Abstract
We previously reported that immunodepletion of Greatwall kinase prevents Xenopus egg extracts from entering or maintaining M phase due to the accumulation of inhibitory phosphorylations on Thr14 and Tyr15 of Cdc2. M phase–promoting factor (MPF) in turn activates Greatwall, implying that Greatwall participates in an MPF autoregulatory loop. We show here that activated Greatwall both accelerates the mitotic G2/M transition in cycling egg extracts and induces meiotic maturation in G2-arrested Xenopus oocytes in the absence of progesterone. Activated Greatwall can induce phosphorylations of Cdc25 in the absence of the activity of Cdc2, Plx1 (Xenopus Polo-like kinase) or mitogen-activated protein kinase, or in the presence of an activator of protein kinase A that normally blocks mitotic entry. The effects of active Greatwall mimic in many respects those associated with addition of the phosphatase inhibitor okadaic acid (OA); moreover, OA allows cycling extracts to enter M phase in the absence of Greatwall. Taken together, these findings support a model in which Greatwall negatively regulates a crucial phosphatase that inhibits Cdc25 activation and M phase induction.
INTRODUCTION
The induction of mitosis or meiosis requires the coordinated regulation of multiple protein kinases and phosphatases that together promote a sequential program of M phase entry. The rate-limiting step in this process is the activation of Cdc2/cyclin B, also called M phase–promoting factor (MPF). The activation of Cdc2/cyclin B at the G2/M transition is achieved by the removal of inhibitory phosphorylations on Thr14 and Tyr15 of Cdc2, which in turn results from activation of the protein phosphatase Cdc25 and inactivation of the opposing Wee1 family kinases (reviewed by Perry and Kornbluth, 2007). A complex regulatory network, not yet fully understood, controls Cdc25 and Wee1 activities and thus the dephosphorylation of Cdc2 and M phase induction. Experimental manipulation of any of several key positive and negative regulators within this network affects the rates of Cdc2 activation and M phase induction. Another prominent feature of this regulatory network is its positive feedback regulation by the end product Cdc2/cyclin B. Once a catalytic amount of Cdc2/cyclin B is activated, it can influence the network in a manner that promotes the activation of additional Cdc2/cyclin B, resulting in a switch-like, all-or-none pattern of M phase induction (Xiong and Ferrell, 2003).
Remarkably, it appears that the opposing activities of Cdc25 and Wee1 are controlled in large part by similar regulatory strategies and by several of the same effectors (reviewed by Stark and Taylor, 2006; Perry and Kornbluth, 2007). During interphase, the phosphorylation of Cdc25 (at Ser287 in Xenopus) and Wee1 (at Ser549) induces the binding of both to 14-3-3 proteins, resulting in the inactivation of Cdc25 and the activation of Wee1. Several kinases (including Ca2+/calmodulin kinase II [CamKII], protein kinase A [PKA], C-TAK, and the checkpoint kinases Chk1 and Cds1) target Ser287 of Cdc25; Chk1 and perhaps other kinases target Ser459 of Wee1. During the G2/M transition, the regulation of Cdc25 and Wee1 is reversed due to the action of several mitosis-promoting kinases. Plx1 (Xenopus polo-like kinase), Cdc2, and mitogen-activated protein kinase (MAPK) can phosphorylate various activating sites on Cdc25 (Wang et al., 2007). Interestingly, the phosphorylation of Wee1 by two of the same kinases (Plx1 and Cdc2) leads to Wee1 inactivation and degradation. The positive autoregulation of MPF at M phase entry results in large part from Cdc2's mirror-image effects that activate Cdc25 and inactivate Wee1. However, recent results excitingly suggest that this feedback regulation is reinforced at an additional level: key M phase–specific phosphorylations on Cdc25 and Wee1 may also be controlled by phosphatases that are activated during interphase and suppressed during M phase (Margolis et al., 2006a; Mochida and Hunt, 2007).
We have recently reported multiple lines of evidence indicating that the novel, conserved Ser/Thr protein kinase Greatwall is a critical component of the network that regulates the inhibitory phosphorylations of Cdc2. The G2/M transition is retarded in Drosophila cells homozygous for null alleles of the greatwall gene (Yu et al., 2004). Immunodepletion of Greatwall from M phase–arrested Xenopus egg extracts (so-called cytostatic factor [CSF] extracts) results in rapid inactivation of Cdc2/cyclin B associated with the accumulation of inhibitory phosphorylations on Cdc2 (Yu et al., 2006). Immunodepletion of Greatwall from interphase Xenopus extracts prevents Cdc2 dephosphorylation and inhibits mitotic entry (Yu et al., 2006). In addition, Greatwall itself is activated by M phase–specific phosphorylations, which mostly appear to be directly catalyzed by Cdc2/cyclin B itself (Yu et al., 2006). These results taken in total indicate direct or indirect roles of Greatwall in the regulation of Cdc25 and/or Wee1 and further position Greatwall in a Cdc2 autoactivation loop. However, the precise relationship of Greatwall to the network controlling MPF remains undefined.
The most direct approach to a further investigation of Greatwall function would be to identify the substrate(s) Greatwall kinase can phosphorylate. We have tested many obvious candidate substrates in vitro and tried several approaches to define consensus sites targeted by Greatwall, but unfortunately these efforts have been unsuccessful to date. In this article, we have instead attempted to establish an experimental framework in which to evaluate Greatwall's roles in M phase entry and maintenance. We show first that activated Greatwall is not only necessary for the G2/M transition, but it is also sufficient to cause the premature onset of mitosis and meiosis. We then systematically ask whether the removal or inactivation of known mitotic effectors interferes with the ability of activated Greatwall to promote activating phosphorylations on the Cdc25 phosphatase.
The results reported here allow us to narrow the possible mechanisms through which Greatwall might act on Cdc25. We show evidence that Greatwall does not work by regulating uniquely any one of the kinases known to be involved in Cdc25 activation during M phase. Instead, active Greatwall can potentiate Cdc25 phosphorylation in the absence or near absence of Cdc2, Plx1, or MAPK functions. We find that Greatwall also influences Cdc25 by counteracting both the inhibitory phosphorylation at the Ser287 site of Cdc25 and an unknown inhibitory mechanism involving PKA. The manifold effects of Greatwall on Cdc25 may potentially be explained by findings that the behavior of extracts in the presence of activated Greatwall parallels that of extracts treated with the phosphatase inhibitor okadaic acid (OA) and that under certain conditions, the addition of OA can compensate for the absence of Greatwall. We propose that Greatwall might thus negatively regulate an OA-sensitive phosphatase that must be “turned off” during M phase, allowing the accumulation on Cdc25 and other proteins of phosphorylations that are mediated by a variety of mitotic kinases.
MATERIALS AND METHODS
Reagents
Antibody against Xenopus laevis Greatwall was generated and purified as previously described (Yu et al., 2006). Anti-Cdc2 (sc-54) antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against Phospho-MAPK (9106) and Phospho-Cdc2 (Tyr15) (9111) were from Cell Signaling Technology (Beverly, MA). Plx1 mAb and anti-p90Rsk were from Zymed (South San Francisco, CA). Cdc25C antibody was the gift of Dr. William Dunphy (California Institute of Technology, Pasadena, CA; Kumagai and Dunphy, 1992). Antibody against Cyclin B1 was from Dr. James Maller (University of Colorado, Denver, CO), and anti-cyclin A was the kind gift of Dr. Tim Hunt (Cancer Research UK, South Mimms, Hertfordshire, United Kingdom). Antibody against phosphorylated Ser287 on Cdc25C was generously provided by Drs. Joan Ruderman and Eunah Chung (Harvard Medical School, Boston, MA; Duckworth et al., 2002).
OA was from Alexis Biochemicals (San Diego, CA). Roscovitine, U0126, and the CamKII inhibitor 281-309 were all from EMD Calbiochem (San Diego, CA). 8-Br-cAMP was purchased from Biosource International (Camarillo, CA).
Xenopus Egg Extracts
Cycling and CSF extracts were prepared as described (Murray and Kirschner, 1989; Murray, 1991). On preparation, cycling extracts are in the interphase after meiotic exit and can then undergo repeated mitotic cycles. CSF extracts are arrested in meiotic M phase (corresponding to metaphase of meiosis II). Interphase extracts were used instead of cycling extracts in certain experiments (shown in Figures 3B, 5, and 6; and in Supplementary Figure S1, B and C) to assess mitotic entry when high concentrations of glycerol or DMSO in added reagents inhibited the progression of cycling extracts. To make interphase extracts, CaCl2 was added to CSF extracts (0.5 mM final concentration); the extracts were then incubated at 23°C for 40 min to induce mitotic exit. Control interphase extracts typically reenter mitosis ∼120 min from the start of incubation (data not shown). Although cycling and interphase extracts are in roughly the same cell cycle state upon their preparation, they differ in two important ways. First, interphase extracts that enter mitotic M phase become arrested in a metaphase-like state and cannot subsequently exit M phase, whereas cycling extracts enter and exit mitotic M phase several times. Second, Mos becomes degraded during the preparation of cycling extracts, so the activity of the MAPK pathway upon M phase entry remains much lower than that seen when interphase extracts (which do not degrade Mos) enter mitosis.
Figure 3.
Greatwall can influence Cdc25 phosphorylation independent of MPF activity. (A) Greatwall promotes mitotic entry in the presence of the Cdc2 inhibitor roscovitine (Ros). Cycling extracts were incubated at 23°C (starting at t = 0) for 30 min before the addition of 250 μM Ros as indicated. Excess active Greatwall (5×) or an equal volume of buffer was added 10 min later. Extracts were processed every 10 min thereafter for Western blot analysis and Histone H1 kinase assay. (B) Greatwall promotes Cdc25 phosphorylation in the absence of MPF. To eliminate MPF activity, CSF extracts were first induced into interphase (at t = 0) by adding CaCl2 to a final concentration of 0.5 mM and then incubated at 23°C for 15 min. Cycloheximide (CHX) was then added (100 μg/ml final concentration) to inhibit protein synthesis as indicated. After another 15 min, exogenous cyclin B was supplemented to the indicated groups. At t = 40 min, active Greatwall was added as shown, and samples were collected every 10 min and processed for immunoblotting. In untreated control extracts, mitotic entry occurs at ∼120 min after CaCl2 addition (data not shown). Note that in the presence of active Greatwall, extracts enter mitosis prematurely at 50–60 min when cyclin B concentrations are low. In addition, a partial mobility shift of Cdc25 occurs at this time even when cyclin B is undetectable and (as shown in Figure 6 below) H1 kinase activity is extremely low due to CHX.
Figure 5.
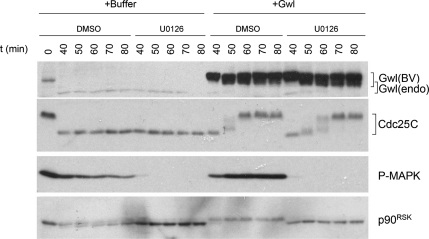
The MAPK pathway is not critical for Greatwall-induced activation of Cdc25. The involvement of the MAPK pathway in Greatwall-induced mitotic entry was tested in interphase extracts released from CSF arrest by the addition of CaCl2 at t = 0. Extracts were then incubated at 23°C for 30 min before U0126 was supplemented to a final concentration of 400 μM as shown. After 10 min of incubation, excess active Greatwall was added as indicated. Immunoblot analysis reveals that U0126 shuts down signaling through the MAPK pathway, but does not interfere with Greatwall's ability to induce Cdc25 phosphorylation and precocious mitotic entry.
Figure 6.
Greatwall induces the phosphorylation of Cdc25 by several mitotic kinases. Interphase extract was prepared by addition of CaCl2 to CSF extract a t = 0 and incubated at 23°C for 20 min. The extract was then supplemented with the MEK inhibitor U0126 (400 μM) and/or the protein synthesis inhibitor CHX (100 μM) or DMSO, followed by another 20-min incubation at 23°C. Extracts were then subjected to Plx1 depletion or mock depletion as indicated. One-microliter aliquots were collected immediately after the depletion and every 10 min thereafter for immunoblotting and H1 kinase assay.
Immunodepletion was performed as described (Yu et al., 2006) using Affi-prep protein A beads (Bio-Rad Laboratories, Hercules, CA) coated with affinity-purified anti-Greatwall. Mock-depleted extracts were treated with protein A beads alone. Analysis of extracts by Western blotting was previously described (Yu et al., 2006). The Institutional Animal Care and Use Committee at Cornell University monitored frog husbandry.
Expression and Purification of Recombinant Proteins
Greatwall protein was expressed and purified from Sf9 cells using the Bac-to-Bac expression system (Invitrogen, Carlsbad, CA) as previously described (Yu et al., 2006). Active Greatwall was produced by treating the infected cells with OA to a final concentration of 100 nM for 12 h before harvesting.
Microinjection of Xenopus Oocytes
Fully grown prophase I oocytes were obtained from unprimed Xenopus laevis females as described (Jessus et al., 1987). Oocytes were microinjected with 50 nl of either wild-type or Kinase-dead Greatwall (1.5 and 2.4 μg/μl, respectively). When specified, oocytes were incubated for 1 h in 100 μg/ml cycloheximide (CHX) and then microinjected and cultured in the continuous presence of CHX. Control oocytes were induced to mature by 1 μM progesterone (Pg). In some cases, oocytes were injected with a morpholino directed against c-mos mRNA according to Dupre et al. (2002).
RESULTS
Greatwall Is a Key Regulator of Cdc25
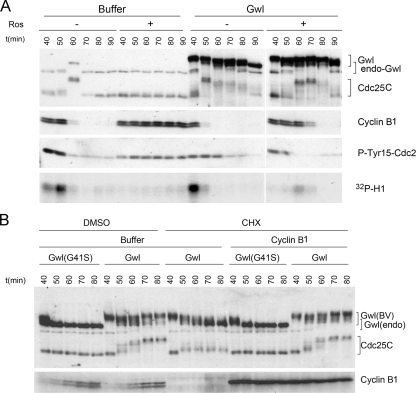
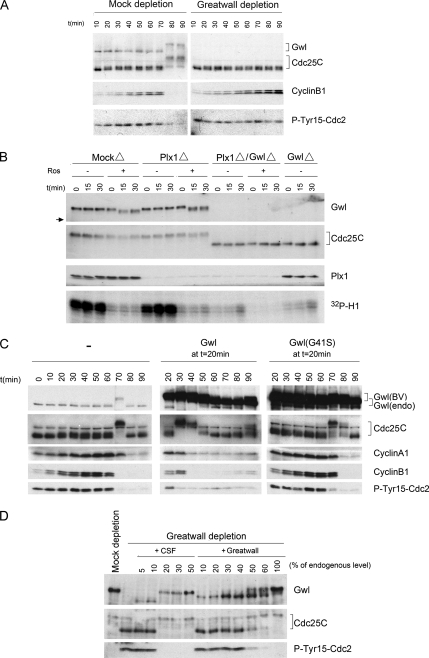
The experiments shown in Figure 1 recapitulate and extend our previous findings indicating the importance of Greatwall to the establishment and maintenance of M phase–specific phosphorylations on Cdc25C required for the function of this phosphatase (Yu et al., 2006). These phosphorylations result in a substantial retardation (upshift) in the electrophoretic mobility of Cdc25C (hereafter Cdc25). Figure 1A demonstrates the effects of removing Greatwall from “cycling” egg extracts that are in interphase upon their preparation, but then have the ability to undergo several rounds of mitosis (Murray and Kirschner, 1989). Immunodepletion of Greatwall from cycling extracts prevents the Cdc25 upshift, resulting in a block to mitotic entry because inactive Cdc25 cannot dephosphorylate Tyr15 on Cdc2. Another marker for the failure of these Greatwall-depleted extracts to enter M phase is their inability to degrade cyclin B (because cyclin B degradation requires prior MPF activation; Rudner and Murray, 2000; Frank-Vaillant et al., 2001). Figure 1A also illustrates the hyperphosphorylation that activates Greatwall itself during M phase.
Figure 1.
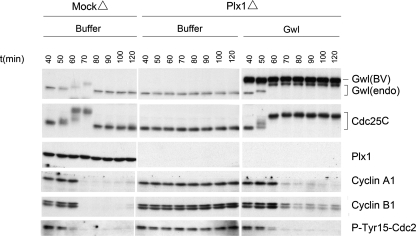
Greatwall regulates Cdc25. (A) Depletion of Greatwall from cycling extracts prevents Cdc25 activation and mitotic entry. Mock-depleted control extracts enter M phase within 80 min, as shown by M phase–specific upshifts of Cdc25 and Greatwall, the loss of the inhibitory Tyr15 phosphorylation of Cdc2, and the rapid degradation of cyclin B. Greatwall-depleted extracts fail to enter mitosis as judged by all of these criteria; similar experiments published previously (Yu et al., 2006) show that the block to mitotic entry continues for at least 60 min beyond the latest time point shown here. (B) Maintenance of mitotic phosphorylations on Cdc25C in CSF extracts requires Greatwall but not Plx1 or Cdc2 activity. Removal of Plx1 by immunodepletion and/or inhibition of Cdc2 activity by 250 μM roscovitine have no detectable impact on Cdc25C mobility (groups of Plx1Δ/−Ros and MockΔ/+Ros). Depletion of Greatwall completely eliminates the migration shift of Cdc25C (groups of Plx1Δ/GwlΔ and GwlΔ). The electrophoretic mobility of Greatwall itself is unaffected by Plx1 depletion, but roscovitine appears to cause the removal of some phosphorylations on Greatwall; this effect is only partial because Greatwall's migration is still significantly retarded (the arrow at left indicates the mobility of fully dephosphorylated Greatwall during interphase). (C) Premature mitotic entry induced by active Greatwall. Freshly made cycling extracts were incubated at 23°C starting at t = 0. Untreated cycling extracts enter mitosis at 70 min. Addition at 20 min of excess (five times the endogenous level) active wild-type Greatwall [Gwl(BV)] purified from OA-treated Sf9 cells promotes premature mitotic entry at t = 30 min, as indicated by the mobility shifts of Cdc25C and endogenous (endo) Greatwall and the dephosphorylation of Tyr15 of Cdc2. Within 20 min of mitotic entry, the extracts exit mitosis (note the degradation of cyclins A1 and B1), indicating that excess active Greatwall does not block mitotic exit. Addition of an even larger excess (7.5 times the endogenous level) of kinase-dead Greatwall (G41S) does not accelerate mitotic entry. Throughout this article, the relative amounts of recombinant and endogenous proteins were determined by Coomassie Blue staining (data not shown); the intensities of the exogenous Greatwall bands on the Western blots in B–D are distorted by the immunoglobulin Z domain in these recombinant proteins as well as some nonlinearity in the relationship between the protein amount and Western blot signal intensity (see Supplementary Figure S1E). The interaction between the Z domain and secondary antibodies accounts for ∼50% of the total signal on Western blots (Supplementary Figure S1E). (D) Greatwall purified from OA-treated Sf9 cells is less active than endogenous Greatwall. CSF egg extracts were subjected to Greatwall depletion or mock depletion (see Yu et al., 2006 for details). Greatwall-depleted egg extract was then supplemented with various amounts of mock-depleted CSF egg extract or with active purified Greatwall. Extracts were prepared for analysis 30 min after supplementation. Purified Greatwall preparations have only about one-third the activity of endogenous mitotic Greatwall in maintaining the M phase status of the extracts.
Figure 1B shows that Cdc25 with already-acquired M phase–specific phosphorylations cannot maintain these phosphorylations in the absence of Greatwall. Immunodepletion of Greatwall from CSF extracts (that is, extracts in M phase prepared from mature oocytes arrested in meiosis II) results in the rapid dephosphorylation of Cdc25. Remarkably, Greatwall is much more important to the maintenance of Cdc25 during M phase than the well-characterized Cdc25 kinases Cdc2 or Plx1: in accordance with previous observations (Izumi and Maller, 1995; Descombes and Nigg, 1998; Qian et al., 2001; Liu and Maller, 2005), neither removal of Plx1 by immunodepletion nor inhibition of Cdc2 by the drug roscovitine (Ros) affects Cdc25 phosphorylation in CSF extracts for extended periods (Figure 1B).
Together with our previous demonstration that MPF can activate Greatwall (Yu et al., 2006), the experiments presented in Figure 1, A and B, position Greatwall within a Cdc2 autoregulatory loop. If this assumption is correct, then the addition of already activated Greatwall to interphase extracts should suffice to promote other events within or dependent upon the loop, including the activations of Cdc25 and Cdc2 as well as M phase entry. To test this possibility, we first purified active Greatwall from insect Sf9 cells that express baculovirus-based Greatwall constructs (Supplementary Figure S1A). These cells are treated with OA, a potent serine/threonine phosphatase inhibitor that causes M phase arrest (Fernandez et al., 2002). Greatwall purified by this method acquires the M phase–specific phosphorylations required for its activity.
To investigate the sufficiency of Greatwall in determining M phase, we added active Greatwall into cycling egg extracts (Figure 1C). We determined the cell cycle stage of these extracts over time by examining several markers. During M phase, Cdc25 and Greatwall are hyperphosphorylated, and the inhibitory Tyr15 phosphorylation on Cdc2 is removed. When the extracts subsequently exit mitosis, the phosphorylations on Cdc25 and Greatwall are lost, and in addition, cyclins A and B are degraded. The entry into M phase is also associated as expected with increased cyclin-dependent kinase (CDK) activity, as measured by histone H1 phosphorylation (see Figure 3A below).
In our hands, cycling extracts normally first enter mitosis 60–80 min after their preparation, and then exit mitosis soon thereafter. However, within 10 min after the addition of active wild-type (WT) Greatwall, Cdc25 is precociously upshifted and the extracts thus enter mitosis as judged by all M phase markers examined (Figure 1C; see also Figure 3A below). If Greatwall is added immediately after the extracts are prepared, mitotic entry can be advanced by as much as 1 h (data not shown). Because the level of cyclin B at the time of Greatwall-induced mitotic entry is much lower than that normally seen at the beginning of M phase, Greatwall apparently lowers the threshold of cyclin B required to promote mitosis. No early mitotic entry is observed in extracts supplemented with similar, or even much larger, amounts of kinase-dead (KD) Greatwall (with the mutation G41S; Yu et al., 2006) that had also been purified from OA-treated Sf9 cells (Figure 1C and Supplementary Figure S1B) or with WT Greatwall made in Sf9 cells that had not been treated with OA (Supplementary Figure S1C). The mitosis-promoting effect is thus indeed due to Greatwall function.
High amounts (about five times the endogenous level) of activated WT Greatwall are required to induce premature M phase entry. The major reason so much protein is needed appears to be that the insect cell–expressed enzyme has only one-fifth to one-third the activity of endogenous mitotic Greatwall (Figure 1D). WT Greatwall prepared in this manner is not fully phosphorylated (Supplementary Figure S1D), potentially explaining the lower activity of the insect-expressed protein. A second likely basis of the need for excess active Greatwall is the presence during interphase of competing phosphatase activities that remove mitotic phosphorylations from Greatwall and thus deactivate it (Supplementary Figure S1B). The method used to quantify the amounts of exogenous active Greatwall added to the extracts relative to the endogenous levels of this protein is illustrated in Supplementary Figure S1E.
Active Greatwall Induces Oocyte Maturation
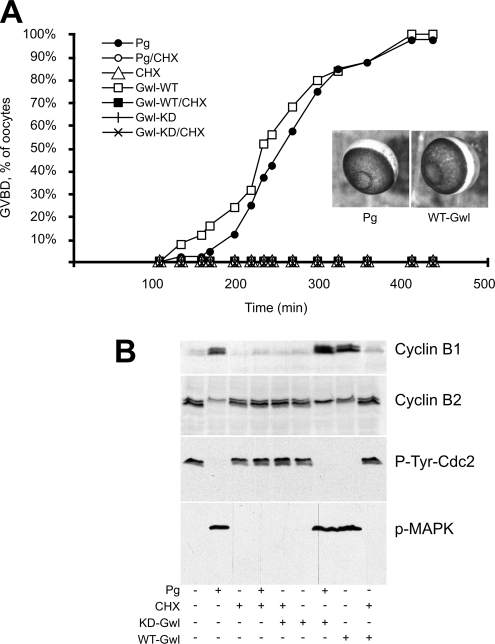
We were interested to know whether Greatwall could also promote meiotic, as well as mitotic, divisions. We first analyzed the expression and phosphorylation level of Greatwall during meiotic maturation of oocytes stimulated by Pg. Greatwall is expressed at a constant level during the prophase I to metaphase II transition and becomes hyperphosphorylated at the time of Cdc2-cyclin B activation (Supplementary Figure S2). We next directly asked whether Greatwall can promote entry into meiosis by injecting active Greatwall protein into immature oocytes arrested in G2. Remarkably, in the absence of Pg the oocytes begin germinal vesicle breakdown (GVBD) 3 h after Greatwall injection and almost all complete GVBD within 7 h; injection of KD Greatwall was without effect (Figure 2A). Western blots examining the Tyr15 phosphorylation level of Cdc2, cyclin B1 expression levels, cyclin B2 mobility, and the phosphorylation of MAPK confirmed that GVBD induced by WT Greatwall corresponds to M phase (Figure 2B). We conclude that WT active Greatwall is sufficient to induce oocyte meiotic M-phase entry even in the absence of Pg.
Figure 2.
Active Greatwall induces oocyte maturation in the absence of progesterone in a protein synthesis–dependent manner. (A) Oocytes were injected with active (WT) or kinase-dead (KD) Greatwall protein. Control oocytes were stimulated by progesterone (Pg). In some cases as indicated, cycloheximide (CHX) was added 1 h before Greatwall injection or progesterone addition. The graph shows the time course of germinal vesicle breakdown (GVBD). Photographs illustrate the external morphology of Greatwall-injected oocytes (Gwl) or progesterone-stimulated oocytes (Pg). (B) Oocytes were homogenized at GVBD, or 7 h after injection or stimulation when GVBD did not occur. Lysates were immunoblotted with the indicated antibodies. Cyclin B1 is expressed at a low level in immature oocytes and its synthesis is stimulated during meiotic maturation; Cyclin B2 migrates as a doublet when MPF is inactive and as a single band when MPF is active (Karaiskou et al., 2004).
It should be noted that Greatwall-induced oocyte maturation requires new protein synthesis because the translation poison CHX completely abolishes the effect of active Greatwall (Figure 2, A and B). This finding suggests that either Mos or cyclin B, both of whose synthesis are important for meiotic maturation (Haccard and Jessus, 2006), could control Greatwall or mediate its effects. To investigate these possibilities, we injected morpholinos against Mos (a kinase synthesized during oocyte meiotic maturation that is required to activate MAPK) into oocytes that were then stimulated with Pg (Supplementary Figure S2). The morpholinos efficiently eliminated Mos protein and thus MAPK activation, yet they did not block Greatwall phosphorylation or meiotic maturation. This result indicates that Greatwall phosphorylation does not depend on the Mos/MAPK pathway. To explain the effects of CHX, Greatwall's meiotic regulation and function could still depend on cyclin B synthesis and be mediated through MPF. This hypothesis seems to be valid since Greatwall phosphorylation is totally prevented when oocytes are microinjected with the Cdc2 inhibitor, p21Cip1 (data not shown).
Greatwall Can Induce Partial Phosphorylation of Cdc25 Independent of MPF
Given current knowledge, the most straightforward model to explain Greatwall's role in M phase entry would suggest that Greatwall is downstream of MPF but upstream of Cdc25 and/or Myt1/Wee1. In other words, MPF activates Greatwall (as we have previously demonstrated; Yu et al., 2006), and then activated Greatwall contributes to the activation of Cdc25 and/or the inactivation of Myt1/Wee1, which in turn complete the loop by mobilizing additional Cdc2. To test this model, we asked whether active Greatwall could influence components of the autoregulatory loop under conditions in which MPF is nonfunctional. The major read-out of these experiments is the hyperphosphorylation of Cdc25 during M phase.
To interfere with MPF activity, we first used the drug roscovitine to inhibit Cdc2 (Figure 3A; Meijer et al., 1997). Although 250 μM Ros prevents normal mitotic entry in control cycling extracts, addition of excess active Greatwall causes a substantial retardation of Cdc25 mobility even in the presence of the drug. Remarkably, the presence of active Greatwall induces Cdc25 hyperphosphorylation when H1 kinase is <10% of the levels normally required for M phase entry.
We next used an alternative approach to turn off MPF more definitively: we added CHX to cycling extracts that had just exited mitosis, thus preventing cyclin synthesis (Figure 3B). CHX treatment prevents the full Cdc25 upshift induced by Greatwall; however, supplementing the drug-treated extract with Xenopus cyclin B1 purified from Sf9 cells overcomes this effect. Importantly, however, addition of active Greatwall causes a partial but clear mobility shift of Cdc25 when cyclin B is not present at detectable levels and H1 kinase activity is essentially absent (Figure 3B, middle group; see also Figure 6 below). This result suggests that Greatwall induces initial steps in the phosphorylation and activation of Cdc25 that are MPF-independent, whereas later M phase phosphorylations of Cdc25 are the direct or indirect result of MPF activity.
Active Greatwall Overrides the Need for Plx1 in Mitotic Entry
The results in the preceding section revealed that active Greatwall induces phosphorylations of Cdc25 that are independent of MPF. Because Cdc25 appears not to be a direct substrate of Greatwall kinase (data not shown), we entertained the hypothesis that Greatwall could potentiate a kinase other than MPF that targets Cdc25 during M phase. Biochemical investigations have revealed three enzymes in addition to MPF that are responsible for the large majority of the Cdc25-directed kinase activity in M phase Xenopus egg extracts: one of these is Plx1, the second is p42 MAPK, and the identity of the third is currently unknown (Wang et al., 2007). We thus investigated whether Greatwall mediates Cdc25 mobility via pathways involving Plx1 or MAPK, starting with Plx1.
As expected from previous studies (Abrieu et al., 1998; Qian et al., 1998), removal of Plx1 from Xenopus cycling extracts by immunodepletion blocks mitotic entry (Figure 4). However, the addition of active Greatwall (at five times the endogenous level) overcomes the effects of Plx1 depletion and induces a substantial upshift of Cdc25. The electrophoretic mobility of Cdc25 under these conditions is intermediate between its normal interphase and M phase positions, consistent with the loss of Plx1-specific M phase phosphorylations. Nonetheless, in the absence of Plx1 but presence of active Greatwall, the behaviors of several other cell cycle markers (endogenous Greatwall mobility and Tyr15 on Cdc2) indicate the extracts can enter an M phase-like state. It thus appears that Greatwall can induce both Cdc25 and Cdc2 activities even if Plx1-specific phosphorylations of Cdc25 are missing. This result confirms the impression from the experiment previously shown in Figure 1B that Greatwall plays a role independent of Plx1 in maintaining certain aspects of M phase. It should be noted in Figure 4 that Plx1-depleted extracts supplemented with active WT Greatwall seem to have difficulties in exiting mitosis. This is expected since Plx1 is known to be involved in the pathway leading to the destruction of cyclins during mitotic exit (Liu and Maller, 2005), and Figure 4 correspondingly shows that the destruction of cyclins is incomplete in the Plx1-depleted extracts.
Figure 4.
Plx1 is not required for Greatwall-induced mitotic entry. Cycling extracts were subjected to mock depletion or Plx1 depletion, and then incubated starting at t = 0 for 40 min at 23°C. The extract was then supplemented with buffer (PBS) or with WT or KD Greatwall purified from OA-treated Sf9 cells. One-microliter aliquots of extracts were subsequently collected every 10 min for immunoblot analysis. As expected, Plx1 depletion causes a defect in mitotic entry. However, Plx1 depletion does not interfere with Greatwall-induced mitotic entry even though Cdc25C does not obtain its full complement of M phase phosphorylations.
Regulation of Cdc25 Phosphorylation by Greatwall Does Not Require the MAPK Pathway
MAPK has recently been identified as a major contributor to the phosphorylation of Cdc25 in M phase extracts (Wang et al., 2007). We used two approaches to elucidate whether MAPK or its downstream effectors could be necessary to Greatwall's phosphorylation and ability to promote the activation of Cdc25 and M phase entry. First, as already mentioned, we injected Mos morpholinos into G2-arrested oocytes that were subsequently stimulated by Pg (Supplementary Figure S2). In the absence of Mos/MAPK pathway signaling, Greatwall was normally phosphorylated and Cdc2 was activated as judged by cyclin B2 electrophoretic mobility. Second, we used the MAP/ERK kinase (MEK) inhibitor U0126 (Gross et al., 2000) to interfere with the activation of MAPK in interphase or cycling extracts (Figure 5 and Supplementary Figure S3, respectively). Addition of U0126 successfully blocks phosphorylation of MAPK and its downstream effector p90RSK. However, the drug does not prevent the activation of Cdc25 induced by Greatwall. Both of these approaches thus reveal that the MAPK pathway is not essential for Greatwall's phosphorylation and its function in promoting Cdc25 phosphorylation and M phase entry. These results do not exclude the possibility that MAPK may still play a minor role in the Greatwall-mediated regulation of Cdc25, because U0126 appears to slow slightly the kinetics of Cdc25 activation in the presence of Greatwall. The control experiments in Figure 5 also interestingly indicate that Greatwall may conversely impact the MAPK pathway by promoting activating phosphorylations on MAPK and p90RSK; we believe that this is an indirect effect associated with the entry of the extracts into M phase when Greatwall is added.
Greatwall Induces the Phosphorylation of Cdc25 by Several Kinases
The results shown to this point indicate that Greatwall can mobilize one or more kinases other than MPF to regulate Cdc25, yet neither Plx1 nor MAPK is uniquely required for Greatwall-induced Cdc25 activation. These findings could be explained in either of two ways. Greatwall could positively regulate another, currently unknown kinase that phosphorylates Cdc25; alternatively, Greatwall could in some manner potentiate the function of several Cdc25-targeting kinases.
To discriminate between these models, we performed manipulations to inactivate pairwise combinations of MPF and either Plx1 or MAPK. It will be recalled from Figure 3B that active Greatwall promotes an upshift of Cdc25 when the function of Cdc2 is abrogated in cycling extracts; however, this upshift is only partial, indicating that some sites on Cdc25 that are normally phosphorylated during M phase remain dephosphorylated. When either Plx1 is removed or MAPK is inhibited in addition to Cdc2, the phosphorylation of Cdc25 is further decreased (Figure 6). It thus appears that both Plx1 and MAPK contribute to the Cdc25 phosphorylation observed in the presence of active Greatwall. However, Cdc25's electrophoretic mobility in both cases still remains slightly retarded relative to that seen during interphase. This is also true even under conditions in which MPF, Plx1, and MAPK are simultaneous inactivated (Figure 6). These small shifts in Cdc25 mobility are consistent with the possibility that Greatwall might also mobilize the activity of a fourth Cdc25 kinase whose existence has been demonstrated (Wang et al., 2007) but remains unidentified; alternatively, these shifts might be explained by low residual levels of some combination of these kinases after the inactivating treatments.
Active Greatwall Overrides Inhibitory Phosphorylations of Cdc25 at Ser287
Under certain conditions, the rate-limiting step for the activation of Cdc25 is the removal of an inhibitory phosphorylation at amino acid Ser287, resulting in the dissociation of Cdc25 from 14-3-3 proteins (Perdiguero and Nebreda, 2004). It is thus plausible that Greatwall might promote the dephosphorylation of Ser287 and/or the dissociation of Cdc25 from 14-3-3. To test this idea, we monitored Ser287 phosphorylation during Greatwall-induced precocious mitotic entry (Figure 7). This phosphorylation disappears soon after Greatwall addition and concurrently with the Cdc25 upshift, so Greatwall counteracts the inhibitory phosphorylation at the Ser287 site of Cdc25. Interestingly, substantial Ser287 phosphorylation was detected on Cdc25 species with retarded mobility, implying that the removal of this phosphorylation is not a precondition for at least some M phase–specific phosphorylations on Cdc25.
Figure 7.
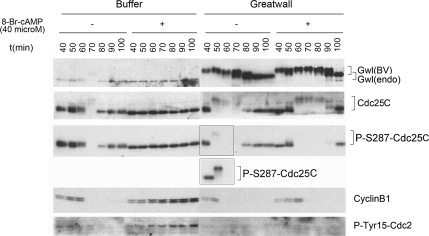
Greatwall overrides the effect of the PKA activator 8-Br-cAMP. Addition of 8-Br-cAMP (40 μM final concentration) effectively blocks mitotic entry in control cycling extracts; Cdc25 remains unphosphorylated, whereas inhibitory Tyr15 phosphorylations accumulate on Cdc2. The addition of excess (five times the endogenous level) active Greatwall at t = 40 min overcomes this effect of 8-Br-cAMP. In controls, the drug does not increase phosphorylation at the S287 residue of Cdc25C; the mechanism by which 8-Br-cAMP blocks mitotic entry remains unknown. The inset shows the result of a similar experiment that emphasizes the existence of Cdc25 species of retarded mobility that retain substantial Ser287 phosphorylation.
To approach this same issue in an alternative manner, we used the drug 8-Br-cAMP to activate PKA, one of the kinases targeting Ser287 (Grieco et al., 1994). Ser287 phosphorylation by PKA is an intrinsic element of the mechanism maintaining G2 arrest before meiosis in immature Xenopus oocytes (Duckworth et al., 2002). We therefore anticipated that 8-Br-cAMP treatment would increase Ser287 phosphorylation and produce a G2 phase arrest in cycling extracts. The drug does in fact arrest cycling extracts in G2, and this is associated with the accumulation of inhibitory Tyr15 phosphorylations on Cdc2 (Figure 7). This effect of 8-Br-cAMP is substantially overridden by active Greatwall, which promotes the efficient removal of the Ser287 phosphorylation on Cdc25, prevents the Tyr15 phosphorylation of Cdc2, and causes rapid mitotic entry within 10–20 min of its addition, even in the presence of this cAMP analog. Greatwall thus counteracts the blockage of M phase entry caused by 8-Br-cAMP. However, contrary to our expectations, 8-Br-cAMP does not increase the levels of Ser287 phosphorylation on Cdc25. The M phase block caused by this drug is thus mediated by mechanisms independent of Ser287 regulation that are currently unknown.
OA Treatment Mimics Greatwall's Mitosis-promoting Function
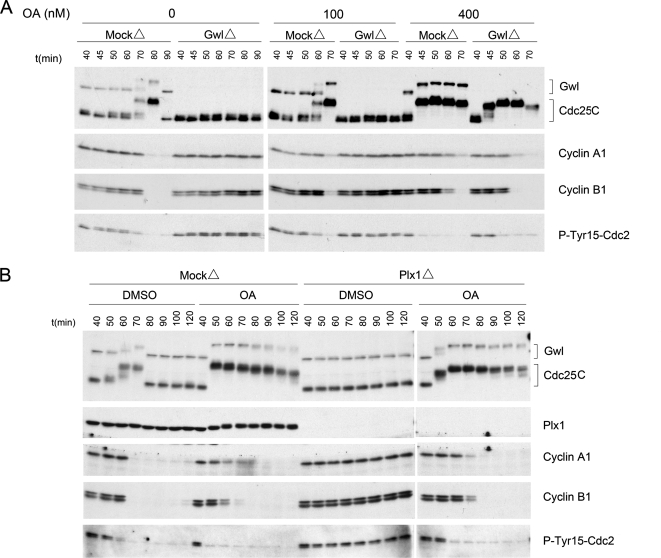
The similarities between the consequences of adding either active Greatwall or the phosphatase inhibitor OA to cycling extracts are striking. Both reagents promote M phase entry with near-identical rapid kinetics, and both are dominant in their effects to treatments that disrupt other cell cycle regulators (this article; Gowdy et al., 1998). It is generally thought that OA affects mitosis by inhibiting a phosphatase, probably a form of phosphatase 2A (PP2A; Lee et al., 1994; Maton et al., 2005; see Margolis et al., 2006b for an alternative view) that normally dephosphorylates and inactivates mitotic phosphoproteins such as Cdc25 (Perry and Kornbluth, 2007). We were thus led to test the hypothesis that Greatwall might target the same PP2A-like phosphatase inhibited by OA. Some of our results are consistent with this idea. Addition of a low concentration of OA (400 nM, which is sufficient to inhibit PP2A, but not PP1; Margolis et al., 2003) not only induces rapid mitotic entry in mock-depleted cycling extracts, but it also overcomes the G2/M arrest caused by Greatwall depletion (Figure 8A). Furthermore, 400 nM OA can induce precocious mitotic entry in a Plx1-independent manner just as active Greatwall, and with similar kinetics (Figure 8B).
Figure 8.
OA overcomes the Greatwall depletion phenotype in cycling extracts. (A) Mock-depleted or Greatwall-depleted cycling extracts were incubated at 23°C for 40 min before supplementation with 100 or 400 nM OA in DMSO or with an equal volume of DMSO alone (0). One-microliter aliquots were collected every 10 min thereafter and processed for immunoblotting. (B) OA rescues the Plx1 depletion phenotype in cycling extracts. Mock-depleted or Plx1-depleted cycling extracts were incubated at 23°C for 40 min before supplementation with 400 nM OA or DMSO.
DISCUSSION
At the outset of these studies, we knew that Greatwall is essential for M phase entry and maintenance and that it operates through a mechanism that influences the regulation of the Tyr15 inhibitory phosphorylation site on Cdc2. Moreover, it was clear that Greatwall is itself activated during M phase by phosphorylations that are probably mediated by MPF (Yu et al., 2006). The results described in this article place Greatwall more firmly within the Cdc2 autoregulatory loop. Not only is Greatwall downstream of MPF, but at least one Greatwall function is upstream of Cdc25, because once it is activated, Greatwall can promote Cdc25 phosphorylations in the absence of detectable MPF activity (Figure 3). The importance of Greatwall to Cdc25 regulation is emphasized by the fact that with a single exception (discussed below), Greatwall is “dominant” to other known components of the autoregulatory loop in a variety of biochemical epistasis tests. Cdc25 acquires activating phosphorylations in the presence of Greatwall and loses these phosphorylations in the absence of Greatwall, regardless of the depletion or inactivation of other cell cycle regulators. Our investigations do not address the possibility that Greatwall might also influence the function of the Wee1/Myt1 kinases that catalyze the Tyr15 Cdc2 phosphorylations removed by Cdc25.
The results reported here eliminate models that posit Greatwall works uniquely through one of the kinases known to phosphorylate and activate the Cdc25 phosphatase during M phase. Not only does Greatwall promote Cdc25 phosphorylation independent of MPF (Figure 3), but Greatwall also induces Cdc25 when Plx1 is removed by immunodepletion (Figure 4) or when MAPK is inactivated (Figure 5 and Supplementary Figure S3). These findings must of course be considered in light of the caveat that undetectable but nonetheless significant residual activity of MPF, Plx1, and/or MAPK may have survived the treatments we used to deplete or inactivate these enzymes.
It is striking that Cdc25 activity under the conditions of Plx1 depletion or MAPK inactivation appears to be sufficient to promote M phase entry, although it should be emphasized that this conclusion has been inferred indirectly from effects on various cell cycle markers rather than through direct measurements of Cdc25 itself. These results have interesting implications concerning the functional significance of various Cdc25 phosphorylations. First, our results verify previous conclusions that in Xenopus, the MAP kinase pathway is not required for entry into mitosis or meiosis (Takenaka et al., 1997), although it does play a critical role in maintaining M phase (Guadagno and Ferrell, 1998) and a redundant role in oocyte maturation (Haccard and Jessus, 2006). The ability of Greatwall to induce Cdc25 in the absence of MAPK function also fits with our previous finding that when Greatwall is depleted from M phase (CSF) extracts, Cdc25 is dephosphorylated rapidly even while the MAPK pathway remains active (Yu et al., 2006). Second, the experiment in Figure 4 presents a condition in which Cdc25 can be activated in the absence of Plx1-specific phosphorylations. This result was unexpected since these phosphorylations are normally required for Cdc25 function in Xenopus eggs and extracts (Qian et al., 1998, 2001; Karaiskou et al., 1999, 2004) and because recent models suggest that the phosphorylation of Cdc25 by Plx1 is a late event that requires previous “priming” phosphorylations of Cdc25 by CDKs or MAPK (Elia et al., 2003; Liu et al., 2004). The idea that Plx1-specified phosphorylations are not absolutely required for Cdc25 function if other activating sites on Cdc25 are phosphorylated may help explain recent findings in human tissue culture cells challenging the notion that Plk1 (human Plx1) is truly required for Cdc25 activation and mitotic entry (Barr et al., 2004).
Paradoxically, even though Greatwall does not depend upon any one kinase to regulate Cdc25, Greatwall mobilizes at least three, and possibly four, kinases that can target Cdc25 relatively independently of each other (Figure 6). The simplest explanation for this conclusion is that Greatwall might directly regulate all of these enzymes; however, we have found to date no evidence that any of these kinases is a direct substrate of Greatwall. Another possibility is that Greatwall might induce the activity of an unknown “master” kinase that can substitute for the function of MPF in targeting a variety of M phase substrates. The literature contains reports suggestive of the existence of such an enzyme (Kuang et al., 1991), but it has not been identified or characterized to date.
The finding in Figure 7 that Greatwall counteracts the inhibitory Ser287 phosphorylation on Cdc25 not only illustrates another role for Greatwall in the regulation of Cdc25, but it also suggests a third possible explanation for Greatwall's manifold effects. Greatwall appears to contribute to the dephosphorylation of this site and/or the release of Cdc25 from 14-3-3 proteins, perhaps indirectly by influencing the phosphorylation of the Thr138 site required for these events (Margolis et al., 2006a). One could imagine that in this manner Greatwall controls an early, rate-limiting step in Cdc25 activation. Once this step is completed, the various kinases responsible for later stages in Cdc25 activation would then be mobilized to target Cdc25. However, we strongly believe that such a mechanism cannot explain all the effects of Greatwall. Ser287 is indeed phosphorylated in interphase during early embryonic mitosis (Figure 7; Hutchins et al., 2002; Stanford and Ruderman, 2005), but it has never been clearly demonstrated that Ser287 is a major factor in the regulation of normal cell cycles in which the DNA damage checkpoint is not active. Moreover, it has recently been reported that addition of Cdc25 with an Ser287Ala mutation (that cannot be phosphorylated at this site) accelerates M phase entry in cycling extracts only by a few minutes (Chun et al., 2005), an effect much more modest than the robust consequences of adding active Greatwall. The results shown in Figure 7 also suggest that the dephosphorylation of Ser287 is not a precondition for M phase–specific phosphorylations of Cdc25. Finally, we conducted an additional experiment to assess whether S287 dephosphorylation is essential to Greatwall function. Because CamKII is known to provide the major Ser287-directed activity in egg extracts when the DNA damage checkpoint is not active (Hutchins et al., 2003), we used the CamKII inhibitor 281-309 (Smyth et al., 2002) to reduce levels of S287 phosphorylation (Supplementary Figure S4). Addition of 400 μM CamKII inhibitor 281-309 to cycling extracts accelerates mitotic entry by ∼20 min, consistent with a reduction in S287 phosphorylation. However, 291-309 cannot overcome the mitotic entry defect caused by Greatwall depletion, suggesting that Greatwall does not uniquely function by promoting S287 dephosphorylation or by inhibiting CamKII.
In our view, the hypothesis that best matches the currently available information is that Greatwall antagonizes the activity of an OA-sensitive phosphatase (most likely a form of PP2A; Lee et al., 1994; Maton et al., 2005) that removes M phase–specific modifications from mitotic phosphoproteins such as Cdc25. It has been known for many years that the phosphatase(s) targeting at least some activating phosphorylations on Cdc25 is (are) down-regulated specifically during M phase (Kumagai and Dunphy, 1992; Clarke et al., 1993; Lee et al., 1994); furthermore, Mochida and Hunt (2007) have recently obtained evidence that phosphatase(s) affecting several mitotic phosphoproteins are turned off during M phase. The idea that Greatwall might mediate this phosphatase down-regulation is extremely attractive. The kinetics of precocious GVBD and mitotic entry caused by excess active Greatwall are similar to those seen upon the addition of OA (compare Figures 1C and 8A). Moreover, both treatments are “dominant” to a variety of conditions that alter the activities of other cell cycle regulators: for example, OA (or microcystin, another phosphatase inhibitor) as well as Greatwall can promote Cdc25 phosphorylation in the absence of Plx or Cdc2 function (Figures 4 and 8B; Izumi and Maller, 1995). Most persuasively, OA is the only reagent that we found to be able to overcome the G2 arrest caused by Greatwall depletion from Xenopus cycling extracts (Figure 8A).
However, several observations do not easily fit a model in which a phosphatase is the major substrate of Greatwall. The effects of OA and Greatwall are not absolutely identical: OA promotes M phase entry in immature oocytes independently of protein synthesis, but the induction of meiotic maturation by Greatwall requires protein synthesis (Figure 2). Moreover, in preliminary studies using meiotic prophase extracts from immature oocytes (described by Maton et al., 2005), OA cannot rescue the effects of Greatwall depletion on M phase entry (data not shown). One could still reconcile these observations with the hypothesis if OA inhibits a broader spectrum of phosphatases than does Greatwall, if Greatwall participates in additional regulatory pathways or if Greatwall phosphorylates different substrates during mitosis and meiosis.
Our work clarifies the importance of Greatwall in M phase entry and sets a framework for understanding Greatwall's function. To understand the precise mechanism of Greatwall action, it will be necessary to identify the substrates of this kinase. Unfortunately, in vitro tests have thus far failed to demonstrate the ability of Greatwall to phosphorylate the most obvious candidate proteins, including Plx1, Plx3, Cdc2, cyclin B, Cdc25C, Cdc25A, Chk1, Wee1, Myt1, Pin1, and various components of PP2A (subunits A and C, and the regulatory subunits B55 and B56). Additional approaches may thus be necessary to solve this intriguing unsolved mystery in cell cycle regulation.
Supplementary Material
ACKNOWLEDGMENTS
We thank the investigators named in Materials and Methods for generously providing antibodies. This work was supported by National Institutes of Health Grant GM48430 to M.L.G.; funding from the Centre Nationale de la Recherche Scientifique, Université Pierre et Marie Curie, Association pour la Recherche sur le Cancer (Grant 3969), and Agence Nationale de la Recherche (BLAN07-3 185404) to C.J. and O.H., and National Institutes of Health, National Cancer Institute Grant R01 CA93941 to J.K.
Abbreviations used:
- CamKII
Ca2+/calmodulin kinase II
- CDK
cyclin-dependent kinase
- CHX
cycloheximide
- CSF
cytostatic factor
- GVBD
germinal vesicle breakdown
- KD
kinase dead
- MAPK
mitogen-activated protein kinase
- MPF
mitosis-promoting factor
- OA
okadaic acid
- PKA
protein kinase A
- PP2A
protein phosphatase 2A
- Ros
roscovitine
- WT
wild type.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-11-1099) on January 16, 2008.
REFERENCES
- Abrieu A., Brassac T., Galas S., Fisher D., Labbe J., Doree M. The Polo-like kinase Plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs. J. Cell Sci. 1998;111:1751–1757. doi: 10.1242/jcs.111.12.1751. [DOI] [PubMed] [Google Scholar]
- Barr F. A., Sillje H.H.W., Nigg E. A. Polo-like kinases and the orchestration of cell division. Nat. Rev. Mol. Cell Biol. 2004;5:429–441. doi: 10.1038/nrm1401. [DOI] [PubMed] [Google Scholar]
- Chun J., Chau A. S., Maingat F. G., Edmonds S. D., Ostergaard H. L., Shibuya E. K. Phosphorylation of Cdc25C by pp90Rsk contributes to a G2 cell cycle arrest in Xenopus cycling egg extracts. Cell Cycle. 2005;4:148–154. doi: 10.4161/cc.4.1.1323. [DOI] [PubMed] [Google Scholar]
- Clarke P. R., Hoffmann I., Draetta G., Karsenti E. Dephosphorylation of cdc25-C by a type-2A protein phosphatase: specific regulation during the cell cycle in Xenopus egg extracts. Mol. Biol. Cell. 1993;4:397–411. doi: 10.1091/mbc.4.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descombes P., Nigg E. A. The polo-like kinase Plx1 is required for M phase exit and destruction of mitotic regulators in Xenopus egg extracts. EMBO J. 1998;17:1328–1335. doi: 10.1093/emboj/17.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth B. C., Weaver J. S., Ruderman J. V. G2 arrest in Xenopus oocytes depends on phosphorylation of cdc25 by protein kinase A. Proc. Natl. Acad. Sci. USA. 2002;99:16794–16799. doi: 10.1073/pnas.222661299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupre A., Jessus C., Ozon R., Haccard O. Mos is not required for the initiation of meiotic maturation in Xenopus oocytes. EMBO J. 2002;21:4026–4036. doi: 10.1093/emboj/cdf400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia A. E., Rellos P., Haire L. F., Chao J. W., Ivins F. J., Hoepker K., Mohammad D., Cantley L. C., Smerdon S. J., Yaffe M. B. The molecular basis for phosphodependent substrate targeting and regulation of Plks by the Polo-box domain. Cell. 2003;115:83–95. doi: 10.1016/s0092-8674(03)00725-6. [DOI] [PubMed] [Google Scholar]
- Fernandez J. J., Candenas M. L., Souto M. L., Trujillo M. M., Norte M. Okadaic acid, useful tool for studying cellular processes. Curr. Med. Chem. 2002;9:229–262. doi: 10.2174/0929867023371247. [DOI] [PubMed] [Google Scholar]
- Frank-Vaillant M., Haccard O., Ozon R., Jessus C. Interplay between Cdc2 kinase and the c-Mos/MAPK pathway between metaphase I and metaphase II in Xenopus oocytes. Dev. Biol. 2001;231:279–288. doi: 10.1006/dbio.2000.0142. [DOI] [PubMed] [Google Scholar]
- Gowdy P., Anderson H., Roberge M. Entry into mitosis without Cdc2 kinase activation. J. Cell Sci. 1998;111:3401–3410. doi: 10.1242/jcs.111.22.3401. [DOI] [PubMed] [Google Scholar]
- Grieco D., Avvedimento E., Gottesman M. A role for cAMP-dependent protein kinase in early embryonic divisions. Proc. Natl. Acad. Sci. USA. 1994;91:9896–9900. doi: 10.1073/pnas.91.21.9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross S. D., Schwab M. S., Taieb F. E., Lewellyn A. L., Qian Y.-W., Maller J. L. The critical role of the MAP kinase pathway in meiosis II in Xenopus oocytes is mediated by p90Rsk. Curr. Biol. 2000;10:430–438. doi: 10.1016/s0960-9822(00)00425-5. [DOI] [PubMed] [Google Scholar]
- Guadagno T. M., Ferrell J. E., Jr Requirement for MAPK activation for normal mitotic progression in Xenopus egg extracts. Science. 1998;282:1312–1315. doi: 10.1126/science.282.5392.1312. [DOI] [PubMed] [Google Scholar]
- Haccard O., Jessus C. Redundant pathways for Cdc2 activation in Xenopus oocytes: either cyclin B or Mos synthesis. EMBO Rep. 2006;7:321–325. doi: 10.1038/sj.embor.7400611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins J.R.A., Dikovskaya D., Clarke P. R. Dephosphorylation of the inhibitory phosphorylation site S287 in Xenopus Cdc25C by protein phosphatase-2A is inhibited by 14-3-3 binding. FEBS Lett. 2002;528:267–271. doi: 10.1016/s0014-5793(02)03327-6. [DOI] [PubMed] [Google Scholar]
- Hutchins J.R.A., Dikovskaya D., Clarke P. R. Regulation of Cdc2/cyclin B activation in Xenopus egg extracts via inhibitory phosphorylation of Cdc25C phosphatase by Ca2+/Calmodium-dependent kinase II. Mol. Biol. Cell. 2003;14:4003–4014. doi: 10.1091/mbc.E03-02-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T., Maller J. L. Phosphorylation and activation of the Xenopus Cdc25 phosphatase in the absence of Cdc2 and Cdk2 kinase activity. Mol. Biol. Cell. 1995;6:215–226. doi: 10.1091/mbc.6.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessus C., Thibier C., Ozon R. Levels of microtubules during the meiotic maturation of the Xenopus oocyte. J. Cell Sci. 1987;87:705–712. doi: 10.1242/jcs.87.5.705. [DOI] [PubMed] [Google Scholar]
- Karaiskou A., Jessus C., Brassac T., Ozon R. Phosphatase 2A and polo kinase, two antagonistic regulators of cdc25 activation and MPF auto-amplification. J. Cell Sci. 1999;112:3747–3756. doi: 10.1242/jcs.112.21.3747. [DOI] [PubMed] [Google Scholar]
- Karaiskou A., Lepretre A.-C., Pahlavan G., Du Pasquier D., Ozon R., Jessus C. Polo-like kinase confers MPF autoamplification competence to growing Xenopus oocytes. Development. 2004;131:1543–1552. doi: 10.1242/dev.01050. [DOI] [PubMed] [Google Scholar]
- Kuang J., Penkala J. E., Wright D. A., Saunders G. F., Rao P. N. A novel M phase-specific H1 kinase recognized by the mitosis-specific monoclonal antibody MPM-2. Dev. Biol. 1991;144:54–64. doi: 10.1016/0012-1606(91)90478-l. [DOI] [PubMed] [Google Scholar]
- Kumagai A., Dunphy W. G. Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell. 1992;70:139–151. doi: 10.1016/0092-8674(92)90540-s. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Turck C., Kirschner M. W. Inhibition of cdc2 activation by INH/PP2A. Mol. Biol. Cell. 1994;5:323–338. doi: 10.1091/mbc.5.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Lewellyn A. L., Chen L. G., Maller J. L. The polo box is required for multiple functions of Plx1 in mitosis. J. Biol. Chem. 2004;279:21367–21373. doi: 10.1074/jbc.M400482200. [DOI] [PubMed] [Google Scholar]
- Liu J., Maller J. L. Xenopus Polo-like kinase Plx 1, a multifunctional mitotic kinase. Oncogene. 2005;24:238–247. doi: 10.1038/sj.onc.1208220. [DOI] [PubMed] [Google Scholar]
- Lorca T., Cruzalegui F. H., Fesquet D., Cavadore J. C., Mery J., Means A., Doree M. Calmodulin-dependent protein kinase II mediates inactivation of MPF and CSF upon fertilization of Xenopus eggs. Nature. 1993;366:270–273. doi: 10.1038/366270a0. [DOI] [PubMed] [Google Scholar]
- Margolis S. S., et al. Role for the PP2A/B56delta phosphatase in regulating 14-3-3 release from Cdc25 to control mitosis. Cell. 2006a;127:759–773. doi: 10.1016/j.cell.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis S. S., Perry J. A., Weitzel D. H., Freel C. D., Yoshida M., Haystead T. A., Kornbluth S. A role for PP1 in the Cdc2/cyclin B-mediated positive feedback activation of Cdc25. Mol. Biol. Cell. 2006b;17:1779–1789. doi: 10.1091/mbc.E05-08-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis S. S., Walsh S., Weiser D. C., Yoshida M., Shenolikar S., Kornbluth S. PP1 control of M phase entry exerted through 14-3-3-regulated Cdc25 dephosphorylation. EMBO J. 2003;22:5734–5745. doi: 10.1093/emboj/cdg545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maton G., Lorca T., Girault J.-A., Ozon R., Jessus C. Differential regulation of Cdc2 and Aurora-A in Xenopus oocytes: a crucial role of phosphatase 2A. J. Cell Sci. 2005;118:2485–2494. doi: 10.1242/jcs.02370. [DOI] [PubMed] [Google Scholar]
- Meijer L., Borgne A., Mulner O., Chong J. P., Blow J. J., Inagaki N., Inagaki M., Delcros J. G., Moulinoux J. P. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- Mochida S., Hunt T. Calcineurin is required to release Xenopus egg extracts from meiotic M phase. Nature. 2007;449:336–340. doi: 10.1038/nature06121. [DOI] [PubMed] [Google Scholar]
- Murray A. W. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- Murray A. W., Kirschner M. W. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989;339:275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- Perdiguero E., Nebreda A. R. Regulation of Cdc25C activity during the meiotic G2/M transition. Cell Cycle. 2004;3:733–737. [PubMed] [Google Scholar]
- Perry J. A., Kornbluth S. Cdc25 and Wee1, analogous opposites? Cell Div. 2007;2:12. doi: 10.1186/1747-1028-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y.-W., Erikson E., Li C., Maller J. L. Activated polo-like kinase Plx1 is required at multiple points during mitosis in Xenopus laevis. Mol. Cell. Biol. 1998;18:4262–4271. doi: 10.1128/mcb.18.7.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y.-W., Erikson E., Taieb F. E., Maller J. L. The Polo-like kinase Plx1 is required for activation of the phosphatase Cdc25C and Cyclin B-Cdc2 in Xenopus oocytes. Mol. Biol. Cell. 2001;12:1791–1799. doi: 10.1091/mbc.12.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh N. R., Schmidt A., Bormann J., Nigg E. A., Mayer T. U. Calcium triggers exit from meiosis II by targeting the APC/C inhibitor XErp1 for degradation. Nature. 2005;437:1048–1052. doi: 10.1038/nature04093. [DOI] [PubMed] [Google Scholar]
- Rudner A. D., Murray A. W. Phosphorylation by Cdc28 activates the Cdc20-dependent activity of the anaphase-promoting complex. J. Cell Biol. 2000;149:1377–1390. doi: 10.1083/jcb.149.7.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth J. T., Abbott A. L., Lee B., Sienaert I., Kasri N. N., De Smedt H., Ducibella T., Missiaen L., Parys J. B., Fissore R. A. Inhibition of the inositol trisphosphate receptor of mouse eggs and A7r5 cells by KN-93 via a mechanism unrelated to Ca2+/Calmodulin-dependent protein kinase II antagonism. J. Biol. Chem. 2002;277:35061–35070. doi: 10.1074/jbc.M202928200. [DOI] [PubMed] [Google Scholar]
- Stanford J. S., Ruderman J. V. Changes in regulatory phosphorylation of Cdc25C Ser287 and Wee1 Ser549 during normal cell cycle progression and checkpoint arrests. Mol. Biol. Cell. 2005;16:5749–5760. doi: 10.1091/mbc.E05-06-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark G. R., Taylor W. R. Control of the G2/M transition. Mol. Biotechnol. 2006;32:227–248. doi: 10.1385/MB:32:3:227. [DOI] [PubMed] [Google Scholar]
- Takenaka K., Gotoh Y., Nishida E. MAP kinase is required for the spindle assembly checkpoint but is dispensable for the normal M phase entry and exit in Xenopus egg cell cycle extracts. J. Cell Biol. 1997;136:1091–1097. doi: 10.1083/jcb.136.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., He G., Nelman-Gonzalez M., Ashorn C. L., Gallick G. E., Stukenberg P. T., Kirschner M. W., Kuang J. Regulation of Cdc25C by ERK-MAP kinases during the G2/M transition. Cell. 2007;128:1119–1132. doi: 10.1016/j.cell.2006.11.053. [DOI] [PubMed] [Google Scholar]
- Xiong W., Ferrell J. E., Jr A positive-feedback-based bistable ‘memory module’ that governs a cell fate decision. Nature. 2003;426:460–465. doi: 10.1038/nature02089. [DOI] [PubMed] [Google Scholar]
- Yu J., Fleming S. L., Williams B., Williams E. V., Li Z., Somma P., Rieder C. L., Goldberg M. L. Greatwall kinase: a nuclear protein required for proper chromosome condensation and mitotic progression in Drosophila. J. Cell Biol. 2004;164:487–492. doi: 10.1083/jcb.200310059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Zhao Y., Li Z., Galas S., Goldberg M. L. Greatwall kinase participates in the Cdc2 autoregulatory loop in Xenopus egg extracts. Mol. Cell. 2006;22:83–91. doi: 10.1016/j.molcel.2006.02.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.