Abstract
Paxillins are a family of conserved LIM domain-containing proteins that play important roles in the function and integrity of the actin cytoskeleton. Although paxillins have been extensively characterized by cell biological and biochemical approaches, genetic studies are relatively scarce. Here, we identify and characterize a paxillin-related protein Pxl1p in the fission yeast Schizosaccharomyces pombe. Pxl1p is a component of the fission yeast actomyosin ring, a structure that is essential for cytokinesis. Cells deleted for pxl1 display a novel phenotype characterized by a splitting of the actomyosin ring in late anaphase, leading to the formation of two rings of which only one undergoes constriction. In addition, the rate of actomyosin ring constriction is slower in the absence of Pxl1p. pxl1Δ mutants display strong genetic interactions with mutants defective in IQGAP-related protein Rng2p and mutants defective in components of the fission yeast type II myosin machinery. Collectively, these results suggest that Pxl1p might cooperate with type II myosin and Rng2p-IQGAP to regulate actomyosin ring constriction as well as to maintain its integrity during constriction.
INTRODUCTION
Paxillins are a class of focal adhesion proteins that play important roles in linking the extracellular matrix with the actin cytoskeleton in mammalian cells (Brown and Turner, 2004). In recent years, paxillins have also been identified in unicellular organisms, such as the budding yeast Saccharomyces cerevisiae and the slime mold Dictyostelium discoideum (Gao et al., 2004; Mackin et al., 2004; Bukharova et al., 2005). Previous studies in different organisms have shown that paxillins are required for cell migration and polarized cell growth (Brown and Turner, 2004). Our understanding of the function of paxillins has been advanced mostly by cellular and biochemical approaches. However, genetic analysis of paxillin function is relatively scarce.
In recent years, the fission yeast Schizosaccharomyces pombe has become an attractive model organism for the study of the actin cytoskeleton, because of its uniform actin-dependent growth pattern and division using an actomyosin-based contractile ring (Chang and Peter, 2003; Balasubramanian et al., 2004). The ability to carry out genetic analysis as well as the availability of mutants affecting the actin cytoskeleton have furthered the use of fission yeast as an attractive organism for the study of the actin cytoskeleton.
S. pombe cells are cylindrical and grow from the cell ends and divide in the medial region (Marks et al., 1986). During growth F-actin is detected at the cell ends as patches and in the form of cables along the long axis of the cell. Maintenance of cylindrical shape and directionality of growth requires F-actin and proteins such as Bud6p, For3p, Cdc42p, and Sla2p, which interact with or affect the actin cytoskeleton (Miller and Johnson, 1994; Feierbach and Chang, 2001; Glynn et al., 2001; Nakano et al., 2002; Castagnetti et al., 2005; Ge et al., 2005). Mutants defective in the function of these genes display abnormal growth patterns, leading to the formation of bent, branched, and spherical cells. On entry into mitosis, actin patches disappear from the cell ends, and reorganization of F-actin leads to the formation of an actomyosin ring structure in the middle of the cell. Position of the actomyosin ring is determined by the position of interphase nucleus and requires an anillin-related protein, Mid1p, which can shuttle out of the nucleus and interact with the type II myosin (Chang et al., 1996; Sohrmann et al., 1996; Motegi et al., 2004). Assembly of the actomyosin ring requires a number of proteins including Cdc3p, Cdc4p, Cdc8p, Cdc12p, Cdc15p, Myo2p, Rlc1p, Rng2p, and Rng3p, and these proteins are incorporated into the ring structure in a strict order (Wu et al., 2003; Balasubramanian et al., 2004). Constriction of the actomyosin ring requires a conserved GTPase-driven signaling pathway called septation initiation network (SIN). SIN components include a GTPase Spg1p, a GTPase-activating protein complex composed of Cdc16p and Byr4p, several protein kinases and regulatory subunits such as Cdc7p, Sid1p, Sid2p, Cdc14p, and Mob1p, and two scaffold proteins Sid4p and Cdc11p (Simanis, 2003; Krapp et al., 2004; Wolfe and Gould, 2005). After ring constriction a division septum grows inward from the cell cortex upon invagination of the cell membrane (Ishiguro, 1998). Physical cell separation occurs under strict transcriptional control and also requires vesicle trafficking and the integrity of F-actin (Bahler, 2005; Sipiczki, 2007).
In this study, we report the identification and characterization of a novel paxillin-related protein Pxl1p in fission yeast. We show that Pxl1p, a component of the actomyosin ring, is important for the integrity of the ring as well as for its proper constriction. Genetic analyses indicate that Pxl1p might regulate actomyosin ring function by interacting with myosin II and the IQGAP-related protein Rng2p.
MATERIALS AND METHODS
Strains, Media, and Growth Conditions
All S. pombe strains used in this study are listed in Table 1. Yeast cells were grown in rich medium (YES) or minimal medium (MM) with appropriate supplements. Genetic crosses were performed on mating plates (YPD) and double mutants were constructed by tetrad analysis. Yeast transformation was done by lithium acetate method (Okazaki et al., 1990). Other general yeast techniques were carried out using standard methods (Moreno et al., 1991). For experiments using plasmids containing the nmt81 promoter, cells were grown to log-phase in minimal medium containing 2.5 μM thiamine and then were washed three times and reinoculated in fresh minimal medium (without thiamine). The exception is the strain pxl1Δ with the plasmid pREP81-GFP-pxl1-C (see below), which was completely grown in minimal medium with 2.5 μM thiamine. Latrunculin A (LatA; BIOMOL Research Lab, Plymouth Meeting, PA) was added in cell culture at a final concentration of 25 μM to disrupt actin structures, and 100 μg/ml brefeldin A (BFA; B-7450; Molecular Probes, Eugene, OR) was used to block vesicle trafficking.
Table 1.
S. pombe strains used in this study
| Name | Genotype | Source |
|---|---|---|
| MBY102 | ade6-M210 ura4-D18 leu1-32 h+ | Laboratory collection |
| MBY103 | ade6-M216 ura4-D18 leu1-32 h− | Laboratory collection |
| MBY192 | ura4-D18 leu1-32 h− | Laboratory collection |
| MBY664 | rlc1-GFP::leu1+leu1-32 ura4-D18 ade6-M210 h+ | Laboratory collection |
| MBY977 | clp1::ura4+ura4-D18 leu1-32 ade6-M21X h+ | Laboratory collection |
| MBY3953 | pxl1::ura4+ura4-D18 leu1-32 ade6-M21X h+ | This study |
| MBY3958 | pxl1::ura4+ura4-D18 leu1-32 ade6-M21X h− | This study |
| MBY3967 | rlc1-GFP::leu1+pxl1::ura4+h− | This study |
| MBY3989 | clp1::ura4+pxl1::ura4+ura4-D18 leu1-32 | This study |
| MBY4010 | pxl1-5Gly-GFP::ura4+ade6-M210 ura4-D18 leu1-32 h+ | This study |
| MBY4011 | pxl1-5Gly-GFP::ura4+ura4-D18 leu1-32 h− | This study |
| MBY4024 | pxl1-5Gly-GFP::ura4+sid4-GFP::KanR | This study |
| MBY4025 | pxl1-5Gly-GFP::ura4+nda3-KM311 ura4-D18 | This study |
| MBY4067 | pxl1-5Gly-GFP::ura4+cdc25-22 ura4-D18 | This study |
| MBY4108 | pREP81 plasmid in pxl1::ura4+ | This study |
| MBY4109 | pREP81-pxl1 plasmid in pxl1::ura4+ | This study |
| MBY4110 | pREP81-pxl1-N plasmid in pxl1::ura4+ | This study |
| MBY4111 | pREP81-pxl1-C plasmid in pxl1::ura4+ | This study |
| MBY4144 | ace2::KanR ura4-D18 leu1-32 h− | C. R. Vázquez de Aldana |
| MBY4169 | pREP81-GFP-pxl1 plasmid in pxl1::ura4+ | This study |
| MBY4170 | pREP81-GFP-pxl1-N plasmid in pxl1::ura4+ | This study |
| MBY4171 | pREP81-GFP-pxl1-C plasmid in pxl1::ura4+ | This study |
| MBY4173 | pxl1-LIM::ura4+leu1-32 ura4-D18 h− | This study |
| MBY4185 | pxl1::ura4+myp2::his7+ura4-D18 leu1-32 ade6-M21X | This study |
| MBY4215 | pxl1::ura4+rlc1-GFP::leu1+cdc7-GFP::ura4+ | This study |
| MBY4293 | pxl1::ura4+cdc3-124 ura4-D18 ade6-M21X | This study |
| MBY4294 | pxl1::ura4+cdc8-110 ura4-D18 leu1-32 ade6-M21X | This study |
| MBY4295 | pxl1::ura4+rng3-65 (FTS65) leu1-32 ade6-M21X | This study |
| MBY4296 | pxl1::ura4+rng2-D5 (BC2) ura4-D18 leu1-32 ade6-M21X | This study |
| MBY4297 | pxl1::ura4+cdc12-112 ura4-D18 leu1-32 | This study |
| MBY4298 | pxl1::ura4+cdc15-140 ura4-D18 leu1-32 ade6-M21X | This study |
| MBY4299 | pxl1::ura4+rlc1::ura4+ura4-D18 leu1-32 ade6-M21X | This study |
| MBY4638 | pxl1-5Gly-GFP::ura4+cdc15-140 | This study |
| MBY4639 | pxl1-5Gly-GFP::ura4+cdc3-124 | This study |
| MBY4662 | clp1::ura4+ace2::KanR | This study |
| MBY4907 | rlc1-mCherry::ura4+agn1-GFP::KanR pxl1::ura4+ | This study |
| MBY4908 | rlc1-mCherry::ura4+mid1-4GFP::leu+mid1::ura4+pxl1::ura4+ | This study |
| MBY4914 | rlc1-mCherry::ura4+spn1-GFP::KanR pxl1::ura4+ | This study |
| MBY5058 | pxl1-5Gly-GFP::ura4+myo2-E1 | This study |
| MBY5060 | pxl1-5Gly-GFP::ura4+rng2-D5 | This study |
| MBY5061 | pxl1-5Gly-GFP::ura4+cdc12-112 | This study |
| MBY5124 | rlc1-GFP::leu1+pxl1::ura4+nda3-KM311 | This study |
| MBY5125 | rlc1-GFP::leu1+nda3-KM311 | This study |
Gene Deletion, GFP/mCherry-Tagging, and Plasmids
The entire pxl1 open reading frame was replaced by ura4 gene using homologous recombination (Bahler et al., 1998). The primers MOH2601 (TGATTTCTTAAGGTTGTCAACGATCCTGAATTCTTTCCGTTGATTAGTGTCCTGATAGTTGAGAAGCATATCAGCAAAGTAATACGACTCACTATAGGGC) and MOH2602 (TCCTTATAAAGCTAAAATCGATGAAAGAAGTGAAAAAATAACAAAAAAAAAAGAAAAAGAAAGTGATGAGTAAGTCTGAGAATTAACCCTCACTAAAGGG) were used to amplify the ura4 cassette from the plasmid pCDL126 (containing ura4 gene in pBSK+ vector). PCR products were transformed into a diploid wild-type strain (ura4-D18/ura4-D18, leu1-32/leu1-32, ade6-210/ade6-216). A haploid pxl1Δ strain was obtained by tetrad dissection. To delete the C-terminal LIM domains of pxl1, we first cloned a genomic fragment (nucleotide sequence between 182 and 821 base pairs) of pxl1 plus a stop codon into the pJK210 vector with the restriction enzyme sites XhoI/XbaI using the primers MOH2612 (CCGCTCGAGTAAGATGGAGCACTTAACTTTAC) and MOH2613 (GCTCTAGATTATTCAGAATTGCCTCTATAAAG), and the obtained plasmid pCDL1162 was linearized with EcoRI and transformed into a haploid wild-type strain (MBY192). For the pxl1-5Gly-GFP strain, a 0.8-kb C-terminal fragment of pxl1 was amplified using the primers MOH2577 (CGGGGTACCTAATTGTTGATCATGATTGCATTG) and MOH2618 (TCCCCCGGGTCCTCCTCCTCCTCCATCCAAATTAAACTTGACTG), and it was subcloned into the pJK210-GFP vector after being digested with restriction enzymes KpnI/SmaI. The obtained plasmid pCDL1139 was linearized with AflII and transformed into a haploid wild-type strain (MBY102 or MBY192). For the rlc1-mCherry strain, a 0.5-kb fragment of rlc1 was amplified using the primers MBY461 (GAGAGCTGGTACCTGAATGTTCTCTTCGAAGGAA) and MBY462 (GAGAGTGCCCGGGATTGCTATCTTTTGACCC), and it was subcloned into the pJK210-mCherry vector (a gift from S. Oliferenko, Temasek Life Sciences Laboratory, Singapore) after being digested with restriction enzymes KpnI/SmaI. The obtained plasmid pCDL1269 was linearized by partial digestion with BamHI and transformed into a haploid wild-type strain (MBY102 or MBY103). Correct transformants were confirmed by colony PCR. To construct the plasmid pREP81-pxl1 (pCDL1166), the full-length of pxl1 cDNA was amplified from a cDNA library using the primers MOH2614 (CGGCATATGCATTCACCAATTCCAG) and MOH2615 (CGCGGATCCTTAATCCAAATTAAACTTG) and subcloned into the pREP81 vector after digested with restriction enzymes NdeI/BamHI. Similarly, two other plasmids pREP81-pxl1-N (pCDL1167, N-terminal part: 1-765 base pairs) and pREP81-pxl1-C (pCDL1168, C-terminal part: 766-1317 base pairs) were constructed using the primers MOH2614 and MOH2616 (CGCGGATCCTTATTCAGAATTGCCTCTATAAAG), and the primers MOH2617 (CGGCATATGAAATCCTGTCATAGTTGCG) and MOH2615, respectively. To construct the green fluorescence protein (GFP) expression plasmids, a GFP fragment was inserted into the NdeI site of these plasmids to yield the plasmids pREP81-GFP-pxl1 (pCDL1183), pREP81-GFP-pxl1-N (pCDL1184), and pREP81-GFP-pxl1-C (pCDL1185). All plasmids were transformed into the pxl1Δ strain (MBY3953).
Microscopy
Fluorescence microscopy was performed as described previously (Balasubramanian et al., 1997). Epifluorescence was observed in live cells or in formaldehyde fixed cells. Cells were grown to log-phase, and samples were taken for live cell imaging or were fixed with 7% formaldehyde for immunofluorescence staining. DNA, septum, and F-actin were stained with 4′6,-diamidino-2-phenylindole (DAPI, Sigma, St. Louis, MO), aniline blue/calcofluor white (Sigma), and Alexafluor-488–conjugated phalloidin (Molecular Probes), respectively. To detect Pxl1p-GFP and Cdc4p, we used α-GFP (ab1218, Abcam, Cambridge, MA) and α-cdc4 (McCollum et al., 1995) as primary antibodies, and α-rabbit and α-mouse IgG-conjugated with either Alexa Fluor-594 or Alexa Fluor-488 (Molecular Probes) as secondary antibodies. Fluorescence microscopy was done with Olympus IX71 microscope (Melville, NY) equipped with a Photometrics CoolSnap ES camera (Tucson, AZ). Images were acquired and processed with Metamorph software (Universal Imaging, West Chester, PA). For confocal laser scanning microscopy, cells were observed on a Zeiss Meta Inverted Laser Scanning Confocal microscope (LSM510; Thornwood, NY). Images were captured and analyzed with LSM 5 browser software. Z-stack images were taken with 0.5-μm intervals and reconstructed in three dimensions using the projection module. For time-lapse imaging, cells were grown to log-phase and spotted on top of a 2% agar pad (Tran et al., 2004).
RESULTS
The S. pombe pxl1 Gene Encodes a Paxillin-related Protein and Localizes to the Division Site in an Actin-dependent Manner
Pxl1p (encoded by SPBC4F6.12) was identified from the S. pombe genome database based on its amino acid sequence similarity to the paxillin-like protein Pxl1p in S. cerevisiae (Gao et al., 2004; Mackin et al., 2004). Paxillin is a multidomain adaptor protein involved in focal adhesion signaling (Brown and Turner, 2004). Figure 1A shows the alignment of the amino acid sequence of Pxl1p from S. pombe and S. cerevisiae, as well as paxillin from chicken. LIM (Lin-11, Isl-1, Mec-3) domains are highly conserved double-zinc finger motifs present at the C-termini of paxillin family proteins, and they contain the consensus sequence C-x(2)-C-x(16,23)-H-x(2)-[CH]-x(2)-C-x(2)-C-x(16,21)-C-x(2,3)-[CHD], where x is any amino acid (Freyd et al., 1990; Michelsen et al., 1993). Similar to other proteins in the paxillin family, Pxl1p has three LIM domains at its C-terminal region, and they are located between residue 258 and 309, 318 and 360, and 378 and 428 (Figure 1B). However, no obvious conservation was detected between the N-terminal parts of these proteins.
Figure 1.
Pxl1p is a paxillin-related protein in fission yeast. (A) Sequence alignment between Pxl1p and its related proteins. ClustalW (1.82) and Boxshade (3.21) programs were used for multiple sequence alignment and shading, respectively. Identical residues are shaded in black and conservative substitutions are shaded in gray. Sp, Schizosaccharomyces pombe; Sc, S. cerevisiae; Gg, Gallus gallus. (B) Schematic representation of Pxl1p showing three C-terminal LIM domains. The location and amino acid sequences of these LIM domains are shown at the bottom. X represents any amino acid.
To determine the intracellular localization of Pxl1p, we made a pxl1-5Gly-GFP strain (referred to as pxl1-GFP, whose expression was under control of the native promoter sequence) in which one pentaglycine linker and the GFP sequence were fused to the C-terminal part of the pxl1 coding sequence. Pxlp was detected at the division site in cells undergoing cytokinesis (Figure 2A). Localization of Pxl1p is different from that of its budding yeast homolog Pxl1p, which localizes to both polarized growth sites and the cell division plane (Gao et al., 2004; Mackin et al., 2004). The intracellular distribution of Pxl1p was reminiscent of the components of the contractile actomyosin ring. To address if Pxl1p assembled into a ring structure, we used confocal microscopy and acquired Z-section images of cells expressing pxl1-GFP. Three-dimensional (3D) reconstruction of these images showed that Pxl1p-GFP indeed formed ring structures of varying diameters in cells undergoing cytokinesis (Figure 2B, cell a displays a larger ring, whereas cell b displays a smaller ring). Based on this observation, Pxl1p-GFP appeared to form a contractile ring structure during cytokinesis. To test this, we also monitored the dynamics of Pxl1p-GFP by time-lapse confocal microscopy using a fission yeast strain expressing pxl1-GFP and sid4-GFP, a spindle pole body protein that served as a marker for cell cycle progression (Chang and Gould, 2000). Time-lapse imaging showed that Pxl1p-GFP accumulated at the division site and constricted during septation (Figure 2C). Although the myosin light chain Cdc4p assembled into a ring structure earlier than Pxl1p (Figure 2D, cells a and b), these proteins colocalized during late stages of mitosis and cytokinesis (Figure 2D, cells c–f). Taken together, these data indicated that Pxl1p localizes to the division site as a ring that constricts during cytokinesis.
Figure 2.
Pxl1p localizes to the division site in cells undergoing cytokinesis. (A) Localization of Pxl1p in wild-type cells. Cells expressing pxl1-GFP were grown in YES medium at 24°C and visualized by confocal microscopy. (B) Pxl1p-GFP forms a ring structure. Z-stack images were taken for two individual cells from A and processed for 3D reconstruction, and the obtained images are shown at different angles. (C) The Pxlp ring constricts during cytokinesis. Cells expressing pxl1-GFP sid4-GFP were grown on a YES agar pad at 24°C and visualized by time-lapse confocal microscopy, and Z-stack images were taken at different time points. 3D reconstruction images with maximal projection are shown. (D) Colocalization of Pxl1p and Cdc4p at late stages of cytokinesis. Cells expressing pxl1-GFP were grown in YES medium at 24°C, and samples were fixed and stained with DAPI, α-GFP antibodies, and α-cdc4 antibodies to visualize nuclei, Pxl1p and Cdc4p, respectively. Time is indicated in min::sec. BF, bright field. Scale bar, 5 μm.
Because Pxl1p localizes to the division site and appears to be one component of the actomyosin ring, we asked whether the actin cytoskeleton and other actomyosin ring components were required for its localization to the medial ring. To test the role of F-actin in Pxl1p localization, we synchronized cdc25-22 cells expressing pxl1-GFP at G2/M by shift to the restrictive temperature of 36°C for 3.5 h and synchronously released to the permissive temperature of 24°C in the presence of LatA, which prevents actin polymerization (DMSO was used as a solvent control). After 1 h, the Pxl1p-GFP signal appeared in the medial region in most cells treated with DMSO, but this signal was not detected in the medial cortex of cells treated with LatA (Figure 3A). In addition, although Pxl1p was able to localize to actomyosin rings in wild-type cells cultured at 36°C, Pxl1p was unable to localize to the medial cortex in cdc3-124 (profilin), cdc12-112 (formin), cdc15-140 (F-BAR/FCH domain protein), rng2-D5 (IQGAP-related), and myo2-E1 (type II myosin heavy chain) mutants cultured at 36°C (Figure 3B). These data suggested that the actin cytoskeleton and a stable actomyosin ring are essential for the accumulation of Pxl1p at the division site. Pxl1p localized normally in cells treated with BFA (an inhibitor of vesicle trafficking) and in cells lacking microtubules (Figure 3C). Collectively, our experiments indicated that Pxl1p localizes to the division site in a vesicle-trafficking and microtubule-independent manner and requires F-actin and other actomyosin ring proteins for its assembly at the division site.
Figure 3.
Localization of Pxl1p depends on F-actin and essential components of the acytomyosin ring. (A) F-actin is required for the medial localization of Pxl1p. cdc25-22 cells expressing pxl1-GFP were grown at 24°C and shifted to 36°C for 3.5 h to block cells at G2 and then were released to 24°C for 1 h in the presence of latrunculin A (LatA) or DMSO. (B) Mutations in several components of the actomyosin ring disrupt Pxl1p distribution in the division site. Wild-type and mutant cells such as cdc3-124, cdc12-112, cdc15-140, rng2-D5, and myo2-E1 expressing pxl1-GFP were grown at 24°C and shifted to 36°C for 3–4 h. Images were taken both at 24 and 36°C. (C) Localization of Pxl1p is independent of vesicle trafficking and microtubules. Left panels, cdc25-22 cells expressing pxl1-GFP were treated similarly as in A except for release in the presence of brefeldin A (BFA) or ethanol. Right panels, nda3-KM311 cells expressing pxl1-GFP were grown at 32°C and shifted to 19°C for 6.5 h. Scale bar, 5 μm.
pxl1Δ Cells Display Cell Separation Defects That Might Arise from Compromised Actomyosin Ring Function
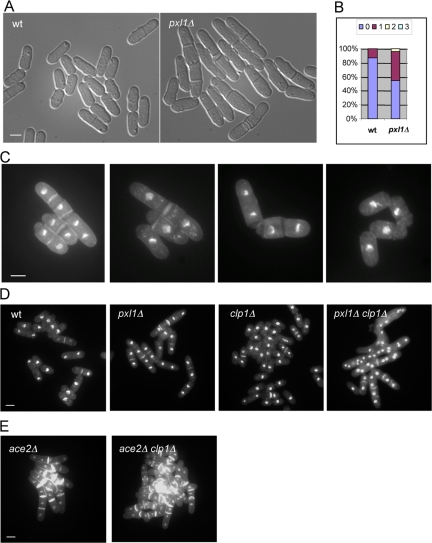
To understand the function of Pxl1p in S. pombe, a pxl1 deletion strain (referred to as pxl1Δ hereafter) was generated using PCR-based homologous recombination. The entire pxl1 open reading frame was deleted and replaced with the ura4+ marker. Cells lacking Pxl1p were viable and did not show any obvious growth defect on agar plates or in liquid medium at temperatures ranging from 24 to 36°C. Microscopic observation, however, revealed that the percentage of septated cells was significantly higher in pxl1Δ cells, compared with wild-type cells, suggesting a role for Pxl1p in cell separation (Figure 4A). To analyze this cell separation phenotype more carefully, we stained wild-type and pxl1Δ cells with DAPI and aniline blue to visualize nuclei and septum, and quantified the percentage of cells with septa as well as the number(s) of septa in individual cells. Interestingly, 42% of asynchronously growing pxl1Δ cells displayed one septum, whereas only 13% of wild-type cells possessed a septum under the same conditions (Figure 4B). In addition, 3% of pxl1Δ cells had two or three septa (Figure 4B). Figure 4C shows several examples of pxl1Δ mutant cells stained with DAPI and aniline blue. We also noticed that the cell separation phenotype varied at different temperatures and was much stronger when grown in fresh medium after reinoculation (our unpublished data). From these observations, we concluded that Pxl1p is required for efficient cell separation in S. pombe.
Figure 4.
pxl1Δ cells have cell separation defects and display genetic interaction with clp1Δ. (A) Cell separation defects in pxl1Δ mutant. Wild-type and pxl1Δ mutant cells were grown in YES medium at 24°C and observed by light microscopy. (B) Quantification of the percentage of cells with septa as well as the number(s) of septa in wild-type and pxl1Δ mutant cells (n = 500). (C) Examples of abnormal cells in the pxl1Δ mutant. pxl1Δ cells were grown at 24°C, and samples were fixed and stained with aniline blue and DAPI to visualize septa and nuclei, respectively. (D) pxl1Δ clp1Δ double mutant cells accumulate multiple nuclei in the same compartment. Cells of indicated genotypes were grown in YES medium (or YES plus 1.2 M sorbitol medium) at 24°C. Samples were fixed and stained with aniline blue and DAPI to visualize septa and nuclei, respectively. (E) ace2Δ clp1Δ double mutant cells do not accumulate multiple nuclei in the same compartment. Cells of indicated genotypes were treated as D. Scale bar, 5 μm.
The phenotype of pxl1Δ cells is similar to that of mutants defective in known components of the cell separation machinery, such as septins, Mid2p, Agn1p, and Eng1p (Berlin et al., 2003; Martin-Cuadrado et al., 2003; Tasto et al., 2003; An et al., 2004; Dekker et al., 2004; Garcia et al., 2005; Martin-Cuadrado et al., 2005). Because the septin ring structure is important for physical separation of S. pombe cells, we first examined the distribution of septin proteins in pxl1Δ mutant cells. A pxl1Δ strain expressing Spn1p-GFP fusion protein was generated and the localization of Spn1p was examined by confocal microscopy. We found that the localization of Spn1p, which localizes to single or double rings in wild-type cells, was unaffected in pxl1Δ mutant cells (Figure S1A). Furthermore, the septin rings in wild-type and pxl1Δ cells did not show any obvious differences in stability (Figure S1B). Localization of Mid2p, a protein that regulates septin dynamics, was also unaffected in cells deleted for pxl1 (Figure S1C). These data together suggest that the septin ring and its regulators are organized properly in pxl1Δ cells. The glucanases Agn1p and Eng1p localize to the division site after septation and are important for degradation of the primary septum and cell separation. We found that the localization of both Agn1p and Eng1p was unaffected in pxl1Δ cells (Figure S1, D and E). These observations established that pxl1Δ cells are not defective in localizing several known components of the cell separation machinery.
We then set out to understand the basis of the cell separation defects in pxl1Δ cells. Previous studies have shown that a defect in cell separation can arise from mutations that partially affect assembly and/or function of the actomyosin ring. For example, cells defective for the type II myosin heavy-chain Myp2p and the myosin regulatory light-chain Rlc1p exhibit a strong defect in cell separation that arises from improper assembly and function of the actomyosin ring (Bezanilla et al., 1997; Motegi et al., 1997; Le Goff et al., 2000; Naqvi et al., 2000). We therefore considered the possibility that pxl1Δ cells might be defective for actomyosin ring function. Previous studies have shown the protein phosphatase Clp1p plays an essential role in promoting fidelity of cytokinesis in cells in which the cytokinetic machinery is partially compromised (Cueille et al., 2001; Trautmann et al., 2001; Mishra et al., 2004). For example, although cells lacking Myp2p or Rlc1p are viable, loss of these proteins in the absence of Clp1p function leads to a strong and deleterious effect characterized by the accumulation of multinucleate cells and lethality (Mishra et al., 2004). We therefore tested if combined loss of Pxl1p and Clp1p led to a synthetic interaction. To this end we created double mutants lacking Pxl1p and Clp1p. Interestingly, we found that the double mutant grew more slowly compared with either single mutant (our unpublished data). To examine the phenotype of the double mutants closely, these cells were grown in YES plus 1.2 M sorbitol medium and fixed and stained with aniline blue and DAPI. We found that 37% of the pxl1Δ clp1Δ double mutant cells accumulated more than four nuclei in one compartment (n = 500), which was never found in either of the single mutants (Figure 4D). Other cell separation mutants such as ace2Δ did not display deleterious interactions with clp1Δ mutants (Figure 4E). These genetic studies, indirectly, pointed to a role for Pxl1p in actomyosin ring function.
Pxl1p Is Important for Actomyosin Ring Integrity during Cytokinesis
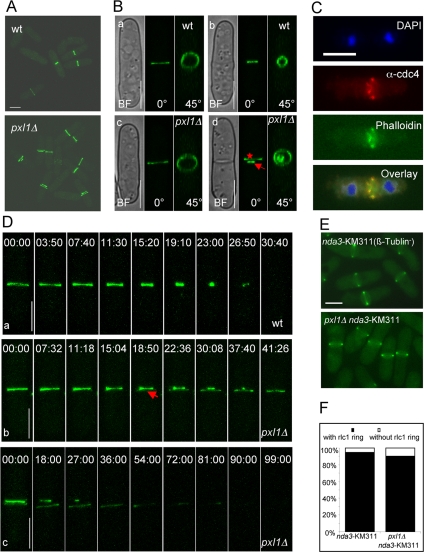
Genetic evidence from the pxl1Δ clp1Δ double mutant revealed that the actomyosin ring was not fully functional in pxl1Δ mutant cells. We therefore investigated the organization of the actomyosin rings in pxl1Δ cells. To visualize the actomyosin ring, we first introduced rlc1-GFP into the pxl1Δ mutant (Naqvi et al., 2000). Interestingly, we found that organization of Rlc1p-GFP was abnormal compared with wild-type cells. In wild-type cells, Rlc1p-GFP appeared as a broad band or a single ring structure in the medial region of the cell (Figure 5A). However, in pxl1Δ mutant cells, Rlc1p-GFP frequently appeared as a double ring structure (Figure 5A). Quantification showed that 15% of cells undergoing cytokinesis displayed this double ring structure that was never observed in wild-type cells (n = 100). Closer examination by confocal microscopy also showed that pxl1Δ cells had only a single unconstricted actomyosin ring in early mitosis, which is similar to what we observed in wild-type cells (Figure 5B, cells a and c). However, at later stages of the cell cycle, pxl1Δ cells contained one constricting ring and one unconstricted ring, which were close to each other (Figure 5B, cells b and d). In the remainder of the study, we refer to the constricting ring as the primary ring (arrow in Figure 5B, cell d), and the unconstricted ring as the secondary ring (star in Figure 5B, cell d).
Figure 5.
pxl1Δ mutant cells have a defect in the organization of the actomyosin ring. (A) pxl1Δ mutant cells have a double ring structure. Wild-type and pxl1Δ mutant cells expressing rlc1-GFP were grown at 24°C and observed by confocal microscopy. Z-stack images were taken and 3D reconstruction images with maximal projection are shown. (B) pxl1Δ mutant cells have a single unconstricted ring at early mitosis and a double ring at late stages. Individual cells from A were observed by confocal microscopy, Z-stack images were taken, and 3D reconstruction images are shown at different angles. Cells a and c, early stage. Cells b and d, late stage. Arrow indicates the primary ring and star indicates the secondary ring. (C) Cdc4p and F-actin are present in the double ring structure. pxl1Δ cells were grown in YES medium at 24°C. Samples were fixed and stained with DAPI, α-cdc4 antibodies, and phalloidin to visualize nuclei, Cdc4p, and F-actin, respectively. (D) Formation of the secondary ring structure in pxl1Δ mutant cells. Wild-type cells expressing rlc1-GFP were grown at 24°C and observed by time-lapse confocal microscopy. Z-stack images were taken at different time points, and 3D reconstruction images with maximal projection are shown panel a. pxl1Δ cells expressing rlc1-GFP were treated and observed as above (b and c). Arrow indicates the appearance of the secondary ring structure. (E) Formation of metaphase actomyosin ring is normal in pxl1Δ mutant cells. nda3-KM31 and nda3-KM311 pxl1Δ cells expressing rlc1-GFP were grown at 32°C to log-phase and shifted to 19°C for 6.5 h to arrest cells at metaphase. Images were taken by fluorescence microscopy. (F) Quantification of the number of metaphase actomyosin rings in wild-type and pxl1Δ mutant cells. Cells from E were quantified for the presence of actomyosin rings (n = 200). Time is indicated in min::sec. BF, bright field. Scale bar, 5 μm.
In fission yeast, the actomyosin ring structure is composed of more than 20 proteins, including F-actin (Wu et al., 2003; Balasubramanian et al., 2004). We therefore wondered whether the secondary ring structure also contains F-actin. To this end, we performed immunofluorescence studies to observe Cdc4p (the essential light chain of myosin II; McCollum et al., 1995) and F-actin simultaneously in pxl1Δ mutant cells. DAPI, α-cdc4 antibodies and phalloidin were used to visualize nuclei, Cdc4p and F-actin, respectively. We observed that both Cdc4p and F-actin were present in the primary and secondary ring structures in pxl1Δ mutant cells (Figure 5C). This suggested that the secondary ring structure indeed contains F-actin and myosin.
This unusual appearance of a double ring structure in pxl1Δ cells suggested two possibilities. It was possible that the secondary ring was assembled de novo. Alternatively, it was possible that splitting of a fully formed actomyosin ring generated the secondary ring. To distinguish between these possibilities, we monitored the dynamics of the actomyosin ring during the cell cycle by time-lapse microscopy in wild-type and pxl1Δ cells. In wild-type cells, actomyosin rings assembled early in mitosis and underwent orderly constriction upon mitotic exit (Figure 5D, cell a). Interestingly, in pxl1Δ cells, although assembly of the actomyosin ring occurred as in wild-type cells, these rings were found to split concomitant with initiation of constriction of the fully formed ring leading to the formation of the primary (constricting) and secondary (unconstricted) rings (Figure 5D, cell b). We also observed that the secondary ring was disassembled at later time points (Figure 5D, cell c). These results indicated that the Pxl1p is required to maintain integrity of the actomyosin ring during its constriction. The observation that metaphase arrested pxl1Δ, like metaphase arrested wild-type cells, assembled normal actomyosin rings (Figure 5, E and F), further indicated that Pxl1p function might be important only during later stages of cytokinesis.
Assessment of Localization of Various Cytokinetic Proteins to the Secondary Rings
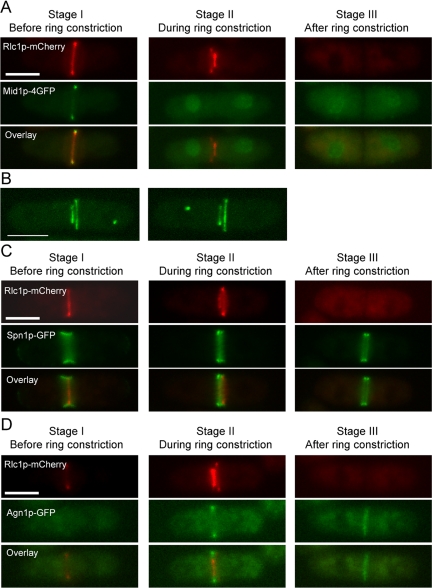
We then tested whether several proteins known to regulate various steps of cytokinesis localized to the secondary rings during constriction of the primary ring. Mid1p, a PH domain protein known to be important for ring positioning, localizes to the ring until before constriction and localizes to the nucleus during ring constriction (Chang et al., 1996; Sohrmann et al., 1996; Paoletti and Chang, 2000). Mid1p was detected in the nucleus during constriction of the primary ring and was not detected in the secondary ring (Figure 6A). Previous studies have shown that the Cdc7p-kinase localized to one spindle pole body during later stages of cytokinesis (Sohrmann et al., 1998; Krapp et al., 2004). Given the presence of two rings in pxl1Δ cells we assessed if Cdc7p was found proximal to either the primary or secondary rings. Cdc7p was randomly distributed with respect to the primary rings (Figure 6B; of 11 cells counted Cdc7p was proximal to the primary ring in six cases and to the secondary ring in five cases). Two components of the cell separation apparatus (Spn1p-septin, which assembles double rings) (Berlin et al., 2003; Tasto et al., 2003) and Agn1p (α-glucanase; Dekker et al., 2004; Garcia et al., 2005) were only found in the vicinity of the primary ring and were not obviously visualized near the secondary rings (Figure 6, C and D). These experiments established that upon constriction of the primary ring, the secondary ring remained “inert” and did not undergo constriction and did not support accumulation of proteins acting in late steps in cytokinesis.
Figure 6.
Assessment of localization of various cytokinetic proteins to the secondary rings in pxl1Δ mutant cells. (A) Mid1p localizes to the nucleus during the primary ring constriction in pxl1Δ mutant cells. pxl1Δ cells expressing rlc1-mcherry mid1-4GFP were grown at 24°C to log-phase. Samples were taken and observed by fluorescence microscopy. (B) Cdc7p was distributed randomly with respect to the primary rings in pxl1Δ mutant cells. pxl1Δ cells expressing rlc1-GFP cdc7-GFP were grown at 24°C to log phase. Samples were taken and observed by confocal laser microscopy. Z-stack images were taken and 3D reconstruction images with maximal projection are shown. (C) Spn1p localizes to the primary ring in pxl1Δ mutant cells. pxl1Δ cells expressing rlc1-mcherry spn1-GFP were treated as A. (D) Agn1p localizes to the primary ring in pxl1Δ mutant cells. pxl1Δ cells expressing rlc1-mcherry agn1-GFP were treated as A. Scale bar, 5 μm.
Pxl1p Regulates Actomyosin Ring Constriction and Exhibits Genetic Interactions with Type II Myosin
Asynchronous cultures of pxl1Δ are enriched for septating/septated cells. Because pxl1Δ cells were not defective in the localization of several cell separation molecules, we considered the possibility that Pxl1p might be important for optimal constriction of the actomyosin ring. If this were true, the percentage of cells with an actomyosin ring would be higher in mutant cells. In wild-type cells, 21% of cells from an asynchronous culture displayed an actomyosin ring. Interestingly, however, the percentage of pxl1Δ cells with an actomyosin ring was increased to 49% (Figure 7A). The presence of a higher proportion of cells with rings was consistent with the idea that the rings in pxl1Δ cells might constrict more slowly compared with those in wild-type cells. To examine this more precisely, we measured the actomyosin ring constriction rate in wild-type and pxl1Δ cells. The rate of ring closure in wild-type cells was ∼0.130 μm/min (n = 10). However, the rate in pxl1Δ mutant cells was ∼0.072 μm/min (n = 10; including three constricting rings in cells with a double ring structure; Figure 7, B and C). These data indicated that Pxl1p might play a role in proper constriction of the actomyosin ring.
Figure 7.
Rate of actomyosin ring constriction slows down in pxl1Δ mutant cells. (A) Percentage of cells with Rlc1p ring is higher in pxl1Δ mutant. Wild-type and pxl1Δ mutant cells expressing rlc1-GFP were grown at 24°C and observed by fluorescence microscopy. The number of cells with Rlc1p ring was quantified (n = 500). (B) Rate of ring constriction is constant in both wild-type and pxl1Δ mutant cells. Wild-type cells expressing rlc1-GFP were grown at 24°C and observed by time-lapse confocal microscopy. Z-stack images were taken at different time points. 3D-reconstruction images are shown at a 45° angle. The diameter of the Rlc1p ring was measured and plotted over time. Two examples from wild-type and pxl1Δ mutant cells are shown, and the rate of ring constriction was calculated using the stated formula. dn represents the diameter of the actomyosin ring at the time tn. (C) Rate of ring constriction is slower in pxl1Δ mutant cells. Wild-type and pxl1Δ mutant cells expressing rlc1-GFP were treated and observed as in B, and the calculation of the rate of ring constriction was based on the formula in B (n = 10).
To identify molecules that might interact with Pxl1p, we carried out genetic crosses between pxl1Δ and actomyosin ring assembly/function mutants cdc3-124, cdc4-8, cdc8-110, cdc12-112, cdc15-140, rng2-D5, rng3-65, myo2-E1, rlc1Δ, and myp2Δ. After examination of these double mutants, we found that pxl1Δ displays synthetic lethality with cdc4-8 and myo2-E1. In addition, we observed strong genetic interactions between pxl1Δ and rng2-D5 as well as pxl1Δ and rlc1Δ (Table 2). pxl1Δ cells did not exhibit appreciable genetic interactions with mutants such as cdc3-124, cdc8-110, cdc12-112, and cdc15-140, which are known to be defective in F-actin assembly. Thus, it appeared that Pxl1p might interact with components of the type II myosin machinery and Rng2p to regulate aspects of cytokinesis.
Table 2.
Genetic interactions
| Mutant | Cell separation phenotype (24°C) |
|---|---|
| pxl1Δ | X |
| cdc3-124 pxl1Δ | X |
| cdc4-8 pxl1Δ | SL |
| cdc8-110 pxl1Δ | XX |
| cdc12-112 pxl1Δ | X |
| cdc15-140 pxl1Δ | X |
| myo2-E1 pxl1Δ | SL |
| rng2-D5 pxl1Δ | XXXX |
| rng3-65 pxl1Δ | XXX |
| rlc1Δ pxl1Δ | XXXX |
| myp2Δ pxl1Δ | XX |
X represents the degree of cell separation defects, with XXXX being most severe defect. SL, synthetic lethal.
LIM Domains Are Required for Pxl1p Function
Pxl1p, like other members of the paxillin family, contains three tandem LIM domains at its C terminus. To determine the function of these conserved LIM domains, we generated a C-terminal truncated protein by introducing ura4+ marker into the pxl1 coding region. The strain we obtained was named pxl1-limΔ, in which the three LIM domains were deleted. Microscopic examination of pxl1-limΔ cells showed that they had the same cell separation defect as pxl1Δ cells (Figure 8A). This result indicates that LIM domains are necessary for Pxl1p function. To investigate whether LIM domains are sufficient for Pxl1p function, we also tested if the LIM domains could rescue the cell separation phenotype in the pxl1Δ mutant. To this end, several plasmids that contain pxl1 full-length, N-terminal part and C-terminal part were constructed and transformed into pxl1Δ mutant cells. Interestingly, the plasmid pREP81-pxl1-N did not rescue the mutant phenotype, whereas pREP81-pxl1-C and pREP81-pxl1 completely rescued the cell separation defect (Figure 8B). Figure 8C shows the quantification of the percentage of cells with septa as well as the number(s) of septa. Thus, LIM domains themselves are sufficient to carry out Pxl1p function. In addition, we also made GFP-tagged constructs for different parts of Pxl1p. Surprisingly, we found that both N- and C-terminal truncated proteins localized to the division site in septating cells, as did full-length Pxl1p (Figure 8D). In addition, the C-terminal part of Pxl1p that contains the three LIM domains was also present in the nucleus (Figure 8D, arrow). This suggests that targeting of Pxl1p to the division site can occur through its N-terminal part or through the LIM domains at its C terminus. Together, these data indicated that LIM domains are essential for Pxl1p function.
Figure 8.
LIM domains are required for Pxl1p function. (A) pxl1-limΔ cells show a cell separation phenotype as in pxl1Δ. Wild-type, pxl1Δ, and pxl1-limΔ cells were grown at 24°C and observed by light microscopy. (B) LIM domains can rescue the pxl1Δ phenotype. pxl1Δ cells bearing the plasmids pREP81, pREP81-pxl1, pREP81-pxl1-N, and pREP81-pxl1-C were grown at 24°C. Samples were taken and stained with aniline blue to visualize septa. (C) Quantification of the number of septa. Cells from B were quantified for the number of septa (n = 500). (D) Localization of N- and C-terminal parts of Pxl1p. pxl1Δ cells bearing the plasmids pREP81-GFP-pxl1, pREP81-GFP-pxl1-N, and pREP81-GFP-pxl1-C were grown at 24°C and observed by fluorescence microscopy. Images with Calcoflour white staining of the cell wall are shown at the bottom. Arrow indicates the nucleus localization of GFP-Pxl1p-C. Scale bar, 5 μm.
DISCUSSION
S. pombe Paxillin-related Protein, Pxl1p, Is a Component of the Actomyosin Ring
In this study, we have characterized the single paxillin-related protein from the fission yeast, Pxl1p, which like other members of this family contains 3 LIM domains. Interestingly, previous microarray analyses have shown that the transcript of Pxl1 peaks during late mitosis, suggesting a role for this protein in the regulation of actin and/or myosin function during cytokinesis (Rustici et al., 2004; Oliva et al., 2005; Peng et al., 2005). Although pxl1-transcript has been shown to undergo sixfold increase during mitosis and cytokinesis, the protein levels fluctuated less dramatically and could be at best seen to fluctuate in less than a twofold range (Figure S2). We have found that S. pombe Pxl1p is a component of the actomyosin ring, a structure that assembles during mitosis and is essential for cell division. Unlike its budding yeast, Dictyostelium, and mammalian counterparts, however, S. pombe Pxl1p was not detected at sites of cellular polarization. Because fission yeast cells lacking Pxl1p are defective in cytokinesis, but not in polarized growth, it is likely that Paxillins might be used for different physiological end points in different organisms. Whether mammalian Paxillins participate in cytokinesis has not been addressed.
In S. pombe, assembly of the actomyosin ring occurs in a series of distinct steps (Wu et al., 2003, 2006). The earliest detected protein is Mid1p, which is detected at the medial cortex, almost throughout the cell cycle. A large number of proteins, including the formin-Cdc12p, Fer-Cip domain protein Cdc15p, type II myosin components, Myo2p, Cdc4p, and Rlc1p assemble into nodes and/or spot-like structures late in G2 (Wu et al., 2006). On entry into mitosis, nodes composed of the above mentioned proteins and F-actin compact into an orthogonal actomyosin ring (Wu et al., 2006), which constricts upon completion of anaphase. Interestingly, we have been unable to detect Pxl1p in nodes in G2 cells and in rings in early mitotic (metaphase and anaphase A) cells. Thus Pxl1p localizes relatively later in mitosis compared with elements of the type II myosin machinery. Localization of Pxl1p to the division site in fission yeast requires the function of an intact F-actin cytoskeleton and other actomyosin ring components and is independent of vesicle trafficking and microtubules. Thus Formin mediated assembly of F-actin early in mitosis might help recruit Paxillin to the division site.
It has been shown that the LIM domains of paxillin can assemble at the focal adhesion site themselves, suggesting an important role of LIM domains in the distribution of paxillin (Brown and Turner, 2004). Consistent with this, we have found that the three LIM domains of Pxl1p can accumulate at the division site. Interestingly, the N-terminal region of Pxl1p also localizes to the division site. We suspect that the ability of both N-terminal part and C-terminal LIM domains to target Pxl1p to the division site helps this protein to localize efficiently. In addition, the LIM domains of Pxl1p also display strong nucleus localization. Previous studies have shown that LIM domain-containing proteins can shuttle from the nucleus to the cytoplasm, which might play a role in transcription (Brown and Turner, 2004). It is not known whether the LIM domains of Pxl1p have a function in the regulation of transcription in S. pombe.
Pxl1p Functions to Stabilize the Actomyosin Ring during Cytokinesis
What is the function of Pxl1p in the actomyosin ring? Cells lacking Pxl1p are viable. However, asynchronous cultures of pxl1Δ contain a high proportion of septating and/or septated cells. Curiously, a number of known components of the cell separation machinery display normal localization in the absence of Pxl1p, indicating that, despite the cell separation–defective phenotype, Pxl1p is unlikely to be involved in the organization of cell separation apparatus. Interestingly, a simultaneous loss of Pxl1p and the protein phosphatase Clp1p, a key element of the cytokinesis checkpoint, leads to a severe cytokinesis-defective phenotype characterized by the accumulation of a high proportion of multinucleate cells. Previous studies have shown that mutants defective in components of the actomyosin ring, such as the type II myosin heavy and light chains, display cell separation defects and similar genetic interactions with clp1Δ mutants (Mishra et al., 2004). Because mutants defective in bona fide elements of the cell separation machinery, such as ace2Δ does not give rise to multinucleated cells in combination with clp1Δ, it is likely that Pxl1p might regulate aspects of actomyosin ring function directly. Because genetic interactions are detected between pxl1Δ and myo2-E1, rng2-D5, rlc1Δ, and cdc4-8, but not with Cdc3-profilin and Cdc8-tropomyosin, it is likely that Pxl1p might affect the function of type II myosin and Rng2p during cytokinesis.
Analysis of actomyosin ring dynamics in pxl1Δ cells revealed a novel phenomenon characterized by splitting of the actomyosin ring during constriction. This, to our knowledge, is the first instance in which such a ring-splitting phenotype has been observed. We have shown that Cdc7p, which localizes to one SPB during cytokinesis is distributed randomly with respect to the primary and secondary rings. We have also shown that Mid1p, which normally leaves the actomyosin ring during constriction, is not detected in the secondary rings, upon constriction of the primary rings. Finally, we have shown that the elements of the cell separation machinery, such as Spn1p-septin and a-glucan synthase are recruited to the primary, but not the secondary, rings. Although the basis of this phenotype is not fully understood, we consider two possible explanations. First, it is possible that the actomyosin ring is composed of two or more concentric rings. Second, it is possible that the actomyosin ring may be organized as a spiral in which certain positions might be more sensitive to severing. In both of these scenarios, Pxl1p might prevent unraveling and severing of the ring structure. It also indicates that the properties of the actomyosin ring may be different before and during its constriction, and the late ring structure may be more sensitive to perturbation compared with the early mitotic ring structure.
We have also shown that the rate of actomyosin ring constriction is slower in pxl1Δ mutant cells. Cells with a single (unsplit) ring also display slow constriction, suggesting that the slow constriction is unlikely to be due to splitting of the actomyosin ring. Because genetic interactions have been detected between pxl1Δ and type II myosin mutants, Pxl1p, by modulating myosin II function, might regulate proper constriction of the actomyosin ring. However, the mechanism of this regulation is unclear because, presently, we have been unable to detect any physical interaction between Pxl1p and myosin II (our unpublished data). Because pxl1Δ cells also display genetic interactions with rng2-D5 mutants, the slow down of ring constriction rates might also reflect potential interactions between Pxl1p and Rng2p that might influence ring contractility.
In summary, we have shown that fission yeast Paxillin-related protein plays a key role in modulating myosin II and Rng2p-IQGAP function during cytokinesis. In addition, Pxl1p also ensures integrity of the actomyosin ring during cytokinesis. Future studies should investigate the potential interactions between Pxl1p, Rng2p, and myosin II, as well as to examine the molecular basis of actomyosin ring splitting in fission yeast cells lacking Pxl1p. It will also be of interest to determine if paxillins perform similar functions in metazoans.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. E. Bi (University of Pennsylvania School of Medicine, Philadelphia, PA), K. Gould (Vanderbilt University School of Medicine, Nashville, TN), I. Mabuchi (University of Tokyo, Japan), S. Oliferenko, A. Paoletti (Institut Curie, Centre de Recherche and CNRS UMR 144, Paris, France), J. Pringle (Stanford University School of Medicine, Stanford, CA), C. R. Vázquez de Aldana (Instituto de Microbiología Bioquímica, Spain), and J. Wu (The Ohio State University, Columbus, OH) for yeast strains and plasmids. We especially thank Dr. Pilar Perez for communication before publication. We also thank T. G. Chew, Y. Huang, and other members of the Temasek Life Sciences Laboratory (TLL) yeast and fungal laboratories for discussions. Special thanks are due to Dr. V. Wachtler, H. Yan, and M. Mishra for critical reading of the manuscript. This work was supported by research funds from the TLL. W.G. was supported by a fellowship from the Singapore Millennium Foundation.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-07-0715) on February 13, 2008.
REFERENCES
- An H., Morrell J. L., Jennings J. L., Link A. J., Gould K. L. Requirements of fission yeast septins for complex formation, localization, and function. Mol. Biol. Cell. 2004;15:5551–5564. doi: 10.1091/mbc.E04-07-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahler J. A transcriptional pathway for cell separation in fission yeast. Cell Cycle. 2005;4:39–41. doi: 10.4161/cc.4.1.1336. [DOI] [PubMed] [Google Scholar]
- Bahler J., Wu J. Q., Longtine M. S., Shah N. G., McKenzie A., 3rd, Steever A. B., Wach A., Philippsen P., Pringle J. R. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 1998;14:943–951. doi: 10.1002/(SICI)1097-0061(199807)14:10<943::AID-YEA292>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Balasubramanian M. K., Bi E., Glotzer M. Comparative analysis of cytokinesis in budding yeast, fission yeast and animal cells. Curr. Biol. 2004;14:R806–R818. doi: 10.1016/j.cub.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Balasubramanian M. K., McCollum D., Gould K. L. Cytokinesis in fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1997;283:494–506. doi: 10.1016/s0076-6879(97)83039-x. [DOI] [PubMed] [Google Scholar]
- Berlin A., Paoletti A., Chang F. Mid2p stabilizes septin rings during cytokinesis in fission yeast. J. Cell Biol. 2003;160:1083–1092. doi: 10.1083/jcb.200212016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla M., Forsburg S. L., Pollard T. D. Identification of a second myosin-II in Schizosaccharomyces pombe: Myp2p is conditionally required for cytokinesis. Mol. Biol. Cell. 1997;8:2693–2705. doi: 10.1091/mbc.8.12.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Turner C. E. Paxillin: adapting to change. Physiol. Rev. 2004;84:1315–1339. doi: 10.1152/physrev.00002.2004. [DOI] [PubMed] [Google Scholar]
- Bukharova T., Weijer G., Bosgraaf L., Dormann D., van Haastert P. J., Weijer C. J. Paxillin is required for cell-substrate adhesion, cell sorting and slug migration during Dictyostelium development. J. Cell Sci. 2005;118:4295–4310. doi: 10.1242/jcs.02557. [DOI] [PubMed] [Google Scholar]
- Castagnetti S., Behrens R., Nurse P. End4/Sla2 is involved in establishment of a new growth zone in Schizosaccharomyces pombe. J. Cell Sci. 2005;118:1843–1850. doi: 10.1242/jcs.02311. [DOI] [PubMed] [Google Scholar]
- Chang F., Peter M. Yeasts make their mark. Nat. Cell Biol. 2003;5:294–299. doi: 10.1038/ncb0403-294. [DOI] [PubMed] [Google Scholar]
- Chang F., Woollard A., Nurse P. Isolation and characterization of fission yeast mutants defective in the assembly and placement of the contractile actin ring. J. Cell Sci. 1996;109(Pt 1):131–142. doi: 10.1242/jcs.109.1.131. [DOI] [PubMed] [Google Scholar]
- Chang L., Gould K. L. Sid4p is required to localize components of the septation initiation pathway to the spindle pole body in fission yeast. Proc. Natl. Acad. Sci. USA. 2000;97:5249–5254. doi: 10.1073/pnas.97.10.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueille N., Salimova E., Esteban V., Blanco M., Moreno S., Bueno A., Simanis V. Flp1, a fission yeast orthologue of the S. cerevisiae CDC14 gene, is not required for cyclin degradation or rum1p stabilisation at the end of mitosis. J. Cell Sci. 2001;114:2649–2664. doi: 10.1242/jcs.114.14.2649. [DOI] [PubMed] [Google Scholar]
- Dekker N., Speijer D., Grun C. H., van den Berg M., de Haan A., Hochstenbach F. Role of the alpha-glucanase Agn1p in fission-yeast cell separation. Mol. Biol. Cell. 2004;15:3903–3914. doi: 10.1091/mbc.E04-04-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierbach B., Chang F. Roles of the fission yeast formin for3p in cell polarity, actin cable formation and symmetric cell division. Curr. Biol. 2001;11:1656–1665. doi: 10.1016/s0960-9822(01)00525-5. [DOI] [PubMed] [Google Scholar]
- Freyd G., Kim S. K., Horvitz H. R. Novel cysteine-rich motif and homeodomain in the product of the Caenorhabditis elegans cell lineage gene lin-11. Nature. 1990;344:876–879. doi: 10.1038/344876a0. [DOI] [PubMed] [Google Scholar]
- Gao X. D., Caviston J. P., Tcheperegine S. E., Bi E. Pxl1p, a paxillin-like protein in Saccharomyces cerevisiae, may coordinate Cdc42p and Rho1p functions during polarized growth. Mol. Biol. Cell. 2004;15:3977–3985. doi: 10.1091/mbc.E04-01-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia I., Jimenez D., Martin V., Duran A., Sanchez Y. The alpha-glucanase Agn1p is required for cell separation in Schizosaccharomyces pombe. Biol. Cell. 2005;97:569–576. doi: 10.1042/BC20040096. [DOI] [PubMed] [Google Scholar]
- Ge W., Chew T. G., Wachtler V., Naqvi S. N., Balasubramanian M. K. The novel fission yeast protein Pal1p interacts with Hip1-related Sla2p/End4p and is involved in cellular morphogenesis. Mol. Biol. Cell. 2005;16:4124–4138. doi: 10.1091/mbc.E04-11-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn J. M., Lustig R. J., Berlin A., Chang F. Role of bud6p and tea1p in the interaction between actin and microtubules for the establishment of cell polarity in fission yeast. Curr. Biol. 2001;11:836–845. doi: 10.1016/s0960-9822(01)00235-4. [DOI] [PubMed] [Google Scholar]
- Ishiguro J. Genetic control of fission yeast cell wall synthesis: the genes involved in wall biogenesis and their interactions in Schizosaccharomyces pombe. Genes Genet. Syst. 1998;73:181–191. doi: 10.1266/ggs.73.181. [DOI] [PubMed] [Google Scholar]
- Krapp A., Gulli M. P., Simanis V. SIN and the art of splitting the fission yeast cell. Curr. Biol. 2004;14:R722–R730. doi: 10.1016/j.cub.2004.08.049. [DOI] [PubMed] [Google Scholar]
- Le Goff X., Motegi F., Salimova E., Mabuchi I., Simanis V. The S. pombe rlc1 gene encodes a putative myosin regulatory light chain that binds the type II myosins myo3p and myo2p. J. Cell Sci. 2000;113(Pt 23):4157–4163. doi: 10.1242/jcs.113.23.4157. [DOI] [PubMed] [Google Scholar]
- Mackin N. A., Sousou T. J., Erdman S. E. The PXL1 gene of Saccharomyces cerevisiae encodes a paxillin-like protein functioning in polarized cell growth. Mol. Biol. Cell. 2004;15:1904–1917. doi: 10.1091/mbc.E04-01-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks J., Hagan I. M., Hyams J. S. Growth polarity and cytokinesis in fission yeast: the role of the cytoskeleton. J. Cell Sci. Suppl. 1986;5:229–241. doi: 10.1242/jcs.1986.supplement_5.15. [DOI] [PubMed] [Google Scholar]
- Martin-Cuadrado A. B., Duenas E., Sipiczki M., Vazquez de Aldana C. R., del Rey F. The endo-beta-1,3-glucanase eng1p is required for dissolution of the primary septum during cell separation in Schizosaccharomyces pombe. J. Cell Sci. 2003;116:1689–1698. doi: 10.1242/jcs.00377. [DOI] [PubMed] [Google Scholar]
- Martin-Cuadrado A. B., Morrell J. L., Konomi M., An H., Petit C., Osumi M., Balasubramanian M., Gould K. L., Del Rey F., de Aldana C. R. Role of septins and the exocyst complex in the function of hydrolytic enzymes responsible for fission yeast cell separation. Mol. Biol. Cell. 2005;16:4867–4881. doi: 10.1091/mbc.E04-12-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum D., Balasubramanian M. K., Pelcher L. E., Hemmingsen S. M., Gould K. L. Schizosaccharomyces pombe cdc4+ gene encodes a novel EF-hand protein essential for cytokinesis. J. Cell Biol. 1995;130:651–660. doi: 10.1083/jcb.130.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum D., Feoktistova A., Morphew M., Balasubramanian M., Gould K. L. The Schizosaccharomyces pombe actin-related protein, Arp3, is a component of the cortical actin cytoskeleton and interacts with profilin. EMBO J. 1996;15:6438–6446. [PMC free article] [PubMed] [Google Scholar]
- Michelsen J. W., Schmeichel K. L., Beckerle M. C., Winge D. R. The LIM motif defines a specific zinc-binding protein domain. Proc. Natl. Acad. Sci. USA. 1993;90:4404–4408. doi: 10.1073/pnas.90.10.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. J., Johnson D. I. Cdc42p GTPase is involved in controlling polarized cell growth in Schizosaccharomyces pombe. Mol. Cell. Biol. 1994;14:1075–1083. doi: 10.1128/mcb.14.2.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra M., Karagiannis J., Trautmann S., Wang H., McCollum D., Balasubramanian M. K. The Clp1p/Flp1p phosphatase ensures completion of cytokinesis in response to minor perturbation of the cell division machinery in Schizosaccharomyces pombe. J. Cell Sci. 2004;117:3897–3910. doi: 10.1242/jcs.01244. [DOI] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Motegi F., Mishra M., Balasubramanian M. K., Mabuchi I. Myosin-II reorganization during mitosis is controlled temporally by its dephosphorylation and spatially by Mid1 in fission yeast. J. Cell Biol. 2004;165:685–695. doi: 10.1083/jcb.200402097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motegi F., Nakano K., Kitayama C., Yamamoto M., Mabuchi I. Identification of Myo3, a second type-II myosin heavy chain in the fission yeast Schizosaccharomyces pombe. FEBS Lett. 1997;420:161–166. doi: 10.1016/s0014-5793(97)01510-x. [DOI] [PubMed] [Google Scholar]
- Nakano K., Imai J., Arai R., Toh E. A., Matsui Y., Mabuchi I. The small GTPase Rho3 and the diaphanous/formin For3 function in polarized cell growth in fission yeast. J. Cell Sci. 2002;115:4629–4639. doi: 10.1242/jcs.00150. [DOI] [PubMed] [Google Scholar]
- Naqvi N. I., Wong K. C., Tang X., Balasubramanian M. K. Type II myosin regulatory light chain relieves auto-inhibition of myosin-heavy-chain function. Nat. Cell Biol. 2000;2:855–858. doi: 10.1038/35041107. [DOI] [PubMed] [Google Scholar]
- Okazaki K., Okazaki N., Kume K., Jinno S., Tanaka K., Okayama H. High-frequency transformation method and library transducing vectors for cloning mammalian cDNAs by trans-complementation of Schizosaccharomyces pombe. Nucleic Acids Res. 1990;18:6485–6489. doi: 10.1093/nar/18.22.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva A., Rosebrock A., Ferrezuelo F., Pyne S., Chen H., Skiena S., Futcher B., Leatherwood J. The cell cycle-regulated genes of Schizosaccharomyces pombe. PLoS Biol. 2005;3:e225. doi: 10.1371/journal.pbio.0030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti A., Chang F. Analysis of mid1p, a protein required for placement of the cell division site, reveals a link between the nucleus and the cell surface in fission yeast. Mol. Biol. Cell. 2000;11:2757–2773. doi: 10.1091/mbc.11.8.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X., et al. Identification of cell cycle-regulated genes in fission yeast. Mol. Biol. Cell. 2005;16:1026–1042. doi: 10.1091/mbc.E04-04-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustici G., Mata J., Kivinen K., Lio P., Penkett C. J., Burns G., Hayles J., Brazma A., Nurse P., Bahler J. Periodic gene expression program of the fission yeast cell cycle. Nat. Genet. 2004;36:809–817. doi: 10.1038/ng1377. [DOI] [PubMed] [Google Scholar]
- Simanis V. Events at the end of mitosis in the budding and fission yeasts. J. Cell Sci. 2003;116:4263–4275. doi: 10.1242/jcs.00807. [DOI] [PubMed] [Google Scholar]
- Sipiczki M. Splitting of the fission yeast septum. FEMS Yeast Res. 2007;7(6):761–770. doi: 10.1111/j.1567-1364.2007.00266.x. [DOI] [PubMed] [Google Scholar]
- Sohrmann M., Fankhauser C., Brodbeck C., Simanis V. The dmf1/mid1 gene is essential for correct positioning of the division septum in fission yeast. Genes Dev. 1996;10:2707–2719. doi: 10.1101/gad.10.21.2707. [DOI] [PubMed] [Google Scholar]
- Sohrmann M., Schmidt S., Hagan I., Simanis V. Asymmetric segregation on spindle poles of the Schizosaccharomyces pombe septum-inducing protein kinase Cdc7p. Genes Dev. 1998;12:84–94. doi: 10.1101/gad.12.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasto J. J., Morrell J. L., Gould K. L. An anillin homologue, Mid2p, acts during fission yeast cytokinesis to organize the septin ring and promote cell separation. J. Cell Biol. 2003;160:1093–1103. doi: 10.1083/jcb.200211126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran P. T., Paoletti A., Chang F. Imaging green fluorescent protein fusions in living fission yeast cells. Methods. 2004;33:220–225. doi: 10.1016/j.ymeth.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Trautmann S., Wolfe B. A., Jorgensen P., Tyers M., Gould K. L., McCollum D. Fission yeast Clp1p phosphatase regulates G2/M transition and coordination of cytokinesis with cell cycle progression. Curr. Biol. 2001;11:931–940. doi: 10.1016/s0960-9822(01)00268-8. [DOI] [PubMed] [Google Scholar]
- Wang H., Tang X., Liu J., Trautmann S., Balasundaram D., McCollum D., Balasubramanian M. K. The multiprotein exocyst complex is essential for cell separation in Schizosaccharomyces pombe. Mol. Biol. Cell. 2002;13:515–529. doi: 10.1091/mbc.01-11-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe B. A., Gould K. L. Split decisions: coordinating cytokinesis in yeast. Trends Cell Biol. 2005;15:10–18. doi: 10.1016/j.tcb.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Wu J. Q., Kuhn J. R., Kovar D. R., Pollard T. D. Spatial and temporal pathway for assembly and constriction of the contractile ring in fission yeast cytokinesis. Dev. Cell. 2003;5:723–734. doi: 10.1016/s1534-5807(03)00324-1. [DOI] [PubMed] [Google Scholar]
- Wu J. Q., Sirotkin V., Kovar D. R., Lord M., Beltzner C. C., Kuhn J. R., Pollard T. D. Assembly of the cytokinetic contractile ring from a broad band of nodes in fission yeast. J. Cell Biol. 2006;174:391–402. doi: 10.1083/jcb.200602032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.