, essential role for ILK in hair follicle morphogenesis and epidermal integrity, both at the dermo-epidermal junction and within the suprabasal layers.
Ablation of ILK in interfollicular epidermis did not cause significant perturbations in keratinocyte proliferation or stratification, but gave rise to the formation of microblisters characterized by detachment of the basal layer from the underlying tissue. Of note, we observed small blisters in all the tissue sections analyzed from more than 15 ILK-deficient animals, but did not find them in any of the tissue sections obtained from ILK-expressing littermates, consistent with the concept that they were not simply the result of tissue processing. This phenotype is reminiscent of the alterations caused by loss of α3β1 integrin function in the epidermis of mice with targeted inactivation of α3 integrin (Di Persio et al., 1997). ILK is a well-established downstream target of β1 integrins. Given that α3β1 integrins not only contribute to keratinocyte attachment, but also are necessary to establish and maintain the integrity of the laminin 332-containing basement membrane, our studies indicate that ILK may be an important intracellular transducer of α3β1 integrin activity in the epidermis. Notably, ILK-deficient epidermis exhibited alterations in hair follicle morphogenesis absent in α3β1-null mice, but present in animals with targeted inactivation of the β1 integrin gene, which lack three integrin complexes present in the epidermis (α2β1, α3β1, and α5β1; Brackebusch et al., 2000; Raghavan et al., 2000). Specifically, both ILK- and β1-deficient epidermis exhibit substantial decreases in the abundance and developmental stage reached by the hair follicles, as well as in proliferative capacity of follicular keratinocytes. β1 integrins are implicated in the ability of developing hair follicles to remodel the ECM and invaginate into the underlying dermal tissue, although virtually nothing is known about the molecular events that ensue upon integrin activation. Laminin-integrin interactions contribute to hair development and to the expression of early hair markers, such as sonic hedgehog and Gli1 (Li et al., 2003). On the basis of the striking similarities between β1- and ILK-deficient follicles, we propose ILK as a likely important mediator of laminin/β1 integrin–modulated developmental cues during folliculogenesis. ILK is involved in integrin-independent functions in keratinocytes, such as formation of cell–cell junctions, and consequently, our studies do not rule out a role for ILK in hair follicle morphogenesis independent of integrin activation. Abnormalities in hair follicle development were also reported recently in a different mutant mouse strain, in which conditional inactivation of a loxP-flanked Ilk gene was induced by expression of Cre recombinase driven by the keratin 5 promoter (Lorenz et al., 2007). In stark contrast with the enormous reduction in hair follicle numbers observed in out Ilkf/f;K14Cre mice, few, if any, alterations in hair follicles were reported in newborn ILK-K5 mice. In contrast to Ilkf/f;K14Cre mice, which die perinatally, ILK-K5 mice live to adulthood, and their epidermis exhibits alteration in differentiation marker expression and keratinocyte proliferation profiles, accompanied by inflammation. Similarly, we find that Ilkf/f;K14Cre mice exhibit pronounced alterations in the distribution of hemidesmosomal components, such as integrins α6 and β4, again in contrast with the minimal alterations reported in ILK-K5 mice. One of the differences between these two mouse strains is likely the time of activation of Cre recombinase gene expression. Specifically, Cre expression in the K14Cre transgenic mice used to generate Ilkf/f;K14Cre mice in the present study is first detected at 11.5 d of gestation, preceding epidermal stratification and folliculogenesis (Dassule et al., 2000). In contrast, the K5Cre transgenic mouse used to generate ILK-K5 animals is reported to show Cre expression much later, at ∼15.5 d of gestation, a time when the epidermis has stratified and hair follicle formation is well under way (Lorenz et al., 2007). Thus, the differences between the phenotypes of these two mouse strains may be due to distinct penetrance or to differences in the abundance and/or timing during embryogenesis of Cre transgene expression and Ilk gene inactivation. Regardless, our studies demonstrate a key role for ILK in the early events that specify hair follicle formation.
Keratinocytes isolated from Ilkf/f;K14Cre mice and subsequently cultured have adhesion and proliferation defects. Those reduced proliferation rates are reminiscent of defects observed in hair follicle, but not in interfollicular, cells. Primary keratinocytes placed in culture require signaling through α3β1 integrins for normal proliferation (Manohar et al., 2004), and our data and other studies place ILK as a crucial mediator of responses to β1 integrins. Given that a majority of primary cultured keratinocytes isolated from epidermis are transit-amplifying or committed progenitor cells, the impairment in proliferation observed in cultured Ilkf/f;K14Cre keratinocytes likely arises, at least in part, from defects in β1 integrin-mediated processes in this cell population, although defects in stem cell proliferation cannot be ruled out and would constitute an important area for future studies.
We have characterized adhesion and migration in cultured Ilkf/f keratinocytes in which the Ilk gene was inactivated after isolation by exogenous expression of Cre recombinase. We first examined in these cells the role of ILK in polarized cell migration in the context of leading keratinocytes in a scraped monolayer and found a pronounced impairment in forward cell movement and Rac1 activation, indicating that ILK is a limiting factor for these two events. These alterations occurred irrespective of the extracellular matrix (ECM) substrate provided. Polarization and migration on these substrates depends on ligation of αβ1 integrins, and our studies now place ILK as a central downstream effector in these signaling pathways.
Interactions with laminin 332 ECM are mediated by α3β1 and α6β4 integrins in keratinocytes. The former mediate adhesion and spreading and are involved in migration, whereas the latter contribute to formation of stable adhesions through hemidesmosome assembly (Borradori and Sonnenberg, 1999; Choma et al., 2004). The involvement of ILK in cell migration is well recognized, and our studies begin to address this aspect of ILK function in epidermal keratinocytes, with the demonstration that ILK is required for distribution of active Cdc42 at the leading edge, and to modulate the extent and kinetics of Rac1 activation, as well as formation of stable polarized lamellipodia, which trigger directional cell movements. Given the scaffold functions of ILK, it is not entirely surprising that its deficiency is linked to so many diverse alterations. A key event for cell polarization appears to be the activation of Rac1 specifically at the leading edge of the cell, which is essential for establishing and stabilizing the lamellipodia that direct movement. In keratinocytes, α3β1 integrins regulate cell adhesion during migration on laminin 332, playing a poorly understood role in induction of cell polarization. Our studies show that cell interactions with laminin 332 trigger a downstream cascade of events crucially dependent on ILK, which results in Rac1 activation, lamellipodia formation, and polarized migration.
Rac1 is activated in response to laminin 332 but, notably, the extent and duration of its activation is impaired in the absence of ILK. Thus, ILK functions as an essential link between integrin activation and cell migration through Rac1 activation in wounded epidermal sheets. Significantly, we were able to restore cell spreading and forward movement in single ILK-deficient keratinocytes through various Rac1-activating stimuli, including exogenously expressing a constitutively active Tiam 1 mutant, as well as RhoG. Tiam1 has been identified in keratinocytes as an essential and specific component of a signaling cascade that results in Rac1 activation during laminin 332-induced polarized cell migration (Hamelers et al., 2005). The phenotype of keratinocytes with a targeted inactivation of the Tiam1 gene is remarkably similar to that observed in ILK-deficient cells, and the ability of C1199 Tiam1 to rescue the phenotype of ILK-deficient cells suggests the possibility that ILK and Tiam1 may cooperate. Rac1 is also activated through additional mechanisms, including those that involve RhoG- and Pix-modulated pathways. ILK interacts with other complexes that modulate directional movement, including those containing paxillin, parvins, and Pix (Filipenko et al., 2005; Nishiya et al., 2005). Although the mechanism whereby RhoG rescues the defects in ILK-deficient cells remains to be elucidated, it is possible that it involves activation of Rac1. The relative contributions of different ILK-containing modules to cell polarization likely depend on cell type and context, as well as precise stimuli, and a role for such modules in keratinocyte migration has yet to be determined. Our studies establish that ILK plays a central role in the extent and duration of Rac1 activation during migration of leading keratinocytes, and it will be important to determine how ILK recruits and regulates temporally and spatially specific signaling modules during directional cell movement.
The abnormalities in Rac1 activation we observed in ILK-deficient epithelial sheets differ from the reported normal Rac1 activation in ILK-deficient K5-ILK keratinocytes that had been suspended and replated on a laminin 332 matrix as single cells, although both strains of keratinocytes show impaired polarization and directional migration (Lorenz et al., 2007). This apparent discrepancy may result from the fact that many but not all signaling events are similar in leading keratinocytes in a contiguous sheet and in individually migrating cells. For example, although p38 mitogen-activated protein kinase is phosphorylated in leading keratinocytes and upon detachment of the cells from their substrate, reattachment of the latter to laminin 332 results in its dephosphorylation, without loss of cell migratory properties (Harper et al., 2005). Further, activation of Rac1 upon adhesion to laminin 332 involves multiple input pathways, including signaling from α3β1 integrin to focal adhesion and src kinases (Choma et al., 2006), as well as to the Rac1 activator Tiam 1 (Hamelers et al., 2005). Thus, in the context of single keratinocytes, ILK may not be limiting for Rac1 activation.
In summary, conditional Ilk gene inactivation in murine epidermis has provided major new insights into the multiple roles that ILK plays in this tissue, underscoring a critical, unanticipated and distinct role for ILK in hair follicle morphogenesis, in epidermal–dermal and intraepidermal adhesion, and in polarized keratinocyte migration. In the absence of ILK, hair follicle downgrowth and epidermal integrity are severely disrupted, and Rac1 activation and F-actin organization exhibit pronounced abnormalities, which hamper keratinocyte-orchestrated movements. The next challenge will be to further define the molecular pathways activated by ILK in each of these cellular responses.
Supplementary Material
ACKNOWLEDGMENTS
We thank S. A. Charlesworth for help with immunohistochemistry experiments and J. Kidder and R. Kothary for helpful comments on the manuscript. This work was supported with funds from the Canadian Institutes of Health Research (CIHR) to L.D. and S.J.A.D. and from the Natural Sciences and Engineering Research Council to L.D. S.J.A.D. is a CIHR New Investigator. S.D. acknowledges financial support from the National Cancer Institute of Canada and the Canadian Breast Cancer Research Alliance.
Abbreviations used:
- BSA
bovine serum albumin
- GFP
green fluorescent protein
- GST
glutathione S-transferase
- ILK
integrin-linked kinase
- RFP
monomeric red fluorescent protein.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-06-0526) on January 30, 2008.
REFERENCES
- Benard V., Bohl B. P., Bokoch G. M. Characterization of Rac and Cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J. Biol. Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- Benink H. A., Bement W. M. Concentric zones of active RhoA and Cdc42 around single cell wounds. J. Cell Biol. 2005;168:429–439. doi: 10.1083/jcb.200411109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borradori L., Sonnenberg A. Structure and function of HDs: more than simple adhesion complexes. J. Invest. Dermatol. 1999;112:411–418. doi: 10.1046/j.1523-1747.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- Brackebusch C., et al. Skin and hair follicle integrity is crucially dependent on β1 integrin expression in keratinocytes. EMBO J. 2000;19:3990–4003. doi: 10.1093/emboj/19.15.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgeson R. E., Christiano A. M. The dermal-epidermal junction. Curr. Opin. Cell Biol. 1997;9:651–658. doi: 10.1016/s0955-0674(97)80118-4. [DOI] [PubMed] [Google Scholar]
- Chang F., Lemmon C. A., Park D., Romer L. H. FAK potentiates Rac1 activation and localization to matrix adhesion sites: a role for βPIX. Mol. Biol. Cell. 2007;18:253–264. doi: 10.1091/mbc.E06-03-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choma D. P., Milano V., Pumiglia K., DiPersio C. M. Integrin α3β1-dependent activation of FAK/Src regulates Rac1-mediated keratinocyte polarization on laminin-5. J. Invest. Dermatol. 2006;127:31–40. doi: 10.1038/sj.jid.5700505. [DOI] [PubMed] [Google Scholar]
- Choma D. P., Pumiglia K., DiPersio C. M. Integrin α3β1 directs the stabilization of a polarized lamellipodium in epithelial cells through activation of Rac1. J. Cell Sci. 2004;117:3947–3959. doi: 10.1242/jcs.01251. [DOI] [PubMed] [Google Scholar]
- Cooper B., Brimer N., Stoler M., Vande Pol S. B. Suprabasal overexpression of beta-1 integrin is induced by bovine papilomavirus type I. Virology. 2006;355:102–114. doi: 10.1016/j.virol.2006.06.032. [DOI] [PubMed] [Google Scholar]
- D'Souza S.J.A., Pajak A., Balazsi K., Dagnino L. Ca+2 and BMP-6 signalling regulate E2F during epidermal keratinocyte differentiation. J. Biol. Chem. 2001;276:23531–23538. doi: 10.1074/jbc.M100780200. [DOI] [PubMed] [Google Scholar]
- Dassule H. R., Lewis P., Bei M., Maas R., McMahon A. P. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development. 2000;127:4775–4785. doi: 10.1242/dev.127.22.4775. [DOI] [PubMed] [Google Scholar]
- Di Persio C. M., Hodivala-Dilke K. M., Jaenish R., Kreidberg J. A., Hynes R. O. α3β1 integrin is required for normal development of the epidermal basement membrane. J. Cell Biol. 1997;137:729–742. doi: 10.1083/jcb.137.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer C. S., Watt F. M., Speight P. M. Loss of alpha 6 and beta 4 integrin subunits coincides with loss of basement membrane components in oral squamous cell carcinomas. J. Pathol. 1993;171:183–190. doi: 10.1002/path.1711710306. [DOI] [PubMed] [Google Scholar]
- Filipenko N. R., Attwell S., Roskelley C., Dedhar S. Integrin-linked kinase activity regulates Rac- and Cdc42-mediated actin cytoskeleton reorganization via a-PIX. Oncogene Epub. 2005 doi: 10.1038/sj.onc.1208737. [DOI] [PubMed] [Google Scholar]
- Frank D. E., Carter W. G. Laminin 5 deposition regulates keratinocyte polarization and persistent migration. J. Cell Sci. 2004;117:1351–1363. doi: 10.1242/jcs.01003. [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse E., Mesaddeq N., Yehia G., Cadalbert L., Dierich A., Le Meur M. Absence of integrin α6 leads to epidermolysis bullosa and neonatal death in mice. Nat. Genet. 1996;13:370–373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- Giancotti F. G., Ruoshlati E. Integrin signalling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Grashoff C., Aszodi A., Sakai T., Hunziker E. B., Fassler R. Integrin-linked kinase regulates chondrocyte shape and proliferation. EMBO Rep. 2003;4:432–438. doi: 10.1038/sj.embor.embor801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grashoff C., Thievessen I., Lorenz K., Ussar S., Fassler R. Integrin-linked kinase: integrin's mysterious partner. Curr. Opin. Cell Biol. 2004;16:565–571. doi: 10.1016/j.ceb.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Hamelers I.H.L., Olivo C., Mertens A.E.E., Pegtel D. M., van der Kammen R. A., Sonnenberg A., Collard J. H. The Rac activator Tiam1 is required for α3β1-mediated laminin-5 deposition, cell spreading and cell migration. J. Cell Biol. 2005;171:871–881. doi: 10.1083/jcb.200509172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan G. E., Troussard A. A., Dedhar S. Integrin-linked kinase: A cancer therapeutic target unique amongst its ilk. Nat. Rev. Cancer. 2005;5:51–63. doi: 10.1038/nrc1524. [DOI] [PubMed] [Google Scholar]
- Harper E. G., Alvarez S. M., Carter W. G. Wounding activates p38 map kinase and activation transcription factor 3 in leading keratinocytes. J. Cell Sci. 2005;118:3471–3485. doi: 10.1242/jcs.02475. [DOI] [PubMed] [Google Scholar]
- Hertle M. D., Kubler M. D., Leigh I. M., Watt F. M. Aberrant integrin expression during epidermal wound healing and in psoriatic epidermis. J. Clin. Invest. 1992;89:1892–1901. doi: 10.1172/JCI115794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman D., Weiner O. D., Goosney D. L., Sedat J. W., Finlay B. B., Abo A., Bishop J. M. Enteropathogenic E. coli acts through WASP and Arp2/3 complex to form actin pedestals. Nat. Cell Biol. 1999;1:389–391. doi: 10.1038/14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh H., Hiramoto K., Negishi M. Activation of Rac1 by RhoG regulates cell migration. J. Cell Sci. 2005;119:56–65. doi: 10.1242/jcs.02720. [DOI] [PubMed] [Google Scholar]
- Katoh H., Negishi M. RhoG activates Rac1 by direct interaction with the Dock180-binding protein Elmo. Nature. 2003;424:461–464. doi: 10.1038/nature01817. [DOI] [PubMed] [Google Scholar]
- Legate K. R., Montanez E., Kudlacek O., Fassler R. ILK, PINCH and parvin: The tIPP of integrin signalling. Nat. Rev. Mol. Cell Biol. 2006;7:20–31. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- Li J., et al. Laminin-10 is crucial for hair morphogenesis. EMBO J. 2003;22:2400–2410. doi: 10.1093/emboj/cdg239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz K., Grashoff C., Torka R., Sakai T., Langbein L., Bloch W., Aumalley M., Fassler R. Integrin-linked kinase is required for epidermal and hair follicle morphogenesis. J. Cell Biol. 2007;177:501–513. doi: 10.1083/jcb.200608125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magerl M., Tobin D. J., Muller-Rover S., Hagen E., Lindner G., McKay I. A., Paus R. Patterns of proliferation and apoptosis during murine hair follicle morphogenesis. J. Invest. Dermatol. 2001;116:947–955. doi: 10.1046/j.0022-202x.2001.01368.x. [DOI] [PubMed] [Google Scholar]
- Manohar A., Shome S. G., Lamar J., Stirling L., Iyer V., Pumiglia K., DiPersio C. M. Alpha 3 beta 1 integrin promotes keratinocyte cell survival through activation of a MEK/ERK signaling pathway. J. Cell Sci. 2004;117:4043–4054. doi: 10.1242/jcs.01277. [DOI] [PubMed] [Google Scholar]
- Michiels F., Jord C. S., Hordlijk P., van der Kammen R. A., Ruuls-Van Stalle L., Feltkamp C. A., Collard J. H. Regulated membrane localization of Tiam1, mediated by the NH2-terminal pleckstrin homology domain, is required for Rac-dependent membrane ruffling and C-Jun NH2-terminal kinase activation. J. Cell Biol. 1997;137:387–398. doi: 10.1083/jcb.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morasso M. I., Tomic-Canic M. Epidermal stem cells: the cradle of epidermal determination, differentiation and wound healing. Biol. Cell. 2005;97:173–183. doi: 10.1042/BC20040098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiya N., Kiosses W. B., Han J., Ginsberg M. H. An a4 integrin-paxillin-Arf-GAP complex restricts Rac activation to the leading edge of migrating cells. Nat. Cell Biol. 2005;7:343–352. doi: 10.1038/ncb1234. [DOI] [PubMed] [Google Scholar]
- Nobes C. D., Hall A. Rho, rac and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamelipodia and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Paus R., Muller-Rover S., van der Veen C., Maurer M., Eichmuller S., Ling G., Hoffman U., Foitzik K., Mecklenburg L., Handjiski B. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J. Invest. Dermatol. 1999;113:523–532. doi: 10.1046/j.1523-1747.1999.00740.x. [DOI] [PubMed] [Google Scholar]
- Raghavan S., Bauer C., Mundschan G., Li Q., Fuchs E. Conditional ablation of beta1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J. Cell Biol. 2000;150:1149–1160. doi: 10.1083/jcb.150.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., Parsons J. T., Horwitz A. R. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Sakai T., Li S., Dicheva D., Grashoff C., Sakai K., Kostka G., Braun A., Pfeifer A., Yurchenco P. D., Fassler R. Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion and controlling actin accumulation. Genes Dev. 2003;17:926–940. doi: 10.1101/gad.255603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenwaelder S. M., Burridge K. Bidirectional signalling between the cytoskeleton and integrins. Curr. Opin. Cell Biol. 1999;11:274–286. doi: 10.1016/s0955-0674(99)80037-4. [DOI] [PubMed] [Google Scholar]
- Terpstra L., Prud'homme J., Arabian A., Takeda S., Karsenty G., Dedhar S., St-Arnaud R. Reduced chondrocyte proliferation and chondrodysplasia in mice lacking the integrin-linked kinase in chondrocytes. J. Cell Biol. 2003;162:139–148. doi: 10.1083/jcb.200302066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Neut R., Krimmpenfort P., Calafat J., Niessen C. M., Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin β4 null mice. Nat. Genet. 1996;13:366–369. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]
- Vespa A., D'Souza S.J.A., Dagnino L. A novel role for integrin-linked kinase in epithelial sheet morphogenesis. Mol. Biol. Cell. 2005;16:4084–4095. doi: 10.1091/mbc.E05-02-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vespa A., Darmon A. J., Turner C. E., D'Souza S.J.A., Dagnino L. Ca2+-dependent localization of integrin-linked kinase to cell-cell junctions in differentiating keratinocytes. J. Biol. Chem. 2003;278:11528–11535. doi: 10.1074/jbc.M208337200. [DOI] [PubMed] [Google Scholar]
- Vidal V. P., Chaboissier M. C., Lutzkendorf S., Cotsarelis G., Mill P., Hui C. C., Ortonne N., Ortonne J. P., Schedl A. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr. Biol. 2005;15:1340–1351. doi: 10.1016/j.cub.2005.06.064. [DOI] [PubMed] [Google Scholar]
- Watt F. M. Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J. 2003;21:3919–3926. doi: 10.1093/emboj/cdf399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.