Abstract
In mammals the transfer of passive immunity from mother to young is mediated by the MHC-related receptor FcRn, which transports maternal IgG across epithelial cell barriers. In birds, maternal IgY in egg yolk is transferred across the yolk sac to passively immunize chicks during gestation and early independent life. The chicken yolk sac IgY receptor (FcRY) is the ortholog of the mammalian phospholipase A2 receptor, a mannose receptor family member, rather than an FcRn or MHC homolog. FcRn and FcRY both exhibit ligand binding at the acidic pH of endosomes and ligand release at the slightly basic pH of blood. Here we show that FcRY expressed in polarized mammalian epithelial cells functioned in endocytosis, bidirectional transcytosis, and recycling of chicken FcY/IgY. Confocal immunofluorescence studies demonstrated that IgY binding and endocytosis occurred at acidic but not basic pH, mimicking pH-dependent uptake of IgG by FcRn. Colocalization studies showed FcRY-mediated internalization via clathrin-coated pits and transport involving early and recycling endosomes. Disruption of microtubules partially inhibited apical-to-basolateral and basolateral-to-apical transcytosis, but not recycling, suggesting the use of different trafficking machinery. Our results represent the first cell biological evidence of functional equivalence between FcRY and FcRn and provide an intriguing example of how evolution can give rise to systems in which similar biological requirements in different species are satisfied utilizing distinct protein folds.
INTRODUCTION
The transfer of maternal antibodies to the gestating fetus or neonate allows for the passive acquisition of humoral immunity to antigens encountered by the mother. In mammals, this process is mediated by the neonatal Fc receptor (FcRn). FcRn was first isolated from the neonatal rodent gut (Simister and Rees, 1985) and was later shown by sequence analysis to be related to class I major histocompatibility complex (MHC) molecules (Simister and Mostov, 1989). Subsequent structural analyses demonstrated that FcRn has a narrowed and nonfunctional counterpart of the MHC peptide-binding groove and interacts with Fc in a manner that differs from MHC interactions with peptides, T-cell receptors, and other macromolecules (Burmeister et al., 1994; Martin et al., 2001). FcRn has since been identified in other mammals, including humans (Leach et al., 1996), nonhuman primates (Spiekermann et al., 2002), ruminants (Mayer et al., 2002, 2004), and marsupials (Western et al., 2003). FcRn also plays a key role in serum IgG homeostasis by serving as a protection receptor for IgG internalized via fluid phase endocytosis by the vascular endothelium, causing it to be recycled and released at the plasma membrane rather than lysosomally degraded (for review see Ghetie and Ward, 2002). The expression of FcRn across many different mammalian species coupled with its roles in bidirectional transcytosis and recycling have made it a widely used model for studies of intracellular transport (Praetor et al., 1999; McCarthy et al., 2000, 2001; Zhu et al., 2002; Ward et al., 2003; Claypool et al., 2004; Ober et al., 2004a,b; Lencer and Blumberg, 2005; Tesar et al., 2006).
Transfer of maternal immunoglobulin to offspring has long been known to occur in birds as well as mammals (Brambell, 1970). IgY, the avian and reptilian counterpart of IgG, is packaged into egg yolk and transported across yolk sac membranes during development (Kowalczyk et al., 1985). Yolk sac membranes isolated from chicken exhibit IgY-binding properties similar to those of FcRn for IgG—high-affinity binding at acidic pH and no observable binding at neutral or basic pH (Linden and Roth, 1978; Tressler and Roth, 1987)—suggesting the existence of a functionally equivalent receptor in avian species. Recently, our laboratory isolated a chicken yolk sac IgY receptor (FcRY) that exhibits pH-dependent binding to IgY (West et al., 2004). Surprisingly, FcRY shared no homology with class I MHC molecules or FcRn, but was instead a homolog of the mammalian phospholipase A2 receptor (PLA2R), a member of the mannose receptor (MR) family (East and Isacke, 2002). Mammalian MR family members mediate a variety of functions, including roles in the innate and adaptive immune systems (MR), internalization of soluble PLA2 enzymes (PLA2R), presentation of antigens to T-cells (DEC-205), and remodeling of the extracellular matrix (Endo180). FcRY shares a common domain organization with other MR family members, which consists of an N-terminal cysteine-rich domain, a single fibronectin type II (FNII) domain, 8 to 10 tandem C-type lectin-like domains (CTLDs), a single-pass transmembrane region and a short cytoplasmic tail (East and Isacke, 2002). The presence of tandem lectin-like domains does not necessarily imply lectin activity because only the MR and Endo180 bind to monosaccharides. Indeed, the CTLDs in PLA2R mediate a protein-protein interaction independently of carbohydrates (East and Isacke, 2002), and FcRY is thought to recognize IgY through a protein–protein interaction (West et al., 2004).
None of the mammalian MR family members are known to function as immunoglobulin and/or transcytotic receptors, but all exhibit clathrin-mediated endocytosis, resulting in either recycling back to the plasma membrane or delivery of cargo to the late endosome/lysosomal pathway (East and Isacke, 2002). The cytoplasmic tails of mammalian MR family members contain two putative internalization motifs: an acid-based dihydrophobic motif (Exxxφφ) and a low density lipoprotein receptor (LDLR)-like tyrosine-based internalization motif (φxNxxY; East and Isacke, 2002). The cytoplasmic tail of FcRY also includes these motifs, although the tyrosine in the LDL-like motif is substituted by a phenylalanine. Although FcRY appeared to fill all criteria for being the functional equivalent of mammalian FcRn (West et al., 2004), it had not been directly demonstrated to endocytose and transcytose IgY. Here we report creation of a cell-based model system to probe questions regarding the function of FcRY in relation to other known members of the MR family and present direct evidence that FcRY functions in endocytosis and transcytosis of IgY. These studies establish FcRY as an intriguing model of receptor-mediated transport in polarized cells and demonstrate an increased diversity of functional possibilities within the MR family.
MATERIALS AND METHODS
Antibodies and Proteins
Mouse monoclonal anti-Rab11, anti-clathrin heavy chain, and anti-EEA1 antibodies were purchased from BD Transduction Laboratories (San Jose, CA). Alexa Fluor 546– and 568—conjugated goat anti-mouse and Alexa Fluor 647–conjugated goat anti-chicken secondary antibodies were from Molecular Probes (Eugene, OR). Chicken IgY and FcY were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA) and passed over a Superdex 200 column to remove aggregates.
The purified FcRY ectodomain, expressed in baculovirus-infected insect cells and purified as described (West et al., 2004), was used as an immunogen for the production of polyclonal ascites as described previously (Ou et al., 1993). The Fc fragment of rat IgG2a (Fcγ) was expressed in Chinese hamster ovary cells and purified from culture supernatants as described (Martin and Bjorkman, 1999). For quantitative endocytosis and transport experiments, FcY and Fcγ were iodinated to a specific activity of 30 μCi/μg using Na[125I] (MP Biomedicals, Solon, OH) and IODO-GEN (Pierce, Rockford, IL). Radiolabeled ligands were buffer exchanged into HBS (25 mM HEPES, 150 mM NaCl, pH 7.4) by two subsequent passages over Zeba Desalting spin columns (Pierce). Protein concentrations were determined by BCA assay (Pierce) using bovine γ-globulin as a standard.
Cell Culture and Generation of Stable Cell Lines
Rat inner medullary collecting duct (IMCD) cells (a kind gift from Neil Simister, Brandeis University) were cultured in DMEM supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 mg/ml streptomycin under 5% CO2. Cells were fed every other day and passaged weekly. The cDNA encoding the full-length FcRY gene was subcloned into the pIRES2-EGFP mammalian expression vector (Clontech, Mountain View, CA) and transfected into IMCD cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Stable clones were selected with 500 μg/ml G418 (Invitrogen). For experiments requiring polarized cell monolayers, cells were seeded at superconfluent density (1 × 106 cells/ml) onto 12-mm Transwell polyester filters (0.4-μm pores; Corning Costar, Acton, MA) with 0.5 and 1.5 ml of media in the apical and basolateral reservoirs, respectively. Cells were fed daily beginning 2 d after initial seeding and used for experiments on the fourth or fifth day after plating.
Quantitative Endocytosis Assay
Cells were grown to near-confluence in 12-well tissue culture plates until ∼80–90% confluent. Before incubations with ligand, the cells were serum-starved for 20 min in Hank's balanced salt solution (HBSS), 1.3 mM CaCl2, 0.8 mM MgSO4 (HBSS+), 1% bovine serum albumin (BSA; Sigma, St. Louis, MO), 1 mM KI adjusted to either pH 5.9 or 8.0 with MES or HEPES (20 mM each), respectively. [125I]FcY or [125I]Fcγ was added to a final concentration of 100 nM in HBSS+ at pH 5.9 or 8.0, and plates were incubated in a 37°C circulating water bath for 45 min. For competitor studies, 20 μM unlabeled IgY was added during the preincubation and kept present throughout the experiment. After incubation with the radioligand the cells were cooled on ice, washed four times with ice-cold HBSS+ at pH 8.0 to remove surface-bound ligand, and then lysed in 0.1 N NaOH. Total protein concentration in the lysates was determined by BCA assay and the radioactivity present in the lysates was counted on a Beckman 5500 γ-counter (Fullerton, CA) and converted to picograms of protein using the specific activity of the radiolabeled ligand.
Transcytosis Assay
FcRY-IMCD and untransfected IMCD cell monolayers were grown on permeable filter supports as described previously (McCarthy et al., 2000). Polarization of monolayers was confirmed by measuring a transepithelial electrical resistance (TEER) of 350–500 Ωcm2. Filters were preincubated for 20 min with HBSS+/BSA/KI at pH 5.9 on the loading surface and HBSS+/BSA/KI at pH 8.0 on the nonloading surface. For competition experiments, unlabeled IgY (20 μM) was present in the loading surface medium during the preincubation step. Where indicated, cells were incubated with nocodazole (33 μM) for 1 h at 37°C in both the loading and nonloading surface medium. Nocodazole treatment did not alter the TEER values across the monolayers (data not shown). [125I]FcY or [125I]Fcγ was added directly to the loading media to a final concentration of 100 nM. Plates were incubated for 90 min in a 37°C circulating water bath. Media from the nonloading surface were collected and precipitated with 10% trichloroacetic acid (TCA) at 4°C. TCA-insoluble (intact ligand) fractions were counted on a Beckman 5500 γ-counter.
Recycling Assay
Filter-grown FcRY-IMCD and untransfected IMCD cells were treated as described above for the transcytosis assay. After 90 min of incubation with 125I-ligand, the filters were cooled on ice and washed six times with ice-cold HBSS+/BSA at pH 8. Prewarmed HBSS+/BSA at pH 8 was added, and the cells were returned to 37°C for 1 h. The media from the loading surface were collected, precipitated with TCA, and counted as described above.
Immunofluorescence and Microscopy
For immunofluorescence colocalization studies, FcRY-IMCD cells were grown until polarized on permeable filters. Monolayers were washed once with HBSS+/BSA and treated with IgY or FcY (500 nM in HBSS+/BSA, pH 5.9 or 8.0) for 45 min at 37°C. Cells were cooled on ice, washed twice with HBSS+/BSA, pH 8, and fixed with 4% paraformaldehyde before immunostaining. Immunofluorescence was carried out as described previously (Tesar et al., 2006). Commercial antibodies (anti-EEA1, anti-clathrin heavy chain, and anti-Rab11) were used at 1:100 dilutions. The anti-FcRY polyclonal ascites was used at 10 μg/ml. Secondary antibodies were used at a 1:500 dilution. Samples were imaged on an inverted Zeiss LSM 510 confocal microscope equipped with a Zeiss Plan-Apochromat 100× oil immersion objective (NA 1.4; Thornwood, NY). Green fluorophores were excited with the 488-nm line of an argon ion laser. Orange and far-red dyes were excited with the 543- and 633-nm lines of a He-Ne laser, respectively. Image processing was performed using the Zeiss LSM Examiner software (v. 3.2) and arranged for presentation in Photoshop 7.0 (Adobe Systems, San Jose, CA).
Potassium Depletion
Potassium depletion of FcRY-IMCD cells was performed essentially as described (McGraw and Subtil, 1999). Briefly, filter-grown FcRY-IMCD monolayers were shocked in hypotonic medium (1:1 vol/vol MEM/water) for 10 min before being incubated in potassium-free HBSS+/BSA, pH 5.9, for 30 min. Cells were then incubated for with 500 nM IgY in potassium-free HBSS+/BSA, pH 5.9, for 45 min and processed for immunofluorescence as described above.
RESULTS
Functional Expression of FcRY in IMCD Cells
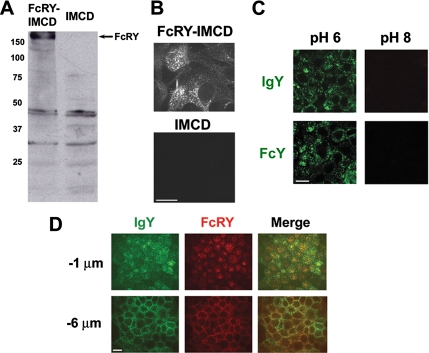
To study the behavior of FcRY in a cellular context, we prepared a polarized cell line stably expressing the full-length FcRY gene. Because an appropriate avian epithelial cell line is not presently available, we considered two mammalian cell lines for FcRY expression: Madin-Darby canine kidney (MDCK) cells and rat IMCD cells. Both cell lines can be grown as polarized monolayers on filter supports and have been used as model systems for studying transcytosis and recycling by FcRn and other receptors (Praetor et al., 1999; McCarthy et al., 2000, 2001; Wu and Simister, 2001; Claypool et al., 2002, 2004; Tesar et al., 2006). We were able to obtain expression of FcRY in IMCD cells, but not MDCK cells (data not shown); thus we proceeded to study FcRY function in IMCD cells using untransfected IMCD cells as a negative control. Western blot analysis of lysates of transfected IMCD cells (hereafter abbreviated as FcRY-IMCD) using a polyclonal ascites raised against the FcRY ectodomain detected a prominent band with an apparent molecular mass of 180 kDa (Figure 1A), corresponding to the anticipated molecular weight of FcRY. Although two nonspecific bands of ∼30 and 45 kDa were detected in FcRY-IMCD and untransfected IMCD cell lysates, confocal immunofluorescence analysis of fixed cells showed that only FcRY-IMCD cells were positively stained by the ascites under identical treatment and imaging conditions (Figure 1, B and D, red fluorescence) and that FcRY-positive staining colocalized with internalized IgY (Figure 1D, green fluorescence and merged panels). Untransfected IMCD cells do not internalize detectable amounts of IgY or FcY when subjected to the same treatment and imaging conditions as FcRY-IMCD cells (data not shown).
Figure 1.
Functional expression of FcRY in IMCD cells. (A) Anti-FcRY Western blot of detergent lysates from FcRY-transfected IMCD and untransfected IMCD cells using a polyclonal ascites raised against the FcRY ectodomain. (B) Confocal immunofluorescence of FcRY-IMCD and untransfected IMCD cells stained with anti-FcRY polyclonal ascites. Images were collected with the same laser power, exposure times, and detector settings. (C) Confocal images of FcRY-IMCD cells incubated with 500 nM Alexa 488–labeled IgY or FcY at pH 6 and 8. Internalized ligand was seen only when incubated at acidic pH. (D) Confocal images demonstrating colocalization of FcRY and IgY. Filter-grown monolayers of FcRY-IMCD cells were incubated with Alexa 488–labeled IgY at pH 6 and stained with anti-FcRY. Optical sections were taken ∼1 μm below the tight junctions (top panels) or ∼6 μm below the tight junctions (bottom panels). Scale bars, 10 μm.
We also examined pH-dependent binding and internalization of FcRY ligands by FcRY-IMCD cells. FcRY-IMCD cells were incubated with intact IgY or the Fc fragment of IgY (FcY) at pH 6 or 8 at a concentration of 500 nM. Fixed cells were imaged by confocal microscopy (Figure 1C). Both IgY and FcY were efficiently internalized at pH 6, but not at pH 8. This is consistent with previous surface plasmon resonance-based binding studies, which showed that FcRY bound to these ligands at acidic, but not basic, pH (West et al., 2004), analogous to the pH-dependent binding properties of FcRn.
Quantitative Analysis of FcRY-mediated Endocytosis
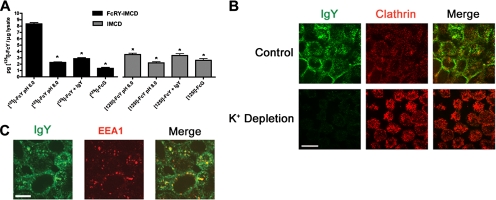
Having demonstrated that FcY and IgY could be internalized by FcRY-IMCD cells (Figure 1, C and D), we next sought to quantify endocytosis using radiolabeled FcY ([125I]FcY). Endocytosis of 100 nM [125I]FcY was observed in FcRY-expressing cells, but not in untransfected cells (Figure 2A) when incubated at pH 6 (see Materials and Methods), but not at pH 8, consistent with our confocal analysis (Figure 1C). FcRY-mediated endocytosis was specific, as we observed no endocytosis of a recombinant Fc fragment from rat IgG2a (Fcγ), which binds to FcRn but not to FcRY (West et al., 2004). As expected for a receptor-mediated process, endocytosis was saturable by addition of a 200-fold excess of unlabeled IgY to the incubation medium (Figure 2A).
Figure 2.
Quantitative endocytosis of FcY and delivery to clathrin- and EEA1-positive compartments. (A) Endocytosis of [125I]FcY by FcRY-IMCD cells. FcRY-IMCD and untransfected IMCD cells were grown to near-confluence in 12-well plates. Cells were incubated at pH 6 or 8 with 100 nM [125I]FcY or [125I]Fcγ in the presence or absence of a 200-fold excess of unlabeled IgY. After washing at pH 8 to remove surface bound ligand, cells were lysed and counted for 125I radioactivity. Bars, the mean ± SEM of triplicate measurements. *p < 0.001 relative to [125I]FcY, pH 6.0. (B) Clathrin-dependent endocytosis of IgY. Control or potassium-depleted FcRY-IMCD cells were incubated with 500 nM IgY at pH 6 and then fixed and stained with antibodies against IgY (left panels, green fluorescence) and clathrin heavy chain (center panels, red fluorescence). (C) Delivery of endocytosed IgY to EEA1-positive endosomes. IgY-treated cells were stained with antibodies against IgY (green fluorescence) and EEA1 (red fluorescence). Scale bars, 10 μm.
Clathrin-mediated Internalization and Delivery to Early Endosomes
To investigate the potential involvement of clathrin in FcRY-mediated endocytosis and the postendocytic fate of IgY, we performed confocal immunofluorescence experiments on polarized FcRY-IMCD monolayers. After incubation with 500 nM FcY at pH 6, cells were fixed and stained with an antibody against chicken IgY to visualize the internalized ligand and with antibodies against either the clathrin heavy chain or the early endosome marker EEA1. As shown in Figure 2, B and C, endocytosed IgY (green fluorescence) is present in discrete compartments throughout the cell. A single confocal section through a medial region of the monolayer showed that some clathrin-positive compartments (Figure 2B, red fluorescence) contained endocytosed IgY (yellow signal in merged image). Some of these doubly-labeled structures may represent clathrin-coated vesicles that had pinched off from the plasma membrane and were en route to deliver their contents to the subcellular endosomal network. To confirm that FcRY-mediated ligand internalization was via a clathrin-dependent pathway, we performed IgY internalization experiments using cells in which clathrin-mediated endocytosis was blocked by potassium depletion (Larkin et al., 1983). When potassium-depleted cells are treated with IgY and imaged under the same conditions as the FcRY-IMCD cells, little or no intracellular IgY was detected (Figure 2B, bottom-left panel). Staining of IgY-treated cells with anti-EEA1 (Figure 2C, red fluorescence) showed many double-positive compartments (yellow signal in merged image). Taken together, these results demonstrated that FcRY ligands were internalized via a clathrin-mediated mechanism and subsequently entered the endosomal trafficking network, most likely through postendocytotic delivery to early endosomes.
FcRY Mediates Bidirectional Transcytosis
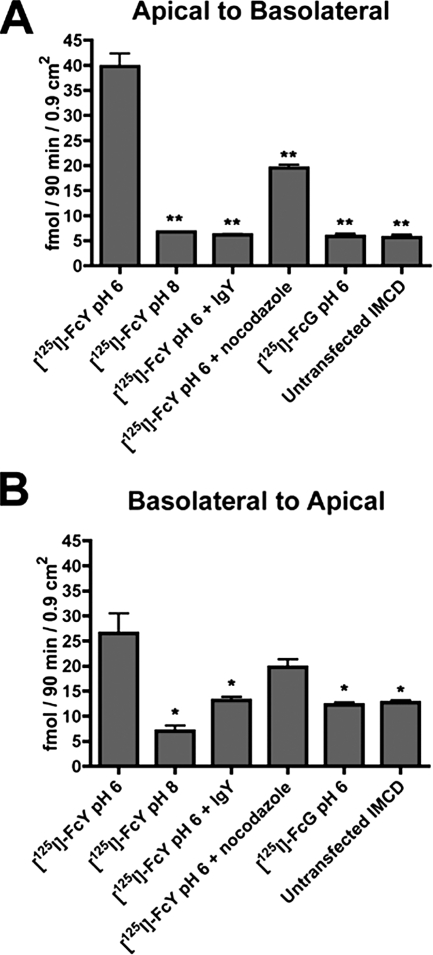
We next asked if FcRY expressed in IMCD cells could recapitulate transcytosis, a canonical in vivo function of its mammalian cognate, FcRn. Radiolabeled FcY was added to the loading surface of filter-grown monolayers, and cells were allowed to transport [125I]-FcY into the nonloading surface medium for 90 min. Transcytosis of [125I]FcY was observed in both the apical-to-basolateral and basolateral-to-apical directions of FcRY-IMCD cells, but not the control untransfected cells (Figure 3, A and B). Buffering the loading surface to pH 8 diminished transcytosis in FcRY-expressing cells to the background levels observed for untransfected cells, as did inclusion of a 200-fold excess of unlabeled competitor IgY. Radiolabeled Fcγ, which does not bind to FcRY (West et al., 2004), was not significantly transcytosed in either direction. Pretreatment of cells with the microtubule-depolymerizing agent nocodazole reduced observable transcytosis in both directions to approximately one-half of the corresponding specific (above-background) level. The reduction was statistically significant in the apical-to-basolateral direction (p = 0.0007), but not in the basolateral-to-apical direction (p = 0.0943). These results suggest that directed movement of cargo-loaded vesicles along microtubule tracts is required for efficient FcRY-mediated apical-to-basolateral transport across polarized cell monolayers.
Figure 3.
Bidirectional transcytosis of FcY. FcRY-IMCD and untransfected IMCD cells were grown as polarized monolayers on permeable filter supports. [125I]FcY or [125I]Fcγ was added to the loading surface to a final concentration of 100 nM with or without unlabeled competitor or nocodazole. Medium from the nonloading surface was collected after 90 min, and levels of [125I] radioactivity in the acid-insoluble fraction were determined. (A) Apical-to-basolateral transcytosis. (B) Basolateral-to-apical transcytosis. Both sets of histograms represent the mean ± SEM of triplicate measurements from a representative experiment. Similar results were obtained in two independent experiments. *p < 0.05, **p < 0.001 relative to [125I]FcY, pH 6.0.
Ligand Recycling by FcRY
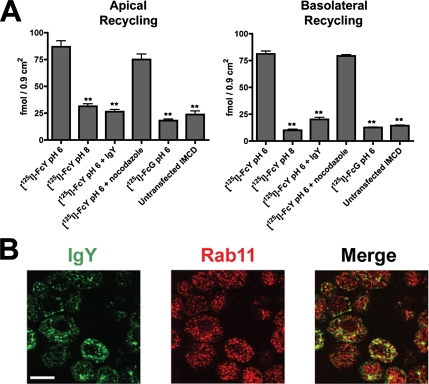
Having shown that FcRY was capable of ligand transport across polarized monolayers, we next sought to determine if endocytosed ligands could be recycled from either the apical or basolateral surfaces. Filter-grown cell monolayers were treated with [125I]FcY as described for the transcytosis assay. Cell were washed and returned to 37°C to allow recycling of internalized ligands back into the loading surface media. As shown in Figure 4A, we observed recycling at both the apical and basolateral surfaces of FcRY-expressing cells that had been incubated with ligand at pH 6. Consistent with these results, confocal immunofluorescence of fixed, IgY-treated cells using a mAb against the recycling endosome marker Rab11 (Figure 4B, red fluorescence) confirmed the presence of internalized IgY (green fluorescence) in Rab11-positive recycling compartments in FcRY-IMCD cells (Figure 4B), but not in untransfected cells (data not shown). No significant amount of recycling was observed when cells were incubated with ligand at pH 8 or in the presence of competitor IgY (Figure 4A). In addition, no recycling was observed in untransfected IMCD cells. Interestingly, treatment with nocodazole had no significant effect on [125I]FcY recycling (Figure 4A), suggesting that recycling of FcY, unlike transcytosis, does not rely on an intact microtubule network.
Figure 4.
Recycling of FcY occurs at both the apical and basolateral surfaces. (A) Recycling of [125I]FcY and [125I]Fcγ by polarized FcRY-IMCD monolayers. Cells grown as polarized monolayers on permeable filters were treated as described for the bidirectional transcytosis assay. After a 90-min incubation with a 125I-ligand, the cells were cooled on ice, washed to remove surface bound 125I-ligand, and returned to 37°C to allow recycling of internalized ligands into the loading surface medium. Data are shown as the mean ± SEM of triplicate measurements from a representative experiment in both panels. **p < 0.001 relative to [125I]FcY pH 6.0. (B) Confocal images demonstrating trafficking of IgY to Rab11-positive recycling endosomes. Filter-grown FcRY-IMCD cells were incubated with 500 nM IgY at pH 6. Cells were washed, fixed, and double-stained for IgY (green fluorescence) and the recycling endosome marker, Rab11 (red fluorescence). Scale bar, 10 μm.
DISCUSSION
The transfer of passive immunity from mother to young is a pervasive feature of most, if not all, orders of mammals. The mammalian neonatal Fc receptor, FcRn, has served as the central model for nearly all biochemical and cell biological investigations of this process to date, although it has been known for many years that this process is not unique to mammals (Brambell, 1970). The molecular characterization of the functional equivalent of FcRn in birds, the yolk sac IgY receptor FcRY, yielded the unexpected result that FcRn and FcRY were structurally distinct; FcRn being a homolog of class I MHC molecules, and FcRY being a homolog of the mammalian MR family member PLA2R (West et al., 2004). Biochemical experiments using a recombinant FcRY ectodomain showed that it bound to the Fc region of IgY with the expected pH dependence of an FcRn analog (West et al., 2004), but whether FcRY could recapitulate FcRn function in a cellular context remained unclear. Here we describe the development of a cell culture model with which to study the cellular function of FcRY using stably-transfected IMCD cells.
Our FcRY-expressing IMCD cells specifically and saturably endocytosed FcY when it was added at pH 6, but not pH 8, as observed previously for endocytosis of Fcγ by FcRn expressed in transfected cell lines (Ellinger et al., 1999; McCarthy et al., 2000; Claypool et al., 2004; Tesar et al., 2006). In addition to specific endocytosis of FcY, FcRY-expressing IMCD cells bidirectionally transcytosed FcY across filter-grown polarized monolayers in a saturable and specific manner. To our knowledge, this represents the first observation of transcytosis by a member of the MR family. Additionally, our results suggest that key components of the cellular endocytotic/transcytotic machinery are conserved in avians and mammals.
FcRn is one of the few transcytotic receptors known to carry out physiologically relevant apical-to-basolateral transcytosis. Quantitative analyses of transcytosis mediated by rat FcRn expressed in IMCD (McCarthy et al., 2000) and MDCK cells (Tesar et al., 2006) have shown that rat FcRn transports significantly more ligand in the apical-to-basolateral than in the basolateral-to-apical direction, consistent with its role in transporting IgG in ingested milk from the luminal (apical) surface of the intestinal epithelium of newborn rodents, where the pH is acidic, to the serosal (basolateral) surface, which is exposed to the slightly basic pH of the bloodstream. By contrast, human FcRn transcytoses IgG more efficiently in the basolateral-to-apical direction when expressed in transfected cells (Claypool et al., 2002, 2004), perhaps owing to its purported role in transferring antibodies from the host circulation into luminal secretions by basolateral-to-apical transcytosis. Once in the lumen, the antibodies can complex with antigens, which can then be presented to dendritic cells in the host circulation after reverse transcytosis (Yoshida et al., 2004). Whether this difference between the human and rat receptors gives rise to differences in the functions of these molecules in their respective in vivo settings remains unclear. In the case of FcRY, a preferred apical-to-basolateral directionality makes sense in light of the fact that the pH-dependent interaction between FcRY and IgY would then dictate unidirectional transport from the apical surface of yolk sac membrane cells, where the pH of the yolk is ∼6 (Cutting and Roth, 1973), to the basolateral surface leading to the circulation of the developing chick, where the pH is ∼7.4 (Dawes and Simkiss, 1969). Accordingly, we have shown that FcRY-expressing IMCD cells transcytose more FcY in the apical-to-basolateral than in the basolateral-to-apical direction (Figure 3, A and B).
Biochemical and biophysical characterization of recombinant FcRY suggested that the molecule undergoes a conformational change at pH 6, assuming a more compact form that binds IgY compared with an elongated form at basic pH that does not bind IgY (West et al., 2004). This is consistent with recent electron microscopy studies of the mannose receptor and Endo180, which revealed that these molecules undergo significant conformational changes upon incubation at acidic pH, with the mannose receptor in particular assuming a more compact conformation at low pH (Rivera-Calzada et al., 2003; Boskovic et al., 2006). Although the functional relevance of the conformational change is unclear for the mammalian MR family members, the body of evidence strongly suggests that the pH-dependent conformational change in FcRY is required for its function in delivery of maternal IgY to the chick. Thus acidic pH, either in endosomes or in egg yolk, would cause FcRY to adopt a conformation that allows IgY binding, whereas the slightly basic pH of the bloodstream would result in a conformation that promotes IgY release. In general, pH-dependent conformational changes in MR family members could serve as a driving force for activity modulation; e.g., by controlling binding and dissociation of a receptor-ligand complex, activation of signal cascades through the receptor, and/or mediation of downstream trafficking events. Unlike FcRY, mammalian MR family members must be capable of binding to their cognate ligands at the neutral/slightly basic pH of the extracellular environment; e.g., PLA2R binds circulating PLA2 at the cell surface. FcRY may be unique among MR family members in that the compact conformation assumed at acidic pH is the functional ligand-binding state, whereas the elongated conformation at neutral/basic pH is not permissive for ligand binding. Because the other MR family members retain binding of their ligands at the cell surface, the lack of a practical mechanism for ligand release makes it unlikely that the mammalian MR proteins function to transcytose ligands across polarized cells.
In addition to its function in bidirectional transcytosis, we found that FcRY promoted ligand recycling at both the apical and basolateral surfaces of IMCD cells (Figure 4A). Consistent with this observation, confocal immunofluorescence experiments showed that internalized IgY was present in Rab11-positive recycling compartments (Figure 4B). Given that recycling is a common feature of MR family members (East and Isacke, 2002), these results were not unexpected. However, combined with the evidence for immunoglobulin transcytosis by FcRY and the role of mammalian FcRn in serum IgG homeostasis (Ghetie and Ward, 2002), this raises the intriguing possibility that serum IgY homeostasis in avian species is regulated by FcRY. Indeed, the observation that a greater amount of FcY was recycled than transcytosed under the same conditions (Figures 3 and 4) suggests that ligand recycling is an important part of the in vivo function of FcRY. Although mammalian FcRn can recycle serum albumin as well as IgG, and is therefore proposed to serve as a protection receptor for both types of ligand (Chaudhury et al., 2003; Kim et al., 2006; Koltun et al., 2005), FcRY does not appear to function as a protection receptor for albumin, as we observed no binding of FcRY to ovalbumin in surface plasmon resonance binding assays conducted at pH 5–6 and at concentrations of up to 10 μM (data not shown).
To investigate the importance of cytoskeletal elements in FcRY-mediated transport, we asked whether intact microtubules were required for FcRY transcytosis and recycling. Although treatment of FcRY-IMCD cells with the microtubule depolymerizing agent nocodazole appeared to reduced specific transcytosis by approximately one-half in both the apical-to-basolateral and basolateral-to-apical directions, only in the apical-to-basolateral direction was this reduction statistically significant. The incomplete inhibition of transcytosis in the absence of an intact microtubule network could indicate that diffusion allows for the proper delivery of some vesicles containing receptor-ligand complexes to the opposite plasma membrane, but that the efficiency of cargo delivery is increased by the presence of intact microtubule tracts. It should be noted, however, that in previous studies of rat FcRn expressed in IMCD cells, nocodazole treatment resulted in more complete inhibition of transcytosis in both directions (McCarthy et al., 2000), perhaps reflecting a difference in the extent to which intact microtubules are required for transcytosis mediated by these two receptors. By contrast to its effect on transcytosis, treatment with nocodazole had no observable effect on the efficiency of FcRY-mediated recycling (Figure 4). Taken together, these results indicate that, in IMCD cells, FcRY mediates trafficking of endocytosed ligands into both a microtubule-dependent transcytotic pathway and a microtubule-independent recycling pathway.
FcRY is an intriguing model for the evolution and function of Fc receptors and of transcytotic receptors in general. Unlike other well-characterized transcytotic receptors, FcRY and its mammalian cognate FcRn are capable of bidirectional transcytosis, owing to the utilization of pH-dependent ligand binding as an efficient mechanism to allow for ligand release upon exposure to the neutral/slightly basic pH of the extracellular environment. Additionally, FcRY represents the incorporation of a new function, transcytosis, into the repertoire of functions performed by members of the MR family. Like its mammalian counterpart FcRn, FcRY evolved from a protein fold whose original function was of no apparent relation to immunoglobulin transport. The comparison of FcRY with other MR family members and with FcRn offers a fascinating glimpse into the evolution of cross-species functional equivalence in molecules that are structurally distinct, illustrating how the key protein player within an essential and intricate system can evolve from more than one functional protein fold and, conversely, how certain folds can evolve as versatile tools to be applied in more than one functional setting.
ACKNOWLEDGMENTS
We thank Anthony West for construction of expression vectors, the Caltech Protein Expression Center for baculovirus expression of FcRY, and members of the Bjorkman lab for critical reading of the manuscript. This work was supported by the National Institutes of Health (2 R37 AI041239-06A1 to P.J.B.).
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-09-0972) on February 6, 2008.
REFERENCES
- Boskovic J., Arnold J. N., Stilion R., Gordon S., Sim R. B., Rivera-Calzada A., Wienke D., Isacke C. M., Martinez-Pomares L., Llorca O. Structural model for the mannose receptor family uncovered by electron microscopy of Endo180 and the mannose receptor. J. Biol. Chem. 2006;281:8780–8787. doi: 10.1074/jbc.M513277200. [DOI] [PubMed] [Google Scholar]
- Brambell F. W. Amsterdam: North-Holland Company; 1970. The Transmission of Passive Immunity from Mother to Young. [Google Scholar]
- Burmeister W. P., Gastinel L. N., Simister N. E., Blum M. L., Bjorkman P. J. Crystal structure at 2.2 A resolution of the MHC-related neonatal Fc receptor. Nature. 1994;372:336–343. doi: 10.1038/372336a0. [DOI] [PubMed] [Google Scholar]
- Chaudhury C., Mehnaz S., Robinson J. M., Hayton W. L., Pearl D. K., Roopenian D. C., Anderson C. L. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J. Exp. Med. 2003;197:315–322. doi: 10.1084/jem.20021829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claypool S. M., Dickinson B. L., Wagner J. S., Johansen F. E., Venu N., Borawski J. A., Lencer W. I., Blumberg R. S. Bidirectional transepithelial IgG transport by a strongly polarized basolateral membrane Fcgamma-receptor. Mol. Biol. Cell. 2004;15:1746–1759. doi: 10.1091/mbc.E03-11-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claypool S. M., Dickinson B. L., Yoshida M., Lencer W. I., Blumberg R. S. Functional reconstitution of human FcRn in Madin-Darby canine kidney cells requires co-expressed human beta 2-microglobulin. J. Biol. Chem. 2002;277:28038–28050. doi: 10.1074/jbc.M202367200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting J. A., Roth T. F. Changes in specific sequestration of protein during transport into the developing oocyte of the chicken. Biochim. Biophys. Acta. 1973;298:951–955. doi: 10.1016/0005-2736(73)90398-2. [DOI] [PubMed] [Google Scholar]
- Dawes C., Simkiss K. The acid-base status of the blood of the developing chick embryo. J. Exp. Biol. 1969;50:79–86. [Google Scholar]
- East L., Isacke C. M. The mannose receptor family. Biochim. Biophys. Acta. 2002;1572:364–386. doi: 10.1016/s0304-4165(02)00319-7. [DOI] [PubMed] [Google Scholar]
- Ellinger I., Schwab M., Stefanescu A., Hunziker W., Fuchs R. IgG transport across trophoblast-derived BeWo cells: a model system to study IgG transport in the placenta. Eur. J. Immunol. 1999;29:733–744. doi: 10.1002/(SICI)1521-4141(199903)29:03<733::AID-IMMU733>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Ghetie V., Ward E. S. Transcytosis and catabolism of antibody. Immunol. Res. 2002;25:97–113. doi: 10.1385/IR:25:2:097. [DOI] [PubMed] [Google Scholar]
- Kim J., Bronson C. L., Hayton W. L., Radmacher M. D., Roopenian D. C., Robinson J. M., Anderson C. L. Albumin turnover: FcRn-mediated recycling saves as much albumin from degradation as the liver produces. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290(2):G352–G360. doi: 10.1152/ajpgi.00286.2005. [DOI] [PubMed] [Google Scholar]
- Koltun M., Nikolovski J., Strong K., Nikolic-Paterson D., Comper W. D. Mechanism of hypoalbuminemia in rodents. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H1604–H1610. doi: 10.1152/ajpheart.00808.2004. [DOI] [PubMed] [Google Scholar]
- Kowalczyk K., Daiss J., Halpern J., Roth T. F. Quantitation of maternal-fetal IgG transport in the chicken. Immunology. 1985;54:755–762. [PMC free article] [PubMed] [Google Scholar]
- Larkin J. M., Brown M. S., Goldstein J. L., Anderson R. G. Depletion of intracellular potassium arrests coated pit formation and receptor-mediated endocytosis in fibroblasts. Cell. 1983;33:273–285. doi: 10.1016/0092-8674(83)90356-2. [DOI] [PubMed] [Google Scholar]
- Leach J. L., Sedmak D. D., Osborne J. M., Rahill B., Lairmore M. D., Anderson C. L. Isolation from human placenta of the IgG transporter, FcRn, and localization to the syncytiotrophoblast: implications for maternal-fetal antibody transport. J. Immunol. 1996;157:3317–3322. [PubMed] [Google Scholar]
- Lencer W. I., Blumberg R. S. A passionate kiss, then run: exocytosis and recycling of IgG by FcRn. Trends Cell Biol. 2005;15:5–9. doi: 10.1016/j.tcb.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Linden C. D., Roth T. F. IgG receptors on foetal chick yolk sac. J. Cell Sci. 1978;33:317–328. doi: 10.1242/jcs.33.1.317. [DOI] [PubMed] [Google Scholar]
- Martin W. L., Bjorkman P. J. Characterization of the 2:1 complex between the class I MHC-related Fc receptor and its Fc ligand in solution. Biochemistry. 1999;38:12639–12647. doi: 10.1021/bi9913505. [DOI] [PubMed] [Google Scholar]
- Martin W. L., West A. P., Jr, Gan L., Bjorkman P. J. Crystal structure at 2.8 A of an FcRn/heterodimeric Fc complex: mechanism of pH-dependent binding. Mol. Cell. 2001;7:867–877. doi: 10.1016/s1097-2765(01)00230-1. [DOI] [PubMed] [Google Scholar]
- Mayer B., Kis Z., Kajan G., Frenyo L. V., Hammarstrom L., Kacskovics I. The neonatal Fc receptor (FcRn) is expressed in the bovine lung. Vet. Immunol. Immunopathol. 2004;98:85–89. doi: 10.1016/j.vetimm.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Mayer B., Zolnai A., Frenyo L. V., Jancsik V., Szentirmay Z., Hammarstrom L., Kacskovics I. Redistribution of the sheep neonatal Fc receptor in the mammary gland around the time of parturition in ewes and its localization in the small intestine of neonatal lambs. Immunology. 2002;107:288–296. doi: 10.1046/j.1365-2567.2002.01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy K. M., Lam M., Subramanian L., Shakya R., Wu Z., Newton E. E., Simister N. E. Effects of mutations in potential phosphorylation sites on transcytosis of FcRn. J. Cell Sci. 2001;114:1591–1598. doi: 10.1242/jcs.114.8.1591. [DOI] [PubMed] [Google Scholar]
- McCarthy K. M., Yoong Y., Simister N. E. Bidirectional transcytosis of IgG by the rat neonatal Fc receptor expressed in a rat kidney cell line: a system to study protein transport across epithelia. J. Cell Sci. 2000;113(Pt 7):1277–1285. doi: 10.1242/jcs.113.7.1277. [DOI] [PubMed] [Google Scholar]
- McGraw T. E., Subtil A. John Wiley & Sons; 1999. Current Protocols in Cell Biology. [Google Scholar]
- Ober R. J., Martinez C., Lai X., Zhou J., Ward E. S. Exocytosis of IgG as mediated by the receptor, FcRn: an analysis at the single-molecule level. Proc. Natl. Acad. Sci. USA. 2004a;101:11076–11081. doi: 10.1073/pnas.0402970101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober R. J., Martinez C., Vaccaro C., Zhou J., Ward E. S. Visualizing the site and dynamics of IgG salvage by the MHC class I-related receptor, FcRn. J. Immunol. 2004b;172:2021–2029. doi: 10.4049/jimmunol.172.4.2021. [DOI] [PubMed] [Google Scholar]
- Ou S. K., Hwang J. M., Patterson P. H. A modified method for obtaining large amounts of high titer polyclonal ascites fluid. J. Immunol. Methods. 1993;165:75–80. doi: 10.1016/0022-1759(93)90108-j. [DOI] [PubMed] [Google Scholar]
- Praetor A., Ellinger I., Hunziker W. Intracellular traffic of the MHC class I-like IgG Fc receptor, FcRn, expressed in epithelial MDCK cells. J. Cell Sci. 1999;112(Pt 14):2291–2299. doi: 10.1242/jcs.112.14.2291. [DOI] [PubMed] [Google Scholar]
- Rivera-Calzada A., Robertson D., MacFadyen J. R., Boskovic J., Isacke C. M., Llorca O. Three-dimensional interplay among the ligand-binding domains of the urokinase-plasminogen-activator-receptor-associated protein, Endo180. EMBO Rep. 2003;4:807–812. doi: 10.1038/sj.embor.embor898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simister N. E., Mostov K. E. An Fc receptor structurally related to MHC class I antigens. Nature. 1989;337:184–187. doi: 10.1038/337184a0. [DOI] [PubMed] [Google Scholar]
- Simister N. E., Rees A. R. Isolation and characterization of an Fc receptor from neonatal rat small intestine. Eur. J. Immunol. 1985;15:733–738. doi: 10.1002/eji.1830150718. [DOI] [PubMed] [Google Scholar]
- Spiekermann G. M., Finn P. W., Ward E. S., Dumont J., Dickinson B. L., Blumberg R. S., Lencer W. I. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: functional expression of FcRn in the mammalian lung. J. Exp. Med. 2002;196:303–310. doi: 10.1084/jem.20020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesar D. B., Tiangco N. E., Bjorkman P. J. Ligand valency affects transcytosis, recycling and intracellular trafficking mediated by the neonatal Fc receptor. Traffic. 2006;7:1127–1142. doi: 10.1111/j.1600-0854.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tressler R. L., Roth T. F. IgG receptors on the embryonic chick yolk sac. J. Biol. Chem. 1987;262:15406–15412. [PubMed] [Google Scholar]
- Ward E. S., Zhou J., Ghetie V., Ober R. J. Evidence to support the cellular mechanism involved in serum IgG homeostasis in humans. Int. Immunol. 2003;15:187–195. doi: 10.1093/intimm/dxg018. [DOI] [PubMed] [Google Scholar]
- West A. P., Jr, Herr A. B., Bjorkman P. J. The chicken yolk sac IgY receptor, a functional equivalent of the mammalian MHC-related Fc receptor, is a phospholipase A2 receptor homolog. Immunity. 2004;20:601–610. doi: 10.1016/s1074-7613(04)00113-x. [DOI] [PubMed] [Google Scholar]
- Western A. H., Eckery D. C., Demmer J., Juengel J. L., McNatty K. P., Fidler A. E. Expression of the FcRn receptor (alpha and beta) gene homologues in the intestine of suckling brushtail possum (Trichosurus vulpecula) pouch young. Mol. Immunol. 2003;39:707–717. doi: 10.1016/s0161-5890(02)00260-2. [DOI] [PubMed] [Google Scholar]
- Wu Z., Simister N. E. Tryptophan- and dileucine-based endocytosis signals in the neonatal Fc receptor. J. Biol. Chem. 2001;276:5240–5247. doi: 10.1074/jbc.M006684200. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Claypool S. M., Wagner J. S., Mizoguchi E., Mizoguchi A., Roopenian D. C., Lencer W. I., Blumberg R. S. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004;20:769–783. doi: 10.1016/j.immuni.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Zhu X., Peng J., Raychowdhury R., Nakajima A., Lencer W. I., Blumberg R. S. The heavy chain of neonatal Fc receptor for IgG is sequestered in endoplasmic reticulum by forming oligomers in the absence of beta2-microglobulin association. Biochem. J. 2002;367:703–714. doi: 10.1042/BJ20020200. [DOI] [PMC free article] [PubMed] [Google Scholar]