Abstract
The signaling from MARKK/TAO1 to the MAP/microtubule affinity-regulating kinase MARK/Par1 to phosphorylated microtubule associated proteins (MAPs) renders microtubules dynamic and plays a role in neurite outgrowth or polarity development. Because hyperphosphorylation of Tau at MARK target sites is a hallmark of Alzheimer neurodegeneration, we searched for upstream regulators by the yeast two-hybrid approach and identified two new interaction partners of MARKK, the regulatory Sprouty-related protein with EVH-1 domain1 (Spred1) and the testis-specific protein kinase (TESK1). Spred1-MARKK binding has no effect on the activity of MARKK; therefore, it does not change microtubule (MT) stability. Spred1-TESK1 binding causes inhibition of TESK1. Because TESK1 can phosphorylate cofilin and thus stabilizes F-actin stress fibers, the inhibition of TESK1 by Spred1 makes F-actin fibers dynamic. A third element in this interaction triangle is that TESK1 binds to and inhibits MARKK. Thus, in Chinese hamster ovary (CHO) cells the elevation of MARKK results in MT disruption (via activation of MARK/Par1 and phosphorylation of MAPs), but this can be blocked by TESK1. Similarly, enhanced TESK1 activity results in increased stress fibers (via phospho-cofilin), but this can be blocked by elevating Spred1. Thus, the three-way interaction between Spred1, MARKK, and TESK1 represents a pathway that links regulation of both the microtubule- and F-actin cytoskeleton.
INTRODUCTION
Microtubule-associated protein (MAP)/microtubule affinity-regulating kinase-activating kinase (MARKK), a serine/threonine kinase that belongs to the Ste20 kinase family, was identified as the activating kinase of MARK/Par-1 in porcine brain (Timm et al., 2003). MARKK is highly homologous to the thousand and one amino acid kinase (TAO-1) (Hutchison et al., 1998), and it contains an amino-terminal catalytic domain, a central substrate binding domain, a spacer, and a tail domain. The target protein MARK phosphorylates MAPs such as Tau or MAP2 at the KXGS motifs in their microtubule binding domain. This leads to the detachment of MAPs from microtubules, and it can cause microtubule breakdown (Drewes et al., 1997). MARK plays a central role in differentiation and neurite outgrowth (Biernat et al., 2002; Timm et al., 2003). However, an uncontrolled activity of the MARKK–MARK–MAP pathway leads to severe changes in the cytoskeleton and impaired cellular functions (Thies and Mandelkow, 2007; Ebneth et al., 1998). One example is the hyperphosphorylation and aggregation of Tau protein in degenerating neurons of Alzheimer's disease (Augustinack et al., 2002).
Besides the function in microtubule dynamics, MARKK/TAO-1 is a regulator of mitotic progression through interaction with the kinase BubR1, a spindle checkpoint component (Draviam et al., 2007). Furthermore MARKK has been implicated in mitogen-activated protein kinase (MAPK) signaling, which consist of three subfamilies (extracellular signal-regulated kinase [ERK], c-Jun NH2-terminal kinase [JNK], and p38). On different stress stimuli and in response to DNA damage, MARKK becomes active, phosphorylates MKK3, and thereby enhances the activity of the MAPK p38 (Hutchison et al., 1998; Raman et al., 2006). The human homologue of MARKK, prostate-derived sterile 20-like kinase (PSK)2 can activate JNK and induce apoptotic changes in the cell (Zihni et al., 2006). PSK1, another close relative, can affect both the microtubule and the actin network (Moore et al., 2000; Mitsopoulos et al., 2003). The localization of PSK1 on microtubules depends on its C-terminal regulatory domain, which has only low similarity to MARKK. The C-terminal domain (spacer and tail) of MARKK contains predicted amphipathic helices that suggest a putative protein interaction region. To identify new MARKK regulators that bind to this domain, we performed a yeast two-hybrid screen and detected Sprouty-related protein with EVH-1 domain (Spred1) as a new interaction partner of MARKK.
Spred1 belongs to the recently identified Sprouty/Spred family. It is a membrane associated protein with high expression levels in brain (Engelhardt et al., 2004; Nonami et al., 2005). Three mammalian members of the Spred subfamily (Spred1-3) are known so far. They all consist of an amino-terminal EVH1 domain, a central c-Kit binding domain (KBD), and a cysteine-rich Sprouty translocation domain (spryTD) at the carboxy terminus. The best-characterized function is the negative regulation of the ERK/MAPK pathway. Suppression of the ERK cascade by Spred1 occurs upon stimulation with different growth factors or cytokines, and it is achieved through interaction of Spred1 with Raf, an upstream kinase of this pathway, which blocks Raf activation (Wakioka et al., 2001; Nonami et al., 2004). There is a debate on the involvement of the cysteine-rich C-terminal spryTD domain for this function (Wakioka et al., 2001; King et al., 2005). This domain is thought to be palmitoylated and important for membrane localization after stimulation (Impagnatiello et al., 2001; Lim et al., 2002; Nonami et al., 2005). In transgenic mouse models, animals are viable if Spred1 or Spred2 are knocked out separately (Inoue et al., 2005; Bundschu et al., 2005), but double knockout results in embryonic lethality, probably caused by an incomplete separation of lymphatic and blood vessels (Taniguchi et al., 2007). Furthermore, Spred1 can inhibit cell motility and reduce actin stress fibers through interaction with the Rho-GTPase RhoA (Miyoshi et al., 2004).
In addition to the interaction of MARKK and Spred1, we show here a direct association of MARKK and Spred1 to the testis-specific protein kinase (TESK1). This is a LIM kinase (LIMK)-related serine/threonine kinase that influences actin organization (Toshima et al., 1995, 2001a). It consists of an amino-terminal catalytic domain and a carboxy-terminal regulatory domain. The name TESK1 originates from the high expression level in testis, but subsequent studies revealed that TESK1 is expressed in a variety of tissues, including brain (Toshima et al., 2001a,b). Its main function is the phosphorylation of cofilin, which leads to a reorganization of the actin cytoskeleton (Toshima et al., 2001a). Activation of TESK1 is achieved by integrin signaling and results in stress fiber formation and enhanced cell spreading (Toshima et al., 2001a; LaLonde et al., 2005; Tsumura et al., 2005). Different studies with the Drosophila homologue of TESK1, center divider, suggest its involvement in various developmental processes, such as spermatogenesis, eye development, axon guidance, and synaptogenesis (Kraut et al., 2001; Raymond et al., 2004; Sese et al., 2006). In contrast to LIMK, which is regulated by the p21-activated kinase PAK1 and -4 and Rho GTPases, TESK1 activity seems to be modulated only indirectly by the GTPase Rac1 (Toshima et al., 2001a; Raymond et al., 2004). Interaction of TESK1 with all known binding partners—Sprouty4, 14-3-3β, and actopaxin—leads to a decrease in kinase activity and increased actin dynamics (Toshima et al. 2001c; Tsumura et al., 2005; LaLonde et al., 2005).
In this study, we report the identification and characterization of interactions between MARKK, TESK1, and Spred1. Our data suggest that interaction of these three proteins affects the F-actin and the microtubule network of the cell.
MATERIALS AND METHODS
Plasmid Construction
The cloning of wild-type (WT) His-tagged MARKK (pVL-His-MARKKWT) and wild-type yellow fluorescent protein (YFP)-tagged MARKK (pEYFP C1-MARKKWT) was reported previously (Timm et al., 2003). The derivatives pGADT7- and pGBKT7-MARKKWT were generated by inserting an NdeI/BamHI-restricted fragment from pEYFP C1-MARKKWT into the two-hybrid vectors. pGADT7- and pGBKT7-MARKKK57A (kinase dead mutant) and the different deletion mutants were constructed by polymerase chain reaction (PCR) by using primers containing the appropriate sequences or point mutations and restriction sites. To generate human Spred1, we performed PCRs using oligonucleotides that are provided with NdeI/XhoI or XhoI/NheI restriction sites to amplify an amino-terminal and carboxy-terminal fragment. A human fetal brain cDNA library (Clontech, Palo Alto, CA) was used as a template. Both fragments were inserted together in pEU-Myc vector over NdeI/NheI restriction sites to obtain full-length Spred1. To generate pGADT7-/pGBKT7-Spred1 NdeI/NheI restriction fragments of pEU-Myc-Spred1 after Klenow treatment were inserted into NdeI/SmaI-restricted vectors. pEYFP C1- and pECFP C1-Spred1 were constructed by inserting SalI/NheI restriction fragments of pEU-Myc-Spred1 after Klenow treatment into SalI/SmaI-restricted vectors. To clone glutathione transferase (GST)-fused Spred1 in pGEX3X expression vector, the coding region of Spred1 was amplified by PCR using oligonucleotides that introduce a BamHI restriction site at the start codon and an EcoRI restriction site behind the Stop codon, and it was subsequently inserted in pGEX3X. Constructs of Spred1 mutants were generated by PCR by using primers containing the appropriate sequences and restriction sites. To obtain the full-length coding sequence of wild-type TESK1 (TESK1WT), a series of multiple successive PCRs with rat brain cDNA library in λZAPII (Stratagene, La Jolla, CA) as template were performed, and the sequence was inserted into the pECFP C1 vector. To generate mRFP-TESK1WT, the coding region of TESK1 was amplified by PCR using oligonucleotides that introduce a XhoI restriction site at the start codon and a NheI restriction site behind the Stop codon. The clone of monomeric red fluorescent protein (mRFP) was provided by Dr. R. Tsien (University of California, San Diego, CA). The expression construct pVL-His-TESK1WT was generated by inserting NdeI/SalI restriction fragment of pECFP C1-TESK1WT after Klenow treatment into NdeI/NheI (also Klenow-treated)-restricted vector. pGADT7- and pGBKT7-TESK1WT was constructed by inserting NdeI/BamHI restriction fragments of pVL-His-TESK1WT into the appropriate vectors. Constructs of TESK1 mutants were generated by PCR using primers containing the appropriate sequences and restriction sites. The coding region of cofilin was amplified by PCR from a human fetal brain cDNA library (Clontech) by using oligonucleotides that introduce NdeI restriction site at the start codon and NheI restriction site behind the Stop codon. All plasmids were verified by DNA sequencing. The sequence of all oligonucleotides used for PCR is available upon request.
Yeast Two-Hybrid Analysis
To screen for MARKK C-terminal interacting proteins, the yeast strain AH109 was transformed with MARKK C-term in pGBKT7-vector and a human fetal brain cDNA library (MATCHMAKER, Clontech; 3 × 106 independent clones) cloned in the pACT2-vector. The yeast two-hybrid screen and direct binding assays were performed according to the manufacturer's instruction for MATCHMAKER GAL4 Two-Hybrid System 3 (Clontech) and as described previously (Matenia et al., 2005).
Cell Culture, Transfection, and Immunofluorescence
Cell culture and transfections were performed with CHO cells following standard protocols. Further details are described in Supplemental Material.
Antibodies and Markers
We raised a rat anti-TESK antibody against a TESK1 C-terminal 21-amino acid peptide (CHRGHHAKPPTPSLQLPGARS) (Toshima et al., 1998) (Eurogentec, Seraing, Belgium). The antibody was purified on antigenic peptide-conjugated column. A rabbit anti-Spred1 antibody was raised against two peptides (peptide 1, FDRGIRRAIEDISQGC; peptide 2, ETVVTSEPYRSSNIRP) (Eurogentec), and it was purified on antigenic peptide-conjugated column. Mouse monoclonal antibodies anti-tubulin DM1A, anti-actin (clone AC-40), anti-β1/β2 Adaptin, anti-γ-Adaptin (clone 10/3), and anti-Vimentin (clone V9) were obtained from Sigma-Aldrich (St. Louis, MO). Antibody 12E8 against phosphorylated Ser262 and Ser356 in the KXGS motifs of Tau was a gift from P. Seubert (Elan Pharma, South San Francisco, CA). Mouse monoclonal Golgi antibody K58 was from Abcam (Cambridge, United Kingdom). Mouse monoclonal c-Myc antibody (9E10) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal antibody against MARKK/TAO1 was from BD Biosciences (Franklin Lakes, NJ). Secondary antibodies from Dako Denmark (Glostrup, Denmark) for immunoblotting were horseradish peroxidase-conjugated rabbit anti-rat, goat anti-mouse, and goat anti-rabbit. Dyes to stain mitochondria (MitoFluorRed 589), lysosomes (Lyso-Tracker Red DND-99), and endosomes (FM 4-64FX) were purchased from Invitrogen (Carlsbad, CA). Secondary cyanine (Cy)5-conjugated goat anti-mouse fluorescent antibody was from Dianova (Hamburg, DE).
Pull-Down Assays, Immunoprecipitation, and Immunoblot Analysis
Pull-down assays of TESK1 or MARKK with GST-Spred1 were performed as described previously (Matenia et al., 2005; see details in Supplemental Material). Myc-tagged MARKKWT and His-tagged TESK1WT were expressed in Sf9 cells, coimmunoprecipitated, and analyzed by standard procedures.
Protein Purification
Recombinant proteins MARKK, TESK1, Spred1, and cofilin were prepared as described previously (Matenia et al., 2005; see details in Supplemental Material).
In Vitro Activity Assay
The activity of MARKK was assayed as described previously (Timm et al., 2003), by using a substrate peptide from the activation loop of MARK2 (LIP-peptide: GNKLDTFCGSPPYAAPELFQGKK). Performance of kinase assays with TESK1 is described in Supplemental Material.
RESULTS
Identification of Spred1 as a New Interaction Partner of MARKK
Kinases play a key role in regulating the organization of the cytoskeleton. Members of the MARK family influence the microtubule network through phosphorylation of MAPs such as Tau (Drewes et al., 1997). The activity of MARK is regulated by upstream kinases, such as MARKK (Timm et al., 2003). To identify the signaling pathway that acts upstream of MARKK, we screened a human fetal brain cDNA library for interaction partners by using a yeast two-hybrid approach. The carboxy-terminal fragment of MARKK (amino acids 432-1001), comprising spacer and tail domains, was used as the bait. Of 5 × 105 initial transformants, 43 clones were selected for being positive in growing on adenine- and histidine-selective plates. These clones were further analyzed by DNA sequencing. One plasmid insert contained DNA encoding the carboxy terminus of Spred1 (aa 159–444). Spred1 is a member of the Sprouty/Spred family of regulatory proteins, with main functions in the ERK pathway (Wakioka et al., 2001; for review, see Bundschu et al., 2006).
To analyze the binding of Spred1 to MARKK in more detail, we cloned Spred1 from the fetal cDNA library. In addition, different domains and mutants of both MARKK and Spred1 were generated. The interacting regions within MARKK and Spred1 were mapped by direct interaction tests in the two-hybrid system (Figure 1A). Both proteins interact via their C-terminal domains. The catalytic N-terminal domain of MARKK seems not to be involved in binding, because this domain alone shows no interaction with Spred1 constructs, and mutation within the catalytic domain (K57A, catalytic inactive) does not affect Spred1 binding. For Spred1, the interaction with MARKK is mediated via the carboxy-terminal spryTD domain, which is important for binding to known partners (Bundschu et al., 2006).
Figure 1.
Interaction between MARKK and Spred1. (A) Mapping the interaction domains of MARKK and Spred1 by direct yeast two-hybrid tests. Constructs are shown schematically; domains are distinguished by different colors (for MARKK: CAT, kinase catalytic domain; SBD, substrate binding domain, spacer domain, and tail domain; for Spred1: EVH1, Enabled/VASP homology 1 domain; KBD, c-Kit binding domain; and SpryTD, Sprouty translocation domain. Results of the two-hybrid analysis are indicated by +++ (very strong interaction), ++ (strong), + (weak), and − (no interaction). Note that MARKK interacts via its C-terminal half (spacer and tail domains), but not with the kinase domain. Spred1 interacts also via its C-terminal half, but not via the EVH1 domain. (B) Interaction of MARKK and Spred1 determined by GST pull-down assay. His-tagged MARKKWT purified from Sf9 cells (0.3 μM) was incubated with GST (0.6 μM) or GST-Spred1 (0.17 μM). The pull-down fractions were analyzed by SDS-PAGE followed by Coomassie staining (GST-constructs) or immunoblotting with anti-MARKK antibody. Immunoblots show that MARKK binds strongly to GST-Spred1, but not to GST alone.
To confirm the results of the two-hybrid tests, a GST pull-down binding assay was carried out. For this, Spred1 fused to GST was purified from Escherichia coli, and His-tagged MARKK was expressed in Sf9 cells. Purified MARKKWT protein was incubated together with GST or GST-Spred1 bound to glutathione-Sepharose beads. Bound proteins were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) and immunoblotted with anti-MARKK antibody. The analysis revealed a strong interaction between MARKKWT and Spred1 (Figure 1B).
Spred1 Does Not Influence MARKK Activity
The best studied function of Spred1 is the regulation of the ERK/MAPK signal transduction pathway (Wakioka et al., 2001; King et al., 2005). To analyze whether Spred1 is also able to affect the MARKK–MARK–MAP pathway, we examined the effect of Spred1 binding on MARKK activity. For in vitro activity assays, both proteins were incubated together with a MARKK substrate (the Lip peptide derived from the MARK2 activation loop) and [32P]ATP. As a control, phosphorylation of the LIP peptide was also measured in presence of different amounts of GST. GST alone had no effect on MARKK activity (data not shown). Similar results were obtained with increasing concentration of GST-tagged Spred1 (Figure 2A). Detection of the phosphorylation status of MARKK and Spred1 in these assays showed no change of MARKK autophosphorylation, and it proved that Spred1 is not phosphorylated by MARKK (Figure 2B). These results suggest that Spred1 does not affect the kinase activity of MARKK in vitro, although it binds to MARKK.
Figure 2.
Spred1 does not influence MARKK activity. (A) To examine the influence of Spred1 on MARKK activity both proteins were incubated together with the target LIP peptide derived from MARK2. MARKK activity is analyzed by the phosphorylation of the LIP-peptide (100 μM). The kinase activity of MARKKWT was normalized to 100%. Increasing amounts of Spred1 have no effect on the MARKK activity (triplicate experiments showing mean ± SE). (B) To analyze the phosphorylation status of MARKKWT and Spred1 after the activity assay, proteins were separated by SDS-PAGE and gels stained with Roti-Blue (Roth, Karlsruhe, Germany). Autoradiograms (ARs) show no change in MARKKWT autophosphorylation and no phosphorylation of Spred1 by MARKK.
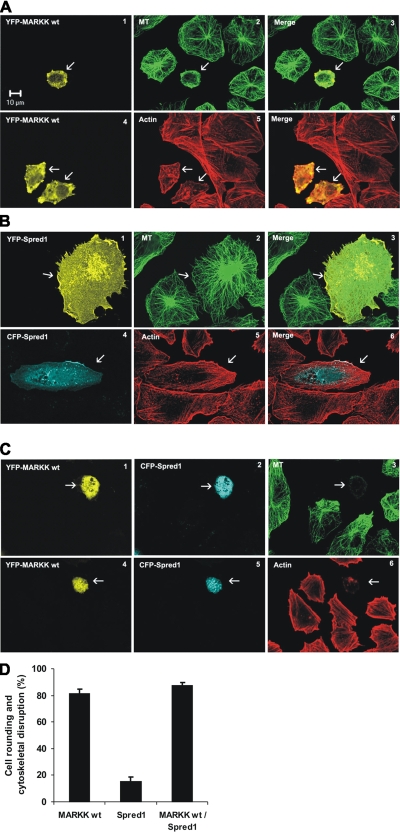
Next, we analyzed the effects of the MARKK–Spred1 interaction in cells. Chinese hamster ovary (CHO) cells were singly or jointly transfected with plasmids for YFP-MARKKWT and YFP/CFP-Spred1. First, we examined in singly transfected cells the effects of MARKKWT and Spred1 on the actin and microtubule networks (Figure 3, A and B). We had shown earlier that overexpression of MARKK in CHO cells leads to dramatic microtubule disruption caused by the increase in MARK activation, which results in elevated phosphorylation of MAP4 and detachment from microtubules (Figure 3A, 1–3; Timm et al., 2003). In addition to the microtubule breakdown, the actin stress fibers are destroyed (Figure 3A, 4–6). This disturbance of the cytoskeleton leads to cell rounding and eventually to cell death. By contrast, cells overexpressing Spred1 do not display any changes in their microtubule and actin network (Figure 3B), suggesting that Spred1 by itself has no influence on cytoskeletal organization. YFP- or cyan fluorescent protein (CFP)-tagged Spred1 protein was mainly located in the cytosol, around the centrosome, and partially at the plasma membrane (Figure 3B, 1 and 4). Cells cotransfected with MARKKWT and Spred1 possess the same phenotype as in the MARKKWT single transfection (Figure 3C). These cells are rounded up and show clear disruption of their microtubule and actin network. In addition, the effects of the expression of MARKK and Spred1 on cell morphology and cytoskeletal organization were analyzed quantitatively (Figure 3D). From the results of the in vitro activity assays and the expression studies in CHO cells, we conclude that Spred1 does not influence the kinase activity of MARKK.
Figure 3.
Subcellular localization of MARKK and Spred1 and their effects on the stability of the microtubule and actin filament network. CHO cells transfected with YFP-MARKKWT, YFP-Spred1, or CFP-Spred1 were cultured for 16 h, fixed, and costained with DM1A (MT-staining; green) or β-actin (Actin-staining; red) primary antibody and Cy5 secondary antibody. Transfected cells are indicated by arrows. (A) In YFP-MARKKWT–expressing cells, both microtubules and actin filaments are disturbed (3 and 6). (B) In contrast, cells expressing Spred1 show no change in MT or actin network (3 and 6). (C) Coexpression of YFP-MARKKWT and CFP-Spred1 leads to the same effects as MARKKWT alone—disruption of MT and actin filament network (3 and 6). (D) Quantitative analysis of the effects of MARKK and Spred1 on cell morphology and the cytoskeleton. Percentage of transfected cells that are rounded up and show a destroyed microtubule network is summarized in a histogram (triplicate experiments showing mean ± SD).
Interaction between Spred1 and Kinase TESK1
The question arose whether the newly identified interaction between MARKK and Spred1 had a biological function. This pointed the way to the kinase TESK1 because of the known connection between TESK1 and its inhibitor Sprouty4, a protein related to Spred1 (Leeksma et al., 2002; Tsumura et al., 2005). Because of the similarity between the C-terminal domains of Spred1 and Sprouty4, we suspected a possible inhibitory role of Spred1 on TESK1. Because Spred1 was identified as an interaction partner of the kinase MARKK, we hypothesized that Spred1 functions as a scaffold between MARKK and TESK1. This possibility would be interesting because it would provide a link between the actin-regulatory TESK1 and the microtubule-regulatory MARKK. To investigate whether TESK1 interacts with Spred1, we analyzed their binding in direct yeast two-hybrid tests. As illustrated in Figure 4A, we detected a strong interaction of TESK1 with full-length Spred1 and the C-terminal region, but not with the N-terminal region containing the EVH-1 and KBD domains. Thus, TESK1 interacts with Spred1 through the carboxy-terminal cysteine-rich spryTD domain, in analogy with Sprouty4. Conversely, TESK1 exhibits a more complicated binding pattern, because the whole molecule, and the N- and C-terminal halves, interact strongly with Spred1 (Figure 4A). From our data, we suggest at least two binding regions, possibly for structural reasons resulting from a closed conformation of the kinase.
Figure 4.
Direct interaction between Spred1 and TESK1. (A) Interaction of Spred1 and TESK1 mapped by yeast two-hybrid system. Diagrams of Spred1, TESK1, and their deletion constructs used for direct yeast two-hybrid assays are shown schematically; domains are indicated by different colors (Spred1: EVH1, Enabled/VASP homology 1 domain; KBD, c-Kit binding domain; and SpryTD, Sprouty translocation domain; TESK1: CAT, kinase catalytic domain; and CR, conserved regions). Interactions are classified by +++ (very strong), ++ (strong), + (weak), and − (no interaction). For Spred1, the interaction resides mainly in the C-terminal domain (SpryTD). For TESK1, all constructs interact equally well. (B) Interaction of Spred1 and TESK1 determined by GST pull-down assay. The lysates of His-tagged TESKWT-expressing Sf9 cells were incubated with GST (0.6 μM) or GST-Spred1 (0.17 μM). The pull-down fractions were analyzed by SDS-PAGE followed by Coomassie staining (GST-constructs) or immunoblotting with anti-MARKK antibody. TESK1 binds specifically to Spred1, but not to GST, which was used as a control.
The interaction between Spred1 and TESK1 was verified by a GST pull-down assay (Figure 4B, input lanes 2 and 4). Purified GST-tagged Spred1 protein or GST alone were incubated together with cell lysates of His-tagged TESK1WT-overexpressing Sf9 cells. Immunoblot analysis of Sf9 cell lysates with anti-TESK1 antibody confirmed equal amounts of TESK1WT protein in each approach. After precipitation, TESK1 was only detected in the presence of Spred1, but not in the GST control (Figure 4B, lane 4, TESK-blot). The results suggest that Spred1 interacts with TESK1 and binding is mediated via the C-terminal spryTD domain.
TESK1 Activity Is Inhibited by Spred1
To determine whether the binding between TESK1 and Spred1 has functional consequences, we performed in vitro activity assays. TESK1WT activity was examined by the phosphorylation of the substrate cofilin. Both proteins were purified from Sf9 cells and incubated together with [32P]ATP. TESK1 activity significantly decreased in the presence of increasing amounts of GST-Spred1 (Figure 5, lanes 2 and 7). GST alone had no effect on the TESK1 activity (data not shown). We did not observe a phosphorylation of Spred1 by TESK1, suggesting that Spred1 is not a substrate of the kinase TESK1. From these results, we conclude that Spred1 inhibits TESK1 activity by binding to it, similar to Sprouty4.
Figure 5.
Spred1 inhibits TESK1 kinase activity. The influence of Spred1 on TESK1 activity was determined by activity assays with the TESK1 substrate cofilin (7 μM). For analyzing the phosphorylation status of cofilin the proteins were separated by SDS-PAGE and gels stained with Roti-Blue (Roth). Phosphorylation of cofilin was measured from the autoradiogram (AR); the kinase activity of TESK1WT alone was normalized to 100% (lane 2). Increasing amounts of Spred1 decreased the TESK1 activity (triplicate experiments showing mean ± SE).
Subcellular Localization of TESK1 and Effects on the Cytoskeleton
To examine the cellular distribution of TESK1, the CFP-tagged wild-type kinase and the kinase dead mutant TESK1D170A were overexpressed in CHO cells. A strong localization in vesicle-like structures can be observed, which are dispersed in the cytosol (Figure 6A and Supplemental Figure S2). To identify the nature of these structures, different types of vesicles or organelles were labeled with specific antibodies or dyes for lysosomes, mitochondria, and endosomes (Supplemental Figure S2, A and B). All tested compartments revealed no or only very faint colocalization with TESK1 vesicles. In addition, TESK1WT-transfected cells were immunostained for vimentin to check for aggresome formation (Kopito, 2000). We did not observe the formation of a vimentin cage that is characteristic for aggresomes (Supplemental Figure S2A, 13–16). Attempts to detect endogenous TESK1 protein in different cell types (e.g., neurons) via immunofluorescence staining were unsuccessful, probably because of the low TESK1 concentration and the antibody quality. However, the presence of TESK1, Spred1, and MARKK in these cells (CHO cells and cortical neurons) was confirmed by immunoblotting (Supplemental Figure S1, A and B). Furthermore the distribution of TESK1WT in CHO cells was monitored after different expression times (Supplemental Figure S2C). Localization in vesicle-like structures occurs as early as after 8 h, even at low expression levels (Supplemental Figure S2C, 1 and 4).
Figure 6.
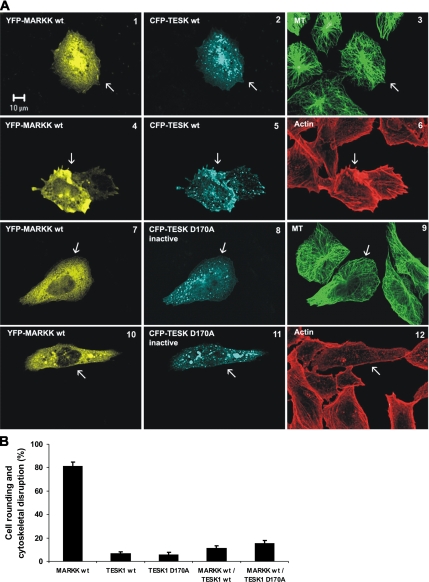
Subcellular localization of TESK1 and the inhibitory effect of Spred1 on TESK1. Transfected CHO cells were cultured for 16 h, fixed, and costained with DM1A (MT-staining, green) or β-actin (Actin-staining, red) primary antibody and Cy5 secondary antibody. Transfected cells are indicated by arrows. (A) CFP-TESK1WT singly transfected cells show no change in microtubule network but strongly enhanced actin stress fiber formation (3 and 6). By contrast, the kinase-dead mutant TESK1D170A reduces stress fiber formation in transfected cells (12). Both TESK1WT and TESK1D170A are distributed in vesicle-like dots (1, 4, 7, and 10). (B) Coexpression of CFP-TESK1WT and YFP-Spred1 results in an inhibition of TESK1 and hence an unaltered actin filament network (6). Additionally, a strong colocalization of both proteins in vesicular dots can be observed (1, 2 and 4, 5). (C) Quantitative analysis of the effects of TESK1 and Spred1 on the organization of the actin network. Percentage of transfected cells that contain increased actin stress fibers is summarized in a histogram (triplicate experiments showing mean ± SD).
TESK1 is mainly involved in the organization of the actin filament network in the cell. By phosphorylation of the actin-depolymerizing factor cofilin, TESK1 induces actin stress fiber formation (Toshima et al., 2001a). This is illustrated in Figure 6A (4–6, arrows) for CFP-tagged TESK1WT overexpressed in CHO cells. By contrast, microtubules in these cells showed no change of their network structure (Figure 6A, 1–3, arrows). As expected, the kinase dead mutant TESK1D170A does not increase actin stress fibers, and it has no influence on microtubules either (Figure 6A, 7–12, arrows).
To prove that the formation of TESK1WT-induced actin stress fibers depends on the regulation of cofilin activity, we examined the effect of the constitutively active mutant cofilinS3A. Cofilin is an actin-depolymerizing factor whose activity is blocked via phosphorylation of serine 3 by LIMK or TESK. The used mutant cofilinS3A is not phosphorylatable, leading to a constitutively active molecule. In contrast to cells overexpressing TESK1WT alone that show stress fibers (Supplemental Figure S3, 1–3), cells coexpressing TESK1WT and cofilinS3A show no change in their actin organization, suggesting that inhibition of cofilin by TESK1 is sufficient to induce stress fibers (Supplemental Figure S3).
Spred1 Colocalizes with TESK1 and Inhibits Its Effect on Actin Stress Fiber Formation
To verify the effect of the TESK1–Spred1 interaction in cells, CHO cells were transfected with YFP-tagged Spred1 and CFP-tagged TESK1WT. Surprisingly, a strong colocalization of both proteins in vesicular dots can be observed (Figure 6B, 1–2 and 4–5). Thus, it seems that coexpression of TESK1 forces Spred1 to redistribute from a homogeneous cytosolic distribution to vesicle-like TESK1-containing structures (compare Figure 3B, 1 and 4 with Figure 6B, 1 and 4). When looking at the organization of actin fibers and microtubules in these cells, we found that both cytoskeletal networks were unaltered (Figure 6B, 3 and 6). The lack of enhanced actin stress fibers indicates the inhibition of TESK1 activity via interaction with Spred1 (for a quantitative analysis, see Figure 6C). These data suggest that Spred1 binds to the TESK1 kinase and that this interaction inhibits TESK1 activity, reminiscent of the inhibitory action of Sprouty4. It supports the hypothesis that Spred1 functions as a scaffold to connect MARKK and TESK1, regulators of microtubules and actin filaments, respectively. However, the further analysis (below) revealed a direct connection between the kinases MARKK and TESK1.
TESK1 Interacts with MARKK, Inhibits It, and Prevents Microtubule Breakdown
The interaction between the two kinases MARKK and TESK1 was analyzed by yeast two-hybrid assays, by using different combinations of domains (Figure 7A). The results suggest a complex pattern: The N-terminal and C-terminal halves of MARKK interact mainly with the C-terminal half of TESK1; however, the full-length molecules do not interact. This is best explained by a folded conformation of the full-length molecules (Timm, unpublished data).
Figure 7.
Interaction between MARKK and TESK1. (A) Mapping the interaction domains of MARKK and TESK1 by direct yeast two-hybrid tests. Domains are indicated by different colors as mentioned in Figures 1 and 4. Results of the two-hybrid analysis are indicated by +++ (very strong interaction), ++ (strong), + (weak), and − (no interaction). Note that full-length MARKK shows no interactions, but the N- or C-terminal halves do, indicating that interaction sites are occluded in the full-length protein. The interactions of TESK1 are mediated mainly by the regulatory C-terminal domain. (B) In vivo association of MARKK and TESK1. Sf9 cells were transfected with either myc-tagged MARKKWT cDNA alone or together with His-tagged TESK1WT cDNA. Cell lysates were subjected to immunoprecipitation with anti-myc antibody. Immunoprecipitated proteins and the expression of MARKK and TESK1 were analyzed by immunoblotting with anti-MARKK and anti-TESK antibody.
To investigate the biological relevance of the MARKK–TESK1 interaction in cells, we performed coimmunoprecipitation experiments. Myc-tagged MARKKWT was overexpressed together with His-tagged TESK1WT in Sf9 cells and subjected to immunoprecipitation with anti-Myc antibody (Figure 7B). TESK1WT was coprecipitated specifically in the presence of MARKKWT, confirming that both kinases can bind to each other in cells.
These results were followed up by in vitro activity assays. We detected phosphorylation of cofilin by TESK1 in the presence of increasing amounts of MARKK. Recombinant proteins were purified from Sf9 cells and incubated together with [32P]ATP. Figure 8A shows that there is no influence of MARKK toward TESK1 activity. Conversely, we examined the MARKK activity in the presence of TESK1 by using the LIP peptide of MARK as substrate. Here, we observed a dramatic decrease of MARKK activity (Figure 8B), showing that the interaction with TESK1 inhibits MARKK kinase activity. Because MARKK and TESK1 are kinases, we expected that inhibition was caused by phosphorylation. However, examination of the phosphorylation states of both kinases revealed that this is not the case. MARKK, and TESK1 show only autophosphorylation. Whereas the phosphorylation level of TESK1 does not change (data not shown), we found a significant decrease of MARKK autophosphorylation to 50% due to the presence of TESK1 (Figure 8C). To confirm that inhibition of MARKK by TESK1 is due to binding and not to phosphorylation, we performed activity assays with the kinase dead mutant of TESK1. As seen in Figure 8D inactive TESK1D170A inhibits MARKK activity to the same degree as TESK1WT. We conclude that interaction of the kinases MARKK and TESK1 leads to reduced MARKK activity caused by binding of TESK1 and not by phosphorylation.
Figure 8.
MARKK has no influence on the TESK1 activity, but TESK1 inhibits the MARKK activity. (A) Influence of MARKK on TESK1 activity was determined by activity assays with the TESK1 substrate cofilin (10 μM). Cofilin phosphorylation was measured from the AR, and kinase activity of TESK1WT alone was normalized to 100%. Increasing amounts of MARKK have no effect on the TESK1 activity (triplicate experiments showing mean ± SE). (B) Inhibition of MARKKWT by recombinant TESK1WT was analyzed via the phosphorylation of the MARK2 Lip peptide (100 μM) by MARKK. The kinase activity of MARKKWT alone was normalized to 100%. Addition of different amounts of TESK1WT reduces MARKK activity. (C) Autoradiogram of MARKK autophosphorylation. Increasing the TESK1WT concentration in the assay leads to a reduced autophosphorylation of MARKK, indicating that TESK1 does not phosphorylate MARKK in order to decrease MARKK activity. (D) The inhibitory effect of TESK1WT and TESK1D170A (kinase dead mutant) on MARKK activity was analyzed via the phosphorylation of the MARK2 Lip peptide by MARKK. The kinase activity of MARKKWT (100 nM) alone was normalized to 100%. TESK1WT (525 nM) and the kinase inactive mutant TESK1D170A (550 nM) can inhibit MARKKWT activity to the same degree, showing that the activity of TESK1 is not important for the inhibition of MARKK, but the binding.
To determine how MARKK and TESK1 can influence each other in the regulation of the cytoskeleton, we coexpressed YFP-tagged MARKKWT and CFP-tagged TESK1WT or kinase dead TESK1D170A in CHO cells (Figure 9). MARKK and TESK1 colocalized in vesicular structures and partly in the cytosol. Consistent with the data from the in vitro studies, MARKK activity is also inhibited in cells, so that transfected cells show a normal morphology and a normal microtubule network (Figure 9, 1–3; for quantitative analysis, see Figure 9B). Similar results were obtained when MARKK was coexpressed with the inactive mutant TESK1D170A (Figure 9, 7–9). This supports the view that MARKK inhibition is due to binding to TESK1. Conversely, TESK1 is not inhibited by MARKK, similar to the in vitro assays. Cells coexpressing MARKK and TESK1WT clearly show an enhancement of actin stress fibers, indicating increased actin stability (Figure 9, 4–6). These data confirm that interaction between MARKK and TESK1 leads only to inhibition of MARKK kinase activity.
Figure 9.
Coexpression of MARKKWT and TESK1 inhibits MARKK effects on the cytoskeleton. (A) CHO cells coexpressing YFP-MARKKWT and CFP-TESK1WT or CFP-TESK1D170A inactive mutant were cultured for 16 h, fixed, and costained with DM1A (MT staining; green) or β-actin (Actin-staining; red) primary antibody and Cy5 secondary antibody. Transfected cells are indicated by arrows. In cotransfected cells, TESK1WT and TESK1D170A inactive mutant localize predominantly in vesicular structures (2, 5, 8, and 11), whereas the distribution of MARKKWT ranges from mainly cytosolic (1 and 4) to vesicle-like structures (7 and 10). Coexpression of MARKKWT and TESK1WT or TESK1D170A inactive mutant results in an inhibition of MARKK and stabilization of MT (3 and 9). Organization of the actin filament network depends on TESK1 activity so that TESK1WT induces formation of actin stress fibers (6), whereas TESK1D170A inactive mutant leads to the dissolution of actin stress fibers in the cell (12). (B) Quantitative analysis of the effects of MARKK and TESK1 on cell morphology and the cytoskeleton. Percentage of transfected cells that are rounded up and show a destroyed microtubule network is summarized in a histogram (triplicate experiments showing mean ± SD).
Ternary Interactions of MARKK, TESK1, and Spred1
Our data show that the two new interaction partners of MARKK, Spred1, and TESK1 can bind to each other. Attempts to rebuild an interaction complex with all three proteins in vitro failed. We, therefore, investigated the effects of a possible complex formation of all three proteins in cells. CHO cells were used as a cell model in which very low levels of endogenous MARKK, TESK1, and Spred1 could be detected via immunoblotting (Supplemental Figure S1). Cell morphology and the cytoskeletal organization of microtubules and actin filaments were normal in CHO cells coexpressing MARKKWT, TESK1WT and Spred1 (Figure 10A), suggesting that the MARKK and the TESK1 activity is inhibited.
Figure 10.
Ternary interactions of MARKK, TESK1, and Spred1 (A) CHO cells coexpressing YFP-MARKKWT, CFP-Spred1, and mRFP-TESK1WT were cultured for 16 h, fixed, and costained with DM1A (MT staining; green) or β-actin (Actin-staining; green) primary antibody and Cy5 secondary antibody. Transfected cells are indicated by arrows. Cotransfected cells show normal cell morphology and normal actin and microtubule organization, indicating that MARKK and TESK1 activity are inhibited. (B) To examine the influence of Spred1 (500 nM) and TESK (500 nM) on MARKK (100 nM) activity, the proteins were incubated together with the target LIP peptide derived from MARK2. MARKK activity is analyzed by the phosphorylation of the LIP-peptide (100 μM). The kinase activity of MARKKWT was normalized to 100%. MARKK activity is inhibited by TESK1, but not by Spred1. Incubation of all three proteins together results in a decrease of MARKK activity similar to the effect of TESK1 alone (triplicate experiments showing mean ± SE). (C) The influence of Spred1 (500 nM) and MARKK (500 nM) on TESK1 (100 nM) activity was determined by activity assays with the TESK1 substrate cofilin (10 μM). For analyzing the phosphorylation status of cofilin, the proteins were separated by SDS-PAGE, and gels were stained with Roti-Blue (Roth). Phosphorylation of cofilin was measured from the AR; the kinase activity of TESK1WT alone was normalized to 100%. TESK1 activity decreases in the presence of Spred1, whereas MARKK has no influence. Incubation of all three proteins leads to the same decrease in TESK1 activity as seen with Spred1 alone (triplicate experiments showing mean ± SE).
These observations were followed up in more detail by in vitro kinase assays. To evaluate MARKK activity, we monitored the phosphorylation of the MARKK substrate peptide LIP, whereas TESK1 activity was examined by the phosphorylation of cofilin. As shown in Figure 10B, MARKK activity is reduced in the presence of TESK1, but it does not change if Spred1 is added. Incubation of all three proteins together leads to inhibition of MARKK activity indistinguishable from the effect seen with TESK1 alone. Spred1 seems not to interfere with the inhibitory effect of TESK1 on MARKK. A similar result was obtained for the TESK1 activity (Figure 10C). The activity of TESK1 is reduced by Spred1, but not by MARKK. This inhibition of the TESK1 by Spred1 was not influenced by the presence of MARKK. The formation of a ternary complex in which the effects on the MARKK and TESK1 activity are not affected by the respective third component might be possible, but it needs to be analyzed in more detail.
DISCUSSION
Many kinases are multifunctional and affect different cell processes. This is exemplified by the interactions of the kinase MARKK/TAO1 reported here. We were originally interested in clarifying the regulation of microtubule dynamics by MARKK. Activation of MARK by MARKK or LKB1 leads to the destabilization of microtubules (Timm et al., 2003; Lizcano et al., 2004), and the phosphorylation sites on Tau are among the earliest observed in Alzheimer neurodegeneration (Augustinack et al., 2002). We, therefore, searched for upstream regulators of MARKK, and we identified Spred1 as a new interaction partner. Because Spred1 exerts regulatory functions in different pathways, we suspected that binding to MARKK also results in an alteration of its activity (Wakioka et al., 2001; Miyoshi et al., 2004). However, we did not observe a significant influence of the Spred1-MARKK binding on the microtubule-regulating activity of MARKK. In contrast, both proteins are involved in different branches of the MAPK pathways, and they may mediate the cross-talk between them. MARKK/TAO1 phosphorylates MKK3 after stress stimulation, which leads to p38/MAPK activation (Hutchison et al., 1998), and Spred1-mediated Raf inhibition down-regulates ERK/MAPK signaling (Wakioka et al., 2001).
Because Spred1 shows some homology with the Sprouty proteins, and because Sprouty4 inhibits the actin-regulating kinase TESK1, we became interested in TESK1 because we suspected a similar connection between Spred1 and TESK1 (Leeksma et al., 2002; Tsumura et al., 2005). Besides its actin-regulating function, TESK1 was also shown to enhance Tau toxicity in a Drosophila model (Shulman and Feany, 2003). Therefore, it was of special interest to investigate the connection between TESK1 and the MARKK–MARK–MAP cascade.
We initially hypothesized that Spred1 is a scaffold of the two kinases MARKK and TESK1. We confirmed that Spred1 indeed interacts with TESK1, in analogy with the interaction between Sprouty4 and TESK1 (Tsumura et al., 2005). In both cases, the binding is mediated through the carboxy-terminal spryTD domain of Spred1 or Sprouty4. This binding blocks the activity of TESK1 and thus decreases the phosphorylation of cofilin at Ser3 by TESK1 (Tsumura et al., 2005), raising the level of unphosphorylated cofilin and rendering the actin network more dynamic.
This was confirmed in CHO cells where expression of TESK1 leads to a strong increase in stress fibers, consistent with the phosphorylation and inactivation of cofilin (Toshima et al., 2001a). By contrast, cells coexpressing TESK1 and Spred1 show an unaltered actin cytoskeleton due to the inhibition of TESK1 by Spred1. In transfected CHO cells, both proteins largely colocalize in punctate structures. This implies a dramatic redistribution of Spred1, because in singly transfected cells Spred1 is localized evenly throughout the cytoplasm. Localization in dot-like structures had previously been shown for TESK1 alone in HeLa cells (Tsumura et al., 2005). To characterize these structures, we counterstained with different antibodies and dyes (e.g., Golgi, lysosomes, mitochondria, and endosomes), but we did not find a consistent colocalization.
The reorganization of the actin network plays an essential role in cell differentiation and migration where Spred1 exerts a regulatory function through its interaction with the GTPase RhoA (Wakioka et al., 2001; Miyoshi et al., 2004). This blocks the activation of the downstream effector kinase Rho kinase (ROCK) and decreases LIMK activity, enhances active cofilin, and results in inhibition of cell motility (Miyoshi et al., 2004). Inactivation of cofilin by LIMK is essential in differentiation and migration (Dawe et al., 2003; Nishita et al., 2005). Actin stress fibers are important for focal adhesion formation and cell spreading, two events that precedes migration (Machesky and Hall, 1997; Kaverina et al., 2002). Like LIMK, TESK1 influences actin organization by promoting stress fibers, enhancing adhesion, and increasing cell spreading (Arber et al., 1998; Yang et al., 1998; Toshima et al., 2001a; Tsumura et al., 2005). Similar to the effect of the Spred1–RhoA interaction, the binding of Spred1 to TESK1 leads to elevated level of active cofilin followed by enhanced actin dynamics. Therefore, the TESK1–Spred1 interaction identified here represents an additional mechanism to decrease actin stability and focal adhesions locally and temporally.
In addition, we detected a direct interaction between the actin-regulating kinase TESK1 and the microtubule-regulating kinase MARKK. This suggests a new mechanism for coordinating the actin filament and microtubule organization in the cell. When mapping the interacting domains within both kinases, we detected binding between all tested fragments, i.e., the catalytic and the regulatory domains of MARKK and TESK1 interact both with the catalytic and with the regulatory domain of the partner. Surprisingly, the full-length kinases do not bind to each other, possibly for structural reasons. Many kinases such as ROCK or Ca2+/calmodulin-dependent protein kinase exist in a closed conformation in the inactive state where N- and C-terminal domains fold over each other (Goldberg et al., 1996; Amano et al., 1999). Direct yeast two-hybrid tests and GST pull-down experiments with MARKK and TESK1 support this hypothesis. By contrast, interaction of both full-length proteins occurs in cells, as shown by coimmunoprecipitation. Activity assays with both kinases revealed unexpected results. Interaction of MARKK and TESK1 results in a unidirectional inhibition of MARKK, whereas TESK1 activity is not altered. Notably, this inhibition is mediated by binding and not by phosphorylation. Such a connection between two kinases is unusual, but we observed a similar relation between MARK2 and PAK5 in a previous study (Matenia et al., 2005). Interestingly, both complexes are composed of one kinase that influences actin organization and another that affects the microtubule network. In both cases, the microtubule-regulating kinase (MARKK or MARK) is inhibited by the binding of the actin-regulating kinase (TESK1 or PAK5), but not vice versa.
Expression of MARKK and TESK1 in CHO cells results in clearly distinguishable effects on the cytoskeleton. MARKK-transfected cells show microtubule disruption and an impaired actin network that leads to cell degeneration. Conversely, TESK1WT overexpression stabilizes F-actin stress fibers, whereas the microtubule organization is unaltered. Consequently, cotransfection of both kinases results in normal microtubules but enhanced stress fibers. The same effect on the microtubule network is observed in cells that overexpress MARKK and inactive TESK1D170A. This proves that inhibition is achieved by binding and not through phosphorylation. The novel MARKK–TESK1 interaction leads to stability of both microtubule and F-actin networks. In developmental processes such as neuritogenesis, the rearrangements of actin and microtubules need to be tightly coupled (for review, see Bradke and Dotti, 2000; Dent and Gertler, 2003). MARKK and TESK1 have been implicated in such events. MARKK is needed for neurite outgrowth and TESK1 influences axon guidance and synaptogenesis (Kraut et al., 2001; Timm et al., 2003). Thus, the binding between MARKK and TESK1 links the organization of the microtubule and actin network.
Here, we identified two partners of MARKK, Spred1 and TESK1, that also bind to each other. It is possible that there is a temporary interplay of all three proteins to coordinate their function (Figure 11). Such signaling molecules often form complexes to be operable, which might also be the case for MARKK, TESK1, and Spred1. The permanent reorganization and the spatiotemporal restrictive nature of protein complexes make it difficult to detect all proteins involved. Thus, different types of studies are required to obtain information on signaling complexes, to identify false positives and negatives, and to judge the significance of interactions for the cross-talk between different signaling cascades.
Figure 11.
Diagram of effects of MARKK, TESK1, and Spred1 on the organization of the microtubule and F-actin cytoskeleton. For details, see Discussion.
Supplementary Material
ACKNOWLEDGMENTS
We thank Annika Bülow and Anja Thiessen for excellent technical assistance, Drs. Jacek Biernat and Bettina Griesshaber for expert help with technical procedures, and Dr. Eckhard Mandelkow for advice on the manuscript. We are grateful to Dr. P. Seubert for providing 12E8 antibody and to Dr. R. Tsien for the mRFP-plasmid. This project was supported by the Deutsche Forschungsgemeinschaft.
Abbreviations used:
- MAP
microtubule-associated protein
- MARK
MAP/microtubule affinity-regulating kinase
- MARKK
MARK-activating kinase
- MT
microtubule
- Spred
Sprouty related protein with EVH-1 domain
- TESK
testis-specific protein kinase.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-07-0730) on January 23, 2008.
REFERENCES
- Amano M., Chihara K., Kaibuchi K. The COOH terminus of Rho-kinase negatively regulates Rho-kinase activity. J. Biol. Chem. 1999;274:32418–32424. doi: 10.1074/jbc.274.45.32418. [DOI] [PubMed] [Google Scholar]
- Arber S., Barbayannis F. A., Hanser H., Schneider C., Stanyon C. A., Bernard O., Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- Augustinack J., Schneider A., Mandelkow E.-M., Hyman B. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer's disease. Acta Neuropathol. 2002;103:26–35. doi: 10.1007/s004010100423. [DOI] [PubMed] [Google Scholar]
- Biernat J., Wu Y.-Z., Timm T., Zheng-Fischhöfer Q., Mandelkow E., Meijer L., Mandelkow E.-M. Protein kinase MARK/PAR-1 is required for neurite outgrowth and establishment of neuronal polarity. Mol. Biol. Cell. 2002;13:4013–4028. doi: 10.1091/mbc.02-03-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradke F., Dotti C. G. Establishment of neuronal polarity: lessons from cultured hippocampal neurons. Curr. Opin. Neurobiol. 2000;10:574–581. doi: 10.1016/s0959-4388(00)00124-0. [DOI] [PubMed] [Google Scholar]
- Bundschu K., Knobeloch K.-P., Ullrich M., Schinke T., Amling M., Engelhardt C. M., Renne T., Walter U., Schuh K. Gene disruption of Spred2 causes dwarfism. J. Biol. Chem. 2005;280:28572–28580. doi: 10.1074/jbc.M503640200. [DOI] [PubMed] [Google Scholar]
- Bundschu K., Walter U., Schuh K. The VASP-Spred-sprouty domain puzzle. J. Biol. Chem. 2006;281:36477–36481. doi: 10.1074/jbc.R600023200. [DOI] [PubMed] [Google Scholar]
- Dawe R. H., Minamide L. S., Bamburg J. R., Cramer L. P. ADF/cofilin controls cell polarity during fibroblast migration. Curr. Biol. 2003;13:252–257. doi: 10.1016/s0960-9822(03)00040-x. [DOI] [PubMed] [Google Scholar]
- Dent E. W., Gertler F. B. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40:209–227. doi: 10.1016/s0896-6273(03)00633-0. [DOI] [PubMed] [Google Scholar]
- Draviam V. M., Stegmeier F., Nalepa G., Sowa M. E., Chen J., Liang A., Hannon G. J., Sorger P. K., Harper J. W., Elledge S. J. A functional genomic screen identifies a role for TAO1 kinase in spindle-checkpoint signaling. Nat. Cell Biol. 2007;5:556–564. doi: 10.1038/ncb1569. [DOI] [PubMed] [Google Scholar]
- Drewes G., Ebnet A., Preuss U., Mandelkow E.-M, Mandelkow E. MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell. 1997;89:297–308. doi: 10.1016/s0092-8674(00)80208-1. [DOI] [PubMed] [Google Scholar]
- Ebneth A., Godemann R., Stamer K., Illenberger S., Trinczek B., Mandelkow E.-M., Mandelkow E. Overexpression of tau protein alters kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implication for Alzheimer's disease. J. Cell Biol. 1998;143:777–794. doi: 10.1083/jcb.143.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt C. M., Bundschu K., Messerschmitt M., Renne T., Walter U., Reinhard M., Schuh K. Expression and subcellular localization of Spred proteins in mouse and human tissues. Histochem. Cell Biol. 2004;122:527–538. doi: 10.1007/s00418-004-0725-6. [DOI] [PubMed] [Google Scholar]
- Goldberg J., Nairn A. C., Kuriyan J. Structural basis for the autoinhibition of calcium/calmodulin-dependent protein kinase I. Cell. 1996;84:875–887. doi: 10.1016/s0092-8674(00)81066-1. [DOI] [PubMed] [Google Scholar]
- Hutchison M., Berman K. S., Cobb M. H. Isolation of TAO1, a protein kinase that activates MEKs in stress-activated protein kinase cascades. J. Biol. Chem. 1998;273:28625–28632. doi: 10.1074/jbc.273.44.28625. [DOI] [PubMed] [Google Scholar]
- Impagnatiello M.-A., Weitzer S., Gannon G., Compagni A., Cotten M., Christofori G. Mammalian Sprouty-1 and -2 are membrane-anchored phosphoprotein inhibitors of growth factor signaling in endothelial cells. J. Cell Biol. 2001;152:1087–1098. doi: 10.1083/jcb.152.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H., et al. Spred1 negatively regulates allergen-induced airway eosinophilia and hyperresponsiveness. J. Exp. Med. 2005;201:73–82. doi: 10.1084/jem.20040616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaverina I., Krylyshkina O., Small J. V. Regulation of substrate adhesion dynamics during cell motility. Int. J. Biochem. Cell Biol. 2002;34:746–761. doi: 10.1016/s1357-2725(01)00171-6. [DOI] [PubMed] [Google Scholar]
- King J. A., et al. Distinct requirements for the sprouty domain for functional activity of Spred proteins. Biochem. J. 2005;388:445–454. doi: 10.1042/BJ20041284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopito R. R. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 2000;10:524–530. doi: 10.1016/s0962-8924(00)01852-3. [DOI] [PubMed] [Google Scholar]
- Kraut R., Menon K., Zinn K. A gain-of-function screen for genes controlling motor axon guidance and synaptogenesis in Drosophila. Curr. Biol. 2001;11:417–430. doi: 10.1016/s0960-9822(01)00124-5. [DOI] [PubMed] [Google Scholar]
- LaLonde D. P., Brown M. C., Bouverat B. P., Turner C. E. Actopaxin interacts with TESK1 to regulate cell spreading on fibronectin. J. Biol. Chem. 2005;280:21680–21688. doi: 10.1074/jbc.M500752200. [DOI] [PubMed] [Google Scholar]
- Leeksma O. C., et al. Human Sprouty4, a new Ras antagonist on 5q31, interacts with the dual specificity kinase TESK1. Eur. J. Biochem. 2002;269:2546–2556. doi: 10.1046/j.1432-1033.2002.02921.x. [DOI] [PubMed] [Google Scholar]
- Lim J., Yusoff P., Wong E. S., Chandramouli S., Lao D.-H., Fong C. W., Guy G. R. The cysteine-rich Sprouty translocation domain targets mitogen-activated protein kinase inhibitory proteins to phosphatidylinositol 4,5-bisphosphate in plasma membranes. Mol. Cell Biol. 2002;22:7953–7966. doi: 10.1128/MCB.22.22.7953-7966.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizcano J. M., et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky L. M., Hall A. Role of actin polymerization and adhesion to extracellular matrix in Rac- and Rho-induced cytoskeletal reorganization. J. Cell Biol. 1997;138:913–926. doi: 10.1083/jcb.138.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matenia D., Griesshaber B., Li X.-Y., Thiessen A., Johne C., Jiao J., Mandelkow E., Mandelkow E.-M. PAK5 kinase is an inhibitor of MARK/Par-1, which leads to stable microtubules and dynamic actin. Mol. Biol. Cell. 2005;16:4410–4422. doi: 10.1091/mbc.E05-01-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsopoulos C., Zihni C., Garg R., Ridley A. J., Morris J. D. The prostate-derived Sterile 20-like kinase (PSK) regulates microtubule organization and stability. J. Biol. Chem. 2003;278:18085–18091. doi: 10.1074/jbc.M213064200. [DOI] [PubMed] [Google Scholar]
- Miyoshi K., Wakioka T., Nishinakamura H., Kamio M., Yang L., Inoue M., Hasegawa M., Yonemitsu Y., Komiya S., Yoshimura A. The Sprouty-related protein, Spred, inhibits cell motility, metastasis, and Rho-mediated actin reorganization. Oncogene. 2004;22:5567–5576. doi: 10.1038/sj.onc.1207759. [DOI] [PubMed] [Google Scholar]
- Moore T. M., Garg R., Johnson C., Coptcoat M. J., Ridley A. J., Morris J. D. PSK, a novel Ste20-like kinase derived from prostatic carcinoma that activates the c-Jun N-terminal kinase mitogen-activated protein kinase pathway and regulates actin cytoskeletal organization. J. Biol. Chem. 2000;275:4311–4322. doi: 10.1074/jbc.275.6.4311. [DOI] [PubMed] [Google Scholar]
- Nishita M., Tomizawa C., Yamamoto M., Horita Y., Ohashi K., Mizuno K. Spacial and temporal regulation of cofilin activity by LIM kinase and slingshot is critical for directional cell migration. J. Cell Biol. 2005;171:349–359. doi: 10.1083/jcb.200504029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonami A., Kato R., Taniguchi K., Yoshiga D., Taketomi T., Fukuyama S., Harada M., Sasaki A., Yoshimura A. Spred1 negatively regulates Interleukin-3-mediated ERK/MAP kinase activation in hematopoietic cells. J. Biol. Chem. 2004;279:52543–52551. doi: 10.1074/jbc.M405189200. [DOI] [PubMed] [Google Scholar]
- Nonami A., Taketomi T., Kimura A., Saeki K., Takaki H., Sanada T., Taniguchi K., Harada M., Kato R., Yoshimura A. The Sprouty-related protein, Spred1, localizes in lipid raft/caveola and inhibits ERK activation in collaboration with caveolin-1. Genes Cells. 2005;10:887–895. doi: 10.1111/j.1365-2443.2005.00886.x. [DOI] [PubMed] [Google Scholar]
- Raman M., Earnest S., Zhang Y., Cobb M. TAO kinases mediate activation of p38 in response to DNA damage. EMBO J. 2007;26:2005–2014. doi: 10.1038/sj.emboj.7601668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond K., Bergeret E., Avet-Rochex A., Griffin-Shea R., Fauvarque M.-O. A screen for modifiers of RacGAP(84C) gain-of-function in the Drosophila eye revealed the LIM kinase Cdi/TESK1 as a downstream effector of Rac1 during spermatogenesis. J. Cell Sci. 2004;117:2777–2789. doi: 10.1242/jcs.01123. [DOI] [PubMed] [Google Scholar]
- Sese M., Corominas M., Stocker H., Heino T., Hafen E., Serras F. The Cdi/TESK1 kinase is required for sevenless signaling and epithelial organization in the Drosophila eye. J. Cell Sci. 2006;119:5047–5056. doi: 10.1242/jcs.03294. [DOI] [PubMed] [Google Scholar]
- Shulman J. M., Feany M. B. Genetic modifiers of tauopathy in Drosophila. Genetics. 2003;165:1233–1245. doi: 10.1093/genetics/165.3.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Kohno R., Ayada T., Kato R., Ichiyama K., Morisada T., Oike Y., Yonemitsu Y., Maehara Y., Yoshimura A. Spreds are essential for embryonic lymphangiogenesis by regulating vascular endothelial growth factor receptor 3 signaling. Mol. Cell. Biol. 2007;27:4541–4550. doi: 10.1128/MCB.01600-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies E., Mandelkow E.-M. Missorting of tau in neurons causes degeneration of synapses that can be rescued by MARK2/Par-1. J. Neurosci. 2007;27:2896–2907. doi: 10.1523/JNEUROSCI.4674-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timm T., Li X.-Y., Biernat J., Jiao J., Mandelkow E., Vandekerckhove J., Mandelkow E.-M. MARKK, a Ste20-like kinase, activates the polarity-inducing kinase MARK/PAR-1. EMBO J. 2003;22:5090–5101. doi: 10.1093/emboj/cdg447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timm T., Matenia D., Li X.-Y., Griesshaber B., Mandelkow E.-M. Signaling from MARK to tau: regulation, cytoskeletal crosstalk and pathological phosphorylation. Neurodegener. Dis. 2006;3:207–217. doi: 10.1159/000095258. [DOI] [PubMed] [Google Scholar]
- Toshima J., Koji T., Mizuno K. Stage-specific expression of testis-specific protein kinase 1 (TESK1) in rat spermatogenic cells. Biochem. Biophys. Res. Commun. 1998;249:107–112. doi: 10.1006/bbrc.1998.9099. [DOI] [PubMed] [Google Scholar]
- Toshima J., Ohashi K., Okano I., Nunoue K., Kishioka M., Kuma K., Miyata T., Hirai M., Baba T., Mizuno K. Identification and characterization of a novel protein kinase, TESK1, specifically expressed in testicular germ cells. J. Biol. Chem. 1995;270:31331–31337. doi: 10.1074/jbc.270.52.31331. [DOI] [PubMed] [Google Scholar]
- Toshima J., Toshima J. Y., Amano T., Yang N., Narumiya S., Mizuno K. Cofilin phosphorylation by protein kinase testicular protein kinase 1 and its role in the integrin-mediated actin reorganisation and focal adhesion formation. Mol. Biol. Cell. 2001a;12:1131–1145. doi: 10.1091/mbc.12.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshima J., Toshima J. Y., Suzuki M., Noda T., Mizuno K. Cell-type specific expression of a TESK1 promoter-linked lacZ gene in transgenic mice. Biochem. Biophys. Res. Commun. 2001b;286:566–573. doi: 10.1006/bbrc.2001.5404. [DOI] [PubMed] [Google Scholar]
- Toshima J. Y., Toshima J., Watanabe T., Mizuno K. Binding of 14–3-3β regulates the kinase activity and subcellular localization of testicular protein kinase1. J. Biol. Chem. 2001c;276:43471–43481. doi: 10.1074/jbc.M104620200. [DOI] [PubMed] [Google Scholar]
- Tsumura Y., Toshima J., Leeksma O. C., Ohashi K., Mizuno K. Sprouty-4 negatively regulates cell spreading by inhibiting the kinase activity of testicular protein kinase. Biochem. J. 2005;387:627–637. doi: 10.1042/BJ20041181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakioka T., Sasaki A., Kato R., Shouda T., Matsumoto A., Miyoshi K., Tsuneoka M., Komiya S., Baron R., Yoshimura A. Spred is a Sprouty-related suppressor of Ras signaling. Nature. 2001;412:647–651. doi: 10.1038/35088082. [DOI] [PubMed] [Google Scholar]
- Yang N., Higuchi O., Ohashi K., Nagata K., Wada A., Kangawa K., Nishida E., Mizuno K. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–812. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- Zihni C., Mitopoulos C., Tavares I. A., Ridley A. J., Morris J. D. Prostate-derived Sterile 20-like kinase 2 (PSK2) regulates apoptotic morphology via c-Jun N-terminal kinase and Rho kinase-1. J. Biol. Chem. 2006;281:7317–7323. doi: 10.1074/jbc.M513769200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.