Abstract
To cope with the frequent exposure to carcinogenic UV B (UVB) wavelengths found in sunlight, keratinocytes have acquired extensive protective measures to handle UVB-induced DNA damage. Recent in vitro and epidemiological data suggest one these protective mechanisms is dependent on the functional status of the insulin-like growth factor-1 receptor (IGF-1R) signaling network in keratinocytes. During the normal UVB response, ligand-activated IGF-1Rs protect keratinocytes from UVB-induced apoptosis; however, as a consequence, these keratinocytes fail to proliferate. This adaptive response of keratinocytes to UVB exposure maintains the protective barrier function of the epidermis while ensuring that UVB-damaged keratinocytes do not replicate DNA mutations. In contrast, when keratinocytes are exposed to UVB in the absence of IGF-1R activation, the keratinocytes are more sensitive to UVB-induced apoptosis, but the surviving keratinocytes retain the capacity to proliferate. This aberrant UVB response represents flawed protection from UVB damage potentially resulting in the malignant transformation of keratinocytes. Using normal human keratinocytes grown in vitro, we have demonstrated that activation of the IGF-1R promotes the premature senescence of UVB-irradiated keratinocytes through increased generation of reactive oxygen species (ROS) and by maintaining the expression of the cyclin-dependent kinase inhibitor p21CDKN1A. Furthermore, IGF-1R–dependent UVB-induced premature senescence required the phosphorylation of p53 serine 46. These data suggest one mechanism of keratinocyte resistance to UVB-induced carcinogenesis involves the induction of IGF-1R–dependent premature senescence.
INTRODUCTION
Cellular senescence is defined as an irreversible arrest in cellular replication in otherwise metabolically active cells. First identified as a phenomenon controlling the longevity of cells cultured in vitro (Hayflick and Moorhead, 1961), senescent cells have now been identified in vivo to accumulate in aging tissues (Dimri et al., 1995; Pendergrass et al., 1999; Toussaint et al., 2002; Herbig et al., 2006). Replicatively senescent cells result from the critical erosion of telomeres after repeated cellular divisions (Harley et al., 1990; Kim et al., 2002; Blackburn, 2005). Although single specific cellular markers for senescent cells in vitro can be inconclusive, senescent cells can be identified by their syndromic phenotype: replication failure; a flattened, spreading cellular morphology; sustained expression of cyclin-dependent kinase inhibitors; and the expression of senescence-associated β-galactosidase (Kim et al., 2002; Blackburn, 2005). Senescence can also be induced before replication exhaustion by factors that induce cellular stress, i.e., oxidative stress, DNA damage, inappropriate oncogene activation (Behrend et al., 2005; Ben-Porath and Weinberg, 2005; Dasari et al., 2006; Mallette et al., 2007). The induction of senescence after stress-related injury is a defense mechanism to avert carcinogenesis (Campisi, 2005; Dimri, 2005).
In the skin, keratinocytes are repeatedly exposed to UV B radiation (UVB) in sunlight that if left unchecked can potentially cause widespread skin cancer (Melnikova and Ananthaswamy, 2005). Exposure to UVB causes both DNA damage and oxidative stress in irradiated keratinocytes (Mullenders et al., 1997). Fortunately, keratinocytes have developed many specific mechanisms to handle the unrelenting bombardment of carcinogenic insults. The in vitro-cultured keratinocyte model system has provided us with a wealth of information regarding how keratinocytes respond to UVB irradiation (Cotton and Spandau, 1997; Kumar et al., 1999; Kuhn et al., 1999; Qin et al., 2002; Zhang et al., 2002; Lewis et al., 2003, 2006; Chaturvedi et al., 2004; Lewis and Spandau, 2007a,b). In addition to the severity of UVB-induced DNA damage, events at the cell membrane of the keratinocyte, and their subsequent signal transduction cascades, critically control the fate of UVB-irradiated keratinocytes. We have examined one of these cell surface events, the activation of the insulin-like growth factor-1 receptor (IGF-1R; Kuhn et al., 1999; Lewis and Spandau, 2007b), and we described three key features affecting the cellular outcome of UVB-irradiated human keratinocytes: 1) ligand-activation of the IGF-1R promotes protection from UVB-induced apoptosis; 2) although UVB-irradiated keratinocytes with activated IGF-1Rs survive, they are incapable of further cellular replication; and 3) in the absence of IGF-1R activation, keratinocytes are more sensitive to UVB-induced apoptosis, but the keratinocytes that do survive retain the capacity to proliferate (Kuhn et al., 1999). We hypothesize that the first two observations were part of the normal protective response of human skin to UVB exposure, i.e., maintaining the integrity of the protective barrier function of the epidermis while ensuring that UVB-damaged keratinocytes are not permitted to replicate DNA mutations. The third observation represents flawed protection from UVB damage, and the consequences of failed UVB protection may include malignant transformation of keratinocytes. Previously, we have reported that the survival pathway controlling the IGF-1R–dependent UVB response in human keratinocytes involved the nuclear factor-κB and p38 signaling pathways (Lewis and Spandau, 2007b). We now report that UVB irradiation induces premature senescence in human keratinocytes that is dependent on a functional IGF-1R and subsequent signaling through p53.
MATERIALS AND METHODS
Cell Culture
Normal human keratinocytes were isolated from neonatal foreskin tissue (Kuhn et al., 1999). Isolated keratinocytes were grown in EpiLife Complete media (Cascade Biologics, Portland, OR) supplemented with human keratinocyte growth supplement (Cascade Biologics) and 1000 U of penicillin-streptomycin (Roche Molecular Biochemicals, Indianapolis, IN). EpiLife NoIn media is identical to EpiLife Complete except it contains no insulin. Subsequently, keratinocytes grown in EpiLife NoIn media are described as (−)IGF-1R keratinocytes, and cells grown in EpiLife Complete media are referred to as (+)IGF-1R keratinocytes (referring to the functional status of the IGF-1R in those keratinocytes; Lewis and Spandau, 2007b). All experiments were conducted using subconfluent, low-passage primary normal human keratinocytes. The n-tert and lif-tert keratinocytes (Dickson et al., 2000) were treated identically to normal keratinocytes. The n-tert cell lines expressing p53 mutants were clonally selected after the transduction of the parental n-tert keratinocytes. Retroviruses were constructed by inserting the relevant p53 gene from pCMV-p53WT, p53S46A, or p53S46D (Ichwan et al., 2006) into the pBABE-puro vector.
UVB Irradiation
UVB irradiation of normal human keratinocytes was accomplished using two Philips FS20T12 UVB broadband light sources. The intensity of the UVB source was measured before each experiment by using an IL1700 radiometer and a SED240 UVB detector (International Light, Newburyport, MA) at a distance of 8 cm from the UVB source to monolayer of cells. Normal human keratinocytes were irradiated in EpiLife media (Cascade Biologics), and then they were returned to standard incubation conditions (37°C and 5% CO2). EpiLife medium absorbs all of the UVC wavelengths emanating from the light source without absorbing significant amounts of UVB wavelengths.
Senescence-associated β-Galactosidase Assays
Keratinocytes were washed twice with phosphate-buffered saline (PBS) and fixed with 2% formaldehyde/0.2% glutaraldehyde at room temperature for 10 min. After two additional washes with PBS, 2 ml of staining solution (150 mM sodium chloride, 25.2 mM sodium phosphate dibasic, 7.36 mM citric acid, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 2 mM magnesium chloride, and 1 ng/ml 5-bromo-4-chloro-3-indolyl-β-d-galactoside, pH 6.0; Dimri et al., 1995) were added to the cells, and they were incubated at 37°C overnight. The cells were again washed with PBS and photographed by bright field microscopy to count blue cells and phase contrast microscopy to count total cells. At least four fields (100× magnification; ∼200–600 cells/field) were counted for each plate of cells; at least two plates of cells for each condition (or cell type) were assayed in each experiment.
Immunoblotting
Cell cultures were washed twice with ice-cold PBS and lysed with radioimmunoprecipitation assay buffer (150 mM sodium chloride, 50 mM Tris-HCl, pH 8.0, 0.1% SDS, 0.5% sodium deoxycholate, and 1% NP-40) containing complete protease inhibitor (Roche Molecular Biochemicals), 10 mM sodium orthovanadate, and 1 mM sodium fluoride for 20 min on ice. Keratinocytes were further disrupted by a sonic dismembranator, seven 3-s bursts on ice. Cellular debris was eliminated by two rounds of centrifugation. The total protein concentration of the clarified cell lysate was then determined (Bio-Rad Protein Assay Reagent; Bio-Rad, Hercules, CA). Forty micrograms of cell lysate was separated on an SDS-polyacrylamide gel, and the proteins were transferred to Immobilon P (Millipore, Billerica, MA) membranes by semidry electrophoresis (Bio-Rad, Melville, NY). After transfer, the membranes were incubated in TSB buffer (150 mM sodium chloride and 100 mM Tris-base, pH 7.5) and 5% nonfat dry milk for 2 h at room temperature. Antibodies were diluted in TSB plus milk (mouse antibodies) or TSB and 5% bovine serum albumin (rabbit antibodies), and they were added to the membrane overnight at 4°C. The membranes were washed three times with TSB containing 0.1% Tween 20. Secondary antibody conjugated to horseradish peroxidase was diluted in TSB plus milk and incubated on the membrane for 30 min. The membrane was washed as described above, and then specific protein bands were identified by chemiluminescence (ECL Plus; GE Healthcare, Piscataway, NJ).
Caspase-3 Assay
Caspase-3 proteolytic activity was measured in keratinocyte cell lysates by using a synthetic fluorogenic substrate (DEVD-AMC; Alexis Laboratories, San Diego, CA) as described previously (Kuhn et al., 1999). Briefly, keratinocyte cell pellets were suspended in lysis buffer (50 mM 1,4-piperazinediethanesulfonic acid, pH 7.0, 50 mM KCl, 5 mM EGTA, 2 mM MgCl2, and 1 mM dithiothreitol [DTT]) for 30 min on ice. Epidermal and cellular debris were removed by centrifugation. An aliquot of the cell lysate was added to a caspase-3 reaction buffer (100 mM HEPES, pH 7.5, 10% sucrose, 0.1% 3-[(3-cholamidopropyl)dimethylammonio]propanesulfonate, 10 mM DTT, 0.1 mg/ml bovine albumin, and 50 μM DEVD-AMC substrate) and incubated at 37°C for 1 h. Release of the fluorescent 7-amino-4-methylcoumarin (AMC) moiety was measured using a Hitachi F2000 spectrophotofluorimeter (excitation, 380 nm; emission, 460 nm). The fluorescent intensity was converted to picomoles of AMC by comparison with the fluorescent intensity of standards of AMC (Invitrogen, Carlsbad, CA). The specific activity of caspase-3 in cell lystates was then determined after the total protein concentration of the cell lysates was measured (Bio-Rad Protein Assay Reagent).
Reactive Oxygen Species (ROS) Assays
Keratinocytes were washed with PBS, and the cells were loaded with 5-(and-6-)-chloromethyl-2′-7′-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFDA; Invitrogen; 10 μM dye solution in HEPES-balanced saline) for 15 min. Keratinocytes were irradiated with desired dose of UVB, incubated an additional 15 min, and then washed and harvested in fresh HEPES-balanced saline. Fluorescence of the H2DCFDA was read at 480-nm excitation/520-nm emission in the Hitachi F2000 spectrophotofluorimeter. The approximate cell number in each sample was determined by subsequent staining with 4,6-diamidino-2-phenylindole (DAPI) (fluorescence read at 358-nm excitation/461-nm emission).
RESULTS
Low Doses of UVB Induce Cellular Senescence Only in Keratinocytes with a Functional IGF-1R
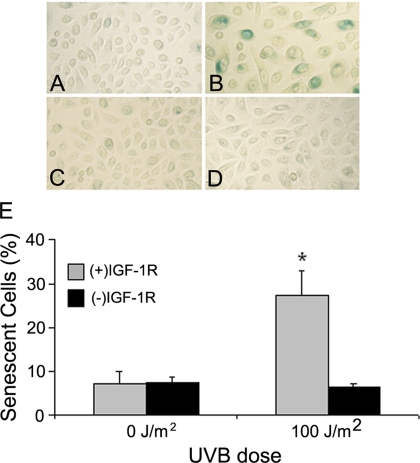
To determine whether the IGF-1R is involved in UVB-induced premature senescence in keratinocytes, normal human keratinocytes were grown in EpiLife Complete [(+)IGF-1R] or EpiLife NoIn [(−)IGF-1R] media for 24 h. The keratinocytes were then irradiated with a dose of 0 or 100 J/m2 UVB. Fifteen hours after irradiation, the media on both (+)IGF-1R and (−)IGF-1R keratinocytes were replaced with EpiLife Complete media. At 72 h after irradiation, the keratinocytes were assayed for senescent-associated β-galactosidase activity, indicated by the development of blue pigment in senescent cells (Dimri et al., 1995). Figure 1, A–D, depicts representative photomicrographs of the stained keratinocyte cultures from these assays. UVB irradiation of (+)IGF-1R keratinocytes resulted in a high frequency of β-galactosidase–positive cells (Figure 1B), and the flattened morphology of these keratinocytes was also indicative of senescent cells. In contrast, UVB irradiation of (−)IGF-1R keratinocytes failed to induce a demonstrable increase in blue-stained cells (Figure 1D) compared with unirradiated keratinocytes (Figure 1, A and C). When the percentages of senescent keratinocytes were tabulated for each experimental group, only UVB-irradiated (+)IGF-1R keratinocytes had a significant increase in senescent keratinocytes. These results correlate with our previous data demonstrating the failure of UVB-irradiated (+)IGF-1R keratinocytes to proliferate, whereas UVB-irradiated (−)IGF-1R keratinocyte retained the ability to proliferate (Kuhn et al., 1999).
Figure 1.
Low doses of UVB irradiation induce cellular senescence only in keratinocytes containing functional IGF-1 receptors. Normal human keratinocytes were grown in EpiLife Complete media [(+)IGF-1R, A and B] or EpiLife NoIn media [(−)IGF-1R, C and D] for 24 h. At that time, keratinocytes were irradiated with 0 (A and C) or 100 (B and D) J/m2 UVB. Eighteen hours after irradiation, the media on all of the dishes was replaced with EpiLife Complete media. Seventy-two hours after irradiation, the keratinocytes were assayed for the expression of senescence-associated β-galactosidase. (A–D) Photomicrographs of keratinocyte cultures stained for the presence of senescence-associated β-galactosidase activity (positive cells are blue). (E) Compiled percentage of senescent cells from each culture condition. Error bars indicate the SEM; the asterisk indicates significant difference between irradiated (+)IGF-1R and (−)IGF-1R keratinocyte percentages (p < 0.01; t test). The data represent three independent assays.
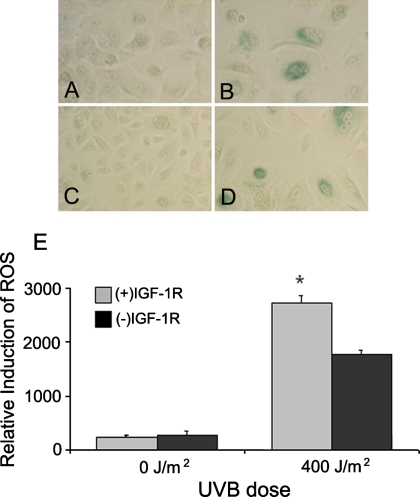
The induction of a stress-induced senescent phenotype can be caused by oxidative stress (Behrend et al., 2005; Ben-Porath and Weinberg, 2005; Dasari et al., 2006). We hypothesized that the failure of (−)IGF-1R keratinocytes to undergo stress-induced senescence after UVB irradiation was due to either the inability of these cells to respond to UVB-induced oxidative stress or a deficiency in the generation of ROS after UVB irradiation. The first possibility was examined by stressing (+)IGF-1R and (−)IGF-1R keratinocytes with hydrogen peroxide and monitoring the induction of senescence. Both (+)IGF-1R and (−)IGF-1R keratinocytes responded to exogenous oxidative stress by undergoing stress-induced senescence (Figure 2, B and D) compared with untreated keratinocytes (Figure 2, A and C), indicating that both types of keratinocytes were capable of undergoing senescence. The induction of ROS by UVB irradiation was then examined by loading (+)IGF-1R and (−)IGF-1R keratinocytes with an indicator dye (CM-H2DCFDA) to measure the generation of ROS after UVB irradiation. As seen in Figure 2E, keratinocytes with functionally active IGF-1Rs generated higher levels of ROS after UVB irradiation than keratinocytes with inactive IGF-1Rs. These data were consistent with the induction of a senescent phenotype in UVB-irradiated (+)IGF-1R keratinocytes. In contrast, (−)IGF-1R keratinocytes accumulate lower levels of ROS after UVB irradiation, and subsequently, they do not undergo premature senescence.
Figure 2.
The IGF-1R regulates the production of ROS following UVB irradiation. Keratinocytes were grown in EpiLife Complete [(+)IGF-1R] or EpiLife NoIn [(−)IGF-1R] media for 24 h. (A–D) Both (+)IGF-1R keratinocytes (A and B) and (−)IGF-1R keratinocytes (C and D) were treated with 0 (A and C) or 600 μM H2O2 (B and D) for an additional 24 h. At that time, keratinocyte were examined for the induction of senescence by assaying for the presence of senescence-associated β-galactosidase activity. (E) To measure the production of ROS, both (+)IGF-1R and (−)IGF-1R keratinocytes were loaded with CM-H2DCFDA dye for 15 min before UVB irradiation. Cells were harvested at 15 min after irradiation, and the fluorescence was measured. The relative induction of ROS was standardized for total cell number by the subsequent staining and measurement of DAPI fluorescence. Error bars indicate the SE of the mean; the asterisk indicates significant difference between irradiated (+)IGF-1R and (−)IGF-1R keratinocyte percentages (p < 0.01, t test). The data represent three independent assays.
Sustained Induction of p21CDKN1A after UVB Irradiation Occurs Only in Keratinocytes Containing a Functional IGF-1R
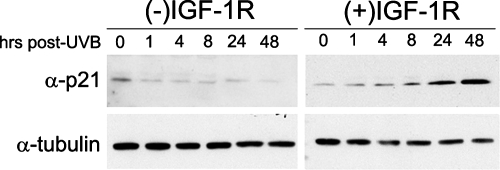
The induction of a senescent phenotype is accompanied by the sustained expression of at least one member of the cyclin-dependent kinase inhibitor family of proteins, namely, p16CDKN2A, p21CDKN1A, or p27CDKN1B (Pajalunga et al., 2007). Therefore, we examined the expression of these proteins after UVB irradiation in both (+)IGF-1R and (−)IGF-1R keratinocytes. In our cultured keratinocyte model, we could not reproducibly detect p16CDKN2A or p27CDKN1B protein, either before or subsequent to UVB irradiation (data not shown). However, consistent with the experiments assaying UVB-induced senescence, the activation status of the IGF-1R influenced the stability of the CDKI p21CDKN1A after UVB irradiation (Figure 3). The p21CDKN1A protein was rapidly degraded within 1 h after UVB irradiation of (−)IGF-1R keratinocytes. In contrast, the level of p21CDKN1A in (+)IGF-1R keratinocytes after UVB irradiation steadily increased over the entire 48-h assay. Again, these data are consistent with our model for the normal UVB response in keratinocytes; the induction of stress-induced premature senescence, which is perhaps maintained by persistent levels of the growth inhibitory p21CDKN1A protein. When the IGF-1R is inactive in keratinocytes, an inappropriate response to UVB irradiation occurs. These cells do not become senescent and they can continue to proliferate, which is consistent with the rapid degradation of the growth inhibitory protein p21CDKN1A.
Figure 3.
Keratinocytes containing a functionally inactive IGF-1R fail to accumulate p21CDKN2A after UVB irradiation. Normal human keratinocytes were grown in EpiLife Complete [(+)IGF-1R] or EpiLife NoIn [(−)IGF-1R] media for 24 h. At that time, the keratinocytes were irradiated with a dose of 400 J/m2 UVB. At the indicated times after irradiation, keratinocytes were harvested and assayed for the expression of p21CDKN2A and α-tubulin via immunoblot analysis.
Keratinocytes without a Functional IGF-1R Fail to Phosphorylate Serine 46 on p53
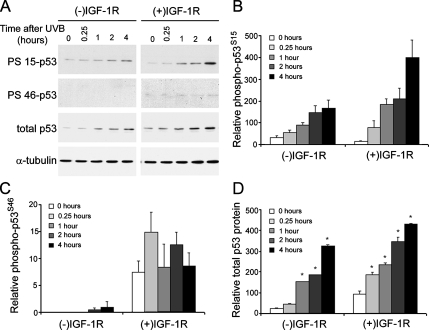
The IGF-1R–dependent protein kinase signaling cascade was exhaustively examined after UVB irradiation. Surprisingly, no differences in the activation of AKT, BCL-XL, BAX, inhibitor of nuclear factor-κBα, RELA/p65 (data not shown), extracellular signal-regulated kinase, p38, or c-Jun NH2-terminal kinase (Lewis and Spandau, 2007b) were observed between (−)IGF-1R and (+)IGF-1R keratinocytes. However, the activation of p53 after UVB irradiation was influenced by the activation status of the IGF-1R (Figure 4A). Activation of the IGF-1R resulted in higher levels of UVB-induced total p53 protein, increased phosphorylation of serine 15 on p53 (p53S15), and increased phosphorylation of serine 46 on p53 (p53S46). Interestingly, the phosphorylation of p53S15 (Figure 4B) and the induction of total p53 protein (Figure 4D) were only marginally higher in (+)IGF-1R keratinocytes compared with (−)IGF-1R keratinocytes. However, detectable phosphorylation of p53S46 only occurred in (+)IGF-1R keratinocytes, and it was generally not observed in (−)IGF-1R keratinocytes (Figure 4C). Unlike p53S15 phosphorylation, the phosphorylation of p53S46 was not influenced by UVB irradiation.
Figure 4.
A functional IGF-1R is required for phosphorylation of serine 46 on p53. (A) Keratinocytes were treated as described in Figure 3 and harvested at the indicated times after irradiation (UVB, 400 J/m2). Keratinocyte cell lysates were analyzed for the presence of phosphorylated serines at amino acids 15 (PS 15-p53) and 46 (PS 46-p53) on p53 by using phospho-specific antibodies. The expression of p53 protein (independent of phosphorylation status) and α-tubulin were also assayed. Densitometry (ImageJ, National Institutes of Health; http://rsb.info.nih.gov/ij/) of the expression of phospho-p53S15 (B), phospho-p53S46 (C), or total p53 protein (D) relative to α-tubulin expression in either (−)IGF-1R keratinocytes or (+)IGF-1R keratinocytes harvested at the indicated time after a UVB dose of 400 J/m2. The data represent three independent assays. Error bars indicate the SEM; the asterisks indicates significant difference between unirradiated keratinocytes (p < 0.05, t test).
IGF-1R–dependent UVB Response of Human Keratinocytes Requires Phosphorylation of p53S46
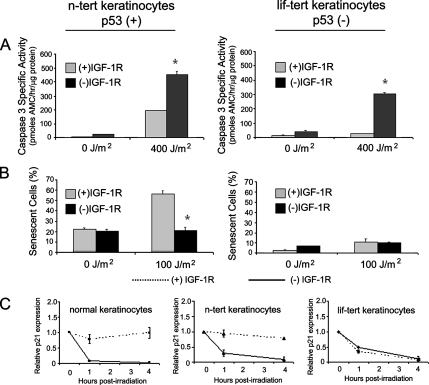
The limited replicative life span of normal human keratinocytes complicates the introduction and analysis of mutant proteins via retroviral transduction. Therefore, it would be advantageous to find an immortal human keratinocyte cell line that could serve as a model for the IGF-1R–dependent UVB response of normal human keratinocytes. Previously, we have demonstrated that the HaCaT immortal keratinocyte cell line was not an appropriate model for the response of normal human keratinocytes to UVB irradiation (Lewis et al., 2006). Subsequently, we have compared normal human keratinocytes with n-tert keratinocytes (Dickson et al., 2000) that were immortalized by ectopic expression of the catalytic subunit of telomerase hTERT. Similar to normal keratinocytes, (−)IGF-1R n-tert cells were more sensitive to UVB-induced apoptosis (Figure 5A; Kuhn et al., 1999; Lewis and Spandau, 2007b). Furthermore, (−)IGF-1R n-tert cells do not undergo UVB-induced premature senescence (Figure 5B, compare with Figure 1E). In addition, the changes in protein expression in n-tert keratinocytes after UVB irradiation (p53, p53pS15, p53pS46, and p21) were very similar to those changes observed in normal keratinocytes (data not shown). Our previous data had suggested that p53 might be a component of the IGF-1R–dependent UVB response. We tested the role of p53 in this process by comparing the UVB response of n-tert keratinocytes with a similarly hTERT-immortalized keratinocyte cell line derived from an individual with Li-Fraumeni syndrome (lif-tert). In the lif-tert keratinocyte cell line, both p53 alleles are disrupted, resulting in no functional p53 activity (Dickson et al., 2000). Comparison of the response of n-tert keratinocytes and lif-tert keratinocytes (Figure 5, A and B) validate the importance of p53 in the normal (+)IGF-1R response of keratinocytes to UVB irradiation. In contrast to normal human keratinocytes and n-tert keratinocytes, (+)IGF-1R lif-tert keratinocytes do not undergo measurable UVB-induced apoptosis (Figure 5A) and (+)IGF-1R lif-tert keratinocytes also do not become senescent after low dose UVB irradiation (Figure 5B). Similarly, p21 expression is lost in lif-tert keratinocytes after UVB irradiation regardless of the functional status of the IGF-1R (Figure 5C). These data validated the use of n-tert keratinocytes as a surrogate for normal human keratinocytes and suggested the importance of p53 in the IGF-1R–dependent UVB response.
Figure 5.
UVB-induced senescence requires p53. (A) The n-tert and lif-tert keratinocytes were grown in EpiLife Complete [(+)IGF-1R] or EpiLife NoIn [(−)IGF-1R] media for 24 h. The keratinocytes were then irradiated with the indicated dose of UVB. Six hours after irradiation, the keratinocytes were harvested and assayed for caspase-3–specific activity. Error bars indicate the SEM; the asterisks indicates significant difference between irradiated (+)IGF-1R and (−)IGF-1R keratinocyte caspase-3–specific activities (p < 0.01, t test). The data represent three independent assays. (B) The n-tert and lif-tert keratinocytes were grown in EpiLife Complete [(+)IGF-1R] or EpiLife NoIn [(−)IGF-1R] media for 24 h. The keratinocytes were then irradiated with the indicated dose of UVB. Fifteen hours after irradiation, the media on all of the keratinocytes was replaced with EpiLife Complete media. Seventy-two hours after irradiation, the keratinocytes were assayed for senescence-associated β-galactosidase activity. The graphs represent the compiled percentage of senescent cells from each culture condition. Error bars indicate the SEM; the asterisk indicates significant difference between irradiated (+)IGF-1R and (−)IGF-1R keratinocyte percentages (p < 0.01, t test). The data represent three independent assays. (C) Normal human keratinocytes, n-tert keratinocytes, and lif-tert keratinocytes were grown in EpiLife Complete [(+)IGF-1R, dotted lines] or EpiLife NoIn [(−)IGF-1R, solid lines] media for 24 h. At that time, the keratinocytes were irradiated with a dose of 400 J/m2 UVB. At the indicated times after irradiation, keratinocytes were harvested and assayed for the expression of p21CDKN2A and α-tubulin via immunoblot analysis. Relative expression of p21CDKN2A was determined from autoradiographs by using ImageJ software (http://rsb.info.nih.gov/ij/) and normalization with α-tubulin expression. The graphs represent data compiled from at least three independent assays.
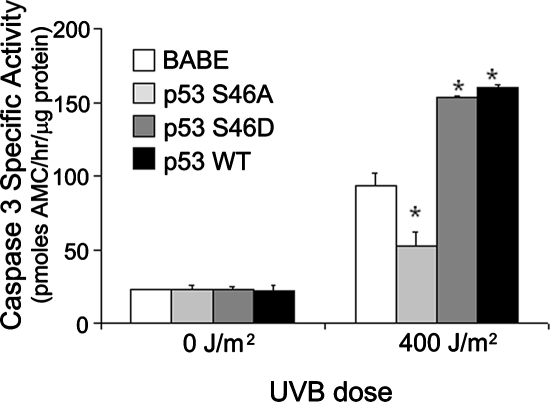
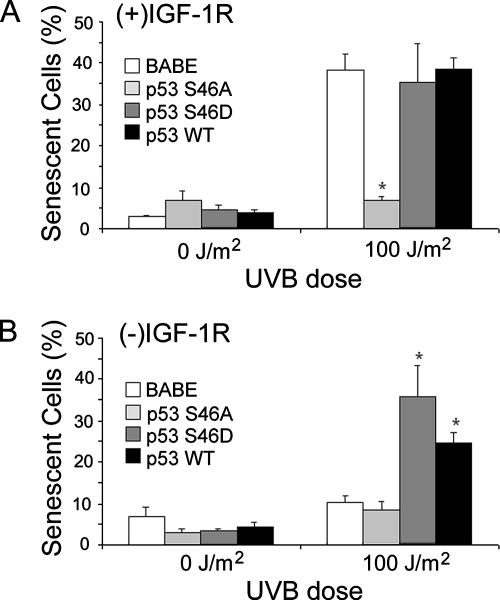
Based on the differential phosphorylation of p53S46 in (+)IGF-1R keratinocytes in contrast to (−)IGF-1R keratinocytes (Figure 4), we created clones of n-tert keratinocytes expressing p53 proteins altered at amino acid 46 (Ichwan et al., 2006). These mutants include p53S46A, in which the p53 gene was altered so that the wild-type serine found at amino acid 46 is replaced by an alanine. Due to this mutation, the p53S46A mutant protein cannot be phosphorylated at amino acid 46 (an inactivating mutation). The mutant p53S46D contains a p53 gene in which the serine at amino acid 46 was replaced by an aspartic acid residue. This substitution results in an amino acid side chain that resembles a phosphorylated serine residue; thus, this mutant will function in a constitutively activated manner (an activating mutation). The n-tert cells transduced with only the retrovirus vector (BABE) or a retrovirus containing the wild-type p53 gene (p53WT) served as negative and positive controls. As seen in Figure 6, in keratinocytes containing a functional IGF-1R, the p53S46A mutation rendered keratinocytes resistant to UVB-induced apoptosis. This result is consistent with experiment shown in Figure 5A, which demonstrates that inactivation of p53 disrupts the UVB-induced apoptotic signal in (+)IGF-1R keratinocytes. If p53 expression was removed from normal control (p53S46D and p53WT clones), n-tert keratinocytes became more sensitive to UVB-induced apoptosis (Figure 6). As seen in Figure 7A, phosphorylation of p53S46 was also essential for UVB-induced senescence in (+)IGF-1R keratinocytes. Low-dose UVB irradiation of n-tert (+)IGF-1R p53S46A keratinocytes failed to become senescent. The activating mutation (p53S46D) or overexpression of p53 (p53WT) did not significantly alter the percentage of senescent cells after UVB irradiation compared with the vector-only (BABE) (+)IGF-1R keratinocytes (Figure 7A). No phosphorylation of p53S46 was observed in n-tert (−)IGF-1R keratinocytes (similar to results in Figure 4), so it was not surprising that n-tert p53S46A (−)IGF-1R keratinocytes were identical to control (BABE) n-tert (−)IGF-1R keratinocytes in failing to become senescent after low-dose UVB irradiation. However, either activating mutations at serine 46 (p53S46D) or ectopic expression of p53 (p53WT) in (−)IGF-1R keratinocytes restored low-dose UVB-induced senescence (Figure 7B). These data indicate that UVB-induced senescence in human keratinocytes requires signaling through a functional IGF-1R and subsequent phosphorylation of p53S46.
Figure 6.
Phosphorylation of serine 46 on p53 is necessary for UVB-induced apoptosis. Independent clones of n-tert keratinocytes transduced with retroviruses expressing the native p53 gene (p53 WT), a p53 gene containing an inactivating mutation resulting from a substitution of an alanine for the serine at amino acid 46 (p53 S46A), a p53 gene containing an activating mutation resulting from a substitution of an aspartic acid for the serine at amino acid 46 (p53 S46D), or containing only the vector sequences (BABE) were irradiated with the indicated dose of UVB. Six hours after irradiation, the keratinocytes were harvested and assayed for caspase-3–specific activity. Error bars indicate the SEM; the asterisks indicate significant differences between irradiated BABE clones and p53 clones keratinocyte specific activities (p < 0.01, t test). The graph represents data compiled from at least three independent clones of each cell type.
Figure 7.
Phosphorylation of serine 46 on p53 is critical for IGF-1R–dependent UVB-induced senescence. The n-tert keratinocyte clones described in Figure 6 were grown in EpiLife Complete [(+)IGF-1R; A] or EpiLife NoIn [(−)IGF-1R; B] media for 24 h. The keratinocytes were then irradiated with the indicated dose of UVB. Fifteen hours after irradiation, the media on all of the keratinocytes were replaced with EpiLife Complete media. Seventy-two hours after irradiation, the keratinocytes were assayed for senescence-associated β-galactosidase activity. The graphs represent the compiled percentage of senescent cells from each culture condition by using at least two independent clones of each cell type. Error bars indicate the SE M; the asterisks indicate significant differences between irradiated BABE clones and p53 clones percentages (p < 0.01, t test).
DISCUSSION
To better understand the biological consequences of UVB-irradiated skin, we have used an in vitro model system to probe the response of normal human keratinocytes to UVB exposure. Using this model system, we have reported previously that the response of human keratinocytes to UVB irradiation was dependent on the activation status of the IGF-1R (Kuhn et al., 1999; Lewis and Spandau, 2007b). In the presence of a functionally active IGF-1R, the response of keratinocytes to UVB irradiation was dependent on the dose of UVB delivered. At low doses of UVB, keratinocytes are able to completely repair all of the UVB-damaged DNA and continue normal cellular functions. At high doses of UVB, the UVB-induced DNA damage is extensive and it cannot be completely repaired by the keratinocytes. At these high doses of UVB, the keratinocytes respond to the severely damaged DNA by undergoing apoptosis. In between these extreme doses of UVB, if the DNA damage is significant enough that the keratinocyte cannot completely repair the damaged DNA, the keratinocyte undergoes mitotic arrest. We have now demonstrated that this mitotic arrest is in fact premature senescence. Furthermore, we have shown that the induction of senescence by UVB requires a functionally active IGF-1R at the time of UVB irradiation.
If we can extrapolate the findings we observed with cultured keratinocytes in vitro to the response of keratinocytes in the skin, they would correlate with these expected outcomes. To prevent UVB-induced carcinogenesis, replicating keratinocytes cannot be permitted to pass UVB-induced mutations to their progeny. Therefore, if possible, UVB-damaged DNA must be repaired. If the damage to the genome is too great to completely repair, keratinocytes must prevent the passage of damaged DNA via alternative mechanisms. One mechanism would be by inducing UVB-induced apoptosis. However, if this was the default mechanism in the absence of complete DNA repair, too many keratinocytes would be lost from the epidermis, and the barrier function of the epidermis would be severely compromised. Therefore, the induction of premature senescence would be a preferred response to intermediate exposure of UVB, allowing for the continued presence of UVB-damaged keratinocytes and disallowing propagation of UVB-damaged DNA.
In the skin, keratinocytes express the IGF-1R, but they do not synthesize IGF-1 (Tavakkol et al., 1992). Dermal fibroblasts support the proliferation of keratinocytes in the epidermis by secreting IGF-1. Interestingly, as dermal fibroblasts become senescent in vitro, their capacity to produce IGF-1 is severely diminished (Ferber et al., 1993); therefore, in aged skin keratinocytes may be provided with a reduced supply of IGF-1. We think this decrease in IGF-1 expression with advancing age is a major component of the increase in nonmelanoma skin cancer seen in geriatric patients. A corollary of this hypothesis would be that individuals with an increased activation of the IGF-1R might have some protection from UVB-induced skin cancer that could be detected by a decrease in skin cancer incidence. IGF-1 and insulin have very similar molecular structures, and high concentrations of insulin will activate the IGF-1R. Patients with type II diabetes frequently have to take exogenous systemic insulin to overcome their insulin resistance. We hypothesized that the high systemic insulin levels in these patients would compensate for an aging-dependent decline in IGF-1 in the skin by inadvertently maintaining IGF-1R activation despite declining levels of IGF-1. If this were true, our control population would have an increasing incidence of skin cancer with advancing age, whereas the type 2 insulin-using diabetic patient cohort would not see a change in skin cancer incidence with age. In fact, the use of insulin was protective of the age-dependent increase in skin cancer incidence (Chuang et al., 2005).
The role of p53 in regulating apoptosis has been well documented in a variety of tissues in response to a plethora of cellular insults (Meek, 2004; Yee and Vousden, 2005). More recently, p53 has been reported to be essential for the control of cellular senescence, both replicative- and stress-induced (Itahana et al., 2001). The outcome of p53 activation, be it apoptosis or senescence, may be determined by the specific type of activation via posttranslational modification (Brooks and Gu, 2003; Xu, 2003; Moll et al., 2005). One of the most important posttranslational modifications of p53 responsible for inducing apoptosis is the phosphorylation of serine 46 on p53. Phosphorylation of p53S46 has been described to alter specific p53-dependent gene expression (Mayo et al., 2005); disrupt interactions with HMD2, leading to p53 stabilization (Xu, 2003; Sullivan et al., 2004); and be essential for p53-dependent apoptosis (Sun et al., 2007; Wesierska-Gadek et al., 2007). The data we have presented concur with a role for p53S46 phosphorylation inducing apoptosis after high doses of UVB. Additionally, we have now demonstrated that phosphorylation of p53S46 in keratinocytes was dependent on the functional activation of the IGF-1R. Failure to phosphorylate p53S46 in (+)IGF-1R keratinocytes resulted in an inhibition of UVB-induced apoptosis. However, UVB-induced apoptosis in (−)IGF-1R keratinocytes was independent of p53S46 phosphorylation. In contrast to previous reports (Sun et al., 2007; Wesierska-Gadek et al., 2007), our data also demonstrated that UVB-induced premature senescence after low-dose UVB exposure required p53S46 phosphorylation.
At least two kinases have been reported to phosphorylate p53S46, the p38 MAPK (Xu, 2003; Sun et al., 2007) and HIPK2 (DiStefano et al., 2005; Wesierska-Gadek et al., 2007). We have previously demonstrated that the activation status of the IGF-1R does not influence UVB-induced activation of p38 MAPK (Lewis and Spandau, 2007b). However, the functional status of the IGF-1R does regulate the activity of UVB-activated p38 MAPK (Lewis and Spandau, 2007b). It is provocative to think that one of the downstream effectors regulating p38 MAPK-dependent signaling would be p53S46 phosphorylation. However, as of yet we have no information linking IGF-1R function and the substrate specificity of the p38 MAPK.
In summary, we have shown that low doses of UVB can induce premature senescence in normal human keratinocytes in vitro. The induction of senescence by UVB was only observed in keratinocytes with functionally active IGF-1 receptors. Ligand-activated IGF-1R resulted in p54S46 phosphorylation, which was required for UVB-induced senescence. We hypothesize that to evade the carcinogenic potential of UVB exposure to the skin, the normal UVB response of human keratinocytes is to undergo premature senescence. Furthermore, this protective response to UVB irradiation requires a functionally active IGF-1R and the subsequent downstream phosphorylation of p53S46.
ACKNOWLEDGMENTS
We are grateful to Dr. Masa-Aki Ikeda (Tokyo Medical and Dental University, Tokyo, Japan) for the generous gift of the p53S46A and p53S46D plasmids. This work was supported by grants from the National Institutes of Health grants R01 ES-11155 (to D.F.S.) and R01 HL-062996 (to J.B.T.) and VA Merit Award (to J.B.T.).
Abbreviations used:
- IGF-1R
insulin-like growth factor-1 receptor
- PBS
phosphate-buffered saline
- UVB
ultraviolet B.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-10-1041) on January 23, 2008.
REFERENCES
- Behrend L., Mohr A., Dick T., Zwacka R. M. Manganese superoxide dismutase induces p53-dependent senescence in colorectal cancer cells. Mol. Cell. Biol. 2005;25:7758–7769. doi: 10.1128/MCB.25.17.7758-7769.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Porath I., Weinberg R. A. The signals and pathways activating cellular senescence. Int. J. Biochem. Cell Biol. 2005;37:961–976. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005;579:859–862. doi: 10.1016/j.febslet.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Brooks C. L., Gu W. Ubiquitination, phosphorylation and acetylation: the molecular basis for p53 regulation. Curr. Opin. Cell Biol. 2003;15:164–171. doi: 10.1016/s0955-0674(03)00003-6. [DOI] [PubMed] [Google Scholar]
- Campisi J. Suppressing cancer: the importance of being senescent. Science. 2005;309:886–887. doi: 10.1126/science.1116801. [DOI] [PubMed] [Google Scholar]
- Chaturvedi V., Qin J. Z., Stennett L., Choubey D., Nickoloff B. J. Resistance to UV-induced apoptosis in human keratinocytes during accelerated senescence is associated with functional inactivation of p53. J. Cell. Physiol. 2004;198:100–109. doi: 10.1002/jcp.10392. [DOI] [PubMed] [Google Scholar]
- Chuang T. S., Lewis D. A., Spandau D. F. Decreased incidence of non-melanoma skin cancer in type 2 diabetes mellituspatients using insulin: a pilot study. Br. J. Dermatol. 2005;153:552–557. doi: 10.1111/j.1365-2133.2005.06738.x. [DOI] [PubMed] [Google Scholar]
- Cotton J., Spandau D. F. Ultraviolet B dose influences the induction of apoptosis and p53 in human keratinocytes. Radiat. Res. 1997;147:148–155. [PubMed] [Google Scholar]
- Dasari A., Bartholomew J. N., Volonte D., Galbiati F. Oxidative stress induces premature senescence by stimulating caveolin-1 gene transcription through p38 mitogen-activated protein kinase/Sp1-mediated activation of two GC-rich promoter elements. Cancer Res. 2006;66:10805–10814. doi: 10.1158/0008-5472.CAN-06-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson M. A., Hahn W. C., Ino Y., Ronfard V., Wu J. Y., Weinberg R. A., Louis D. N., Li F. P., Rheinwald J. G. Human keratinocytes that express hTERT and also bypass a p16INK4a-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol. Cell. Biol. 2000;20:1436–1447. doi: 10.1128/mcb.20.4.1436-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri G. P. What has senescence got to do with cancer? Cancer Cell. 2005;7:505–512. doi: 10.1016/j.ccr.2005.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri G. P., et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiStefano V., Soddu S., Sacchi A., D'Orazi G. HIPK2 contributes to PCAF-mediated p53 acetylation and selective transactivation of p21Waf1 after nonapoptotic DNA damage. Oncogene. 2005;24:5431–5442. doi: 10.1038/sj.onc.1208717. [DOI] [PubMed] [Google Scholar]
- Ferber A., et al. Failure of senescent human fibroblasts to express the insulin-like growth factor-1 gene. J. Biol. Chem. 1993;268:17883–17888. [PubMed] [Google Scholar]
- Harley C. B., Futcher A. B., Greider C. W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Hayflick L., Moorhead P. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961;25:385–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Herbig U., Ferreira M., Condel L., Carey D., Sedivy J. M. Cellular senescence in aging primates. Science. 2006;311:1257. doi: 10.1126/science.1122446. [DOI] [PubMed] [Google Scholar]
- Ichwan S.J.A., Yamada S., Sumrejkanchanakij P., Ibrahim-Auerkari E., Eto K., Ikeda M. A. Defect in serine 46 phosphorylation of p53 contributes to acquisition of p53 resistance in oral squamous cell carcinoma cells. Oncogene. 2006;25:1216–1224. doi: 10.1038/sj.onc.1209158. [DOI] [PubMed] [Google Scholar]
- Itahana K., Dimri G., Campisi J. Regulation of cellular senescence of p53. Eur. J. Biochem. 2001;268:2784–2791. doi: 10.1046/j.1432-1327.2001.02228.x. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Kaminker P., Campisi J. Telomeres, aging, and cancer: in search of a happy ending. Oncogene. 2002;21:503–511. doi: 10.1038/sj.onc.1205077. [DOI] [PubMed] [Google Scholar]
- Kuhn C., Kumar M., Hurwitz S. A., Cotton J., Spandau D. F. Activation of the insulin-like growth factor-1 receptor promotes the survival of human keratinocytes following ultraviolet B irradiation. Int. J. Cancer. 1999;80:431–438. doi: 10.1002/(sici)1097-0215(19990129)80:3<431::aid-ijc16>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Kumar M., Hurwitz S. A., Cotton J., Spandau D. F. Subphysiological concentrations of extracellular calcium sensitize normal human keratinocytes to UVB-induced apoptosis. Arch. Dermatol. Res. 1999;291:37–46. doi: 10.1007/s004030050381. [DOI] [PubMed] [Google Scholar]
- Lewis D. A., Hurwitz S. A., Spandau D. F. UVB-induced apoptosis in normal human keratinocytes: role of the erbB receptor family. Exp. Cell Res. 2003;284:316–327. doi: 10.1016/s0014-4827(02)00043-5. [DOI] [PubMed] [Google Scholar]
- Lewis D. A., Hengeltraub S., Gao F., Leivant M. A., Spandau D. F. Aberrant NF-κB activity in HaCaT cells alters their response to UVB signaling. J. Invest. Dermatol. 2006;126:1885–1892. doi: 10.1038/sj.jid.5700333. [DOI] [PubMed] [Google Scholar]
- Lewis D. A., Spandau D. F. UVB activation of NF-κB in normal human keratinocytes occurs via a unique mechanism. Arch. Dermatol. Res. 2007a;299:93–101. doi: 10.1007/s00403-006-0729-2. [DOI] [PubMed] [Google Scholar]
- Lewis D. A., Spandau D. F. UVB-induced activation of NF-κB is regulated by the IGF-1R and dependent on p38 MAPK. J. Invest. Dermatol. 2007b doi: 10.1038/sj.jid.5701127. (in press). doi: 10.1038/sj.jid5701127. [DOI] [PubMed] [Google Scholar]
- Mallette F. A., Gaumont-Leclerc M. F., Ferbeyre G. The DNA damage signaling pathway is a critical mediator of oncogene-induced senescence. Genes Dev. 2007;21:43–48. doi: 10.1101/gad.1487307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo L. D., Seo Y. R., Jackson M. W., Smith M. L., Guzman J. R., Korgaonkar C. K., Donner D. B. Phosphorylation of human p53 at serine 46 determines promoter selection and whether apoptosis is attenuated or amplified. J. Biol. Chem. 2005;280:25953–25959. doi: 10.1074/jbc.M503026200. [DOI] [PubMed] [Google Scholar]
- Meek D. W. The p53 response to DNA damage. DNA Repair. 2004;3:1049–1056. doi: 10.1016/j.dnarep.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Melnikova V. O., Ananthaswamy H. N. Cellular and molecular events leading to the development of skin cancer. Mutat. Res. 2005;571:91–106. doi: 10.1016/j.mrfmmm.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Moll U. M., Wolff S., Speidel D., Deppert W. Transcription-independent pro-apoptotic functions of p53. Curr. Opin. Cell Biol. 2005;17:631–636. doi: 10.1016/j.ceb.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Mullenders L.H.F., Van Hoffen A., Vreeswijk M. P., Ruven H. J., Vrieling H., van Zeeland A. A. Ultraviolet-induced photolesions: repair and mutagenesis. Recent Results Cancer Res. 1997;143:89–99. doi: 10.1007/978-3-642-60393-8_7. [DOI] [PubMed] [Google Scholar]
- Pajalunga D., Mazzola A., Salzano A. M., Biferi M. B., DeLuca G., Crescenzi M. Critical requirement for cell cycle inhibitors in sustaining nonproliferative states. J. Cell Biol. 2007;176:807–818. doi: 10.1083/jcb.200608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergrass W. R., Lane M. A., Bodkin N. L., Hansen B. C., Ingram D. K., Roth G. S., Yi L., Bin H., Wolf N. S. Cellular proliferation potential during aging and caloric restriction in rhesus monkeys (Macaca mulatta) J. Cell. Physiol. 1999;180:123–130. doi: 10.1002/(SICI)1097-4652(199907)180:1<123::AID-JCP14>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Qin J. Z., Chaturvedi V., Denning M. F., Bacon P., Panella J., Coubey D., Nickoloff B. J. Regulation of apoptosis by p53 in UV-irradiated human epidermis, psoriatic plaques and senescent keratinocytes. Oncogene. 2002;21:2991–3002. doi: 10.1038/sj.onc.1205404. [DOI] [PubMed] [Google Scholar]
- Sullivan A., et al. Polymorphism in wild-type p53 modulates response to chemotherapy in vitro and in vivo. Oncogene. 2004;23:3328–3337. doi: 10.1038/sj.onc.1207428. [DOI] [PubMed] [Google Scholar]
- Sun P., et al. PRAK is essential for ras-induced senescence and tumor suppression. Cell. 2007;128:295–308. doi: 10.1016/j.cell.2006.11.050. [DOI] [PubMed] [Google Scholar]
- Tavakkol A., Elder J. T., Griffiths C.E.M., Cooper K. D., Talwar H., Fisher G. J., Keane K. M., Foltin S. K., Voorhees J. J. Expression of growth hormone receptor, insulin-like growth factor 1 (IGF-1) and IGF-1 receptor mRNA and proteins in human skin. J. Invest. Dermatol. 1992;99:343–349. doi: 10.1111/1523-1747.ep12616668. [DOI] [PubMed] [Google Scholar]
- Toussaint O., Royer V., Salmon M., Remacle J. Stress-induced premature senescence and tissue ageing. Biochem. Pharmacol. 2002;64:1007–1009. doi: 10.1016/s0006-2952(02)01170-x. [DOI] [PubMed] [Google Scholar]
- Wesierska-Gadek J., Schmitz M. L., Ranftler C. Roscovitine-activated HIP2 kinase induces phosphorylation of wt p53 at Ser-46 in human MCF-7 breast cancer cells. J. Cell. Biochem. 2007;100:865–874. doi: 10.1002/jcb.21211. [DOI] [PubMed] [Google Scholar]
- Xu Y. Regulation of p53 responses by post-translational modifications. Cell Death Differ. 2003;10:400–403. doi: 10.1038/sj.cdd.4401182. [DOI] [PubMed] [Google Scholar]
- Yee K. S., Vousden K. H. Complicating the complexity of p53. Carcinogenesis. 2005;26:1317–1322. doi: 10.1093/carcin/bgi122. [DOI] [PubMed] [Google Scholar]
- Zhang B., Spandau D. F., Roman A. The E5 protein of human papillomavirus type 16 protects human foreskin keratinocytes from UVB-induced apoptosis. J. Virol. 2002;76:220–231. doi: 10.1128/JVI.76.1.220-231.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]