Abstract
Neurons express two different microtubule-severing proteins, namely P60-katanin and spastin. Here, we performed studies on cultured neurons to ascertain whether these two proteins participate differently in axonal branch formation. P60-katanin is more highly expressed in the neuron, but spastin is more concentrated at sites of branch formation. Overexpression of spastin dramatically enhances the formation of branches, whereas overexpression of P60-katanin does not. The excess spastin results in large numbers of short microtubules, whereas the excess P60-katanin results in short microtubules intermingled with longer microtubules. We hypothesized that these different microtubule-severing patterns may be due to the presence of molecules such as tau on the microtubules that more strongly shield them from being severed by P60-katanin than by spastin. Consistent with this hypothesis, we found that axons depleted of tau show a greater propensity to branch, and that this is true whether or not the axons are also depleted of spastin. We propose that there are two modes by which microtubule severing is orchestrated during axonal branch formation, one based on the local concentration of spastin at branch sites and the other based on local detachment from microtubules of molecules such as tau that regulate the severing properties of P60-katanin.
INTRODUCTION
The formation of axonal branches is critical for the development of the nervous system. In order for axonal branches to form, the cytoskeleton within the parent axon must undergo dramatic remodeling. In particular, the parent axon is dominated by very long microtubules that must be locally chopped into short highly mobile pieces that are able to move into the newly forming branch. Over a decade ago, we reported indirect evidence of the local severing of microtubules at sites of impending branch formation using serial reconstructions of election micrographs (Yu et al., 1994). We were then able to directly visualize the severing of microtubules at branch sites using live-cell imaging (Dent et al., 1999). Since then, we have dedicated a great deal of attention to studying P60-katanin, which is the predominant microtubule-severing protein in developing neurons (Ahmad et al., 1999; Karabay et al., 2004; Yu et al., 2005). Inhibition of P60-katanin results in a dramatic shift toward longer microtubules in the axon and severely compromises the axon's growth. This is presumably due to the fact that long microtubules are immobile, whereas short microtubules, produced by the severing of long microtubules, are the ones that undergo the transport events crucial for axonal growth (Baas et al., 2006). On the basis of all of these findings, we had anticipated that overexpressing P60-katanin in cultured neurons would enhance the propensity of their axons to form branches. However, this has not proven to be the case in studies performed thus far (Karabay et al., 2004; Yu et al., 2005).
Since we began our work on P60-katanin, it has become apparent that neurons express another microtubule-severing protein called spastin (Errico et al., 2002; Evans et al., 2005; Roll-Mecak and Vale, 2005, 2006). Spastin is very similar to P60-katanin in the AAA-region responsible for severing microtubules, but different in other regions of the molecule. P60-katanin is broadly expressed across cell types, whereas spastin expression is more restricted to the nervous system (Baas et al., 2005; Ma et al., 2006; Roll-Mecak and Vale, 2006; Salinas et al., 2007). One possibility is that neurons express an entirely redundant microtubule-severing protein because the severing of microtubules is so crucial for axonal growth, branch formation, and the ongoing transport of microtubules throughout the life of the neuron. Another possibility is that the two severing proteins have overlapping but nonidentical functions, properties, and roles to carry out in the neuron. Such a “division of labor” for microtubule-severing proteins has already been demonstrated in the mitotic spindle (Zhang et al., 2007).
To date, there have been no studies that directly compare the functional properties of P60-katanin and spastin in neurons. In the present study, we have rectified this by analyzing the effects on the microtubule array and on the morphology of cultured neurons produced by manipulation of each of the two severing proteins. We have also compared the capacity of tau to regulate the severing of microtubules by each of the two severing proteins. In conducting these studies, our main goal was to test the hypothesis that spastin and P60-katanin participate differently in the formation of axonal branches.
MATERIALS AND METHODS
DNA Constructs and Polyclonal Antibodies
Mouse cDNA encoding full-length spastin was purchased from Invitrogen (Carlsbad, CA). Mouse cDNA encoding spastin M85 translated from the second start codon (methionine M85) was cloned into mammalian expression vector pEGFP (Clontech, Palo Alto, CA) downstream from the EGFP tag. Rat cDNA encoding full-length P60-katanin was used to prepare a comparable enhanced green fluorescent protein (EGFP) construct, as previously described (Karabay et al., 2004). To prepare two different polyclonal antibodies against spastin, termed Sp/AAA and Sp/Sp5, two different fragments of spastin cDNA encoding amino acids 333-465 or 90-283, respectively, were cloned into the bacterial expression vector pRSET (Invitrogen, Carlsbad, CA). The two peptides were purified, after which rabbit antisera were prepared by Cocalico Biologicals (Reamstown, PA). IgG fractions were purified by protein A-Sepharose chromatography. Antibody specificity was tested by Western blotting and immunostaining of spastin-transfected and nontransfected fibroblasts. Both antibodies were effective for both Western blotting and immunostaining, but Sp/AAA had properties preferable for Western blotting, whereas Sp/Sp5 had properties preferable for immunostaining.
Preparation and Transfection of Cell Cultures
Cultures of rat hippocampal neurons and RFL-6 rat fibroblasts were prepared as previously described (Yu and Baas, 1994; Buster et al., 2002; Yu et al., 2005; Qiang et al., 2006). For transfection of the EGFP-P60-katanin and EGFP-spastin constructs, we either used a modified electroporation device called a Nucleofector (Amaxa Biosystems, Köln, Germany) before plating, or Lipofectamine 2000 (Invitrogen; 11668-027) at some point after plating the cells. For the former, the single-cell suspension of hippocampal neurons or RFL-6 cells was resuspended in Nucleofector solution (Amaxa Biosystems), and the cells were then transfected with EGFP alone (control) or with the EGFP-P60-katanin or EGFP-spastin construct using the nucleofection device according to manufacturer's instructions and recommended settings (Karabay et al., 2004). After nucleofection, the neurons were plated onto poly-l-lysine–treated glass coverslips as previously described (Yu and Baas, 1994), whereas the RFL-6 cells were plated on untreated glass coverslips (Buster et al., 2002). Lipofectamine 2000 was used for transfecting cells in the culture dish. For this procedure, 0.5–1 μg of DNA and 1.25–2.5 μg of Lipofectamine 2000 were used per 35-mm dish. The ratio of DNA to Lipofectamine 2000 was 1:2.5. The cells were incubated in the DNA/lipofectamine medium for 5 h. After transfection, neurons were transferred to 37°C hippocampal neuron plating medium (Neurobasal medium supplemented with 2% B27, 0.3% glucose, 1 mM glutamine, and 5% FBS). Transfection efficiency was generally 5–10% for neuronal cultures and 15–30% for RFL-6 cell cultures, with EGFP fluorescence appearing within the first several hours after transfection. For experiments involving small interfering RNA (siRNA), neurons were transfected with a pool of four sequences specific to rodent spastin (purchased as a “smartpool” from Dharmacon Research, Boulder, CO; Accession number: XM-343018) or with a nonspecific control sequence (Dharmacon Research; Cat. no. D-001206-03-20), using the Nucleofector as previously described (Qiang et al., 2006). A smartpool of tau siRNA (Dharmacon Research, Cat. no. M-089500-00) was used in some experiments, following our previously described protocol (Qiang et al., 2006). An siRNA sequence that does not correspond to any gene in the rat genome, also purchased from Dharmacon, was used in all siRNA experiments as a control. In some experiments, the RFL-6 fibroblasts were induced to express the human four-repeat form of tau (termed tau 4R; provided by K. S. Kosik, University of California, Santa Barbara, CA) together with EGFP-spastin, EGFP-P60-katanin, or EGFP control for 1 d (using Lipofectamine 2000).
Immunofluorescence Analyses
For immunofluorescence studies on microtubule levels and distribution, cultures were briefly washed with 37°C phosphate buffered-saline (PBS) and then simultaneously fixed and extracted with 4% paraformaldehyde, 0.2% glutaraldehyde, and 0.1% Triton X-100 for 15 min. Cultures were washed with PBS three times for 5 min, quenched with 2 mg/ml sodium borohydride three times for 10 min, and then blocked with 10% normal goat serum and 10 mg/ml BSA in PBS for 1 h. After blocking, cultures were exposed to an anti-EGFP rabbit polyclonal antibody (1:4000; ab6556-25; Abcam, Cambridge, MA) overnight at 4°C. Enhancement of EGFP fluorescence by immunofluorescence assisted in discerning the cells expressing the EGFP, EGFP-P60-katanin, and EGFP-spastin from those that were not expressing, given that the fluorescence of the EGFP itself tended to diminish during the fixation and extraction. Cultures were then rinsed three times for 5 min in PBS, followed by exposure to an anti-β-tubulin antibody directly conjugated to Cy3 (1:400; C4585; Sigma, St. Louis, MO) together with Alexa Fluor-488–conjugated secondary goat anti-rabbit antibody (1:200; A11008; Molecular Probes, Eugene, OR). Cells were then rinsed extensively in PBS and mounted in a medium that reduces photobleaching.
Images were obtained on an Axiovert 200 microscope (Carl Zeiss, Thornwood, NY) equipped with a high resolution CCD (Orca ER, Hamamatsu, Hamamatsu City, Japan). All images were obtained using identical camera, microscope, and imaging criteria such as gain, brightness and contrast, and exposure time. Efforts were made to ensure that the images were not saturated and that minimal bleaching of the fluorescence occurred before image acquisition. Digital gray values of image pixels representing arbitrary fluorescence units (AFU) were obtained using AxioVision software. Fluorescence intensity was quantified for cells that had not been induced to express any of the constructs, cells that had been induced to express EGFP alone, and cells that had been induced to express the P60-katanin or spastin constructs. In some cases, fluorescence images were subjected to the “invert” function in Adobe Photoshop(San Jose, CA) that converts blacks to whites and whites to blacks and inverts gray levels proportionally, because we found that fine details were more clearly visualized. The resulting images are referred to as “inverted images.” Statistics were done using the Student's t test.
Western Blotting
Western blot analyses were performed according to established procedures (Sambrook et al., 1989). Neurons cultured in plastic dishes were suspended with cold 1 × PBS and lysed in the sample buffer with SDS, and then the proteins were resolved in 10% SDS-PAGE gels and transferred onto nitrocellulose membranes (90 mA; overnight at 4°C). Transfers were probed with Sp/AAA antibody against spastin. A goat anti-rabbit secondary HRP-conjugated antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) was used. After reaction with chemiluminescent peroxidase substrate (Super Signal; Pierce, Rockford, IL), the blot was covered with x-ray film. We used three different exposure times (5, 15, and 30 s). Films were imaged using an Epson (Long Beach, CA) Perfection 1280 scanner. Protein markers were used to indicate the appropriate molecular weights. GAPDH was used as the internal control in each group to show that the same amount of protein was loaded in each group.
RESULTS
For the studies here, we used cultures of fetal rat hippocampal neurons, which are a well-characterized model for neuronal development (Dotti et al., 1988; Yu et al., 2005). Shortly after they adhere to the substrate, these neurons extend a broad lamellipodium (stage 1) that subsequently coalesces into multiple immature “minor” processes (stage 2). One of these minor processes then becomes the axon, usually by the second day after plating (stage 3), and then, a few days later, the remaining minor processes develop into dendrites (stage 4). Experiments conducted on these neurons utilized a previously described rat EGFP-P60-katanin construct (Karabay et al., 2004; Yu et al., 2005) and a new mouse EGFP-spastin construct developed in our laboratory (see Materials and Methods). In the case of the spastin construct, we used the sequence corresponding to that produced by the second start codon, which is the only detectable form expressed during development (Claudiani et al., 2005; Salinas et al., 2005).
Depletion of Spastin from Cultured Neurons Reduces Axonal Branch Frequency
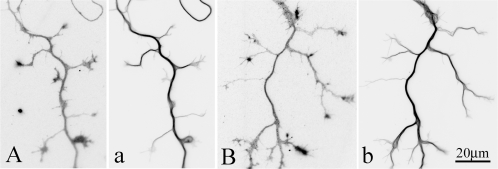
In previous studies, we documented that inhibition of P60-katanin produces profoundly deleterious effects on axonal outgrowth (Ahmad et al., 1999; Karabay et al., 2004). Inhibition of P60-katanin compromises release of microtubules from the centrosome and results in significantly longer microtubules in the cell body and throughout the neuron. These results suggest that the available spastin is insufficient, in the absence of P60-katanin, to meet the microtubule-severing needs of the neuron. The question remains as to whether inhibiting spastin produces a comparable phenotype or produces a phenotype consistent with a more specialized role for spastin in axonal branch formation. To investigate this, we used siRNA to deplete spastin from cultured rat hippocampal neurons. Neurons were treated with either siRNA to spastin or control siRNA, introduced by nucleofection. After allowing 3 d for the spastin protein to be depleted, the neurons were replated and allowed to grow processes anew for 48 h before being fixed. Westerns blots (Figure 1A) confirmed that the spastin siRNA resulted in more than 95% diminution of the spastin protein in the cultures. Spastin-depleted neurons were not dramatically abnormal in their phenotypes, nor did they show indications of being unhealthy. However, as shown in the pie graphs in Figure 1, B and C, 48 h after replating, development was slowed in the neurons depleted of spastin, with more neurons remaining in stages 1 and 2 compared with the control cultures wherein more cells had progressed to stage 3. Comparing cells in stage 3, axons of spastin-depleted neurons were 20% shorter (but had statistically the same number of minor processes) compared with control neurons (Figure 1, D and E). The most dramatic morphological difference was that spastin-depleted neurons displayed 45% fewer axonal branches than control neurons (Figure 1F). Examples of control neurons and spastin-depleted neurons, stained for microtubules, are shown in Figures 1, G and H. More detailed analyses of the microtubule arrays revealed no detectable differences in the overall distribution of microtubules after spastin depletion (data not shown) such as has been reported of neurons with experimentally impaired P60-katanin (Ahmad et al., 1999; Karabay et al., 2004).
Figure 1.
Morphological analysis of control and spastin-depleted hippocampal neurons. (A) Western blot of whole-cell extracts from cultures of rat hippocampal neurons probed with spastin antibody Sp/AAA or GAPDH antibody as an internal control. Cultures were treated with control siRNA or spastin siRNA for 3 d. (B and C) Pie graphs showing the ratio of hippocampal neurons in stage 1, stage 2, or stage 3 in each experimental group (n > 100). More neurons remained in stage 1 or stage 2 in spastin-depleted cultures than in control cultures. (D) Quantitative analyses of the lengths of axons in control and spastin-depleted groups (n > 100). Axonal length of spastin-depleted neurons is 20% shorter than that of the control group (p < 0.01). (E) Quantitative analyses of the number of minor processes in control and spastin-depleted groups (n > 100). There is no significant difference between these two groups (p > 0.05). (E) Quantitative analyses of the number of primary branches per axon (n > 100). For quantification of branches, we chose stage 3 neurons with axons 100–150 μm in length. Depletion of spastin results in 45% reduction of branch numbers (p < 0.001). (G and H) Immunostaining of microtubules in control (G) and spastin-depleted (H) hippocampal neurons. Arrows point to axonal branches. Scale bar, 30 μm.
Collectively, these results indicate that cultured hippocampal neurons do not require spastin in order to grow axons, to develop branches, or to progress from one developmental stage to the next. However, depletion of spastin does slow or diminish all of these aspects of axonal development. In particular, it is noteworthy that the diminution of axonal branch frequency is over twice as great as the diminution of axonal length, which is consistent with a more specialized role for spastin in branch formation.
Overexpression of Spastin in Cultured Neurons Produces a Markedly Different Phenotype than Overexpression of P60-Katanin
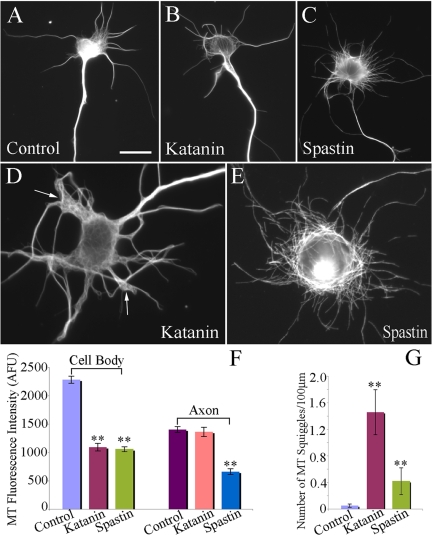
To further test the possibility that the two severing proteins are not identical in their properties, we overexpessed them individually in cultured hippocampal neurons. For controls, neurons were transfected to express EGFP alone. For comparison, neurons were selected from each experimental group that displayed generally similar levels of EGFP fluorescence. For these experiments, neurons were transfected before plating (by nucleofection). Given that high levels of overexpression can severely compromise axonal outgrowth (Karabay et al., 2004), we optimized the parameters of the transfection to achieve levels of expressed P60-katanin or spastin that permitted axons to obtain lengths not dramatically different from controls. These levels were somewhat lower than in our earlier studies (Karabay et al., 2004; Yu et al., 2005). Figure 2, A–C, shows neurons 24 h after transfection, immunostained for microtubules and then displayed as inverted images to make fine details more obvious. Under these conditions, the neurons overexpressing P60-katanin showed 12% diminution in microtubule mass, whereas the neurons overexpressing spastin showed a somewhat greater loss of microtubule mass (30%). The number of minor processes was not significantly different from controls in neurons overexpressing P60-katanin, but was 70% greater in neurons overexpressing spastin. The lengths of the axon and minor processes were statistically indistinguishable from controls in both cases. Figure 2A shows a control neuron in stage 3. Figure 2B shows a neuron overexpressing P60-katanin. Figure 2C shows a typical spastin-overexpressing neuron. Figure 2D shows another spastin-overexpressing neuron but 48 h after plating to demonstrate that the phenotype continues to become more complex over time. The data, shown in Figure 2E, correspond to the 24-h time point. Figure 3 shows neurons immunostained for microtubules and EGFP. It is apparent from the EGFP staining that the processes are not only more numerous in spastin-overexpressing neurons compared with P60-katanin–overexpressing neurons, and the processes also bear large numbers of short lateral extensions deficient in microtubules.
Figure 2.
Morphological analyses of stage 3 hippocampal neurons expressing EGFP, EGFP-P60-katanin, or EGFP-spastin. Control (A) and P60-katanin–overexpressing (B) neurons displayed similar morphologies, with 4–6 minor processes and one axon, whereas neurons overexpressing spastin (C and D) displayed more minor processes than control neurons. Quantitative analysis of the microtubule levels, the number of minor process, the length of minor processes and the length of axons are shown in E (n > 60). The microtubule levels in the P60-katanin–overexpressing and spastin-overexpressing neurons decreased 12% (p < 0.05) and 30% (p < 0.001) respectively compared with control neurons. There is a 70% increase of the number of minor processes in hippocampal neurons overexpressing spastin compared with control and P60-katanin–overexpressing neurons (p < 0.001). There is no significant difference in the length of minor processes and axons between any of the experimental groups (p > 0.5). Scale bar, 40 μm.
Figure 3.
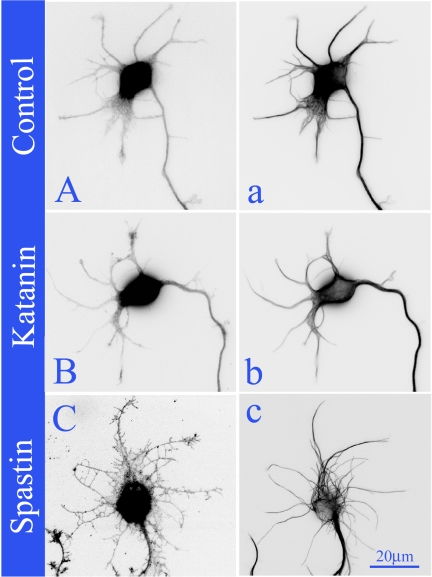
Comparison of hippocampal neurons expressing EGFP (Aa), EGFP-P60-katanin (Bb), or EGFP-spastin (Cc). The left column (A–C) shows EGFP staining, and the right column shows microtubule staining (a–c). To show the cellular morphology in greater detail, the images were displayed as “inverted images” (see Materials and Methods). The neurons expressing EGFP and EGFP-P60-katanin showed an average of about five minor processes with a few filopodia (Aa and Bb), whereas the neuron expressing EGFP-spastin showed a dramatic increase of the number of filopodia on the minor processes (Cc). The microtubule levels in the cell body of the EGFP-spastin–expressing neurons (c) are lower than in the cell body of EGFP-P60-katanin-expressing neurons (b). Scale bar, 20 μm.
The most dramatic observation about phenotype was a marked increase in axonal branching in the spastin-overexpressing neurons but not in the P60-katanin–overexpressing neurons. Figure 4 shows higher magnification images of axons overexpressing spastin for 24 h. The first column shows microtubule stains, whereas the second column shows the EGFP-spastin. The third column is a color overlay of the microtubules in red and the EGFP-spastin in green. Shown are axonal arbors from three different neurons displaying varying degrees of branch formation. We defined branches as somewhat thickened lateral extensions containing multiple microtubules. Thin lateral extensions not (yet) invaded by multiple microtubules were called filopodia. The first row shows the early stages of filopodial formation along the length of the axon. The second row shows longer and more numerous lateral extensions (early branches) into which microtubules have started to invade. The third row shows numerous short branches containing microtubules. Panel D shows quantification of the observations. The number of filopodia along minor processes and axons in spastin-overexpressing neurons increased 8 and 28 times, respectively, compared with control neurons (p < 0.0001). The axonal branches in spastin-overexpressing neurons increased three times relative to control neurons (p < 0.001). There was no significant difference between control and P60-katanin–overexpressing neurons in the number of filopodia along minor processes and axon, or the number of axonal branches (p > 0.05). Figure 5 shows inverted images of neurons overexpressing EGFP-spastin and then immunostained for microtubules and EGFP. The dramatic increase in branches frequency is apparent at 24 h of overexpression (Figure 5Aa). These newly formed branches are not transient (i.e., they do not retract after reaching ∼10 μm in length; see Yu et al., 1994), but rather persist and elongate as shown in images taken at 48 h (Figure 5Bb) and 72 h (Figure 6, Aa and Bb).
Figure 4.
Overexpression of spastin in hippocampal neurons results in a dramatic increase in the number of branches and the number of filopodia on minor processes, axon, and axonal branches. The first column shows microtubule stains, whereas the second column shows the EGFP-spastin. The third column is a color overlay of the microtubules in red and the EGFP-spastin in green. (A, A′, and A″) An axon had many early filopodia along it with no microtubule invasion into the filopodia (small arrows). (B, B′, and B″) An axon with a number of longer filopodia; microtubules have started to invade the filopodia (large arrows). (C, C′, and C″) An axon with several branches. There was an enrichment of EGFP-spastin at the tips of the axons and their branches (arrowheads). Quantitative analyses of the filopodia number on minor processes and axons as well as the number of axonal branches are shown in D (n > 60 for each quantitative analysis). Y-axis shows the number of filopodia or branches per 100 μm minor process or axon. There was no significant difference between control and EGFP-P60-katanin–expressing neurons in terms of the number of filopodia along minor process and axon and the number of axonal branches (p > 0.05). EGFP-spastin, in contrast, caused a dramatic increase of the filopodia number on minor processes and axons (p < 0.001). The number of axonal branches also increased about three times for EGFP-spastin–expressing neurons compared with control neurons (p < 0.001). Scale bar, 25 μm.
Figure 5.
Early stage 3 hippocampal neurons expressing EGFP-spastin, immunostained for EGFP and microtubules. The axons shown here are from the same experiment as shown in Figure 4. Here, two different axons from EGFP-spastin–expressing neurons are displayed as higher magnification “inverted images” to reveal more detail. (A and B) EGFP staining; (a and b) microtubule staining. Aa shows an axon with somewhat shorter branches, whereas the branches of the axon shown in Bb are more developed. The number of filopodia and branches along these axons are much higher than would be observed in controls (see Figure 4). Scale bar, 20 μm.
Figure 6.
Late stage 3 hippocampal neurons expressing EGFP-spastin, immunostained for EGFP and microtubules. Neurons are displayed as “inverted images” to reveal more detail. Aa shows EGFP staining; Bb show microtubule staining. (a and b) Enlarged images from the regions of A and B indicated by rectangles. Note that GFP-spastin is enriched in growth cones (arrowheads) and also (but to a lesser extent) at branch points (arrows). Scale bar, (A and B) 40 μm; (a and b) 15 μm.
Distribution of Spastin in Developing Axons
We previously reported that P60-katanin is relatively evenly distributed along the length of the axon and only slightly enriched in growth cones and branch points of cultured hippocampal neurons (Yu et al., 2005). Our observations thus far described on overexpression of spastin suggest that the distribution of endogenous spastin may be very different from that of P60-katanin, at least during some stages of axonal development. In early axons, the EGFP-spastin appeared to distributed relatively evenly along the axon, but in late stage 3 neurons, with more highly developed axons, it was common for the growth cones to be highly enriched with EGFP-spastin, and the same was true (but to a lesser extent) of many of the sites where early branches emerge (see Figures 4–6). To address the issue of endogenous spastin distribution, we immunostained cultures at various points during development, using the Sp/Sp5 antibody. As shown in Figure 7A, the staining was relatively even along younger axons. In late stage 3 and stage 4 neurons, the immunostains revealed notable enrichments of endogenous spastin in many, but not all, of the growth cones and sites of early branch formation (Figure 7B). Notably, these enrichments, when they occur, are far more dramatic than any of the enrichments ever observed in our previous studies on P60-katanin, with many times the levels of spastin in these growth-related regions compared with other regions along the shaft of the axon. These results are consistent with earlier observations that spastin tends to localize in discrete patches along the length of the axon (Svenson et al., 2005) and also in the distal regions of axons (Errico et al., 2004). We would conclude that, although spastin is less abundant in the neuron than P60-katanin, it has a higher propensity to concentrate at growth-related sites along the axon.
Figure 7.
Immunolocalization of spastin in early stage 3 and stage 4 hippocampal neurons. (A) the axon of an early stage 3 (day 2 culture) hippocampal neuron immunostained with Sp/Sp5 antibody; (B) the axon of a stage 4 (day 10 culture) hippocampal neuron immunostained with Sp/Sp5 antibody. The stage 3 axon shows a relatively even distribution of spastin along its length. The stage 4 axon shows accumulations of spastin in growth cones (arrows), some but not all branch points (upper arrowhead) and at sites that we suspect may soon give rise to new branches (lower arrowhead). Scale bar, 15 μm.
Overexpression of Spastin Produces a Markedly Different Pattern of Microtubule Severing than Overexpression of P60-Katanin
The results described thus far indicate that overexpressed spastin degrades the microtubule array by 15–20% more than a roughly equal amount of overexpressed P60-katanin. However, neurons expressing higher levels of P60-katanin did not show the morphological characteristics of neurons overexpressing spastin (Karabay et al., 2004; Yu et al., 2005). For this reason, we hypothesized that the patterns of microtubule severing produced by each of the two severing proteins might be distinctive for reasons other than just the degree of microtubule loss. As a first step toward testing this hypothesis, we compared the microtubule arrays in cultured rat RFL-6 fibroblasts in which we overexpressed each of the two severing proteins. Fibroblasts are very flat and hence permit more spatial resolution than is possible in neurons. Expression was for 12 h, a time frame selected to optimize detection of differences between the two severing proteins. As shown in Figure 8, control cells contained almost exclusively long microtubules (Figure 8A), whereas P60-katanin–overexpressing cells showed a mixture of short and long microtubules (Figure 8B). In sharp contrast, the spastin-overexpressing cells displayed a much more “evenly chopped” microtubule array, with almost exclusively short microtubules, a few microns in length (Figure 8C). Quantification of microtubule free ends showed a fivefold increase in P60-katanin–overexpressing cells (p < 0.001), but a 39-fold increase in spastin-overexpressing cells (p < 0.00001).
Figure 8.
Different microtubule-severing patterns in fibroblasts produced by overexpression of P60-katanin or spastin. (A–C) RFL-6 fibroblasts immunostained for microtubules. (A) Control fibroblast expressing EGFP displaying long continuous microtubules. (B) Fibroblast expressing EGFP-P60-katanin displaying several obvious long microtubules together very few short ones. Arrows point to short microtubules. Arrowheads point to a microtubule being severed. (C) Fibroblast expressing EGFP-spastin displaying only very short microtubules. (D) Quantitative analyses of the number of microtubule free ends per 104 AFU of microtubules among the control, P60-katanin–overexpressing, and spastin-overexpressing groups (n > 100). Overexpression of P60-katanin creates fivefold more free microtubule ends than in the control group (p < 0.001). Overexpression of spastin creates 39-fold more free microtubule ends than in the control group (p < 0.001). Scale bar, 4 μm.
We next examined neurons overexpressing each of the two severing proteins for 12 h (using Lipofectamine 2000 for transfections). We previously reported that the microtubules in the axon are more resistant to severing over a 12-h bout of overexpression of P60-katanin than microtubules in other compartments of the neuron (Yu et al., 2005; Qiang et al., 2006). Figure 9A shows a control neuron, stained for microtubules. Figures 9, B and C, show neurons overexpressing P60-katanin or spastin, respectively, for 12 h and then stained for microtubules. The neuron overexpressing P60-katanin shows a clear diminution of microtubule mass from the cell body (to about half of control levels), whereas the axon continues to show robust microtubule staining comparable to controls. We then selected spastin-overexpressing neurons that displayed roughly the same degree of diminution of microtubule levels from the cell body, so that we could compare the relative effects on the axon. Unlike neurons overexpressing P60-katanin, these neurons also displayed a reduction of microtubule levels in the axon by about half (Figure 9F). Thus, microtubules in the axon are more resistant than microtubules elsewhere in the neuron to P60-katanin but not to spastin. In addition, a marked difference in the microtubule-severing patterns of the two severing proteins is apparent from the micrographs. As previously reported, neurons overexpressing P60-katanin often show very long microtubules that tend to become twisted into shapes we call “squiggles” (Figure 9D). As shown in Figure 9G, such squiggles occurred far less frequently in neurons overexpressing spastin, which is consistent with the lack of a resistant population of long microtubules that fail to be severed by spastin. By contrast, there are abundant short microtubules in the spastin-overexpressing neurons, whereas short microtubules are less plentiful in the P60-katanin overexpressing neurons (Figure 9, D and E).
Figure 9.
Axonal microtubules are resistant to a 12-h bout of overexpression of P60-katanin, but not spastin. (A–E) The immunostains of microtubules of the hippocampal neurons. (A) Neuron transfected with EGFP control. (B and D) Two neurons transfected to overexpress P60-katanin. (C and E) Two neurons transfected to overexpress spastin. (F) Quantitative analyses of microtubule fluorescence intensity in cell bodies and axons among the three groups (control, overexpression of P60-katanin, and overexpression of spastin). Overexpression of P60-katanin or spastin results in notable microtubule loss from cell bodies compared with control (52% for P60-katanin, p < 0.001; 54% for spastin, p < 0.001). In axons, overexpression of P60-katanin for 12 h results in no significant diminution of microtubules (p > 0.05), whereas overexpression of spastin reduces the microtubule levels by 53% compared with controls (p < 0.001). (G) Quantitative analyses of the number of microtubule “squiggles” along the processes per 100 μm (n > 40). Overexpression of P60-katanin increased the number of microtubule squiggles by 29-fold (p < 0.0001) compared with controls. By contrast, overexpression of spastin increased the number of microtubule squiggles by only eightfold (p < 0.001) compared with controls. Arrows point to the microtubule squiggles in the neuron overexpressing P60-katanin. Scale bar, (A–C) 15 μm; (D and E) 8 μm.
Tau Protects Microtubules Less Well from Spastin than P60-Katanin
The very different patterns of microtubule severing produced by overexpressing each of the two severing proteins suggest that they have different properties or are regulated differently in cells. We previously reported that the ability of P60-katanin to sever microtubules is attenuated by the presence of tau on the microtubules (Qiang et al., 2006). This was evidenced by the fact that depleting tau from neurons before overexpressing P60-katanin rendered the microtubules in the axon equally sensitive to the microtubules in other regions of the neuron. Moreover, microtubules in RFL-6 fibroblasts were rendered highly resistant to severing when tau was coexpressed with P60-katanin. The fact that axonal microtubules are no more resistant to spastin than microtubules elsewhere in the neuron suggests that tau may not be as good at protecting microtubules against spastin. To investigate this, we performed the same type of overexpression experiments on RFL-6 fibroblasts for spastin as we previously conducted for P60-katanin (Qiang et al., 2006). Figure 10, A and B, shows a control fibroblast and a fibroblast expressing tau (specifically, tau 4R), both stained for microtubules. When P60-katanin is overexpressed in control fibroblasts, there is a severe loss of microtubule mass, with the remaining microtubules appearing shorter than in controls (Figure 10C). Fibroblasts expressing tau display no detectable alterations in the microtubule array when P60-katanin is overexpressed (Figure 10D). When spastin is overexpressed in the fibroblasts, there is a 20% greater loss of microtubule mass compared with when P60-katanin is overexpressed, and the individual remaining microtubules are generally shorter than the microtubules in the fibroblasts overexpressing P60-katanin (Figure 10E). When spastin is overexpressed in the fibroblasts expressing tau, the microtubule mass is diminished by about half (Figure 10F). Quantification of microtubule levels is shown in Figure 10G. Taken together, these results indicate that tau affords some protection of microtubules from each of the severing proteins, but that the protection is greater in the case of P60-katanin than spastin.
Figure 10.
Tau provides stronger protection against microtubule severing by P60-katanin than by spastin. (A–F) RFL-6 fibroblasts immunostained for microtubules. (A) Control cell transfected to express EGFP. (B) Cell transfected to express tau 4R. Tau expression causes the formation of dense bundles of microtubules. (C) Cell transfected to overexpress P60-katanin. Microtubule mass is diminished, with remaining microtubules displaying a variety of lengths. (D) Cell transfected to overexpress P60-katanin and express tau 4R. Microtubules in the tau-expressing cell show no indication of severing by overexpression of P60-katanin, and the microtubule mass is not reduced. (E) Cell transfected to overexpress spastin. Microtubules are severed into a large number of very short ones. (F) Cell transfected to express both spastin and tau. There is a reduction of microtubule mass, but several microtubule bundles together with some individual ones are preserved. (G) Quantitative analyses of microtubule mass in fibroblasts induced to overexpress P60-katanin or overexpress spastin together with tau (n > 50). Compared with the control, overexpression of P60-katanin reduces the level of microtubules by 52% (p < 0.01), whereas overexpression of spastin reduces the level by 70% (p < 0.01). Expression of tau increases the microtubule level by 19%. Compared with the microtubule levels of the cells expressing tau alone, the levels of cells expressing tau and overexpressing P60-katanin are not significantly different (p > 0.05); however, expression of both tau and spastin causes 53% loss of microtubule levels compared with the expression of tau alone (p < 0.01). Scale bar, 30 μm.
Depletion of tau from Hippocampal Neurons Enhances Axonal Branch Formation Whether or Not Spastin Is Also Depleted
In previous studies, we documented that P60-katanin's access to the microtubule lattice is restricted by the presence of tau molecules on the surface of the microtubule (Qiang et al., 2006). On this basis, we proposed a “tau-protection” model for the formation of axonal branches in which tau is locally detached from microtubules (presumably by its phosphorylation) at sites of impending branch formation; as a result, P60-katanin is able to avidly sever the microtubules into short pieces, specifically in this locale (Baas and Qiang, 2005; Baas et al., 2006). At the time we proposed this model, we had not yet performed any experiments on spastin, but our current studies (see above) suggest that if a tau-protection–based mechanism does exist for axonal branching, that it would probably pertain to P60-katanin more so than to spastin. To investigate this matter, we tested the prediction that axons of neurons depleted of tau with siRNA would display more branches than control axons. We also combined siRNA for tau with siRNA for spastin, to determine whether such an effect still occurs in the absence of spastin. Neurons were treated with control siRNA, or tau siRNA, or spastin siRNA, or the combination of both tau and spastin siRNA. After allowing 3 d for spastin and tau to be depleted (Qiang et al., 2006), the neurons were replated and allowed to develop processes for 24 h before being fixed.
In all cases, the majority of the neurons were in stage 3 after this experimental regime, except in the spastin-depleted group, in which there were greater numbers of neurons in the earlier stages (Figures 1C and 11A). Interestingly, in the double-knockdown (depletion of tau and spastin) group, the suppression of development induced by spastin siRNA was rescued (Figure 11A). This may be because tau depletion results in an increase in microtubule severing, whereas spastin depletion results in a decrease in microtubule severing. The number of minor processes was increased by 28% as a result of tau depletion (Figure 11B); which is reminiscent of the increases in minor processes observed when either P60-katanin or spastin is overexpressed (see Figure 2; see also Yu et al., 2005). Although spastin depletion within itself did not significantly lower the number of minor processes, the depletion of spastin did appear to prevent the depletion of tau from increasing the number of minor processes. In all experimental groups, axonal lengths were shorter than in controls (Figure 11C).
Figure 11.
Depletion of tau from hippocampal neurons increases the numbers of axonal branches by a mechanism that does not require spastin. Neurons were treated with either control siRNA, tau siRNA, spastin siRNA, or a combination of tau siRNA and spastin siRNA. (A) Pie graphs showing the ratio of hippocampal neurons in stage 1, stage 2, or stage 3 in each experimental group (n > 200). More neurons remained in stage 1 or stage 2 in spastin-depleted cultures than in control cultures. There is no significant difference between the tau-depleted group and the control group or between the spastin+tau-double-depleted group and the control group. (B) Quantitative analyses of the number of minor processes in stage 3 hippocampal neurons. Only in the tau siRNA treated group does the number increase by 28% (p < 0.001). Compared with control, the other two groups showed no significant difference (p > 0.05; n > 200). (C) Quantitative analyses of axonal lengths of stage 3 hippocampal neurons. Compared with control, axonal lengths decrease by 11% (p < 0.01), 12% (p < 0.01), and 16% (p < 0.01) in the spastin-depleted group, the tau-depleted group, and the spastin+tau-double depleted group, respectively (n > 200). (D) Quantitative analyses of number of primary axonal branches in stage 3 hippocampal neurons. For quantification of branches, we chose stage 3 neurons with axons 100–150 μm in length. Compared with the control group, there is a 40% (p < 0.01) decrease in the number of axonal branches in the spastin-depleted group and a 56% (p < 0.0001) increase of the number of axonal branches in the tau-depleted group. There is no significant difference between the control and spastin+tau-double-depleted groups (p > 0.05; n > 200).
With regard to branching, depletion of tau increased the number of branches by 56% (Figure 11D), consistent with the predictions of our tau-protection hypothesis. In neurons depleted of spastin, the number of branches was 40% lower than controls. In neurons depleted of both tau and spastin, the number of branches was indistinguishable for controls, indicating that depletion of tau is able to increase branching even, whereas the depletion of spastin is having the opposite effect on branching. These results indicate that the increase in axonal branching associated with tau depletion occurs whether or not spastin is present and hence support the conclusion that a tau-based mechanism for axonal branching functions mainly via P60-katanin.
DISCUSSION
In various studies, including this one, we have never observed an increase in axonal branch formation associated with experimental elevation of P60-katanin (Karabay et al., 2004; Yu et al., 2005; Qiang et al., 2006). However, here we found a very dramatic and consistent elevation in axonal branch formation associated with overexpression of spastin. Thus we would conclude that, in a direct comparison of the two severing proteins, the properties of spastin are more conducive to the formation of branches. In support of this conclusion, depletion of spastin from neurons results in a fairly modest reduction in axonal length, but a far greater reduction in the formation of axonal branches. In addition, spastin has a far greater capacity to concentrate at sites of branch formation (and growth cones) than does P60-katanin. We envision a model wherein neurons contain high levels of P60-katanin that are absolutely essential for the ongoing severing of microtubules needed for axonal growth and contain much lower levels of spastin designed to participate in specialized duties such as axonal branch formation. Even so, it is noteworthy that branches can still form in the absence of spastin, and this is consistent with previous observations on spastin-compromised animals (Sherwood et al., 2004; Trotta et al., 2004; Orso et al., 2005; Tarrade et al., 2006; Wood et al., 2006) and humans (McDermott et al., 2006; Salinas et al., 2007). Our data, presented here, suggest that there are two different modes by which axonal branches can form, one that utilizes spastin and the other than utilizes P60-katanin.
As noted earlier, the sequences of spastin and P60-katanin are very different in regions other than the AAA region responsible for severing microtubules (Errico et al., 2002; Roll-Mecak and Vale, 2005; White et al., 2007). Thus, it may be that the other domains of spastin enable it to influence branch formation by means other than the severing of microtubules. For example, it is known that branch formation requires actin filaments as well as microtubules (Dent and Kalil, 2001), and therefore it is conceivable that spastin influences the actin cytoskeleton in a way that P60-katanin does not. Indeed, the entire morphology of the neuron is markedly altered after spastin overexpression (i.e., greater numbers of filopodia and branches) in a manner not duplicated by P60-katanin overexpression. In the future, it will be of interest to ascertain whether spastin has domains that directly participate in pathways relevant to remodeling of the actin cytoskeleton.
For now, we are persuaded to believe that the dramatic morphological differences produced by the two severing proteins relate directly to their distinct patterns of microtubule severing. We have noticed in the past that overexpression of P60-katanin in neurons can often result in the appearance of very long microtubules that appear to be quite resistant to severing (Yu et al., 2005), and the same observation was made here. By contrast, spastin overexpression appears to produce a far more consistent “even chopping” of the microtubules into small pieces. In other words, we have not detected any evidence for a population of microtubules that are strongly resistant to severing by spastin. Given these different severing patterns, the expectation would be that spastin is capable of producing high concentrations of short microtubules, whereas P60-katanin's properties are more suited for generating mixtures of long and short microtubules. Concentrations of plus ends of microtubules would be expected to recruit a variety of +tips which could also interact with structures within the actin-based cortical regions of the axon (Kornack and Giger, 2005). Thus, the enhanced formation of filopodia and the remodeling of actin required for branches to form could relate directly to the manner by which spastin severs microtubules.
What accounts for the different microtubule-severing patterns of spastin and P60-katanin? Studies on P60-katanin suggest that its severing properties are regulated by phosphorylation, but probably not phosphorylation of P60-katanin itself (Vale, 1991; McNally et al., 2002; Baas and Qiang, 2005). Rather, it appears that in nonneuronal cells, the phosphorylation state of MAP4 is crucial for regulating the degree to which P60-katanin can sever microtubules (McNally et al., 2002). The binding of MAP4 to the microtubule apparently reduces the accessibility of the microtubule to P60-katanin. Phosphorylation of MAP4 causes it to dissociate from the microtubules, thereby permitting more robust severing by P60-katanin. MAP4 is not expressed in CNS neurons, but recent studies from our laboratory indicate that a comparable role is played by tau in the axon (Qiang et al., 2006). The fact that tau and MAP4 are strong protectors against P60-katanin provides an appealing explanation for the severing pattern produced by P60-katanin in fibroblasts and neurons. P60-katanin would preferentially sever microtubules that are less rich in these protective proteins, which would then cause progressively more depolymerization of these microtubules. The molecules of tau or MAP4 that had been associated with these microtubules would then be available to bind to the microtubules that were already richer in these proteins, thereby rendering them even more resistant to severing and enabling them to achieve even greater lengths. In this manner, the properties of P60-katanin would promote a mixture of short and long microtubules.
Our current studies suggest that tau is a far less influential factor in the regulation of spastin's severing properties. The microtubules in the axon, rich in tau, show no greater resistance to spastin overexpression than the tau-deficient microtubules located in other compartments of the neuron. Moreover, axons depleted of tau undergo markedly more branching, and this effect is independent of whether or not spastin is present. These results are consistent with an alternative mechanism for axonal branching based on the properties of P60-katanin. In this mechanism, tau is locally detached from the microtubules at a site of impending branch formation. As a result, P60-katanin can produce the localized chopping of microtubules needed for the branch to form. This idea, which we have discussed in the past (Baas and Qiang, 2005; Baas et al., 2006), does not require a local concentration of P60-katanin, but rather a focal increase in the sensitivity of the microtubules to P60-katanin. This process would presumably be regulated by kinases and phosphatases that phosphorylate or dephosphorylate tau at motifs required for it to bind microtubules. Although our results suggest that tau is probably the main player in such a scenario, there may be other microtubule-associated proteins in the axon that conspire in regulating the sensitivity of the microtubules to P60-katanin.
In conclusion, we propose that there are two modes by which microtubule severing is orchestrated during axonal branch formation, one based on the local concentration of spastin at branch sites and the other based on local detachment from microtubules of molecules such as tau that regulate the severing properties of P60-katanin (see Figure 12). Each of these modes is presumably under the regulation of signaling cascades that regulate such events as the phosphorylation of tau, the attachment or detachment of other protective proteins, and the localization of spastin to sites of impending branch formation. The formation of a particular branch could utilize one mode or the other, or some combination of the two modes, working in tandem.
Figure 12.
Schematic illustration of two modes by which microtubule severing may be regulated at sites of impending axonal branch formation. Both A and B show the axon undergoing the formation of branch. (A) In the “katanin mode,” microtubule severing is enhanced at sites of branch formation by local detachment from the microtubules of proteins such as tau that would otherwise “protect” the microtubule from being accessed by P60-katanin. In the case of tau, which we posit to be the principal protective molecule in the axon, the detachment is induced by local phosphorylation of tau at a site that causes it to lose its attachment to the microtubule. (B) In the “spastin mode,” microtubule severing is enhanced at sites of branch formation by the concentration of more spastin molecules specifically at the site where the branch is forming. This mode is independent of whether or not molecules such as tau remain attached to the microtubule. Each of these modes is presumably under the regulation of signaling cascades. The two modes, shown here separately, are not mutually exclusive and may work in tandem during branch formation.
ACKNOWLEDGMENTS
We thank Kenneth Kosik (University of California, Santa Barbara) for providing a tau construct that we used for some of the studies reported here and Elena Rugarli (National Neurological Institute, Milan, Italy) for providing a human spastin construct that we used for preliminary studies. This work was funded by grants to P.W.B. from the National Institutes of Health, the Spastic Paraplegia Foundation, the Alzheimer's Association, the Department of Defense, and the Craig H. Neilsen Foundation, and by a grant to W.Y. from the Craig H. Neilsen Foundation.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/10.1091/mbc.E07-10-1015) on January 30, 2008.
REFERENCES
- Ahmad F. J., Yu W., McNally F. J., Baas P. W. An essential role for katanin in severing microtubules in the neuron. J. Cell Biol. 1999;145:305–315. doi: 10.1083/jcb.145.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas P. W., Qiang L. Neuronal microtubules: when the MAP is the roadblock. Trends Cell Biol. 2005;15:183–187. doi: 10.1016/j.tcb.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Baas P. W., Karabay A., Qiang L. Microtubules cut and run. Trends Cell Biol. 2005;15:518–524. doi: 10.1016/j.tcb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Baas P. W., Vidya, Nadar C., Myers K. A. Axonal transport of microtubules: the long and short of it. Traffic. 2006;7:490–498. doi: 10.1111/j.1600-0854.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- Buster D., McNally K., McNally F. J. Katanin inhibition prevents the redistribution of gamma-tubulin at mitosis. J. Cell Sci. 2002;115:1083–1092. doi: 10.1242/jcs.115.5.1083. [DOI] [PubMed] [Google Scholar]
- Claudiani P., Riano E., Errico A., Andolfi G., Rugarli E. I. Spastin subcellular localization is regulated through usage of different translation start sites and active export from the nucleus. Exp. Cell Res. 2005;309:358–369. doi: 10.1016/j.yexcr.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Dent E. W., Kalil K. Axon branching requires interactions between dynamic microtubules and actin filaments. J. Neurosci. 2001;21:9757–9769. doi: 10.1523/JNEUROSCI.21-24-09757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent E. W., Callaway J. L., Szebenyi G., Baas P. W., Kalil K. Reorganization and movement of microtubules in axonal growth cones and developing interstitial branches. J. Neurosci. 1999;19:8894–8908. doi: 10.1523/JNEUROSCI.19-20-08894.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti C. G., Sullivan C. A., Banker G. A. The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errico A., Ballabio A., Rugarli E. I. Spastin, the protein mutated in autosomal dominant hereditary spastic paraplegia, is involved in microtubule dynamics. Hum. Mol. Genet. 2002;11:153–163. doi: 10.1093/hmg/11.2.153. [DOI] [PubMed] [Google Scholar]
- Errico A., Claudiani P., D'Addio. M., Rugarli E. I. Spastin interacts with the centrosomal protein NA14, and is enriched in the spindle pole, the midbody and the distal axon. Hum. Mol. Genet. 2004;13:2121–2132. doi: 10.1093/hmg/ddh223. [DOI] [PubMed] [Google Scholar]
- Evans K. J., Gomes E. R., Reisenweber S. M., Gundersen G. G., Lauring B. P. Linking axonal degeneration to microtubule remodeling by Spastin-mediated microtubule severing. J. Cell Biol. 2005;168:599–606. doi: 10.1083/jcb.200409058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabay A., Yu W., Solowska J. M., Baird D. H., Baas P. W. Axonal growth is sensitive to the levels of katanin, a protein that severs microtubules. J. Neurosci. 2004;24:5778–5788. doi: 10.1523/JNEUROSCI.1382-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornack D. R., Giger R. J. Probing microtubule +TIPs: regulation of axon branching. Curr. Opin. Neurobiol. 2005;15:58–66. doi: 10.1016/j.conb.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Ma D. L., Chia S. C., Tang Y. C., Chang M. L., Probst A., Burgunder J. M., Tang F. R. Spastin in the human and mouse central nervous system with special reference to its expression in the hippocampus of mouse pilocarpine model of status epilepticus and temporal lobe epilepsy. Neurochem. Int. 2006;49:651–664. doi: 10.1016/j.neuint.2006.05.008. [DOI] [PubMed] [Google Scholar]
- McDermott C. J., et al. Clinical features of hereditary spastic paraplegia due to spastin mutation. Neurology. 2006;67:45–51. doi: 10.1212/01.wnl.0000223315.62404.00. [DOI] [PubMed] [Google Scholar]
- McNally K. P., Buster D., McNally F. J. Katanin-mediated microtubule severing can be regulated by multiple mechanisms. Cell Motil. Cytoskelet. 2002;53:337–349. doi: 10.1002/cm.10080. [DOI] [PubMed] [Google Scholar]
- Orso G., Martinuzzi A., Rossetto M. G., Sartori E., Feany M., Daga A. Disease-related phenotypes in a Drosophila model of hereditary spastic paraplegia are ameliorated by treatment with vinblastine. J. Clin. Invest. 2005;115:3026–3034. doi: 10.1172/JCI24694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang L., Yu W., Andreadis A., Luo M., Baas P. W. Tau protects microtubules in the axon from severing by katanin. J. Neurosci. 2006;26:3120–3129. doi: 10.1523/JNEUROSCI.5392-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll-Mecak A., Vale R. D. The Drosophila homologue of the hereditary spastic paraplegia protein, spastin, severs and disassembles microtubules. Curr. Biol. 2005;15:650–655. doi: 10.1016/j.cub.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Roll-Mecak A., Vale R. D. Making more microtubules by severing: a common theme of noncentrosomal microtubule arrays? J. Cell Biol. 2006;175:849–851. doi: 10.1083/jcb.200611149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas S., Carazo-Salas R. E., Proukakis C., Schiavo G., Warner T. T. Spastin and microtubules: functions in health and disease. J. Neurosci. Res. 2007;85:2778–2782. doi: 10.1002/jnr.21238. [DOI] [PubMed] [Google Scholar]
- Salinas S., Carazo-Salas R. E., Proukakis C., Cooper J. M., Weston A. E., Schiavo G., Warner T. T. Human spastin has multiple microtubule-related functions. J. Neurochem. 2005;95:1411–1420. doi: 10.1111/j.1471-4159.2005.03472.x. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E. F., Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sherwood N. T., Sun Q., Xue M., Zhang B., Zinn K. Drosophila spastin regulates synaptic microtubule networks and is required for normal motor function. PLoS. Biol. 2004;2:e429. doi: 10.1371/journal.pbio.0020429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson I. K., Kloos M. T., Jacon A., Gallione C., Horton A. C., Pericak-Vance M. A., Ehlers M. D., Marchuk D. A. Subcellular localization of spastin: implications for the pathogenesis of hereditary spastic paraplegia. Neurogenetics. 2005;6:135–141. doi: 10.1007/s10048-005-0219-2. [DOI] [PubMed] [Google Scholar]
- Tarrade A., et al. A mutation of spastin is responsible for swellings and impairment of transport in a region of axon characterized by changes in microtubule composition. Hum. Mol. Genet. 2006;15:3544–3558. doi: 10.1093/hmg/ddl431. [DOI] [PubMed] [Google Scholar]
- Trotta N., Orso G., Rossetto M. G., Daga A., Broadie K. The hereditary spastic paraplegia gene, spastin, regulates microtubule stability to modulate synaptic structure and function. Curr. Biol. 2004;14:1135–1147. doi: 10.1016/j.cub.2004.06.058. [DOI] [PubMed] [Google Scholar]
- Vale R. D. Severing of stable microtubules by a mitotically activated protein in Xenopus egg extracts. Cell. 1991;64:827–839. doi: 10.1016/0092-8674(91)90511-v. [DOI] [PubMed] [Google Scholar]
- White S. R., Evans K. J., Lary J., Cole J. L., Lauring B. Recognition of C-terminal amino acids in tubulin by pore loops in Spastin is important for microtubule severing. J. Cell Biol. 2007;176:995–1005. doi: 10.1083/jcb.200610072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. D., Landers J. A., Bingley M., McDermott C. J., Thomas-McArthur V., Gleadall L. J., Shaw P. J., Cunliffe V. T. The microtubule-severing protein Spastin is essential for axon outgrowth in the zebrafish embryo. Hum. Mol. Genet. 2006;15:2763–2771. doi: 10.1093/hmg/ddl212. [DOI] [PubMed] [Google Scholar]
- Yu W., Baas P. W. Changes in microtubule number and length during axon differentiation. J. Neurosci. 1994;14:2818–2829. doi: 10.1523/JNEUROSCI.14-05-02818.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Ahmad F. J., Baas P. W. Microtubule fragmentation and partitioning in the axon during collateral branch formation. J. Neurosci. 1994;14:5872–5884. doi: 10.1523/JNEUROSCI.14-10-05872.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Solowska J. M., Qiang L., Karabay A., Baird D., Baas P. W. Regulation of microtubule severing by katanin subunits during neuronal development. J. Neurosci. 2005;25:5573–5583. doi: 10.1523/JNEUROSCI.0834-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang. D., Rogers G. C., Buster D. W., Sharp D. J. Three microtubule severing enzymes contribute to the “Pacman-flux” machinery that moves chromosomes. J. Cell Biol. 2007;17:231–242. doi: 10.1083/jcb.200612011. [DOI] [PMC free article] [PubMed] [Google Scholar]