Abstract
LKB1, a serine/threonine kinase, regulates cell polarity, metabolism, and cell growth. The activity and cellular distribution of LKB1 are determined by cofactors, STRADα and MO25. STRADα induces relocalization of LKB1 from the nucleus to the cytoplasm and stimulates its catalytic activity. MO25 stabilizes the STRADα/LKB1 interaction. We investigated the mechanism of nucleocytoplasmic transport of LKB1 in response to its cofactors. Although LKB1 is imported into the nucleus by importin-α/β, STRADα and MO25 passively diffuse between the nucleus and the cytoplasm. STRADα induces nucleocytoplasmic shuttling of LKB1. STRADα facilitates nuclear export of LKB1 by serving as an adaptor between LKB1 and exportins CRM1 and exportin7. STRADα inhibits import of LKB1 by competing with importin-α for binding to LKB1. MO25 stabilizes the LKB1–STRADα complex but it does not facilitate its nucleocytoplasmic shuttling. Strikingly, the STRADβ, isoform which differs from STRADα in the N- and C-terminal domains that are responsible for interaction with export receptors, does not efficiently relocalize LKB1 from the nucleus to the cytoplasm. These results identify a multifactored mechanism to control LKB1 localization, and they suggest that the STRADβ-LKB1 complex might possess unique functions in the nucleus.
INTRODUCTION
Peutz-Jeghers cancer syndrome is caused by mutations in a gene encoding a serine/threonine kinase called LKB1 (Hemminki et al., 1998). This kinase is closely related to PAR-4, a protein required for polarization of the Caenorhabditis elegans zygote, and it has recently been implicated in the apical/basal polarization of mammalian epithelial cells (Baas et al., 2004). LKB1 is also involved in numerous cell signaling pathways that regulate cellular metabolism (Shaw et al., 2004, 2005; Liang et al., 2007), cell growth, and responses to DNA damage (Wei et al., 2005; Setogawa et al., 2006; Zeng and Berger, 2006). LKB1 can phosphorylate and activate 13 protein kinases from the AMP-activated protein kinase family (Lizcano et al., 2004). However, the biological significance of this phosphorylation is still unclear for many of these kinases. The cellular localization and activity of LKB1 is controlled through its interaction with Ste20 Related Adaptor (STRAD) (Baas et al., 2003) and an adapter protein, MO25 (Boudeau et al., 2003). STRAD, a 48-kDa protein, is a pseudokinase that consists of a STE20-like kinase domain but lacks several residues that are indespensible for catalytic activity. STRAD resembles most closely the STE20 homologues SPAK (Johnston et al., 2000) and ILPIP (Sanna et al., 2002), or PAP kinase (Nishigaki et al., 2003). Whereas SPAK is a true kinase, it was later shown that ILPIP/PAPK is a pseudokinase, similarly to STRAD and that it also interacts with and activates LKB1. Therefore, it was termed STRADβ, whereas the original STRAD became known as STRADα (Boudeau et al., 2003). The complex of LKB1 and STRADα or -β is stabilized by MO25, a 40-kDa protein composed of α-helical repeats that are distantly related to the armadillo repeat domain (Milburn et al., 2004). MO25 binds STRAD by its conserved N-terminal Trp-Glu-Phe sequence. It interacts with LKB1 only in the presence of STRAD, to form a high-affinity ternary complex (Boudeau et al., 2004).
In the presence of STRADα and MO25, LKB1 relocalizes from the nucleus to the cytoplasm, and the catalytic activity of LKB1 toward its substrates is increased 10-fold (Baas et al., 2003; Boudeau et al., 2006). Remarkably, LKB1 translocation from the nucleus to the cytoplasm in response to high levels of STRADα expression can induce the spontaneous polarization of intestinal epithelial cells in the absence of cell–cell contacts (Baas et al., 2004). Nuclear accumulation of LKB1 is thought to be driven by a nuclear localization signal (NLS) in the N-terminal noncatalytic region (residues 38–43) (Nezu et al., 1999; Smith et al., 1999; Tiainen et al., 2002). STRADα, when expressed alone, is predominantly cytoplasmic, and MO25 expressed alone is evenly distributed between the nucleus and the cytoplasm. Significantly, coexpression of STRADα with LKB1 relocalizes the kinase from the nucleus to the cytoplasm, and coexpression with MO25 potentiates this effect, suggesting that nucleocytoplasmic transport plays a key role in the regulation of LKB1 function. Consistent with this idea, 12 mutants of LKB1 (including the SL26 mutant) found in patients with Peutz-Jeghers cancer syndrome fail to interact with STRADα and are constitutively nuclear (Boudeau et al., 2004).
The nuclear envelope that separates the genome from the cytosol is a defining feature of eukaryotic cells, and nucleocytoplasmic transport of proteins across the nuclear envelope participates in the regulation of many cellular signal transduction pathways. The exchange of proteins and RNA between the nucleus and the cytoplasm occurs through the nuclear pore complexes either by passive diffusion (for proteins smaller than ∼40 KDa) or by active transport, which is facilitated by nuclear transport receptors, also known as karyopherins (Macara, 2001; Suntharalingam and Wente, 2003; Weis, 2003; Terry et al., 2007). There are 26 known mammalian karyopherins, which can be subdivided into three groups, the first of which consists of 10 importins that facilitate the import of proteins into the nucleus. The second group, exportins, consists of seven karyopherins that export proteins from the nucleus. One karyopherin, importin13, was shown to function as both an importin and exportin. A third group consists of importin-α isoforms that function as adaptors, which facilitate cargo binding to importin-β. Finally, functions of two karyopherins, RanBP6 and RanBP17, are still unknown (Mosammaparast and Pemberton, 2004; Pemberton and Paschal, 2005).
The best-characterized import pathway is mediated by importin-α and importin-β. Binding of importin-α to importin-β relieves autoinhibition of importin-α and allows it to bind cargo proteins containing an NLS. The trimeric complex of cargo:importin-α:importin-β translocates into the nucleus due to the ability of importin-β to associate with FG-repeat nucleoporins of the nuclear pore complex. Inside the nucleus, binding of RanGTP to importin-β disassembles the import complex. The high nuclear concentration of RanGTP ensures the directionality of the import (Macara, 2001; Pemberton and Paschal, 2005).
The most studied export pathway is mediated by exportin1, also called CRM1 (Stade et al., 1997). CRM1 recognizes short, leucine-rich nuclear export signals (NES) (Fornerod et al., 1997; Fukuda et al., 1997; Ossarehnazari et al., 1997; Stade et al., 1997). Export complexes form in the nucleus because their assembly requires high RanGTP concentration. Trimeric export complexes composed of CRM1, cargo, and RanGTP diffuse out of the nucleus. In the cytoplasm, RanGAP hydrolyzes RanGTP to RanGDP, which disassembles the complex (Stade et al., 1997; Askjaer et al., 1998, 1999; Macara, 2001; Pemberton and Paschal, 2005). Identification of novel cargo proteins for CRM1 has been facilitated by a specific inhibitor of CRM1, Leptomycin B (LMB) (Fornerod et al., 1997; Ossarehnazari et al., 1997; Stade et al., 1997; Kudo et al., 1998, 1999).
Many proteins shuttle constitutively between the nucleus and the cytoplasm. Most commonly, shuttling proteins possess both an NLS and a NES. Posttranslational modifications such as phosphorylation and/or binding of cofactors can affect the availability of these signals, allowing for regulation of the nucleocytoplasmic distribution of the shuttling protein (Fabbro and Henderson, 2003; Burack and Shaw, 2005; Terry et al., 2007).
Little is known about the molecular mechanisms that allow LKB1 to translocate between the nuclear and the cytoplasmic compartments, or how STRADα and MO25 affect this process. STRADα might induce cytoplasmic accumulation of LKB1 by anchoring it in the cytoplasm and/or inhibiting its import by the classical nuclear import pathway. However, it is also possible that STRADα facilitates nuclear export of LKB1. Another intriguing possibility arises from structural similarities between MO25 and karyopherins (Milburn et al., 2004). Could MO25 facilitate nucleocytoplasmic translocation of LKB1 and STRADα? We set out to systematically examine the mechanism of nucleocytoplasmic translocation of each component of the active LKB1 complex.
MATERIALS AND METHODS
Plasmids
Full-length cDNA encoding MO25α was generated by polymerase chain reaction (PCR) amplification from human kidney cDNA library (Clonetech, Mountain View, CA), with primers to the 5′ and 3′ ends of the published sequence (accession no. NM_016289). MO25 was cloned into BamHI and EcoRI restriction sites of pKH3 (hemagglutinin [HA]-tag) and pKCFP vectors and sequenced. pcDNA3-flagSTRADα and pcDNA3-mycLKB1 were gifts from D. Alessi (Baas et al., 2003, 2004; Boudeau et al., 2003, 2004). pQE60-CRM1 was a gift from Ian Mattaj (Fornerod et al., 1997; Askjaer et al., 1998, 1999). pcDNA3-CRM1-His-Myc was a gift from Dr. Dargemont (Black et al., 2001). pcDNA3-exportin7 was generated by PCR amplifying exportin7 from pMT2SR-exportin7, a gift from Dr. Janssen (Koch et al., 2000), and ligating it into pcDNA3 vector. pKRFP-LKB1 was generated by PCR amplifying LKB1 from pcDNA3myc-LKB1 as described above and subcloning it into the pK-RFP vector. pQE32-RanQ69L was a gift from D. Görlich. STRADα mutants pcDNA3-flag-STRADdNES1 (L27A, L29A), STRADdNES2 (L421A, L424A), and STRADddNES (L27A, L29A, L421A, L424A) were generated by site-directed mutagenesis, using the Stratagene Quickchange kit (Stratagene La Jolla, CA).
pKmyc-STRADβ (NM_018571) was PCR amplified from human kidney cDNA by using the following primers: 5′ STRADβ ggcggatccatgtctcttttggattgcttctgcac; 3′ STRADβ gccgcggccgcctagaattcccagtatgagtc; and it was cloned into pKmyc vector using BamHI and NotI restriction sites.
Peptides
The peptides have been described previously (Lindsay et al., 2002), and they have the following sequences: Nup50NLS, CMKNRAVKKAKRRNV; MVMNES, CVDEMTKKFGTLTIHDTEK.
Protein Expression
35S-labeled proteins were expressed using rabbit reticulocyte lysate in in vitro transcription-translation–coupled reactions (Promega, Madison, WI) according to the manufacturer's instructions.
His6-STRAD, CRM1-His6, His6-RanQ69L, and glutathione-S-transferase (GST)-importin-α3 were expressed in Escherichia coli BL21(DE3). Bacterial cultures were grown in Luria broth supplemented with 2% ethanol and appropriate selective antibiotic, to an optical density of ∼0.8 at 600 nm, and then they were induced with 400 μM isopropylthiogalactopyranoside at 18°C overnight. His6-tagged proteins were purified on Ni2+-agarose beads (QIAGEN, Valencia, CA). GST-importin-α3 was purified on glutathione-Sepharose beads (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom)
Cell Culture, Transfection, and Processing
For binding assays, human embryonic kidney (HEK)293T cells were transfected using a calcium phosphate precipitation procedure. Cells were lysed 24 h after transfection. For heterokaryon assays and immunofluorescence analysis, HeLa cells were transfected using FuGENE6 (Roche Diagnostics, Indianapolis, IN). Cells to be analyzed by fluorescence microscopy were fixed with 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS; 137 mM NaCl, 3 mM KCl, 10 mM Na2HPO4, and 2 mM KH2PO4, pH 7.4), permeabilized with 0.2% Triton-100 in PBS, and blocked in 5% bovine serum albumin (BSA)-PBS at room temperature. Myc-tagged proteins were detected with 9E10 monoclonal antibody (mAb) (1:1000), flag-tagged proteins were detected with M2 (Stratagene) mAb (1:1000). DNA was stained with 4′,6′-diamidino-2-phenylindole (DAPI), and the slides were mounted with GelMount (Biomeda, Foster City, CA). Images were captured with a 60× water immersion objective lens (numerical aperture [NA] 1.2) on a Nikon TE200 inverted microscope with Hamamatsu charge-coupled device camera. Immunofluorescence data were obtained with Openlab (Improvision, Coventry, United Kingdom) and processed using Adobe Photoshop software, as described previously (Chen et al., 2004). Nuclear fluorescence was calculated by using DAPI staining of the nuclei to define the nuclear boundaries. Cytoplasmic fluorescence was calculated by subtracting nuclear fluorescence from the total cell fluorescence. Cells coexpressing CFP-MO25, RFP-LKB1, and myc-STRADβ were imaged using a LSM 510 Meta confocal microscope (Carl Zeiss, Jena, Germany). To separate the spectra of CFP-MO25 and Alexa488-labeled STRADβ, emission fingerprinting was used. Emission fingerprinting allows one to deconvolve the emission spectra from each pixel in the confocal image, by comparing the emission profiles with reference spectra of each individual fluorochrome present in the sample.
Crm1 Binding Assays
HEK293 cells were transfected with pcDNA3-flag-STRADα, pcDNA3-flag-STRADdNES1, pcDNA3-flag-STRADdNES2, or pcDNA3-flag-STRADddNES by using calcium phosphate. Twenty-four hours after transfection, cells were trypsinized, washed twice with ice-cold PBS, placed on ice, and lysed by addition of 400 μl of lysis buffer (25 mM HEPES, pH 7.4, 300 mM NaCl, 1.5 mM MgCl2, 1 mM sodium orthovanadate, 0.1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin per ml, and 20 μg of aprotinin per ml). Lysates were cleared by centrifugation (15 min at 14,000 × g; 4°C). The lysate was used for immunoprecipitation with Anti-flag M2 affinity gel (A2220; Sigma-Aldrich, St. Louis, MO) at 4°C for 1 h. Immunoprecipitates were washed two times with PBS, 1% NP-40. The immunoprecipitates were resuspended in binding buffer (20 mM HEPES, pH 7.3, 150 mM potassium acetate, 2 mM magnesium acetate, and 0.1% Tween-20). Crm1-His6 was added to each assay to yield a final concentration of 500 nM. His6-RanQ69L was added as indicated to a final concentration of 5 μM. Samples were incubated with shaking for 2 h at 4°C, and then they were washed three times with binding buffer. Beads were resuspended in 30 μl of Laemmli sample buffer, and the proteins were separated by SDS-PAGE and immunoblotted with horseradish peroxidase (HRP)-conjugated M2 antibody (1:1000 dilution), monoclonal anti-His6 antibody (1:1000 dilution; BabCo, Richmond, CA), and with HRP-conjugated goat anti-mouse antibody (1:10,000 dilution; Jackson ImmunoResearch Laboratories). Proteins were revealed by chemiluminescence (Kirkegaard and Perry Laboratories, Gaithersburg, MD).
35S-labeled proteins were synthesized by in vitro transcription-translation (TNT) according to the manufacturer's instructions (Promega). Each binding reaction was 50 μl total volume, and it contained 5–10 μl of TNT reaction expressing 35S-labeled proteins, 10 μl of appropriate beads, and 5 μM RanQ69L where indicated. Binding buffer was described above. Reactions were incubated, washed, and eluted as described above. Proteins were separated by SDS-PAGE, gels were stained with Coomassie Blue staining, and then they were fixed with 30% methanol, 10% acetic acid, and soaked in Amplify (GE Healthcare) for 30 min. Finally, the gels were dried and exposed to film overnight.
Fluorescence Recovery from Photobleaching Assay (FRAP) Assay
HeLa cells were transfected with pK-CFP or pK-CFP-MO25α using FuGENE6 (Roche Diagnostics) per the manufacturer's instructions. After 24 h, the medium was replaced with RPMI 1640 medium, the cells were imaged at room temperature using confocal microscopy with a 40× oil objective lens (NA 1.2), and then they were subjected to photobleaching. Regions of interest (ROI) were photobleached with the 488-nm laser line at 100% intensity, then the images were collected at 5% intensity every 5–15 s until nuclear-cytoplasmic fluorescence has re-equilibrated or the focal plane had drifted. Mean nuclear fluorescence and background were calculated for each condition. Background photobleaching was negligible in most experiments. Nuclear fluorescence after photobleaching was normalized to prebleach maximum and plotted against postbleach time.
Heterokaryon Fusion Assays
Donor HeLa cells were transiently transfected with pKRFP-LKB1 or pKRFP-LKB1-SL26 12 h before coplating with acceptor cells. Acceptor HeLa cells were labeled with CellTracker Green 5-chloromethylflurescein diacetate (CMFDA) dye (Invitrogen, Carlsbad, CA). Cells were incubated with 500 nM CMFDA in Opti-MEM for 30 min. Cells were then rinsed and incubated in Opti-MEM for another 30 min before coplating with donor cells. Acceptor and donor cells were coplated onto two-well LabTechII slides and grown for 24 h. Before fusion, cells were treated with 50 μM cycloheximide for 30 min. The plasma membranes were then fused with 50% polyethylene glycol (mol. wt. 8000 g/mol) prewarmed to 37°C. Cells were washed five times with Hanks' balanced salt solution and incubated at 37°C for an additional 1 h in the presence of 50 μM cycloheximide. Cells were then fixed with 4% PFA, incubated with 10 ng/ml DAPI, and the coverslips were mounted on glass slides with GelMount (Biomeda). Images of the cells were captured and processed as described above. Images for each set of experiments were obtained using the same camera settings.
Permeabilized Cell Transport Assays
HeLa cells were plated on Lab-Tek coverglass slides (Nalge Nunc International, Rochester, NY), and then they were grown for 24 h. Cells were permeabilized with 0.0025% digitonin in transport buffer (TB), 20 mM HEPES-KOH, pH 7.4, 110 mM potassium acetate, 2 mM magnesium acetate (Adam et al., 1990, 1991, 1992). Permeabilized cells were washed twice with excess of 5% BSA in transport buffer to remove digitonin. Where indicated, cells were treated with 200 μg/ml wheat germ agglutinin (WGA; Sigma Aldrich) for 10 min at room temperature before addition of transport substrates. Transport substrates were dissolved in transport buffer supplemented with 250 mM sucrose and 0.2% gelatin, and substrates were added to permeabilized cells. Then, 70-KDa Texas Red dextran (Invitrogen) was added to 0.2 mg/ml concentration to serve as a control for permeabilization. Five minutes after addition of transport substrates, cells were imaged by confocal microscopy using a 40× oil objective lens (NA 1.2).
Nuclear Transport Assay in the Presence of Rabbit Reticulocyte Lysate
Cells were permeabilized and washed as described above. Transport substrates were solubilized in TB supplemented with 25% nuclease untreated rabbit reticulocyte lysate (Promega), 70-kDa Texas Red fixable dextran was used as a control for permeabilization. Where indicated, 5 μM RanQ69L was added. Cells were incubated 30 min at 30°C, rinsed with TB, and fixed with 4% PFA/PBS. The slides were mounted with GelMount (Biomeda). Images were captured and processed as described above.
Small Interfering RNA (siRNA) Silencing of Exportin7
To knock down exportin7, siGENOME SMART pool M-016571-00-0005 (Dharmacon RNA Technologies, Lafayette, CO) was used. Sequences were as follows: 1) uaucucagcuaaccaaugauu, 2) acaagcacuuauucaguuauu, 3) cuaagcgucuucauaggaauu, and 4) cuacuuggcuugauauugcuu.
Cells were transfected with siRNA SMART pool and control siRNA using OligofectAMINE (Invitrogen) transfection reagent according to manufacturer's instructions. Cells were grown for 48 h, and then they were transfected with pKRFP-LKB1 and pcDNA3flag-STRADα (1:3) by using FuGENE6 (Roche Diagnostics). Twenty-four h after transfection, cells were treated with indicated concentrations of Leptomycin B for 30 min. After 30 min, cells were fixed and immunostained with anti-flag to detect flag-STRADα. Images of the cells expressing both RFP-LKB1 and flag-STRADα were collected, and the ratios of nuclear to cytoplasmic fluorescence of RFP-LKB1 were measured. The ratios were plotted for different Leptomycin B concentrations.
To verify that the XPO7 SMART pool can inhibit expression of exportin7, HEK293T cells were transfected with siRNA as described above. Five h later, the medium was changed, and cells were transfected with pMT2SM-HA-exportin7(human) and pK-GFP control. Cells were lysed after 24 h, and then they were examined for expression of HA-exportin7 and green fluorescent protein (GFP) by Western blotting.
RESULTS
As previously shown, LKB1 expressed in HeLa cells is predominantly nuclear (Figure 1A) (Nezu et al., 1999; Smith et al., 1999). However, in the presence of flag-STRADα, myc-LKB1 relocalizes to the cytoplasm (Figure 1B) (Baas et al., 2003). A fusion of cyan fluorescent protein (CFP) with MO25 is distributed between the nucleus and cytoplasm (Figure 1A) (Boudeau et al., 2003, 2004), but coexpression of flag-STRADα with CFP-MO25 leads to relocation of the CFP-MO25 from the nucleus to the cytoplasm, similarly to myc-LKB1 (Figure 1B) (Boudeau et al., 2003). To examine a possible role of the nuclear export receptor CRM1 in the cytoplasmic localization of LKB1 and MO25 in the presence of STRADα, cells were treated with 200 nM LMB for 1 h. We found that CFP-MO25 and to a lesser degree myc-LKB1, accumulate in the nucleus in response to LMB (Figure 1B). This result suggests that in the presence of STRADα, LKB1, and MO25 are shuttling constitutively between the nucleus and the cytoplasm and that CRM1 is involved in their nuclear export.
Figure 1.
LKB1 shuttles between the nucleus and the cytoplasm in the presence of STRADα. (A) Localization of LKB1, STRADα, and MO25α. HeLa cells expressing mycLKB1, flagSTRADα, and CFP-MO25α were immunostained for myc and flag. Nuclear and cytoplasmic fluorescence were quantified as described in Materials and Methods. The ratios of nuclear fluorescence (NF) to cytoplasmic fluorescence (CF) were plotted for LKB1 (n = 11 cells), STRADα (n = 17), and MO25α (n = 24). The bars indicate SE. (B) LKB1 and MO25α localize to the cytoplasm when coexpressed with STRADα, but accumulate in the nuclei when CRM1-dependent export is inhibited with LMB. HeLa cells expressing myc-LKB1, flag-STRADα, and CFP-MO25α were treated with 200 nM LMB for 1 h at 37°C or left untreated. Cells were immunostained for myc and flag. Ratios of nuclear versus cytoplasmic fluorescence (NF/CF) were calculated as described in A. For MO25α and STRADα coexpression, n = 23; MO25α and STRADα treated with LMB, n = 13; LKB1 and STRADα, n = 19; and LKB1 and STRADα treated with LMB, n = 12. The bars indicate SE. The p values were calculated using an unpaired t test. (C) RFP-LKB1 shuttles between the nucleus and the cytoplasm. Donor cells (D) were transiently transfected with pKRFP-LKB1. Acceptor cells (A) labeled with CellTracker Green CMFDA dye (Invitrogen). Nuclei were stained with DAPI. Cells were coplated and fused with polyethylene glycol as described in Materials and Methods. In the second panel all visible nuclei are a part of one giant heterokaryon cell, and it seems that red fluorescent protein RFP-LKB1 redistributed evenly between all the nuclei of this heterokaryon, including the acceptor nucleus. Only some of the donor cells are marked with arrows. (D) RFP-LKB1(SL26) mutant deficient in STRADα binding does not shuttle between the nucleus and the cytoplasm. Donor cells were transiently transfected with pKRFP-LKB1(SL26), and acceptor cells labeled with CMFDA dye, as in C. Bar, 20 μm.
LKB1 Does Not Shuttle between the Nucleus and the Cytoplasm in the Absence of STRADα
STRADα might affect localization of LKB1 by two mechanisms. It could inhibit a constitutive nuclear import and/or facilitate the nuclear export of LKB1. To distinguish between these mechanisms, we performed a heterokaryon assay. HeLa cells transiently transfected with RFP-LKB1 (donor cells) were coplated with acceptor cells labeled with CellTracker Green CMFDA, a fluorescent, cell-permeant dye that labels intracellular thiol-containing proteins. After 24 h, cells were fused with polyethylene glycol and incubated for 1 h in the presence of 50 μM cycloheximide to prevent new protein synthesis (Chen et al., 2004). On fusion, labeled cytoplasmic proteins diffuse between the acceptor and the donor cells, thus defining the boundaries of the heterokaryon. When multinucleated heterokaryon cells where examined, RFP-LKB1 was consistently found in the nuclei of acceptor cells, confirming that LKB1 shuttles between the nucleus and the cytoplasm (Figure 1C). Next, we transfected donor cells with RFP-LKB1(SL26). This mutant is deficient in binding to STRADα (Baas et al., 2003; Boudeau et al., 2004). LKB1(SL26) was previously found to be nearly exclusively nuclear, unlike wild-type LKB1 (Nezu et al., 1999). When the heterokaryon experiment was performed as described above with RFP-LKB1(SL26), no shuttling was detected, confirming that interaction with STRADα is essential for the nuclear export of LKB1 (Figure 1D).
MO25 Does Not Facilitate Nucleocytoplasmic Shuttling of LKB1
MO25 is structurally related to Armadillo repeat proteins such as importin-α and β-catenin. Because most nuclear transport receptors are Armadillo repeat proteins and β-catenin can diffuse into the nucleus independently of the nuclear transport machinery (Yokoya et al., 1999), we examined whether MO25 might also possess properties that could allow preferential access to the nuclear pore. As a negative control, cells were transfected with cyan fluorescent protein (CFP). CFP is a small protein that can access the nuclear compartment by passive diffusion. Bona fide transport receptors can enter the nucleus much faster than proteins such as CFP due to their ability to associate with FG-repeat nucleoporins (Ribbeck and Gorlich, 2002; Ando et al., 2004). Therefore, if MO25 can also associate with nucleoporins it is expected to diffuse into the nucleus faster than CFP. We expressed CFP and CFP-MO25 in HeLa cells, and then we examined their nucleocytoplasmic shuttling using fluorescence recovery from photobleaching (Figure 2, A and B). First, nuclei of cells expressing either CFP or CFP-MO25 were bleached, and the recovery of nuclear fluorescence was monitored over time. In cells expressing isolated CFP, nuclear fluorescence equilibrated with the cytoplasmic fluorescence within 55 s from the time of the bleach. In contrast, CFP-MO25 remained excluded from the nucleus even after 3 min. Next, the cytoplasm of CFP-MO25–expressing cells was bleached, and nuclear fluorescence was monitored. There was no decrease in the nuclear fluorescence over a 3-min time interval, indicating that CFP-MO25 diffuses out of the nucleus very slowly. Therefore, it seems that MO25 is unlikely to directly facilitate the nucleocytoplasmic shuttling of STRADα and LKB1.
Figure 2.
CFP-MO25α passively diffuses into the nucleus. HeLa cells were transiently transfected with pKCFP or pKCFP-MO25. Twenty-four h after transfection FRAP experiments were performed. The nuclei or the cytoplasm were bleached, and nuclear fluorescence was monitored over time, as described in Materials and Methods. (A) Representative images of FRAP experiment. (B) Quantification shows the change in the nuclear fluorescence (normalized to prebleach maximum) over time for CFP (n = 2), CFP-MO25α nuclear bleach (n = 4), for CFP-MO25α cytoplasmic bleach (n = 1). Bar, 20 μm.
STRADα Inhibits LKB1 Binding to Importin-α
Next, we addressed the question of how LKB1 and STRADα are imported into the nucleus. LKB1 possesses a basic, monopartite NLS. Sequence analysis of STRADα indicated that it also possesses an NLS-like motif (amino acids 34–40: PGDTRRK). To determine whether LKB1 and STRADα bind importins-α and -β, we used recombinant GST-importin-α3 immobilized on glutathione beads. Myc-tagged LKB1, flag-RCC1 (a positive control) and flag-STRADα were each expressed in an in vitro TNT reaction and incubated with GST-importin-α3 on the beads. RCC1 and LKB1 both bound specifically to GST-importinα3. However, flag-tagged STRADα did not bind to GST-importin-α3 (Figure 3A). We also observed that addition of flag-STRADα slightly inhibited myc-LKB1 binding to importin-α3 (Figure 3A, lane 8). To further investigate this effect, we expressed RFP-LKB1, flag-STRADα, and HA-MO25 in TNT reactions, and we tested how addition of STRADα and MO25 affected RFP-LKB1 binding to GST-importin-α3. Only RFP-LKB1 was bound to importin-α3, and addition of flag-STRADα and HA-MO25 significantly inhibited this interaction (Figure 3B). Under the same conditions, M2 anti-flag antibody pulled down a stoichiometric complex of flag-STRADα, RFP-LKB1, and HA-MO25. We conclude that import receptors and STRADα/MO25 compete for binding to LKB1 and that STRADα contributes to nuclear exclusion of LKB1 by inhibiting its binding to the importin-α/β complex.
Figure 3.
STRADα inhibits LKB1 binding to importin-α. (A) LKB1 but not STRADα binds to GST-importin-α3. Myc-LKB1, flag-STRADα, and flag-RCC1 (positive control) were expressed in the rabbit reticulocyte lysate (TNT) system and labeled with [35S]methionine. (B) GST-importin-α3 competes for the binding to LKB1 with flag-STRADα and MO25. RFP-LKB1, flag-STRADα, and HA-MO25α were expressed by TNT and added either to GST or GST-importin-α3 immobilized on glutathione beads, or to M2-agarose.
STRADα Enters the Nucleus by Passive Diffusion
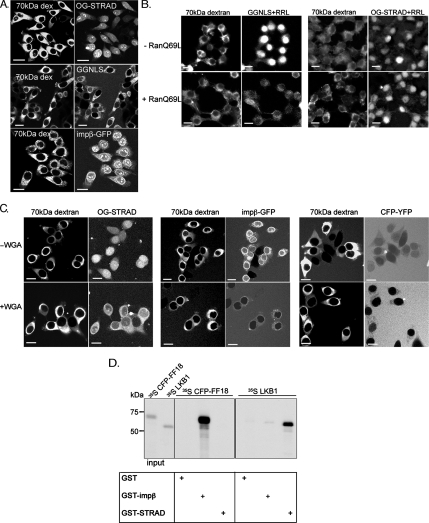
STRADα does not bind importin-α, but it becomes distributed between the nucleus and cytoplasm after LMB treatment (Figure 5A), indicating that it constitutively traverses the nuclear pores. STRADα (48 kDa) is similar in size to mitogen-activated protein kinase, which can diffuse passively through the pores, but there also exist at least 11 importins that might carry STRADα into the nucleus (Khokhlatchev et al., 1998; Matsubayashi et al., 2001; Pemberton and Paschal, 2005). To examine the import mechanism of STRADα, we used recombinant His6-STRADα labeled with OG-STRAD, GST-GFP-NLS (GGNLS), and His6-importin-β-GFP. GGNLS serves as a negative control, because it requires active nuclear import to access the nucleus, whereas importin-β is a positive control due to its ability to associate with nucleoporins. First, OG-STRADα, GGNLS (GGNLS), and importin-β-GFP were added to digitonin-permeabilized HeLa cells in the absence of cytosolic lysate and energy regenerating system. A 70-kDa Texas Red-labeled dextran, which is too large to penetrate the nuclear pores, was used to confirm the intactness of the nuclear envelopes during the assay. We found that, whereas GGNLS was excluded from the nuclei of permeabilized cells, both OG-STRADα and importin-β-GFP were able to accumulate in the nuclei (Figure 4A). Both STRADα and importin-β formed intranuclear aggregates perhaps through association with nucleolar structures. In addition, importin-β bound to the nuclear envelope, because of its affinity for nucleoporins.
Figure 5.
STRADα is exported by CRM1. (A) LMB induces nuclear accumulation of STRADα. HeLa cells expressing flagSTRADα were treated with 200 nM LMB for 1 h as in Figure 1B. Bar, 20 μM. Quantifications were done as in Figure 1. The p value was calculated using unpaired t test. SE bars are shown. (B) STRADα directly binds CRM1 in RanGTP and LMB-sensitive manner. Flag-STRADα and flag-RCC1 were expressed in HEK293T cells and immobilized on M2-agarose. The following reaction components were added as indicated: 500 nM recombinant CRM1-His6, 3 μM recombinant RanQ69L, and 500 nM LMB. (C) Sequence analysis of STRADα showing two NESs. (D) Mutations in NES sequences do not disrupt the ability of STRADα to bind LKB1. Flag-HuR, RFP-LKB1, flag-STRADα, and flag-STRADddNES were expressed in TNT reactions and labeled with [35S]methionine. Flag-tagged proteins were pulled down with M2-agarose. (E) CRM1 binding is disrupted by mutations in NES sequences. Flag-STRADα and flag-STRADddNES were expressed in HEK293T cells and immobilized on M2-agarose. CRM1 and LKB1 were expressed in TNT reaction. We added 5 μM RanQ69L and 500 nM LMB as indicated.
Figure 4.
STRADα passively diffuses into the nucleus. (A) STRADα accumulates in the nuclei of permeabilized cells in the absence of cytosolic extract and energy regenerating system. We added 3 μM recombinant OG-STRADα (His6-STRADα, labeled with Oregon Green maleimide; Invitrogen), GST-GFP-NLS, or His6-importin β-GFP to HeLa cells, permeabilized with digitonin. Cells were examined 5–10 min after addition of import substrates. We used 0.2 mg/ml 70-kDa Texas Red dextran was used as a marker for permeabilization of cytoplasmic membrane. (B) Import of STRADα is not inhibited by RanQ69L. Cells were permeabilized as described above, washed, and incubated in the presence of 3 μM of indicated import substrate, 70-kDa Texas Red fixable dextran, and rabbit reticulocyte lysate supplemented with energy regenerating system for 30 min. Recombinant RanQ69L (5 μM) was added as indicated. Cells were fixed with PFA, and then they were examined by widefield microscopy. (C) WGA inhibits nuclear accumulation of STRADα. OG-STRADα, His6-CFP-YFP, or His6-importin β-GFP was added to digitonin-permeabilized HeLa cells. Cells were incubated for 5–10 min, and then they were examined by confocal microscopy. We used 70-kDa Texas Red dextran as a control for permeabilization. Diffusion was blocked by preincubating cells with 200 μg/ml WGA for 10 min at room temperature before addition of the diffusion substrates. Bar, 20 μm. (D) STRADα does not bind CFP-FF18. GST (negative control), GST-importin-β (positive control), and GST-STRADα were immobilized on GSH-beads, CFP-FF18, and myc-LKB1 were expressed in TNT reaction. Binding reaction was performed as described in Materials and Methods.
These observations suggest that STRADα import does not require soluble transport factors. We next performed the import assay in the presence of a RRL, which contains soluble transport factors and an energy regenerating system. Under these conditions, active import can be blocked by addition of Ran(Q69L), a dominant interfering mutant of Ran, that collapses RanGTP gradient across the nuclear envelope. Both the GGNLS and OG-STRADα, but not the dextran, accumulated in the nuclei upon addition of RRL, demonstrating that the proteins and cells were functional (Figure 4B). However, although GGNLS import was blocked by addition of Ran(Q69L), the nuclear accumulation of STRADα was not inhibited (Figure 4B). We conclude that STRADα enters the nucleus either by passive or facilitated diffusion, but not active import.
We next examined the effect of WGA. WGA is a lectin that binds glycosylated nucleoporins and inhibits facilitated diffusion of transport receptors by obstructing their interactions with nucleoporins. It has been reported to have no effect on the passive diffusion of molecules through the nuclear pores (Finlay et al., 1989). As expected, pretreatment of cells with 200 μg/ml WGA inhibited importin-β-GFP diffusion into the nucleus (Figure 4C). It also inhibited nuclear accumulation of OG-STRADα, suggesting that STRADα might use a facilitated pathway through the pores. However, the inability of WGA to inhibit passive diffusion has been tested only with molecules well below the size cut-off of the nuclear pores (Finlay et al., 1989). STRADα is near this cut-off point. Therefore, we determined the effect of WGA on the nuclear accumulation of a CFP-YFP fusion, which has a molecular mass of ∼54 kDa. Surprisingly, WGA efficiently blocked the import of this protein (Figure 4C), indicating that passive diffusion of larger proteins can be blocked by WGA. Presumably, this inhibition is caused by steric obstruction in the pores. Thus, caution should be applied in the use of WGA as a test for passive versus facilitated nuclear transport mechanisms.
As an alternative approach to determine whether STRADα uses a facilitated diffusion pathway, we asked whether STRADα binds to FF18, an FG-repeat fragment of nucleoporin nsp1p from budding yeast. GST, GST-importin-β, and GST-STRADα were immobilized on glutathione-Sepharose beads, and then they were tested for binding to CFP-FF18 and myc-LKB1. CFP-FF18 bound only to importin-β, whereas LKB1 bound only to STRADα (Figure 4D). Thus, we conclude that STRADα does not detectably interact with FG-repeat nucleoporins, and it most likely diffuses passively into the nucleus.
STRADα Is Exported from the Nucleus by CRM1
Treatment of cells with 200 nM LMB for 1 h leads to an equilibration of STRADα between the nucleus and cytoplasm, consistent with the idea that STRADα can passively diffuse through the nuclear pores but that it localizes predominantly to the cytoplasm at steady-state due to constant export by CRM1 (Figure 5A). To confirm that CRM1 indeed binds STRADα, we immobilized flag-STRADα and flag-RCC1 (as a negative control) on M2-agarose beads, and we tested their binding to recombinant CRM1-His6. STRADα bound CRM1 specifically in the presence of RanGTP, and this interaction was inhibited by LMB (Figure 5B). CRM1 recognizes leucine-rich motifs, and sequence analysis of STRADα revealed two potential NESs, one in the N terminus (NES1) and another in the C-terminal tail (NES2) (Figure 5C). The NES sequences were disrupted by substituting leucines with alanines (L27A, L29A, L421A, L424A), to create STRADddNES. These mutations lie outside of the STRADα pseudokinase domain and LKB1 binding region, but to confirm that the mutant STRADα can still associate with LKB1, we expressed flag-HuR (negative control), flag-STRADα, and flag-STRADddNES in TNT reactions, and we immobilized them on M2 beads. We then added RFP-LKB1, which had been expressed in a separate TNT reaction. RFP-LKB1 coimmunoprecipitated equally well with wild-type and mutant STRADα, but not with HuR, confirming that mutations in the NES sequences did not disrupt STRADα binding to LKB1 (Figure 5D). To determine whether mutations have disrupted CRM1 binding, flag-STRADα and flag-STRADddNES were expressed in HEK293T cells and immobilized on M2 agarose. 35S-CRM1 was expressed in a TNT reaction, and it was mixed with the beads in the presence or absence of RanQ69L and LMB. The CRM1 was unable to bind STRADddNES in the presence of RanQ69L (Figure 5E).
Disruption of CRM1–STRADα Interaction Inhibits LKB1 Export to the Cytoplasm
We coexpressed RFP-LKB1 in HeLa cells with wild-type flag-STRADα (STRADwt) or NES-defective STRAD mutants flag-STRADdNES1 (L27A, L29A), flag-STRADdNES2 (L421A, L424A), or flag-STRADddNES (L27A, L29A, L421A, L424A) at different molar ratios of STRADα to RFP-LKB1 DNA. As expected, when the relative amount of STRADα increased, the nuclear/cytoplasmic ratio of RFP-LKB1 fluorescence decreased. We found that disruption of a single NES was not sufficient to inhibit export of LKB1. STRADdNES1 and STRADdNES2 were as effective as the wild-type STRADα at localizing RFP-LKB1 to the cytoplasm. However, the STRADα mutant in which both NES sequences were disrupted failed to localize LKB1 to the cytoplasm. These results confirm that STRADα serves as an adaptor for CRM1-dependent export of LKB1 (Figure 6, A and B).
Figure 6.
Disruption of both NES sequences of STRADα abolishes its ability to localize LKB1 to the cytoplasm. RFP-LKB1 and flag-STRADα wild-type or NES mutants were transiently expressed in HeLa cells at different ratios of flag-STRADα or its mutants to RFP-LKB1. (A) Cells were fixed and immunostained to visualize flag. Nuclei were stained with DAPI. Bar, 20 μm. (B) Disruption of both NES sequences is necessary to block STRADα-stimulated export of LKB1. RFP-LKB1 fluorescence was quantified, and ratios of nuclear to cytoplasmic fluorescence (N/C) were plotted as a function of molar ratio of transfected STRADα to LKB1. Each data point represents at least 10 cells. SE bars are shown.
STRADα Also Binds to Exportin7
Inhibition of CRM1-mediated export with LMB caused only a partial accumulation of STRADα and LKB1, when coexpressed with STRADα, in the nucleus. We speculated, therefore, that a second export factor might be involved in controlling STRADα localization. To address this possibility, other known mammalian export receptors—importin13, which functions in both import and export; and exportin-t, exportin4, exportin5, exportin6, and exportin7/RanBP16 and its close homologue RanBP17—were tested for STRADα binding (data not shown). These receptors were cloned into expression vectors and produced in coupled TNT reactions, then incubated with flag-STRADα in the presence or absence of GTP-bound Ran. Of the seven exportins that were tested, only exportin7 and CRM1 bound STRADα in the presence of RanQ69L (Figure 7A). LMB inhibited CRM1 binding to STRADα, but not that of exportin7 (Figure 7A). The specificity of this interaction was highlighted by the fact that the two closest mammalian homologues of exportin7, exportin4 and RanBP17, showed no Ran-dependent binding to STRADα (data not shown) (Koch et al., 2000; Kutay et al., 2000; Mingot et al., 2004).
Figure 7.
STRADα is exported from the nucleus by exportin7. (A) Exportin7 binding to STRADα is RanGTP dependent but insensitive to LMB. Flag-STRADα was expressed in HEK293T cells and immobilized on M2-agarose, 35S-CRM1 and 35S exportin7 were expressed in TNT reactions. We added5 μM of recombinant His6-RanQ69L and 500 nM LMB as indicated. (B) Exportin7 binding to flag-STRADα is not inhibited by synthetic NES peptide. Binding assays were performed as in A. Synthetic NES or NLS (negative control) peptides were added as shown. (C) Disruptions of NES sequences of STRADα prevent exportin7 binding to STRADα. Flag-STRADα and NES mutants were expressed in HEK293T cells as above. (D) C-terminal NES (NES2) of STRADα is not sufficient for exportin7 binding. Recombinant GST-C-terminal tail containing NES2 (GST-CT), and C-terminal tail with mutated NES (GST-CTdNES) were bound to beads. Exportin7 and CRM1 (positive control) were added to the beads +/− RanQ69L. (E) Deletion of the N terminus of STRADα that contains basic amino acids does not interfere with CRM1 and exportin7 binding.
The p50RhoGAP, 14-3-3s, and eIF1 are all cargoes for exportin7, but they share no obvious function, structure, or sequence similarities, and the nuclear export signal for exportin7 has not been definitively established. Nonetheless, it is distinct from the short, leucine-rich NES recognized by CRM1, and at least for p50RhoGAP and eIF1 contains positively charged residues (Mingot et al., 2004). Recognition likely involves a particular three-dimensional context, and might require multiple regions of the cargo surface. We were surprised, therefore, to discover that point mutations in the NES motifs of STRADα completely blocked exportin7 binding (Figure 7C). Importantly, however, a synthetic MVM-NES peptide, which has high affinity for CRM1, did not competitively inhibit exportin7 binding to STRADα although, as expected, it did block CRM1 binding (Figure 7B). Additionally, LMB does not inhibit exportin7 binding to STRADα (Figure 7A). The above-mentioned findings indicate that even though an intact hydrophobic sequence is necessary for the interaction of exportin7 with STRADα, it is not sufficient and that although CRM1 and exportin7 binding sites on STRADα overlap, the properties of exportin7 binding are distinct from those expected for binding to a classical NES. Moreover, it seems that, similarly to p50RhoGAP, the exportin7 binding site on STRADα is multipartite.
To confirm this hypothesis, we compared binding of exportin7 and CRM1 to the isolated C-terminal tail of STRADα-tagged with GST (Figure 7D). The GST-WT tail and GST-STRADdNES2 (L421A, L424A) mutant tail were immobilized on glutathione-Sepharose beads, and then they were incubated with export receptors CRM1 or exportin7, in the presence or absence of RanQ69L. CRM1 bound to the GST WT-tail in the presence of RanQ69L, and this binding was disrupted by mutations in the NES sequence. However, exportin7 did not bind to the WT-tail, supporting the idea that intact C-terminal and N-terminal domains are both necessary for exportin7 binding.
An examination of the N-terminus of STRADα revealed a short region containing basic residues. Because it was previously reported that exportin7 binding to eIF1 requires positively charged residues (Mingot et al., 2004), we asked whether the basic residues in STRADα participate in exportin7 binding. Therefore, we deleted the first 19 amino acids of STRADα up to, but excluding, the first leucine-rich NES. Surprisingly, this deletion did not affect exportin7 binding to STRADα (Figure 7E).
Exportin7 Contributes to Nuclear Export of LKB1
To examine whether exportin7 affects localization of LKB1 in the cells, we overexpressed RFP-LKB1 and HA-exportin7 in HEK293T cells (Figure 8A). When expressed in HEK293T cells RFP-LKB1 was almost exclusively nuclear, even though these cells express some endogenous STRADα. Coexpression of exportin7 with RFP-LKB1 increased the cytoplasmic distribution of LKB1, consistent with the notion that exportin7 can function to drive export of the LKB1-STRADα complex.
Figure 8.
(A) Exportin7 enhances cytoplasmic localization of RFP-LKB1 when coexpressed in HEK293T cells. The bars indicate SE. The p values were calculated using an unpaired t test. (B) siRNA knockdown of exportin7 enhances nuclear accumulation of RFP-LKB1 in the nucleus in response to LMB in the presence of flag-STRADα. HeLa cells were transfected with siRNA against exportin7 or control siRNA. After 48 h cells were transfected with RFP-LKB1 and flag-STRAD (1:3 M ratio of DNA). Seventy-two h after siRNA transfection, cells were treated with LMB at indicated concentrations for 30 min, fixed, and examined by widefield microscopy. RFP-LKB1 fluorescence was quantified and ratios of nuclear to cytoplasmic fluorescence (N/C) were plotted as a function of LMB concentration. Each data point represents at least 10 cells. SE bars are shown. (C) siRNA pool inhibit expression of exportin7. HEK293T cells were transfected with siRNA against exportin7 and control siRNA. Cells were transfected with pMT2SM-HA-exportin7(human) and pK-GFP control (7:1 M DNA ratio) 5 h after siRNA transfection. Twenty-four h later, cells were lysed and examined for expression of HA-exportin7 and GFP. siRNA pool against exportin7 inhibited expression of exportin7 by 50% when normalized to GFP expression.
To confirm that exportin7 is involved in regulation of the nucleocytoplasmic distribution of LKB1, we used siRNA against exportin7 in HeLa cells. It was previously shown that HeLa cells express endogenous exportin7, because 14-3-3ς-GFP, a cargo of exportin7, is localized to the cytoplasm of HeLa cells and microinjection of an antibody against exportin7 induced its nuclear accumulation (Mingot et al., 2004). Therefore, we chose to silence exportin7 expression in HeLa cells. Cells were transfected with either an siRNA pool against exportin7 or control siRNA, and 48 h later the cells were transfected with RFP-LKB1 and flag-STRADα, at 1:3M ratio. The cells were treated 24 h later with a range of concentrations of LMB (0, 50, and 100 nM) for 30 min. After fixation, cells were immunostained with anti-flag to visualize STRADα. Cells coexpressing RFP-LKB1 and flag-STRADα were imaged and the ratio of RFP-LKB1 nuclear to cytoplasmic fluorescence was calculated and plotted for each concentration of LMB. We found that at low concentrations of LMB there was a small but significantly higher fraction of cytoplasmic RFP-LKB1 in the cells lacking exportin7. Finally, we verified the ability of the siRNA pool to block expression of exportin7. Because endogenous exportin7 could not be readily detected, we transfected HEK293T cells with siRNA against exportin7 and control siRNA. Five h later, we transfected the same cells with HA-exportin7 and GFP as a control. Twenty-four h later, the cells were lysed and probed for expression of HA-exportin7 and GFP. We found that siRNA pool against exportin7 inhibited expression of exportin7 by 50%, but they did not affect expression of the GFP control.
STRADβ Does Not Mediate LKB1 Export
STRADβ is a widely expressed isoform of STRADα that shares many functional similarities (Boudeau et al., 2003). STRADβ also binds LKB1 and MO25 and induces autophosphorylation and activation of LKB1 (Boudeau et al., 2003). However, the N-terminal and C-terminal domains are not conserved between the two STRAD isoforms (Figure 9A). In particular, those residues necessary for STRADα binding to CRM1 and exportin7 are not conserved in STRADβ. Therefore, we suspected that STRADβ would not be able to efficiently localize LKB1 to the cytoplasm.
Figure 9.
STRADβ interacts with LKB1, but it does not efficiently localize it to the cytoplasm. (A) NES sequences found in STRADα are not conserved in STRADβ. (B) Expression of myc-STRADβ does not efficiently localize RFP-LKB1 to the cytoplasm. HeLa cells were transfected with 5 times excess of STRADβ or STRADα over RFP-LKB1. Nuclear and cytoplasmic fluorescence of RFP-LKB1 was quantified as in Figure 1. (C) In the presence of MO25, STRADβ localizes LKB1 to the cytoplasm less efficiently than STRADα. RFP-LKB1, STRADα, or STRADβ, and CFP-MO25 were expressed in HeLa cells. Images were obtained using emission fingerprinting (Meta; Carl Zeiss) to efficiently resolve the emission spectra of the RFP (LKB1), A488 (STRAD), and CFP (MO25). (D) STRADβ inhibits RFP-LKB1 binding to GST-importin-α3. RFP-LKB1, myc-STRADβ, and HA-MO25 were expressed and labeled with 35S by TNT. GST and GST-importin-α were immobilized on glutathione beads. RFP-LKB1, myc-STRADβ, and HA-MO25 were functional and able to assemble into the trimeric complex as demonstrated by anti-myc pulldown.
We cloned STRADβ from human kidney cDNA and confirmed that it interacts with both LKB1 and MO25 (Figure 9D). However, when STRADβ was expressed in HeLa cells with RFP-LKB1, it failed to localize LKB1 in the cytoplasm (Figure 9B). STRADβ efficiently bound LKB1 in the absence of MO25 when expressed in a TNT reaction and expressed well in HeLa cells. However, because previous report indicated that MO25 was necessary for expression of STRADβ in HEK293T cells and for binding to LKB1 (Boudeau et al., 2003), we coexpressed RFP-LKB1, myc-STRADβ, and CFP-MO25 in HeLa cells, and we examined localization of these proteins. Coexpression of CFP-MO25 with STRADβ and RFP-LKB1 reduced the nuclear/cytoplasmic ratio of LKB1, possibly by inhibition of LKB1 import. However, STRADα is more efficient than STRADβ in localizing LKB1 to the cytoplasm (Figure 9C), consistent with the notion that STRADα possesses additional mechanisms for driving LKB1 to the cytoplasm. Finally, using an in vitro binding assay, we confirmed that STRADβ inhibits LKB1 binding to importin-α3 and that MO25 enhances this effect (Figure 9E). Therefore, whereas both STRADα and STRADβ inhibit import of LKB1, they differ in their ability to drive LKB1 out of the nucleus via interaction with export receptors. Thus, we provide first evidence for functional differences between STRADα and STRADβ.
DISCUSSION
Our results provide insight into the mechanisms that determine the nucleocytoplasmic distribution of the LKB1 protein kinase. A model of this pathway is shown in Figure 10. LKB1 is imported into the nucleus by the classical importin-α/β pathway. In the absence of STRADα, LKB1 is exclusively nuclear, and it does not shuttle between the nucleus and the cytoplasm, consistent with a lack of any nuclear export signals in LKB1. STRADα induces cytoplasmic localization of LKB1 by two mechanisms. First, it diffuses into the nucleus, and it serves as an adaptor to couple LKB1 to two karyopherins, CRM1 and exportin7, which drive the export of LKB1 from the nucleus. Second, STRADα binding to LKB1 blocks reimport of the complex by competitively inhibiting LKB1 binding to importin-α/β. MO25 facilitates the effects of STRADα by enhancing its affinity for LKB1, but MO25 is not directly involved in nuclear transport of the active LKB1 complex. Although both STRADα and MO25α seem to diffuse passively through the nuclear pores, STRADα and MO25-STRADα complex are also actively exported by CRM1 and exportin7. The ability of STRADα to diffuse passively through the nuclear pores allows it to escape the nucleus when its active export is blocked by Leptomycin B, or by mutation of its exportin recognition sequences. Nonetheless, there was no significant reduction in nuclear staining when reticulocyte lysate, which contains Crm1, was added to the permeabilized cells (Figure 4). This absence of detectable export most likely occurs because of the high concentration of OG-STRADα in the incubation mix, and because STRADα binds to nucleoli in the permeabilized cells.
Figure 10.
A model of the mechanism of nucleocytoplasmic shuttling of LKB1 in the presence of STRADα and MO25. LKB1 is imported into the nucleus by importins-α and -β. STRADα and MO25 diffuse into the nucleus by passive diffusion. In the nucleus, STRADα binds exportin7 or CRM1 and is exported to the cytoplasm. STRADα can bind LKB1 both in the nucleus and the cytoplasm and both of these interactions lead to cytoplasmic localization of LKB1. In the nucleus, STRADα serves as an adaptor between LKB1 and export receptors CRM1 and exportin7. In the cytoplasm, STRADα inhibits importin-α and -β binding to LKB1. MO25 stabilizes STRADα–LKB1 complex, but it is not directly involved in its nuclear transport. MO25-STRADα complex can also be exported from the nucleus by CRM1 and exportin7.
STRADα is the first known cargo protein for both CRM1 and exportin7. Remarkably, the exportin7 and CRM1 binding sites overlap, but both the C-terminal and N-terminal domains of STRADα are necessary for binding to exportin7, whereas an individual NES can bind CRM1. We speculate that, as for other known exportin7 cargoes, STRADα is recognized through a multipartite, three-dimensional surface, and that the mutations in the leucine-rich NES perturb this surface. Why some karyopherins use simple linear recognition motifs but others use a more complicated docking site remains to be understood. It is also unclear why two export receptors are necessary and what is the biological significance of exportin7 involvement in the nuclear export of STRADα and LKB1-STRADα complex. Clearly, Crm1 is the major pathway for export, at least in HeLa cells, because mutation of the single N-terminal NES, which will block exportin7 binding, did not significantly alter the nuclear/cytoplasmic ratio of LKB1. However, exportin7 function was revealed when Crm1 was inhibited by addition of LMB.
Exportin7 is a widely expressed protein with the highest expression levels in testis, thyroid and bone marrow (Koch et al., 2000). Interestingly, it was recently found to be one of only three differentially expressed genes in the process of terminal erythroid differentiation (Koury et al., 2007), suggesting a potentially complex role in signaling. An intriguing possibility is that STRADα/LKB1 export is driven primarily by exportin7 in certain tissues, and by Crm1 in others, and that the exportin7 might direct STRADα/LKB1 complex to a specific subset of targets for phosphorylation.
LKB1 seems to be a multitasking protein kinase regulating a variety of biological processes. It is plausible that translocation between various cellular compartments allows for an added level of spatial regulation of its activity (Kau et al., 2004). The activities of many signaling proteins associated with cancer are regulated by nucleocytoplasmic shuttling (Fabbro and Henderson, 2003; Kau et al., 2004; Terry et al., 2007). It was previously thought that inactive LKB1 is sequestered in the nucleus, whereas it is activated and anchored in the cytoplasm by STRADα where it is biologically active. Our findings paint a more complex picture by demonstrating that STRADα does not simply anchor LKB1 in the cytoplasm but that it also stimulates dynamic nucleocytoplasmic shuttling of LKB1. This shuttling implies that there will be a small amount of active LKB1 complex always present in the nucleus, even though the steady-state level is predominantly cytoplasmic. The biological function of this nuclear active LKB1 is not well understood, but recent studies suggest that nuclear LKB1 might function in regulation of p53-dependent gene expression (Zeng and Berger, 2006; Scott et al., 2007). Significantly, STRADα was found to coimmunoprecipitate with LKB1 and p53 in the nuclear fraction of mouse embryonic fibroblast cells (Zeng and Berger, 2006).
STRADα has four splice variants and one isoform, STRADβ. An alignment of the four splice variants of STRADα showed that the variants mainly differ in their N-terminal and C-terminal regions, which are responsible for the nuclear export of STRADα and the LKB1–STRADα complex. Only one STRADα splice variant has full-length C and N termini; therefore, it is exported by both CRM1 and exportin7. The two isoforms, STRADα and STRADβ, have been considered functionally identical (Boudeau et al., 2003). However, sequence comparison revealed that N-terminal and C-terminal regions that interact with CRM1 and exportin7 in STRADα are not conserved in STRADβ. We showed that STRADβ does not localize LKB1 to the cytoplasm as efficiently as STRADα. Therefore, LKB1 complexes with the STRAD variants and isoforms will likely localize differently and engage distinct targets, providing for spatial control of LKB1 activity.
ACKNOWLEDGMENTS
We thank the following people for the generous provision of reagents: L. Pemberton and B. Paschal (University of Virginia, VA), D. Alessi (University of Dundee, United Kingdom), I. Mattaj (European Molecular Biology Organization, Germany), J. W. Janssen (University of Heidelberg, Germany), and D. Görlich (Zentrum für Molekulare Biologie, Universität Heidelberg, Germany). We also thank members of the Macara laboratory for helpful discussions. This work was supported by grant GM-50526 from the National Institutes of Health, Department of Health and Human Services. J.D. was supported in part by Medical Scientist Training Grant T32 GM-07267-27 (G. Owens, principle investigator) from the National Institutes of Health.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-05-0454) on February 6, 2008.
REFERENCES
- Adam S. A., Marr R. S., Gerace L. Nuclear protein import in permeabilized mammalian cells requires soluble cytoplasmic factors. J. Cell Biol. 1990;111:807–816. doi: 10.1083/jcb.111.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam S. A., Sterne-Marr R., Gerace L. In vitro nuclear protein import using permeabilized mammalian cells. Methods Cell Biol. 1991;35:469–482. doi: 10.1016/s0091-679x(08)60584-1. [DOI] [PubMed] [Google Scholar]
- Adam S. A., Sterne-Marr R., Gerace L. Nuclear protein import using digitonin-permeabilized cells. Methods Enzymol. 1992;219:97–110. doi: 10.1016/0076-6879(92)19013-v. [DOI] [PubMed] [Google Scholar]
- Ando R., Mizuno H., Miyawaki A. Regulated fast nucleocytoplasmic shuttling observed by reversible protein highlighting. Science. 2004;306:1370–1373. doi: 10.1126/science.1102506. [DOI] [PubMed] [Google Scholar]
- Askjaer P., et al. RanGTP-regulated interactions of CRM1 with nucleoporins and a shuttling DEAD-box helicase. Mol. Cell Biol. 1999;19:6276–6285. doi: 10.1128/mcb.19.9.6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askjaer P., Jensen T. H., Nilsson J., Englmeier L., Kjems J. The specificity of the CRM1-Rev nuclear export signal interaction is mediated by RanGTP. J. Biol. Chem. 1998;273:33414–33422. doi: 10.1074/jbc.273.50.33414. [DOI] [PubMed] [Google Scholar]
- Baas A. F., Boudeau J., Sapkota G. P., Smit L., Medema R., Morrice N. A., Alessi D. R., Clevers H. C. Activation of the tumour suppressor kinase LKB1 by the STE20-like pseudokinase STRAD. EMBO J. 2003;22:3062–3072. doi: 10.1093/emboj/cdg292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas A. F., Kuipers J., van der Wel N. N., Batlle E., Koerten H. K., Peters P. J., Clevers H. C. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell. 2004;116:457–466. doi: 10.1016/s0092-8674(04)00114-x. [DOI] [PubMed] [Google Scholar]
- Black B. E., Holaska J. M., Levesque L., Ossareh-Nazari B., Gwizdek C., Dargemont C., Paschal B. M. NXT1 is necessary for the terminal step of Crm1-mediated nuclear export. J. Cell Biol. 2001;152:141–155. doi: 10.1083/jcb.152.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudeau J., Baas A. F., Deak M., Morrice N. A., Kieloch A., Schutkowski M., Prescott A. R., Clevers H. C., Alessi D. R. MO25alpha/beta interact with STRADalpha/beta enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. EMBO J. 2003;22:5102–5114. doi: 10.1093/emboj/cdg490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudeau J., Miranda-Saavedra D., Barton G. J., Alessi D. R. Emerging roles of pseudokinases. Trends Cell Biol. 2006;16:443–452. doi: 10.1016/j.tcb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Boudeau J., Scott J. W., Resta N., Deak M., Kieloch A., Komander D., Hardie D. G., Prescott A. R., van Aalten D. M., Alessi D. R. Analysis of the LKB1-STRAD-MO25 complex. J. Cell Sci. 2004;117:6365–6375. doi: 10.1242/jcs.01571. [DOI] [PubMed] [Google Scholar]
- Burack W. R., Shaw A. S. Live cell imaging of ERK and MEK: simple binding equilibrium explains the regulated nucleocytoplasmic distribution of ERK. J. Biol. Chem. 2005;280:3832–3837. doi: 10.1074/jbc.M410031200. [DOI] [PubMed] [Google Scholar]
- Chen T., Brownawell A. M., Macara I. G. Nucleocytoplasmic shuttling of JAZ, a new cargo protein for exportin-5. Mol. Cell Biol. 2004;24:6608–6619. doi: 10.1128/MCB.24.15.6608-6619.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbro M., Henderson B. R. Regulation of tumor suppressors by nuclear-cytoplasmic shuttling. Exp. Cell Res. 2003;282:59–69. doi: 10.1016/s0014-4827(02)00019-8. [DOI] [PubMed] [Google Scholar]
- Finlay D. R., Newmeyer D. D., Hartl P. M., Horecka J., Forbes D. J. Nuclear transport in vitro. J. Cell Sci. Suppl. 1989;11:225–242. doi: 10.1242/jcs.1989.supplement_11.17. [DOI] [PubMed] [Google Scholar]
- Fornerod M., Ohno M., Yoshida M., Mattaj I. W. Crm1 Is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Asano S., Nakamura T., Adachi M., Yoshida M., Yanagida M., Nishida E. Crm1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- Hemminki A., et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–187. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- Johnston A. M., Naselli G., Gonez L. J., Martin R. M., Harrison L. C., DeAizpurua H. J. SPAK, a STE20/SPS1-related kinase that activates the p38 pathway. Oncogene. 2000;19:4290–4297. doi: 10.1038/sj.onc.1203784. [DOI] [PubMed] [Google Scholar]
- Kau T. R., Way J. C., Silver P. A. Nuclear transport and cancer: from mechanism to intervention. Nat. Rev. Cancer. 2004;4:106–117. doi: 10.1038/nrc1274. [DOI] [PubMed] [Google Scholar]
- Khokhlatchev A. V., Canagarajah B., Wilsbacher J., Robinson M., Atkinson M., Goldsmith E., Cobb M. H. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell. 1998;93:605–615. doi: 10.1016/s0092-8674(00)81189-7. [DOI] [PubMed] [Google Scholar]
- Koch P., Bohlmann I., Schafer M., Hansen-Hagge T. E., Kiyoi H., Wilda M., Hameister H., Bartram C. R., Janssen J. W. Identification of a novel putative Ran-binding protein and its close homologue. Biochem. Biophys. Res. Commun. 2000;278:241–249. doi: 10.1006/bbrc.2000.3788. [DOI] [PubMed] [Google Scholar]
- Koury S., et al. Differential gene expression during terminal erythroid differentiation. Genomics. 2007;90:574–582. doi: 10.1016/j.ygeno.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo N., Matsumori N., Taoka H., Fujiwara D., Schreiner E. P., Wolff B., Yoshida M., Horinouchi S. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl. Acad. Sci. USA. 1999;96:9112–9117. doi: 10.1073/pnas.96.16.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo N., Wolff B., Sekimoto T., Schreiner E. P., Yoneda Y., Yanagida M., Horinouchi S., Yoshida M. Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp. Cell Res. 1998;242:540–547. doi: 10.1006/excr.1998.4136. [DOI] [PubMed] [Google Scholar]
- Kutay U., Hartmann E., Treichel N., Calado A., Carmo-Fonseca M., Prehn S., Kraft R., Gorlich D., Bischoff F. R. Identification of two novel RanGTP-binding proteins belonging to the importin beta superfamily. J. Biol. Chem. 2000;275:40163–40168. doi: 10.1074/jbc.M006242200. [DOI] [PubMed] [Google Scholar]
- Liang J., et al. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat. Cell Biol. 2007;9:218–224. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- Lindsay M. E., Plafker K., Smith A. E., Clurman B. E., Macara I. G. Npap60/Nup50 is a tri-stable switch that stimulates importin-alpha:beta-mediated nuclear protein import. Cell. 2002;110:349–360. doi: 10.1016/s0092-8674(02)00836-x. [DOI] [PubMed] [Google Scholar]
- Lizcano J. M., et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macara I. G. Transport into and out of the nucleus. Microbiol. Mol. Biol. Rev. 2001;65:570–594. doi: 10.1128/MMBR.65.4.570-594.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi Y., Fukuda M., Nishida E. Evidence for existence of a nuclear pore complex-mediated, cytosol-independent pathway of nuclear translocation of ERK MAP kinase in permeabilized cells. J. Biol. Chem. 2001;276:41755–41760. doi: 10.1074/jbc.M106012200. [DOI] [PubMed] [Google Scholar]
- Milburn C. C., Boudeau J., Deak M., Alessi D. R., van Aalten D. M. Crystal structure of MO25 alpha in complex with the C terminus of the pseudo kinase STE20-related adaptor. Nat. Struct. Mol. Biol. 2004;11:193–200. doi: 10.1038/nsmb716. [DOI] [PubMed] [Google Scholar]
- Mingot J. M., Bohnsack M. T., Jakle U., Gorlich D. Exportin 7 defines a novel general nuclear export pathway. EMBO J. 2004;23:3227–3236. doi: 10.1038/sj.emboj.7600338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosammaparast N., Pemberton L. F. Karyopherins: from nuclear-transport mediators to nuclear-function regulators. Trends Cell Biol. 2004;14:547–556. doi: 10.1016/j.tcb.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Nezu J., Oku A., Shimane M. Loss of cytoplasmic retention ability of mutant LKB1 found in Peutz-Jeghers syndrome patients. Biochem. Biophys. Res. Commun. 1999;261:750–755. doi: 10.1006/bbrc.1999.1047. [DOI] [PubMed] [Google Scholar]
- Nishigaki K., Thompson D., Yugawa T., Rulli K., Hanson C., Cmarik J., Gutkind J. S., Teramoto H., Ruscetti S. Identification and characterization of a novel Ste20/germinal center kinase-related kinase, polyploidy-associated protein kinase. J. Biol. Chem. 2003;278:13520–13530. doi: 10.1074/jbc.M208601200. [DOI] [PubMed] [Google Scholar]
- Ossarehnazari B., Bachelerie F., Dargemont C. Evidence for a role of Crm1 In signal-mediated nuclear protein export. Science. 1997;278:141–144. doi: 10.1126/science.278.5335.141. [DOI] [PubMed] [Google Scholar]
- Pemberton L. F., Paschal B. M. Mechanisms of receptor-mediated nuclear import and nuclear export. Traffic. 2005;6:187–198. doi: 10.1111/j.1600-0854.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Ribbeck K., Gorlich D. The permeability barrier of nuclear pore complexes appears to operate via hydrophobic exclusion. EMBO J. 2002;21:2664–2671. doi: 10.1093/emboj/21.11.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna M. G., da Silva Correia J., Luo Y., Chuang B., Paulson L. M., Nguyen B., Deveraux Q. L., Ulevitch R. J. ILPIP, a novel anti-apoptotic protein that enhances XIAP-mediated activation of JNK1 and protection against apoptosis. J. Biol. Chem. 2002;277:30454–30462. doi: 10.1074/jbc.M203312200. [DOI] [PubMed] [Google Scholar]
- Scott K. D., Nath-Sain S., Agnew M. D., Marignani P. A. LKB1 catalytically deficient mutants enhance cyclin D1 expression. Cancer Res. 2007;67:5622–5627. doi: 10.1158/0008-5472.CAN-07-0762. [DOI] [PubMed] [Google Scholar]
- Setogawa T., Shinozaki-Yabana S., Masuda T., Matsuura K., Akiyama T. The tumor suppressor LKB1 induces p21 expression in collaboration with LMO4, GATA-6, and Ldb1. Biochem. Biophys. Res. Commun. 2006;343:1186–1190. doi: 10.1016/j.bbrc.2006.03.077. [DOI] [PubMed] [Google Scholar]
- Shaw R. J., Kosmatka M., Bardeesy N., Hurley R. L., Witters L. A., DePinho R. A., Cantley L. C. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. USA. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R. J., Lamia K. A., Vasquez D., Koo S. H., Bardeesy N., Depinho R. A., Montminy M., Cantley L. C. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. P., Spicer J., Smith A., Swift S., Ashworth A. The mouse Peutz-Jeghers syndrome gene Lkb1 encodes a nuclear protein kinase. Hum. Mol. Genet. 1999;8:1479–1485. doi: 10.1093/hmg/8.8.1479. [DOI] [PubMed] [Google Scholar]
- Stade K., Ford C. S., Guthrie C., Weis K. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell. 1997;90:1041–1050. doi: 10.1016/s0092-8674(00)80370-0. [DOI] [PubMed] [Google Scholar]
- Suntharalingam M., Wente S. R. Peering through the pore: nuclear pore complex structure, assembly, and function. Dev. Cell. 2003;4:775–789. doi: 10.1016/s1534-5807(03)00162-x. [DOI] [PubMed] [Google Scholar]
- Terry L. J., Shows E. B., Wente S. R. Crossing the nuclear envelope: hierarchical regulation of nucleocytoplasmic transport. Science. 2007;318:1412–1416. doi: 10.1126/science.1142204. [DOI] [PubMed] [Google Scholar]
- Tiainen M., Vaahtomeri K., Ylikorkala A., Makela T. P. Growth arrest by the LKB1 tumor suppressor: induction of p21(WAF1/CIP1) Hum. Mol. Genet. 2002;11:1497–1504. doi: 10.1093/hmg/11.13.1497. [DOI] [PubMed] [Google Scholar]
- Wei C., Amos C. I., Stephens L. C., Campos I., Deng J. M., Behringer R. R., Rashid A., Frazier M. L. Mutation of Lkb1 and p53 genes exert a cooperative effect on tumorigenesis. Cancer Res. 2005;65:11297–11303. doi: 10.1158/0008-5472.CAN-05-0716. [DOI] [PubMed] [Google Scholar]
- Weis K. Regulating access to the genome: nucleocytoplasmic transport throughout the cell cycle. Cell. 2003;112:441–451. doi: 10.1016/s0092-8674(03)00082-5. [DOI] [PubMed] [Google Scholar]
- Yokoya F., Imamoto N., Tachibana T., Yoneda Y. β-catenin can be transported into the nucleus in a Ran-unassisted manner. Mol. Biol. Cell. 1999;10:1119–1131. doi: 10.1091/mbc.10.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng P. Y., Berger S. L. LKB1 is recruited to the p21/WAF1 promoter by p53 to mediate transcriptional activation. Cancer Res. 2006;66:10701–10708. doi: 10.1158/0008-5472.CAN-06-0999. [DOI] [PubMed] [Google Scholar]