Abstract
Na/H exchange regulatory factor 1 (NHERF1) is a scaffolding protein that regulates signaling and trafficking of several G protein-coupled receptors (GPCRs), including the parathyroid hormone receptor (PTH1R). GPCRs activate extracellular signal-regulated kinase (ERK)1/2 through different mechanisms. Here, we characterized NHERF1 regulation of PTH1R-stimulated ERK1/2. Parathyroid hormone (PTH) stimulated ERK1/2 phosphorylation by a protein kinase A (PKA)-dependent, but protein kinase C-, cyclic adenosine 5′-monophosphate-, and Rap1-independent pathway in Chinese hamster ovary cells stably transfected with the PTH1R and engineered to express NHERF1 under the control of tetracycline. NHERF1 blocked PTH-induced ERK1/2 phosphorylation downstream of PKA. This suggested that NHERF1 inhibitory effects on ERK1/2 occur at a postreceptor locus. Forskolin activated ERK1/2, and this effect was blocked by NHERF1. NHERF1 interacted with AKT and inhibited ERK1/2 activation by decreasing the stimulatory effect of 14-3-3 binding to B-Raf, while increasing the inhibitory influence of AKT negative regulation on ERK1/2 activation. This novel regulatory mechanism provides a new model by which cytoplasmic adapter proteins modulate ERK1/2 activation through a receptor-independent mechanism involving B-Raf.
INTRODUCTION
Mounting evidence indicates that ERK1/2 activity stimulated by G protein-coupled receptors proceeds in a cell-specific and G protein type-dependent manner (Luttrell, 2003). The type I parathyroid hormone (PTH), PTH/PTH-related peptide receptor (PTH1R), a member of class B of seven-transmembrane G protein-coupled receptors (GPCRs) (Horn et al., 2003) mediates PTH and PTHrP actions on extracellular calcium homeostasis and bone turnover. Although signaling through adenylyl cyclase and phospholipase C (PLC) are the best-characterized pathways, the PTH1R also signals its actions through phospholipase D and mitogen-activated protein (MAP) kinases (MAPKs) (Friedman et al., 1999; Lederer et al., 2000; Fujita et al., 2002; Radeff et al., 2004; Singh et al., 2005; Syme et al., 2005; Mahon et al., 2006; Sneddon and Friedman, 2007; Sneddon et al., 2007).
MAPKs are protein serine and threonine kinases that play important roles in cell growth, differentiation, survival, and in many aspects of bone turnover and calcium balance (Ishizuya et al., 1997; Sneddon et al., 2000; Fujita et al., 2002; Ahmed et al., 2003; Schindeler and Little, 2006). Extracellular signal-regulated kinases (ERKs) 1 and 2, c-Jun-NH2-terminal kinase, and p38 kinase lie at the end of parallel MAPK cascades (Cobb, 1999). G protein-coupled receptors activate MAPK through three distinct pathways, including transactivation of the epidermal growth factor (EGF) receptor (EGFR); GPCR internalization; and G protein activation.
The 50-kDa ezrin-binding protein-50, Na/H exchange regulatory factor 1 (NHERF1) is a cytoplasmic adaptor protein (Bretscher et al., 2000; Shenolikar et al., 2004). NHERF1 recruits various cellular receptors, ion transporters, and other proteins to the plasma membrane of epithelia and other cells (Voltz et al., 2001; Bretscher et al., 2002; Mahon et al., 2002). NHERF1 contains tandem postsynaptic density 95/disc-large/zona occludens (PDZ) domains and a merlin-ezrin-radixin-moesin (MERM) domain. The PDZ1 domain is required for its interaction with the carboxy terminus of the PTH1R (Mahon and Segre, 2004). The MERM domain binds to respective actin-associated MERM proteins (Bretscher et al., 2000). NHERF1 tethers the PTH1R to the actin cytoskeleton through the MERM domain.
NHERF1 is involved in growth factor signaling. For example, NHERF1 binds directly to the platelet-derived growth factor (PDGF) receptor (PDGFR) (Takahashi et al., 2006), promotes signaling, (Maudsley et al., 2000), and regulates cell motility (Theisen et al., 2007). The NHERF1-related protein E3KARP (NHERF2) potentiates lysophosphatidic acid-induced ERK activation (Oh et al., 2004). Based on these findings, we theorized that NHERF1 modulates PTH-sensitive ERK phosphorylation. Although this hypothesis was borne out, we unexpectedly found that NHERF1 exerts its regulatory effect at a postreceptor site. NHERF1 interacts directly with AKT and inhibits ERK1/2 activation by converging effects on B-Raf that entail increasing AKT negative regulation of the regulatory domain and displacing 14-3-3 binding within the catalytic domain, thereby reducing the stimulatory action of B-Raf.
MATERIALS AND METHODS
Anti-p44/p42 MAP kinase (ERK1/2) and phospho-p44/42 MAP kinase (pERK1/2) (Thr202/Tyr204) polyclonal antibodies, phospho-AKT(Ser473), total AKT polyclonal antibodies, phospho-mitogen-activated protein kinase kinase (MEK)1/2 (Ser217/221), total MEK1/2 antibodies, and p44/42 MAP kinase assay kit were purchased from Cell Signaling Technology (Beverly, MA). HA.11 ascites monoclonal antibody (mAb) and HA.11 monoclonal affinity matrix were obtained from Covance (Berkeley, CA). NHERF1 polyclonal antibody was purchased from Affinity BioReagents (Golden, CO). NHERF1 mAb was from BD Biosciences (San Jose, CA). 14-3-3 β polyclonal antibody and c-Myc(9E10) mAb were from Santa Cruz Biotechnology (Santa Cruz, CA). Horseradish peroxidase-conjugated goat anti-rabbit antibody was from Pierce Chemical (Rockford, IL). Horseradish peroxidase-conjugated sheep anti-mouse antibody was from GE Healthcare (Little Chalfont, Buckinghamshire, United Kingdom). Tetracycline hydrochloride was purchased from American Bioanalytical (Natick, MA). Lipofectamine 2000, zeocin, blasticidin, Geneticin (G418), Alexa Fluor 546-tagged goat-anti-rabbit second antibody, Alexa Fluor 488-tagged donkey anti-mouse second antibody, and rec-protein G-Sepharose 4B conjugate were obtained from Invitrogen (Carlsbad, CA). FuGENE6 was purchased from Roche Applied Science (Indianapolis, IN). H89, AG1478, AG1295, protease inhibitor cocktail Set I, phosphatase inhibitor cocktail Set II, and anti-pRaf1 (Ser621) were from Calbiochem (San Diego, CA). Human [Nor8,18,Tyr34]PTH(1-34) was purchased from Bachem California (Torrance, CA). All other reagents were from Sigma-Aldrich (St. Louis, MO).
Construction of pcDNA3.1(+)-HA-PTH1R, pcDNA4/TO-NHERF1, pcDNA3.1(+)-HA-PTH1R(M593A), pcDNA3.1(+)-HA-PTH1R-480stop), pcDNA3-HA-B-Raf(S728A), and pcDNA3-HA-B-Raf(S364A,S728A)
Hemagglutinin (HA)-tagged human PTH1R (Dr. Thomas J. Gardella, Massachusetts General Hospital, Boston, MA), previously cloned into pcDNA1, was cut by HindIII and XbaI and subcloned into the mammalian expression vector pcDNA3.1(+), which has a selectable G418 marker.
His-tagged rabbit NHERF1 in pcDNA3.1(+)/Hygro vector was provided by Dr. E. J. Weinman (University of Maryland). The plasmid was cut by Kpn1 and XhoI and a 1.1-kb fragment without epitope was subcloned into the pcDNA4/TO vector, which has two tetracycline operator sequences between the TATA box of the cytomegalovirus promoter and the transcriptional start site.
Mutation of the terminal amino acid of HA-PTH1R from methionine to alanine (M593A) was performed by polymerase chain reaction (PCR) by using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA).
The truncated pcDNA3.1(+)-HA-PTH1R-480stop was prepared in the following manner. The human PTH1R coding for amino acid 1-480 was PCR amplified using the forward primer with HindIII restriction site (GCG TTT AAA CTT AAG CTT GGT ACC GAG CTC), and the reverse primer with XbaI restriction site (GCG GCG TCT AGA TCA TGC CAG TGT CCA GCG). The purified PCR fragment was cut by HindIII and XbaI and subcloned into the pcDNA3.1(+).
Single [(S728A) (S364A)] or double (S364A,S728A) mutations of HA-B-Raf (provided by Dr. D. Altschuler, University of Pittsburgh) were generated by PCR by using the QuikChange kit.
The accuracy of the plasmids was confirmed by sequencing (ABI Prism 377; Applied Biosystems, Foster City, CA) and subsequent sequence alignment (NCBI BLAST) with human PTH1R, rabbit NHERF1, and human B-Raf (GenBank accession nos. L04308, U19815, and M95712, respectively) to ensure the fidelity of the respective constructs.
Stable Expression of pcDNA6-TR, pcDNA4/TO-NHERF1, and HA-PTH1R in Chinese Hamster Ovary (CHO) Cells
CHO-N10, CHO-N10-R3, and CHO-EV6-R4 cells were generated as described previously (Wang et al., 2007). Briefly, T-REx-CHO cells (Invitrogen), transfected with pcDNA6-TR and stably expressing the tetracycline (Tet) repressor protein, were cultured in Ham's F-12 medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10 μg/ml blasticidin in a humidified atmosphere consisting of 5% CO2 and 95% air at 37°C. The cells were then transfected with pcDNA4/TO-NHERF1 or the vector pcDNA/TO (control) by using Lipofectamine 2000, and they were selected with 0.4 mg/ml zeocin. Two cell lines were obtained. The first line, CHO-N10 cells, expresses NHERF1 when Tet is added to the cell culture medium. The second line, CHO-EV6, is a control cell line, in which NHERF1 cannot be induced. These two cell lines were then transfected with pcDNA3.1(+)-HA-PTH1R with Lipofectamine 2000 and selected using 0.75 mg/ml G418. Finally, they were screened by immunoblot to choose two cell lines from all those that grew. The two cell lines (CHO-N10-R3 cells and CHO-EV6-R4 cells) were generated, respectively, simultaneously expressing the PTH1R.
Inducible NHERF1 Expression in CHO-N10-R3 Cells
CHO cells were selected for the present work because there is negligible NHERF1 (Figure 1B; Li et al., 2002) and PTH1R expression or PTH-stimulated cAMP accumulation (data not shown). Graded concentrations of Tet (8–50 ng/ml) were added to the cell culture medium for 48 h. Tet caused concentration-dependent increases of NHERF1 expression (Wheeler et al., 2007). Binding studies with [125I][Nle8,18,Tyr34]PTH(1-34)NH2 revealed that CHO-N10-R3 cells express ∼6.5 × 105 PTH1R/cell, with an average Kd of 14 nM. NHERF1 expression did not alter PTH1R expression (see below; Figure 1B). Tet-induced NHERF1 expression in CHO-N10-R3 was comparable with that of CHO-N10 cells (data not shown).
Figure 1.
PTH-stimulated ERK1/2 phosphorylation and inhibition by NHERF1. (A) Representative time course of PTH-stimulated ERK1/2 phosphorylation in CHO-N10-R3 cells (top). Cells were treated with 10 nM PTH(1-34) for the indicated time. Data from three independent time course and concentration dependence experiments were quantified, normalized to total ERK2, and expressed as the -fold change of basal pERK2. Data are summarized as mean ± SEM (bottom left). At the 10-min time point, PTH (10−11–10−6 M) induced concentration-dependent increases of ERK1/2 phosphorylation (bottom right). (B) NHERF1 inhibition of PTH-stimulated ERK1/2 at graded PTH concentrations. CHO-N10-R3 cells were pretreated where indicated with 50 ng/ml Tet for 48 h. The cells were then serum starved for 3–5 h and incubated at 37°C for 10-min with the indicated concentration of PTH(1-34). Levels of phosphorylated and total ERK1/2, NHERF1 and HA-PTH1R in whole-cell lysates were determined by immunoblotting as described in Materials and Methods. (C) Graded induction of NHERF1 expression causes progressive inhibition of PTH-stimulated ERK stimulation. CHO-N10-R3 cells were pretreated with the indicated concentration of Tet. Cells were then serum starved for 3–5 h, and then they were incubated at 37°C for 10 min with 10 nM PTH(1-34). The figure is representative of three or four independent experiments performed with similar results.
Transient Expression of HA-PTH1R, HA-P1R(M593A), HA-PTH1R-480stop, HA-B-Raf, HA-B-Raf(S364A), HA-B-Raf(S728A), HA-B-Raf(S364A,S728A), HA-B-Raf(T428A,S439A), HA-B-Raf(S364A,T428A,S439A), Flag-B-Raf, and myc-AKT
CHO-N10 cells or CHO-N10-R3 cells were transiently transfected with vector control or the indicated plasmid with FuGENE6. Cells were used for experiments ∼48 h after transfection.
Coimmunoprecipitation
Analysis of the interactions of 14-3-3 with B-Raf or NHERF1 with AKT was performed essentially as described previously (Sneddon et al., 2003). Briefly, six-well plates of CHO-N10-R3 cells were transiently transfected with pcDNA3.1, Flag-B-Raf (Dr. Kathrin Muegge, National Cancer Institute) or myc-AKT (Dr. Daniel Altschuler, University of Pittsburgh). Tet (50 ng/ml) was added as indicated. About 48 h later, the cells were lysed with NP-40 lysis buffer (50 mM Tris, 150 mM NaCl, 5 mM EDTA, and 0.5% NP-40) supplemented with protease inhibitor cocktail I, and they were incubated for 15 min on ice. Solubilized materials were incubated with anti-Flag M2 affinity gel (Sigma-Aldrich), overnight at 4°C or myc mAb for 1 h at 4°C, and then rec-protein G-Sepharose 4B conjugate was added to each sample and incubated overnight at 4°C. Immunoprecipitated proteins were eluted by the addition of SDS sample buffer, and then they were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) and immunoblotted using polyclonal anti-14-3-3β, anti-Flag, anti-NHERF1 polyclonal antibodies or c-myc mAb.
Immunoblot Analysis
CHO-N10-R3 or CHO-N10 cells were seeded on six-well plates. Tet was added as indicated, and selected plasmids were transiently transfected the next day. Forty-eight later, the cells were serum starved for 3–5 h by using DMEM (15017-CV; Mediatech, Herndon, VA). Cells were incubated at 37°C with PTH(1-34) for the noted times. The cells were lysed in 250 μl/well of 0.5% NP-40 lysis buffer supplemented with protease inhibitor cocktail set I and phosphatase inhibitor cocktail set II, and then they were incubated for 15 min on ice. The cell lysates were then drawn four times through a 21-gauge needle attached to a 1-ml syringe, and then they were placed on ice for an additional 15 min. Lysates were centrifuged at 13,000 rpm at 4°C for 20 min. The supernatants were added to an equal amount of 2× Laemmli SDS-PAGE loading buffer (Bio-Rad, Hercules, CA) containing 5% 2-mercaptoethanol. They were then heated at 95°C for 3 min, resolved on 10% SDS-PAGE gels, and transferred to Immobilon-P membranes (Millipore, Billerica, MA) by using the semidry method (Bio-Rad). Membranes were blocked overnight at 4°C with 5% nonfat dried milk in Tris-buffered saline plus Tween 20 (TBST), and then they were incubated with primary antibodies for 2 h at room temperature. The membranes were then washed and incubated with goat anti-rabbit IgG or sheep anti-mouse IgG conjugated to horseradish peroxidase at 1:5000 dilution for 1 h at room temperature. Protein bands were visualized with a luminol-based enhanced chemiluminescence substrate.
p44/42 MAP Kinase Assay
Cells were incubated with Tet for 48 h, where specified, serum starved for 3–5 h, and pretreated with H89 for 10 min followed by a 10-min treatment with PTH or forskolin. Active ERK1/2 in lysates was immunoprecipitated with immobilized phospho-p44/42 MAP kinase (Thr202/Tyr204) antibody and a subsequent in vitro kinase assay using an Elk-1 substrate. Phosphorylated Elk-1 was detected by immunoblotting with a phospho-Elk-1 (Ser338) antibody (Cell Signaling Technology, Danvers, MA).
Cell Fractionation
Cell fractionation was performed by differential centrifugation at 4°C as described previously (Vilardaga et al., 2002). Briefly, CHO-N10-R3 cells were seeded on 10-cm dishes, and then they were incubated with Tet for 48 h. The cells were serum starved for 3–5 h, and then they were incubated 37°C for 10 min with PTH. Cells were detached with cell scraper, pelleted by centrifugation (1000 × g; 10 min), and lysed by sonication in phosphate-buffered saline (PBS) containing protease inhibitor cocktail Set I and phosphatase inhibitor cocktail Set II. The lysates were centrifuged at 1000 × g for 10 min to remove unbroken cells, including large cell debris and some nuclei. The supernatant was further centrifuged at 100,000 × g for 30 min. The resulting supernatant (S100) is the cytosolic fraction, and the pellet (P100) contains the plasma membranes and microsomes. The resulting pellet was solubilized in radioimmunoprecipitation assay buffer (1% Nonidet P-40, 0.5% Na-deoxycholate, 0.1% SDS, 50 mM Tris, pH 7.4, and 150 mM NaCl) supplemented with protease inhibitor cocktail set I and phosphatase inhibitor cocktail set II. Equal amounts of cytosolic and soluble membrane proteins were resolved on 10% SDS-polyacrylamide gels as described above for immunoblot analysis.
Fluorescent Staining
CHO-N10-R3 cells were grown on glass coverslips, and then they were incubated with Tet as indicated. The cells were serum starved for 3–5 h, treated with PTH for 10 min, rinsed in PBS, fixed on 4% paraformaldehyde for 20 min, and then permeabilized with 0.2% Triton X-100 for 15 min at room temperature. Blocking was performed by incubating the cells for 1 h at room temperature in 5% goat serum in PBS. Anti-AKT rabbit polyclonal antibody diluted 1:500 and anti-NHERF1 mouse mAb diluted (1:200) in blocking buffer were applied to the specimens for 1 h at room temperature. Alexa Fluor 546-tagged goat-anti-rabbit second antibody diluted 1:500 and Alexa Fluor 488-tagged donkey anti-mouse second antibody diluted 1:500 were applied under the same conditions as the primary antibody. Coverslips were mounted for immunofluorescence microscopy and analyzed using a Leica confocal microscope with a 63× oil immersion objective.
RESULTS
NHERF1 Inhibition of PTH-stimulated ERK1/2 Phosphorylation
ERK1/2 phosphorylation in CHO cells transfected with PTH1R exhibited time and concentration dependence. Maximal stimulation was achieved at 10 min (Figure 1A) and returned to baseline by 60 min. At the 10-min time point, PTH elicited concentration-dependent increases of ERK1/2 over the range of 10−11 M to 10−6 M (Figure 1A). Induced NHERF1 expression inhibited ERK1/2 phosphorylation at PTH concentrations ranging from 10−10 to 10−8 M (Figure 1B). Graded induction of NHERF1 progressively suppressed ERK1/2 phosphorylation at 10−8 M PTH (Figure 1C). Maximal inhibition of PTH-stimulated ERK1/2 phosphorylation was 62% at 50 ng/ml Tet. Tet itself did not affect ERK1/2 (Supplemental Material).
NHERF1 Inhibits PTH-Stimulated ERK1/2 Activity by a Postreceptor Mechanism
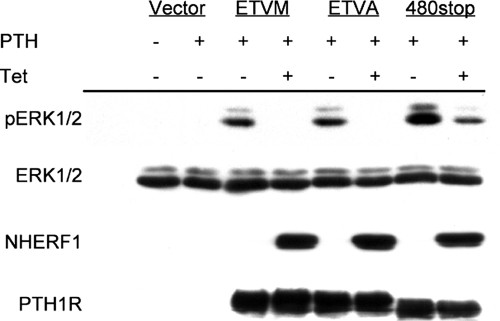
An intact PTH1R carboxy-terminal PDZ recognition motif (ETVM) is required for binding to NHERF1 (Sneddon et al., 2003) and for its effects on receptor signaling and internalization (Mahon et al., 2002; Wang et al., 2007). Therefore, we presumed that inhibition of PTH-induced ERK phosphorylation involves NHERF1 interaction with the PTH1R through its PDZ recognition domain. CHO-N10 cells were transiently transfected with wild-type receptor (PTH1R-ETVM), with a receptor bearing a mutated PDZ recognition motif (PTH1R-ETVA), or with a carboxy-terminally truncated receptor (PTH1R-480stop) lacking most of its intracellular tail. PTH stimulated ERK1/2 phosphorylation in the cells transfected with wild-type as well as mutant and truncated PTH1R (Figure 2). ERK1/2 phosphorylation by PTH1R-480stop was greater than that of wild-type PTH1R. Unexpectedly, however, the inhibitory effect of NHERF1 was as great in cells expressing the PDZ-mutant PTH1R as in cells bearing the wild-type receptor (Figure 2). Moreover, induction of NHERF1 expression did not fully block PTH dependent-ERK1/2 phosphorylation in cells bearing the 480-stop PTH1R mutant. These results suggest that the carboxy-terminal receptor tail is not required for ERK activation and possesses domains that exert an inhibitory influence on ERK1/2 activity. These findings implied that NHERF1 inhibition of PTH-stimulated ERK1/2 phosphorylation either involved PTH1R transactivation or internalization, or occurred at a postreceptor site.
Figure 2.
NHERF1 inhibition of ERK1/2 does not require the PTH1R PDZ recognition domain. CHO-N10 cells were pretreated with 50 ng/ml Tet as indicated, and then they were transiently transfected with 1.0 μg of DNA/well of empty vector (pcDNA3.1+), wild-type PTH1R(ETVM), mutant PTH1R (ETVA), or truncated PTH1R(480stop). After 48 h, the cells were serum starved for 3–5 h, and then they were treated for 10 min with 1 nM PTH. The extent of phosphorylated and total ERK1/2, NHERF1, and PTH1R was determined as described in Materials and Methods. The results represent one of three independent experiments performed with similar results.
NHERF1 Inhibition of PTH-stimulated ERK1/2 Activity Lies Downstream of Protein Kinase A (PKA)
NHERF1 interacts with both PDGF and EGF receptors (Maudsley et al., 2000; Lazar et al., 2004; Theisen et al., 2007). Thus, it was plausible that PTH-stimulated ERK1/2 activation was mediated by transactivation of the EGFR or PDGFR and that NHERF1 interfered with this process. However, neither AG1295 (10 μM 40 min), a PDGF receptor inhibitor, nor AG1478 (500 nM; 15 min), an EGF receptor inhibitor, decreased PTH-stimulated ERK1/2 activity (Figure 3). Gi/o activation was likewise excluded because pretreatment with pertussis toxin (PTX) did not affect ERK1/2 phosphorylation in this cell model (Figure 3).
Figure 3.
NHERF1 inhibition of ERK1/2 activation is not mediated by transactivation of inhibition of Gi/o. CHO-N10-R3 cells were pretreated with 50 ng/ml Tet for 48 h or with 100 ng/ml PTX for 16 h. The cells were then serum-starved for 3–5 h and treated with the PDGF receptor inhibitor AG1295 (10 μM) for 40 min or EGF receptor inhibitor AG1478 (500 nM) for 15 min before addition of 1 nM PTH for 10 min. The extent of phosphorylated and total ERK1/2 and NHERF1 in whole-cell lysates was determined as described in Materials and Methods. The results represent one of three independent experiments performed with similar results.
PTH1R endocytosis, which is inhibited by NHERF1 (Sneddon et al., 2003; Wang et al., 2007), also is capable of stimulating ERK1/2 activation (Syme et al., 2005). However, neither the clathrin binding domain of β-arrestin (β-arrestin[319-418]), nor dominant-negative dynamin (K44A dynamin) altered PTH-stimulated ERK1/2 activation (data not shown). Together, these results excluded an effect of NHERF1 on PTH1R transactivation, internalization, or the inhibitory G protein, Gi, in mediating the inhibitory effects of NHERF1 on ERK1/2 phosphorylation.
PTH-stimulated ERK1/2 activation in CHO cells transfected with PTHR proceeds through a cAMP-mediated pathway that is independent of Ras (Verheijen and Defize, 1997). We hypothesized that direct activation of adenylyl cyclase stimulates ERK1/2 phosphorylation and that NHERF1 interdicts this process. As shown in Figure 4A, forskolin promoted phosphorylation of both the upstream kinase, MEK1/2, and ERK1/2 in CHO-N10-R3 cells. Moreover, NHERF1 blocked both PTH and forskolin-induced MEK1/2 and ERK1/2 phosphorylation. Identical results were obtained in CHO-N10 cells lacking the PTH1R, where NHERF1 blocked forskolin-induced ERK1/2 phosphorylation (data not shown). In view of the novelty of these findings, we verified that the effects of PTH and of forskolin on ERK1/2 phosphorylation and their inhibition by NHERF1 were mirrored by actions on ERK1/2 activity as measured by its ability to phosphorylate Elk-1 (Figure 4B). These results show for the first time that NHERF1 regulates ERK signaling at a postreceptor locus.
Figure 4.
NHERF1 inhibits ERK1/2 at a postreceptor locus. CHO-N10-R3 cells were pretreated with 50 ng/ml Tet for 48 h as indicated. The cells were then serum starved for 3–5 h and treated for 10 min with 20 μM H89 before addition of 10 μM forskolin for 10 min or 1 nM PTH for 10 min. (A) Phosphorylated and total MEK1/2 and ERK1/2, and NHERF1 in whole-cell lysates determined by immunoblotting. (B) ERK1/2 activity (pElk-1) was measured as described in Materials and Methods. The results are representative of three independent experiments performed with similar results.
Because NHERF1 switches PTH1R signaling from adenylyl cyclase in its absence to phospholipase C in its presence (Mahon et al., 2002), we considered this in accounting for the mechanism of inhibition of NHERF1 on ERK1/2 phosphorylation. However, NHERF1 did not alter PTH-induced adenylyl cyclase or PKA activity (data not shown), and inhibition of PKA with H89 blocked PTH-stimulated ERK1/2 activation (Figure 4, A and B). Neither wild-type EPAC nor dominant-negative EPAC affected PTH-stimulated ERK1/2 phosphorylation (data not shown). Thus, PTH-stimulated ERK1/2 activation is PKA dependent, and NHERF1 inhibition of PTH-stimulated ERK1/2 activity lies downstream of PKA.
Decreased 14-3-3 Binding of B-Raf and Reduced PKA-dependent Increases of B-Raf Activity Mediate NHERF1 Inhibition of ERK1/2
B-Raf lies upstream of MEK, and it has been shown to mediate cAMP-dependent ERK1/2 phosphorylation (Fujita et al., 2002). Therefore, we focused our attention on the modulation of ERK activity through the regulatory and catalytic domains of B-Raf. 14-3-3 binds the B-Raf catalytic domain to increase B-Raf and ERK1/2 activity (MacNicol et al., 2000; Qiu et al., 2000). 14-3-3 constitutively interacted with B-Raf (Figure 5A) and PTH increased this association. NHERF1 inhibited the binding of 14-3-3 with B-Raf in the absence or presence of PTH. Moreover, H89 blocked the binding, thus establishing that PKA stimulated B-Raf and mediated ERK1/2 activation (Calipel et al., 2006).
Figure 5.
NHERF1 inhibition of ERK1/2 activation is mediated by decreased of 14-3-3 binding to B-Raf. (A) NHERF1 disrupts 14-3-3 binding to B-Raf. Interaction of 14-3-3 with FLAG-tagged wild-type B-Raf in CHO-N10-R3 cells was determined by coimmunoprecipitation (IP) and immunoblotting (IB) as described in Materials and Methods. (B) B-Raf S728 is critical for activation of ERK1/2. CHO-N10-R3 cells were pretreated with 50 ng/ml Tet as indicated, and then they were transiently transfected with 1.0 μg DNA/well of empty vector, wild-type B-Raf, B-Raf(S364A), B-Raf(S728A), or B-Raf(S364A, S728A). After 48 h, the cells were serum starved for 3–5 h and incubated with 1 nM PTH for 10 min. The extent of phosphorylated and total ERK1/2, NHERF1, and B-Raf in whole-cell lysates was determined by immunoblotting. (C) NHERF1 blocks PTH-stimulated B-Raf S728 phosphorylation. CHO-N10-R3 cells were transfected with HA-tagged wild-type B-Raf or B-Raf(S728A). phospho-Ser728 expression was determined by coimmunoprecipitation with HA monoclonal affinity matrix. The blot was probed with an antibody specific for Raf-1 phospho-Ser621 Raf corresponding to Ser728 in the catalytic domain of B-Raf (Qiu et al., 2000). All figures represent one of three independent experiments performed with comparable results.
Phosphorylation of serines 364 and 728 in B-Raf are critical for association with 14-3-3 (Guan et al., 2000; Zhang and Guan, 2000). S364 is present in the amino-terminal B-Raf regulatory domain, where it forms part of the 14-3-3 binding site, and it is phosphorylated by AKT. S728 forms part of a second 14-3-3 binding motif in the carboxy-terminal catalytic domain of B-Raf, where it is phosphorylated by PKA. We next asked whether PTH-stimulated ERK1/2 activity involved 14-3-3 binding to B-Raf. CHO-N10-R3 cells were transfected with wild-type B-Raf, mutated B-Raf(S364A), B-Raf(S728A), or B-Raf(S364A/S728A) with or without prior induction of NHERF1. Figure 5B shows that in the absence of NHERF1, PTH increased ERK1/2 activity equally in cells transfected with either wild-type B-Raf or B-Raf(S364A). B-Raf expression, therefore, may be rate limiting. Mutation of carboxy-terminal B-Raf(S728A) abolished baseline ERK1/2 phosphorylation and inhibited PTH-stimulated ERK1/2 phosphorylation by 61%. Double mutation of 14-3-3 binding motifs (S364A/S728A) also decreased baseline ERK1/2 phosphorylation, and diminished ERK1/2 phosphorylation but not as strongly as with single S728A. These results show that PTH-stimulated ERK1/2 phosphorylation requires 14-3-3 binding to the carboxy terminus of B-Raf. NHERF1 decreased phosphorylation of B-Raf S728 in the absence or presence of PTH (Figure 5C). B-Raf S364 phosphorylation was unaffected by NHERF1 (data not shown). Interestingly, NHERF1 inhibited PTH-stimulated ERK1/2 phosphorylation in cells transfected with wild-type or mutant B-Raf (Figure 5B), suggesting that NHERF1 must have an additional mechanism by which it blocks ERK1/2 activation other than by decreasing the association of 14-3-3 with B-Raf.
NHERF1 Inhibition of PTH-stimulated ERK1/2 Activity Involves AKT Activation and Translocation
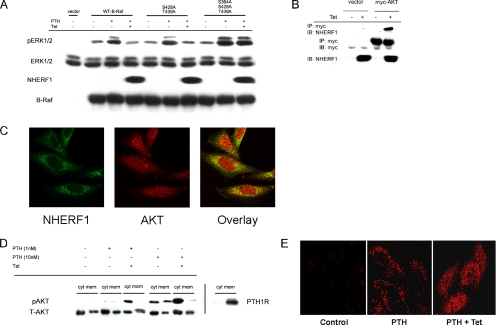
Raf phosphorylation by AKT inhibits ERK1/2 activation (Zimmermann and Moelling, 1999; Guan et al., 2000). Therefore, we predicted that in addition to suppressing PKA-dependent activation of B-Raf, NHERF1 augments AKT-mediated phosphorylation of B-Raf, thereby further inhibiting ERK1/2 phosphorylation. The B-Raf amino-terminal regulatory domain contains three AKT phosphorylation sites: S364, S428, and T439. The first lies within the amino-terminal 14-3-3 binding motif of the B-Raf regulatory domain. Mutation of the first phosphorylation site (S364A) (Figure 5B) or of the last two phosphorylation sites (S428A, T439A) had no effect on PTH-stimulated ERK1/2 activation compared with that of wild-type B-Raf (Figure 6A). Mutation of all three AKT phosphorylation sites, however, dramatically enhanced PTH-stimulated ERK1/2 activation (Figure 6A). Although NHERF1 inhibited PTH-stimulated ERK1/2 phosphorylation in cells transfected with wild-type B-Raf, it had no effect in cells expressing the triple B-Raf phospho-mutant, thereby establishing that the other component of the inhibitory action of NHERF1 is attributable to blockade of AKT-mediated phosphorylation of B-Raf.
Figure 6.
NHERF1 effects on B-Raf regulatory domain. (A) Mutation of the inhibitory B-Raf regulatory domain AKT phosphorylation sites enhances PTH-stimulated ERK1/2 and prevents inhibition by NHERF1. CHO-N10-R3 cells were pretreated with 50 ng/ml Tet as indicated, and then they were transiently transfected with 1.0 μg DNA/well of empty vector, wild-type B-Raf, B-Raf(S428A,T439A), or B-Raf(S364A, S428A, T439A). After 48 h, the cells were serum starved for 3–5 h, and then they were pretreated with 20 μM H89 for 10 min before addition of 1 nM PTH for 10 min. Phosphorylated and total ERK1/2, NHERF1 and B-Raf in whole-cell lysates were determined by immunoblotting (IB). The results are representative of three independent experiments. (B) AKT directly interacts with NHERF1. CHO-N10-R3 cells were treated with 50 ng/ml Tet as indicated, and then they were transfected with myc-AKT. After 48 h, the interaction with NHERF1 was determined by coimmunoprecipitation (IP) and immunoblotting. (C) Colocalization of AKT and NHERF1. CHO-N10-R3 cells were treated with 50 ng/ml Tet for 48 h, and then they were fixed and incubated with polyclonal anti-AKT antibody and monoclonal anti-NHERF1 antibody. Fluorescent staining was analyzed by confocal microscopy as described in Materials and Methods. (D) NHERF1 blocks PTH-induced AKT translocation from membrane to cytosol. The extent of phospho-AKT in cytoplasm (cyt) and membrane (mem) was determined by immunoblotting of the respective fractions that were prepared as described in Materials and Methods. PTH1R was similarly analyzed. (E) Phospho-AKT localization. In control condition, pAKT is barely detectable. PTH (10 nM) for 10 min significantly increased pAKT in membrane punctae. Induction of NHERF1 restricts pAKT to cytoplasm upon PTH application.
NHERF1 and AKT constitutively interact (Figure 6B) and colocalize (Figure 6C). PTH caused concentration-dependent stimulation of AKT phosphorylation in both cell membrane and cytoplasm (Figure 6D), and it was increased by NHERF1. The effect of PTH was greater at 10 nM than at 1 nM. NHERF1 expression increased AKT phosphorylation in the cytosolic fraction and decreased in the membrane fraction, which was especially evident at 10 nM PTH (Figure 6D). As a control for the adequacy of separation, the PTH1R was localized almost exclusively to plasma membranes. NHERF1 promoted translocation of pAKT from membrane to cytoplasm (Figure 6D). Immunofluorescent staining confirmed that PTH-stimulated pAKT was localized in both plasma membrane and cytosol (Figure 6E). In the presence of NHERF1, pAKT was mostly restricted to cytosol. Together, these results show that NHERF1 interacts with AKT, promotes pAKT translocation to cytosol, where it negatively regulates B-Raf activity and inhibits PTH-stimulated ERK1/2 activation.
DISCUSSION
NHERF1 plays a prominent role in regulating the signaling and trafficking of a diverse array of membrane-associated proteins including GPCRs (Voltz et al., 2001; Weinman et al., 2006). The proliferative action of PTH1R activation on osteoblasts and chondrocyte maturation depends on ERK1/2 (Swarthout et al., 2002; Provot et al., 2008). However, the effect of NHERF1 on PTH-stimulated ERK1/2 signaling is unknown. The present results show that NHERF1 regulates PTH-stimulated ERK1/2 activity at a postreceptor site. NHERF1 interacts directly with AKT and inhibits ERK1/2 activation by converging effects on B-Raf that entail increasing AKT negative regulation of the regulatory domain, and displacing 14-3-3 binding within the catalytic domain, thereby reducing the stimulatory action of B-Raf.
Because cell lines derived from defined tissue or from osteosarcomas, or various widely used cell expression systems exhibit variable constitutive levels of NHERF1 expression, we elected to generate a model system wherein the extent of NHERF1 expression could be experimentally manipulated at defined levels of PTH1R abundance. CHO cells were selected as a model because they exhibit negligible NHERF1 expression and the PTH1R is undetectable by radioligand binding and functionally devoid of adenylyl cyclase stimulation (data not shown) or ERK1/2 phosphorylation (Figure 2). CHO cells were engineered to express a Tet repressor system, where application of Tet induces concentration-dependent increases of NHERF1 expression (Wang et al., 2007). Human PTH1R was then introduced at various copy levels. In the present work, we used CHO-N10-R3 cells, which express 6.5 × 105 PTH1R/cell. These cells provide a robust but malleable model, where PTH1R signaling and trafficking can be regulated at different and controllable levels of NHERF1 expression.
Previous studies established that NHERF1 interactions with the PTH1R regulate receptor signaling, membrane tethering, and endocytosis (Mahon et al., 2002; Mahon and Segre, 2004; Wang et al., 2007; Wheeler et al., 2007). Similar phenomena have been described for NHERF1 with other membrane-delimited receptors (Cao et al., 1999; Li et al., 2002; Lazar et al., 2004). The common denominator in these modulatory actions is the interaction of NHERF1 with the PDZ recognition domain of the transmembrane protein (Voltz et al., 2001; Weinman et al., 2006). We describe here a novel means whereby NHERF1 action on ERK1/2 signaling proceeds through a two-site mechanism targeting B-Raf. This effect is entirely independent of the PTH1R and occurs at a downstream, cytoplasmic locus. Other studies established that NHERF1 stabilizes the interaction of E-cadherin with β-catenin, thereby promoting Wnt signaling (Shibata et al., 2003). This receptor independent effect may arise from stabilization of β-catenin at the cell membrane (Kreimann et al., 2007). In contrast, the inhibitory action of NHERF1 on ERK1/2 activation stems from its interaction within the cytoplasm with AKT and attenuation of PKA/B-Raf–dependent signaling.
We initially hypothesized that NHERF1 binding to the PDZ recognition motif of the PTH1R would account for the inhibitory effect of NHERF1 on ERK1/2 phosphorylation. Therefore, we examined the effect on PTH-stimulated ERK1/2 of mutating the PDZ recognition motif from ETVM to ETVA. This replacement abrogates PTH1R interactions with NHERF1 (Sneddon et al., 2003), and of a truncated PTH1R lacking most of its intracellular tail (Wang et al., 2007). Although induction of NHERF1 blocked PTH stimulation of ERK1/2 by wild-type PTH1R as theorized, it unexpectedly also inhibited phosphorylation mediated by the mutated PTH1R-ETVA and by truncated PTH1R-480stop (Figure 2). In fact, PTH elicited greater ERK1/2 phosphorylation by PTH1R-480stop than by wild-type or mutant receptors. Thus, the inhibitory action of NHERF1 is mediated at a site downstream of the PTH1R. We independently confirmed this conclusion by using CHO-N10 cells that lack PTH1R. Here, forskolin stimulated MEK1/2 and ERK1/2 phosphorylation and was blocked by NHERF1.
GPCR mediated ERK1/2 activation involves a variety of independent, but not necessarily exclusive, mechanisms including G protein-mediated signaling, transactivation of tyrosine kinase receptors, and receptor internalization (Luttrell, 2003; Syme et al., 2005). We were able to rule out receptor transactivation and receptor internalization as mediating ERK1/2 phosphorylation. Signals generated by second messenger-dependent protein kinases, such as PKA and protein kinase C, converge on the Raf isoforms Raf-1 and B-Raf. PTH-stimulated ERK1/2 activation does not proceed through Raf-1 because neither RKIP (Raf kinase inhibitory protein) nor dominant negative RKIP affected PTH-stimulated ERK activation (data not shown) (Trakul et al., 2005). Similarly, Rap-GAP and dominant negative Rap1 had no effect on PTH-stimulated ERK1/2 activity (data not shown). This conclusion and the present results are compatible with the finding that A2A-adenosine receptor-induced ERK1/2 stimulation mediated by PKA does not involve Rap1 in CHO cells (Klinger et al., 2002). Accordingly, attention was focused on B-Raf.
B-Raf contains critical serines at positions 364 and 728, corresponding to S259 and S612 in Raf-1 (Hekman et al., 2004). S364 is located within the B-Raf regulatory domain, whereas S728 is in the catalytic domain. PKA can directly phosphorylate B-Raf (Calipel et al., 2006). S259 phosphorylation inhibits Raf-1, whereas S621 phosphorylation is required for Raf-1 activity (Michaud et al., 1995). Consistent with this view, alanine mutation of S364 (S364A) did not impair stimulation of ERK1/2 by PTH (Figure 5B). Mutation of carboxy-terminal B-Raf (S728A), however, diminished basal ERK1/2 levels and decreased PTH-stimulated ERK1/2 activity.
14-3-3 is a Raf-associated protein that binds to phosphoserine residues within the context of the amino acid sequence motif RSXSXP (Aitken et al., 2002). This motif is found in both catalytic and regulatory domains of B-Raf and encompasses S364 and S728 (Hekman et al., 2004). 14-3-3 constitutively bound B-Raf and PTH increased the association (Figure 5B). Importantly, NHERF1 reduced 14-3-3 binding to B-Raf in the absence or presence of PTH. This result implies that NHERF1 inhibits ERK1/2 phosphorylation, in part, by displacing 14-3-3 binding from the B-Raf catalytic domain. Consistent with this view, NHERF1 decreased PKA-dependent B-Raf S728 phosphorylation (Figure 5C). Together with the enhanced inhibition of ERK1/2 phosphorylation in the presence of H89, these findings suggest a possible shift or switch of NHERF1 targeting of PKA from B-Raf to some other protein. Ezrin, a member of the 4.1-ezrin-radixin-moesin family of adapter molecules, contains a binding site for the type II regulatory subunit II (RII) of PKA. It functions as a protein kinase A anchoring protein (AKAP) and links PKA to NHERF1 (Dransfield et al., 1997). However, AKAP St-Ht31, which blocks the interaction between RII and AKAP (Vijayaraghavan et al., 1997), had no detectable effect on PTH-stimulated ERK1/2 phosphorylation or its inhibition by NHERF1 (data not shown). Thus, an association between NHERF1 and PKA mediated by ezrin cannot explain NHERF1 inhibition of PTH-stimulated ERK activation. Because NHERF1 inhibited ERK1/2 phosphorylation in cells transfected with phospho-resistant B-Raf (S728A) (Figure 5C), we inferred that NHERF1 must additionally block ERK1/2 activation at a second site within B-Raf. B-Raf possesses three serine or threonine AKT phosphorylation sites (Guan et al., 2000). One of these sites (S364) is within the amino-terminal 14-3-3 binding motif of B-Raf. AKT negatively regulates B-Raf kinase and ERK1/2 nuclear localization (Guan et al., 2000; Gervais et al., 2006). On stimulation, inactive AKT is recruited to the membrane, where it is phosphorylated and adopts an active conformation. AKT then redistributes either to the cytosol or other cellular compartments, where it phosphorylates its target substrates (Yoeli-Lerner et al., 2005). PTH promoted concentration-dependent AKT activation that was enhanced in the presence of NHERF1 (Figure 6D). We hypothesized that NHERF1 directly interacts with AKT. Indeed, AKT constitutively associates with NHERF1 (Figure 6B), interacting with the PDZ II domain (data not shown).
Mutation of all three AKT phosphorylation sites enhanced PTH-stimulated ERK1/2 activation (Figure 6A). Although NHERF1 inhibited PTH-stimulated ERK1/2 phosphorylation in cells transfected with wild-type B-Raf, it had no effect in cells expressing the triple B-Raf phospho-mutant. Thus, all three AKT phosphorylation sites on B-Raf are implicated in NHERF1 regulation of ERK1/2 activation. In the absence of these requisite AKT phosphorylation sites, the inhibitory effect of NHERF1 is abolished because the AKT–B-Raf–14-3-3 complex no longer forms.
Based on the present findings, and the generally accepted mechanism of B-Raf regulation of ERK1/2 activation, we propose a model (Figure 7) for the inhibitory effect of NHERF1. According to this view, AKT–B-Raf–14-3-3 form a ternary complex. NHERF1 interacts with AKT, thereby inhibiting the association of 14-3-3 with B-Raf. This action enhances the inhibitory effect of AKT on B-Raf, while suppressing the stimulatory effect of PKA. The combination of actions results in virtual elimination of ERK1/2 activation.
Figure 7.
Model of NHERF1 inhibition of PTH-stimulated ERK1/2 activity. PTH binding PTH1R activates adenylyl cyclase and PKA. B-Raf phosphorylated by PKA binds to 14-3-3, thereby activating B-Raf. The activated B-Raf subsequently phosphorylates its substrate MEK1/2 and stimulates ERK1/2 activation. Akt phosphorylates B-Raf in its regulatory domain and decreases B-Raf activation. Higher concentration of PTH stimulates AKT activity, which negatively regulates ERK1/2 activity. The magnitude of PTH-stimulated ERK1/2 phosphorylation is determined by both PKA and AKT activation. In the presence of NHERF1, both B-Raf phosphorylation at position of Ser728 and the association of 14- 3-3 with B-Raf are inhibited. NHERF1 also increases the AKT activity and negatively regulates ERK1/2 activation, thereby inhibiting ERK1/2 phosphorylation.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Bruce Sneddon for constructive input during the course of this work. These studies were supported by grant DK-69998 from the National Institutes of Health.
Abbreviations used:
- CHO
Chinese hamster ovary
- EGFR
epidermal growth factor receptor
- ERK
extracellular signal-regulated kinase
- GPCR
G protein-coupled receptor
- HA
hemagglutinin
- MAPK
mitogen-activated protein kinases
- NHERF1
Na/H exchange regulatory factor 1
- PDGF
platelet-derived growth factor
- PTH
parathyroid hormone
- PTH1R
type 1 parathyroid hormone and parathyroid hormone-related peptide receptor
- Tet
tetracycline
- TO
tandem tetracycline operator sequences
- TR
tetracycline repressor
- PKA
protein kinase A.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-11-1114) on February 13, 2008.
REFERENCES
- Ahmed I., Gesty-Palmer D., Drezner M. K., Luttrell L. M. Transactivation of the epidermal growth factor receptor mediates parathyroid hormone and prostaglandin F2α-stimulated mitogen-activated protein kinase activation in cultured transgenic murine osteoblasts. Mol. Endocrinol. 2003;17:1607–1621. doi: 10.1210/me.2002-0040. [DOI] [PubMed] [Google Scholar]
- Aitken A., Baxter H., Dubois T., Clokie S., Mackie S., Mitchell K., Peden A., Zemlickova E. Specificity of 14-3-3 isoform dimer interactions and phosphorylation. Biochem. Soc. Trans. 2002;30:351–360. doi: 10.1042/bst0300351. [DOI] [PubMed] [Google Scholar]
- Bretscher A., Chambers D., Nguyen R., Reczek D. ERM-Merlin and EBP50 protein families in plasma membrane organization and function. Annu. Rev. Cell Dev. Biol. 2000;16:113–143. doi: 10.1146/annurev.cellbio.16.1.113. [DOI] [PubMed] [Google Scholar]
- Bretscher A., Edwards K., Fehon R. G. ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- Calipel A., Mouriaux F., Glotin A. L., Malecaze F., Faussat A. M., Mascarelli F. Extracellular signal-regulated kinase-dependent proliferation is mediated through the protein kinase A/B-Raf pathway in human uveal melanoma cells. J. Biol. Chem. 2006;281:9238–9250. doi: 10.1074/jbc.M600228200. [DOI] [PubMed] [Google Scholar]
- Cao T. T., Deacon H. W., Reczek D., Bretscher A., von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the β2-adrenergic receptor. Nature. 1999;401:286–290. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- Cobb M. H. MAP kinase pathways. Prog. Biophys. Mol. Biol. 1999;71:479–500. doi: 10.1016/s0079-6107(98)00056-x. [DOI] [PubMed] [Google Scholar]
- Dransfield D. T., Bradford A. J., Smith J., Martin M., Roy C., Mangeat P. H., Goldenring J. R. Ezrin is a cyclic AMP-dependent protein kinase anchoring protein. EMBO J. 1997;16:35–43. doi: 10.1093/emboj/16.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman P. A., Gesek F. A., Morley P., Whitfield J. F., Willick G. E. Cell-specific signaling and structure-activity relations of parathyroid hormone analogs in mouse kidney cells. Endocrinology. 1999;140:301–309. doi: 10.1210/endo.140.1.6462. [DOI] [PubMed] [Google Scholar]
- Fujita T., Meguro T., Fukuyama R., Nakamuta H., Koida M. New signaling pathway for parathyroid hormone and cyclic AMP action on extracellular-regulated kinase and cell proliferation in bone cells. Checkpoint of modulation by cyclic AMP. J. Biol. Chem. 2002;277:22191–22200. doi: 10.1074/jbc.M110364200. [DOI] [PubMed] [Google Scholar]
- Gervais M., Dugourd C., Muller L., Ardidie C., Canton B., Loviconi L., Corvol P., Chneiweiss H., Monnot C. Akt down-regulates ERK1/2 nuclear localization and angiotensin II-induced cell proliferation through PEA-15. Mol. Biol. Cell. 2006;17:3940–3951. doi: 10.1091/mbc.E06-06-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan K. L., Figueroa C., Brtva T. R., Zhu T., Taylor J., Barber T. D., Vojtek A. B. Negative regulation of the serine/threonine kinase B-Raf by Akt. J. Biol. Chem. 2000;275:27354–27359. doi: 10.1074/jbc.M004371200. [DOI] [PubMed] [Google Scholar]
- Hekman M., Wiese S., Metz R., Albert S., Troppmair J., Nickel J., Sendtner M., Rapp U. R. Dynamic changes in C-Raf phosphorylation and 14-3-3 protein binding in response to growth factor stimulation: differential roles of 14-3-3 protein binding sites. J. Biol. Chem. 2004;279:14074–14086. doi: 10.1074/jbc.M309620200. [DOI] [PubMed] [Google Scholar]
- Horn F., Bettler E., Oliveira L., Campagne F., Cohen F. E., Vriend G. GPCRDB information system for G protein-coupled receptors. Nucleic Acids Res. 2003;31:294–297. doi: 10.1093/nar/gkg103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuya T., Yokose S., Hori M., Noda T., Suda T., Yoshiki S., Yamaguchi A. Parathyroid hormone exerts disparate effects on osteoblast differentiation depending on exposure time in rat osteoblastic cells. J. Clin. Invest. 1997;99:2961–2970. doi: 10.1172/JCI119491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger M., Kudlacek O., Seidel M. G., Freissmuth M., Sexl V. MAP kinase stimulation by cAMP does not require RAP1 but SRC family kinases. J. Biol. Chem. 2002;277:32490–32497. doi: 10.1074/jbc.M200556200. [DOI] [PubMed] [Google Scholar]
- Kreimann E. L., Morales F. C., de Orbeta-Cruz J., Takahashi Y., Adams H., Liu T. J., McCrea P. D., Georgescu M. M. Cortical stabilization of β-catenin contributes to NHERF1/EBP50 tumor suppressor function. Oncogene. 2007;26:5290–5299. doi: 10.1038/sj.onc.1210336. [DOI] [PubMed] [Google Scholar]
- Lazar C. S., Cresson C. M., Lauffenburger D. A., Gill G. N. The Na+/H+ exchanger regulatory factor stabilizes epidermal growth factor receptors at the cell surface. Mol. Cell Biol. 2004;15:5470–5480. doi: 10.1091/mbc.E04-03-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer E. D., Sohi S. S., McLeish K. R. Parathyroid hormone stimulates extracellular signal-regulated kinase (ERK) activity through two independent signal transduction pathways: role of ERK in sodium-phosphate cotransport. J. Am. Soc. Nephrol. 2000;11:222–231. doi: 10.1681/ASN.V112222. [DOI] [PubMed] [Google Scholar]
- Li J. G., Chen C., Liu-Chen L. Y. Ezrin-radixin-moesin-binding phosphoprotein-50/Na+/H+ exchanger regulatory factor (EBP50/NHERF) blocks U50,488H-induced down-regulation of the human kappa opioid receptor by enhancing its recycling rate. J. Biol. Chem. 2002;277:27545–27552. doi: 10.1074/jbc.M200058200. [DOI] [PubMed] [Google Scholar]
- Luttrell L. M. ‘Location, location, location’: activation and targeting of MAP kinases by G protein-coupled receptors. J. Mol. Endocrinol. 2003;30:117–126. doi: 10.1677/jme.0.0300117. [DOI] [PubMed] [Google Scholar]
- MacNicol M. C., Muslin A. J., MacNicol A. M. Disruption of the 14-3-3 binding site within the B-Raf kinase domain uncouples catalytic activity from PC12 cell differentiation. J. Biol. Chem. 2000;275:3803–3809. doi: 10.1074/jbc.275.6.3803. [DOI] [PubMed] [Google Scholar]
- Mahon M. J., Bonacci T. M., Divieti P., Smrcka A. V. A docking site for G protein βγ subunits on the parathyroid hormone 1 receptor supports signaling through multiple pathways. Mol. Endocrinol. 2006;20:136–146. doi: 10.1210/me.2005-0169. [DOI] [PubMed] [Google Scholar]
- Mahon M. J., Donowitz M., Yun C. C., Segre G. V. Na+/H+ exchanger regulatory factor 2 directs parathyroid hormone 1 receptor signalling. Nature. 2002;417:858–861. doi: 10.1038/nature00816. [DOI] [PubMed] [Google Scholar]
- Mahon M. J., Segre G. V. Stimulation by parathyroid hormone of a NHERF-1-assembled complex consisting of the parathyroid hormone I receptor, PLCβ, and actin increases intracellular calcium in opossum kidney cells. J. Biol. Chem. 2004;279:23550–23558. doi: 10.1074/jbc.M313229200. [DOI] [PubMed] [Google Scholar]
- Maudsley S., Zamah A. M., Rahman N., Blitzer J. T., Luttrell L. M., Lefkowitz R. J., Hall R. A. Platelet-derived growth factor receptor association with Na+/H+ exchanger regulatory factor potentiates receptor activity. Mol. Cell Biol. 2000;20:8352–8363. doi: 10.1128/mcb.20.22.8352-8363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud N. R., Fabian J. R., Mathes K. D., Morrison D. K. 14-3-3 is not essential for Raf-1 function: identification of Raf-1 proteins that are biologically activated in a 14-3-3- and Ras-independent manner. Mol. Cell Biol. 1995;15:3390–3397. doi: 10.1128/mcb.15.6.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh Y. S., et al. NHERF2 specifically interacts with LPA2 receptor and defines the specificity and efficiency of receptor-mediated phospholipase C-beta3 activation. Mol. Cell Biol. 2004;24:5069–5079. doi: 10.1128/MCB.24.11.5069-5079.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provot S., Nachtrab G., Paruch J., Chen A. P., Silva A., Kronenberg H. M. A-Raf and B-Raf are dispensable for normal endochondral bone development and PTHrP suppresses ERK activation in hypertrophic chondrocytes. Mol. Cell Biol. 2008;28:344–357. doi: 10.1128/MCB.00617-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu W., Zhuang S., von Lintig F. C., Boss G. R., Pilz R. B. Cell type-specific regulation of B-Raf kinase by cAMP and 14-3-3 proteins. J. Biol. Chem. 2000;275:31921–31929. doi: 10.1074/jbc.M003327200. [DOI] [PubMed] [Google Scholar]
- Radeff J. M., Singh A. T., Stern P. H. Role of protein kinase A, phospholipase C and phospholipase D in parathyroid hormone receptor regulation of protein kinase Cα and interleukin-6 in UMR-106 osteoblastic cells. Cell Signal. 2004;16:105–114. doi: 10.1016/s0898-6568(03)00131-1. [DOI] [PubMed] [Google Scholar]
- Schindeler A., Little D. G. Ras-MAPK signaling in osteogenic differentiation: friend or foe? J. Bone Miner. Res. 2006;21:1331–1338. doi: 10.1359/jbmr.060603. [DOI] [PubMed] [Google Scholar]
- Shenolikar S., Voltz J. W., Cunningham R., Weinman E. J. Regulation of ion transport by the NHERF family of PDZ proteins. Physiology. 2004;19:362–369. doi: 10.1152/physiol.00020.2004. [DOI] [PubMed] [Google Scholar]
- Shibata T., Chuma M., Kokubu A., Sakamoto M., Hirohashi S. EBP50, a β-catenin-associating protein, enhances Wnt signaling and is over-expressed in hepatocellular carcinoma. Hepatology. 2003;38:178–186. doi: 10.1053/jhep.2003.50270. [DOI] [PubMed] [Google Scholar]
- Singh A. T., Frohman M. A., Stern P. H. Parathyroid hormone stimulates phosphatidylethanolamine hydrolysis by phospholipase D in osteoblastic cells. Lipids. 2005;40:1135–1140. doi: 10.1007/s11745-005-1477-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon W. B., Friedman P. A. β-arrestin-dependent parathyroid hormone-stimulated ERK activation and PTH1R internalization. Endocrinology. 2007;148:4073–4079. doi: 10.1210/en.2007-0343. [DOI] [PubMed] [Google Scholar]
- Sneddon W. B., Gesek F. A., Friedman P. A. Obligate MAP kinase activation in parathyroid hormone stimulation of calcium transport but not calcium signaling. Endocrinology. 2000;141:4185–4193. doi: 10.1210/endo.141.11.7792. [DOI] [PubMed] [Google Scholar]
- Sneddon W. B., Syme C. A., Bisello A., Magyar C. E., Weinman E. J., Rochdi M. D., Parent J. L., Abou-Samra A. B., Friedman P. A. Activation-independent parathyroid hormone receptor internalization is regulated by NHERF1 (EBP50) J. Biol. Chem. 2003;278:43787–43796. doi: 10.1074/jbc.M306019200. [DOI] [PubMed] [Google Scholar]
- Sneddon W. B., Yang Y., Ba J., Harinstein L., Friedman P. A. Extracellular signal-regulated kinase activation by parathyroid hormone in distal tubule cells. Am. J. Physiol. Renal Physiol. 2007;292:F1028–F1034. doi: 10.1152/ajprenal.00288.2006. [DOI] [PubMed] [Google Scholar]
- Swarthout J. T., D'Alonzo R. C., Selvamurugan N., Partridge N. C. Parathyroid hormone-dependent signaling pathways regulating genes in bone cells. Gene. 2002;282:1–17. doi: 10.1016/s0378-1119(01)00798-3. [DOI] [PubMed] [Google Scholar]
- Syme C. A., Friedman P. A., Bisello A. Parathyroid hormone receptor trafficking contributes to the activation of extracellular signal-regulated kinases but is not required for regulation of cAMP signaling. J. Biol. Chem. 2005;280:11281–11288. doi: 10.1074/jbc.M413393200. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Morales F. C., Kreimann E. L., Georgescu M. M. PTEN tumor suppressor associates with NHERF proteins to attenuate PDGF receptor signaling. EMBO J. 2006;25:910–920. doi: 10.1038/sj.emboj.7600979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisen C. S., Wahl J. K., 3rd, Johnson K. R., Wheelock M. J. NHERF links the N-cadherin/catenin complex to the platelet-derived growth factor receptor to modulate the actin cytoskeleton and regulate cell motility. Mol. Biol. Cell. 2007;18:1220–1232. doi: 10.1091/mbc.E06-10-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trakul N., Menard R. E., Schade G. R., Qian Z., Rosner M. R. Raf kinase inhibitory protein regulates Raf-1 but not B-Raf kinase activation. J. Biol. Chem. 2005;280:24931–24940. doi: 10.1074/jbc.M413929200. [DOI] [PubMed] [Google Scholar]
- Verheijen M.H.G., Defize L.H.K. Parathyroid hormone activates mitogen-activated protein kinase via a cAMP-mediated pathway independent of Ras. J. Biol. Chem. 1997;272:3423–3429. doi: 10.1074/jbc.272.6.3423. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan S., Goueli S. A., Davey M. P., Carr D. W. Protein kinase A-anchoring inhibitor peptides arrest mammalian sperm motility. J. Biol. Chem. 1997;272:4747–4752. doi: 10.1074/jbc.272.8.4747. [DOI] [PubMed] [Google Scholar]
- Vilardaga J. P., Krasel C., Chauvin S., Bambino T., Lohse M. J., Nissenson R. A. Internalization determinants of the parathyroid hormone receptor differentially regulate β-arrestin/receptor association. J. Biol. Chem. 2002;277:8121–8129. doi: 10.1074/jbc.M110433200. [DOI] [PubMed] [Google Scholar]
- Voltz J. W., Weinman E. J., Shenolikar S. Expanding the role of NHERF, a PDZ-domain containing protein adapter, to growth regulation. Oncogene. 2001;20:6309–6314. doi: 10.1038/sj.onc.1204774. [DOI] [PubMed] [Google Scholar]
- Wang B., Bisello A., Yang Y., Romero G. G., Friedman P. A. NHERF1 regulates parathyroid hormone receptor membrane retention without affecting recycling. J. Biol. Chem. 2007;282:36214–36222. doi: 10.1074/jbc.M707263200. [DOI] [PubMed] [Google Scholar]
- Weinman E. J., Hall R. A., Friedman P. A., Liu-Chen L. Y., Shenolikar S. The association of NHERF adaptor proteins with G protein-coupled receptors and receptor tyrosine kinases. Annu. Rev. Physiol. 2006;68:491–505. doi: 10.1146/annurev.physiol.68.040104.131050. [DOI] [PubMed] [Google Scholar]
- Wheeler D. G., Sneddon W. B., Wang B., Friedman P. A., Romero G. Role of NHERF-1 and the cytoskeleton in the regulation of the traffic and membrane dynamics of G-protein-coupled receptors. J. Biol. Chem. 2007;282:25076–25087. doi: 10.1074/jbc.M701544200. [DOI] [PubMed] [Google Scholar]
- Yoeli-Lerner M., Yiu G. K., Rabinovitz I., Erhardt P., Jauliac S., Toker A. Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol. Cell. 2005;20:539–550. doi: 10.1016/j.molcel.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Zhang B. H., Guan K. L. Activation of B-Raf kinase requires phosphorylation of the conserved residues Thr598 and Ser601. EMBO J. 2000;19:5429–5439. doi: 10.1093/emboj/19.20.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann S., Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B) Science. 1999;286:1741–1744. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.