Abstract
We and others previously showed that p38 mitogen-activated protein kinase is indispensable for myogenic differentiation. However, it is less clear which of the four p38 isoforms in the mouse genome participates in this process. Using C2C12 myogenic cells as a model, we showed here that p38α, β, and γ are expressed with distinct expression patterns during differentiation. Knockdown of any of them by small interfering RNA inhibits myogenic differentiation, which suggests that the functions of the three p38 isoforms are not completely redundant. To further elucidate the unique role of each p38 isoform in myogenic differentiation, we individually knocked down one p38 isoform at a time in C2C12 cells, and we compared the whole-genome gene expression profiles by microarrays. We found that some genes are coregulated by all three p38 isoforms, whereas others are uniquely regulated by one particular p38 isoform. Furthermore, several novel p38 target genes (i.e., E2F2, cyclin D3, and WISP1) are found to be required for myogenin expression, which provides a molecular basis to explain why different p38 isoforms are required for myogenic differentiation.
INTRODUCTION
Myogenic differentiation is one of the best models to study the molecular mechanisms by which the cellular programs switch from proliferation to differentiation. This is largely due to our extensive knowledge of key myogenic factors and the availability of both cell culture and animal models. Two families of transcription factors, namely, the myogenic regulatory factors (MRF) and myocyte enhancer binding factor 2s (MEF2s), act cooperatively as master transcription factors to control the expression of an array of cellular genes that collectively contribute to the initiation of the differentiation program and maintenance of the differentiated state (Molkentin and Olson, 1996; Puri and Sartorelli, 2000). MRFs consist of four basic helix-loop-helix (bHLH) proteins (i.e., Myf5, MyoD, myogenin, and MRF4), each heterodimerizing with E proteins (E12 or E47) to efficiently bind to the E box (i.e., CANNTG) in promoters of many muscle-specific genes (Tapscott, 2005). MEF2s consist of four MADS-box-containing proteins (i.e., MEF2A, 2B, 2C, and 2D) that are capable of forming both homo- and heterodimers to bind to a consensus AT-rich sequence (i.e., the MEF2 site) (Black and Olson, 1998).
Both MRFs and MEF2 are in turn regulated by distinct intracellular signaling pathways. We and others previously showed that the p38 mitogen-activated protein kinase (MAPK)-mediated signaling pathway is indispensable for myogenic differentiation (Cuenda and Cohen, 1999; Zetser et al., 1999; Wu et al., 2000b). Accumulating evidence indicates that p38 MAPK can regulate myogenic differentiation through multiple mechanisms. p38 MAPK directly phosphorylates MEF2 and enhances its transcriptional activity (Han et al., 1997; Zhao et al., 1999; Wu et al., 2000a). p38 MAPK also potently enhances the transcriptional activity of MyoD, which is probably due to an increased association of MyoD with E47, because the latter can be directly phosphorylated by p38 MAPK (Wu et al., 2000b; Lluis et al., 2005). Through MyoD and MEF2, which directly bind to the myogenin promoter, p38 MAPK also critically controls the expression of myogenin gene, which is absolutely essential for execution of the differentiation program (Xu et al., 2002). Recently, p38 MAPK was also found to directly phosphorylate BAF60, a component in the SWI/SNF complex, and to target the complex to selected muscle-specific loci (Simone et al., 2004). p38 MAPK also facilitates the recruitment of MyoD and MEF2D to selected promoters of muscle-specific genes (Penn et al., 2004). In addition, p38 MAPK can promote activation of the quiescent muscle satellite cells, and it is also required for proliferation of myoblasts (Jones et al., 2005).
Four p38 isoforms, namely, p38 α, β, γ, and δ, exist in both the human and mouse genomes (Lluis et al., 2005; Keren et al., 2006). So far, many studies on the role of p38 MAPK in myogenic differentiation have relied on the use of SB203580 and SB202190, two pyridinyl imidazole-based small-molecule inhibitors that mainly inhibit p38α and β, but they have no effect on p38γ and δ (Kumar et al., 1997). As both SB203580 and SB202190 potently inhibit myogenic differentiation (Cuenda and Cohen, 1999; Zetser et al., 1999; Wu et al., 2000b), it suggests that p38γ and δ do not play any significant role in myogenic differentiation. In contrast, a previous report showed that p38γ is required for myogenic differentiation, because overexpression of a wild-type p38γ promotes, whereas that of a dominant-negative form of p38γ inhibits myogenic differentiation in C2C12 cells (Lechner et al., 1996). It remains unclear whether p38δ plays any role in myogenic differentiation. To clarify the confusion about the role of p38γ and to clearly understand the role of each p38 isoform in myogenic differentiation, we decide to take the short interfering RNA (siRNA) approach by individually knocking down each p38 isoform followed by assessment of the knockdown effect on myogenic differentiation. Except for p38δ, which is barely detectable in C2C12 cells, we found that p38α, β, and γ are all required for myogenic differentiation. Microarray analysis reveals that some genes are uniquely controlled by a particular p38 isoform, whereas others are coregulated by multiple p38 isoforms. Importantly, several novel p38 target genes were indeed found to be involved in myogenic differentiation, which provides a molecular basis to explain why different p38 MAPKs are required for myogenic differentiation.
MATERIALS AND METHODS
Cell Culture and Antibodies
C2C12 cells (American Type Culture Collection, Manassas, VA) were cultured in DMEM supplemented with 20% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin (growth medium, or GM) in a humidified incubator at 37°C with 5% CO2. To induce differentiation, near confluent C2C12 cells were grown in DMEM supplemented with 2% horse serum (differentiation medium, or DM). Antibodies used in this work were from the following sources: anti-sarcomeric myosin heavy chain (MHC) (MF20) (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA); anti-p38α (Cell Signaling Technology, Danvers, MA); anti-p38β (Zymed Laboratories, South San Francisco, CA); and cyclin D3 (C-16), E2F2 (C-20), anti-myogenin (F5D), anti-β-actin (C-2), and anti-hemagglutinin (HA) (Santa Cruz Biotechnology, Santa Cruz, CA). The polyclonal anti-p38γ was a kind gift from Dr. M. A. Bogoyevitch (University of Western Australia).
DNA Constructs, Transfection, and Cell Lysis
Expression vectors encoding Flag-p38α, Flag-p38β, and Flag-p38γ were kindly provided by Dr. J. Han (Scripps Research Institute, San Diego, CA). G133-luc, 4RE-luc, 3MEF2-luc, and HA-MKK6EE were described previously (Wu et al., 2000b; Xu et al., 2002). Flag-Wnt-induced secreted protein 1 (WISP1) was amplified from C2C12-derived cDNAs by polymerase chain reaction (PCR), and it was cloned into pcDNA3.0 vector. Transient transfection was performed by using LipofectAMINE/PLUS reagents following the manufacturer's instructions (Invitrogen, Carlsbad, CA). Cells were lysed in the lysis buffer (50 mM HEPES, pH 7.6, 10% glycerol, 1% Triton X-100, 150 mM NaCl, 1 mM EGTA, 1.5 mM MgCl2, 100 mM NaF, 20 mM biscyclohexylammonium salt, 20 mM β-glycerol phosphate, 2 mM dithiothreitol, 50 μM sodium vanadate, 0.5 mM phenylmethylsulfonyl fluoride, 2 μg/ml aprotinin, 0.5 μg/ml leupeptin, and 0.7 μg/ml pepstatin) for 10 min on ice. Soluble whole cell extracts (WCEs) were prepared by centrifugation to remove cell debris.
RNA Interference (RNAi)
The following siRNAs (only the top-strand sequence is shown) were synthesized at either Dharmacon RNA Technologies (Lafayette, CO) or RIBOBIO (Guangzhou, China): enhanced green fluorescent protein (5′-GCT GAC CCT GAA GTT CAT C-3′); p38α (1#: 5′-AGC CCA GCA ACC TAG CTG T-3′; 2#: 5′-GAG CCT GAC CTA TGA TGA A-3′); p38β (1#: 5′-TGC TGG TAC TAG ACA GCG A-3′; 2#: 5′-ATT GAG CAG TGA GGC ATT G-3′); p38γ (1#: 5′-GCA TGA GAC CCT GAG TGA A-3′; 2#: 5′-CCT GTT CTT CGG CTT TCG A-3′); E2F2 (1#: 5′-GCA CCT GAC CGA AGA TAA T-3′; 2#: 5′-GTG ACC TCT TCG ACT CCT A-3′); cyclin D3 (1#: 5′-CCA TTC ACC TGT AGC TTG A-3′; 2#:5′-CTG CTT AGC TTC TGT GAT T-3′); and WISP1 (1#:5′-CGG CAG GTC CTA TGG ATT A-3′; 2#:5′-CCA CTA GAG GAA ACG ACT A-3′). For siRNA transfection, 40–60% confluent C2C12 cells were transfected with 100 nM siRNA by LipofectAMINE 2000 following the manufacturer's instructions. Four to 6 h later, the siRNA-liposome mixtures were removed, and fresh GM was added into cell culture plates. Generally, 24 h after transfection, GM was replaced with DM to induce myogenic differentiation.
Reporter Assays
For reporter assays, 50–70% confluent C2C12 cells in 12-well dishes were cotransfected with 0.5 μg of reporter plasmids and 100 nM siRNA together by LipofectAMINE 2000. All the samples were prepared in duplicate or triplicate. Luciferase activity was determined with a LB9507 luminometer (Berthold Technologies, Bad Wilbad, Germany) by adding 10 μl of WCE to 150 μl of freshly made luciferase buffer [0.4 μM luciferin, 13.3 mM ATP, 0.1 M Tris-HCl, pH 7.8, 1 mM EDTA, pH 8.0, and 10 mM Mg(OAc)2]. Luciferase units were normalized against total protein amount present in each sample determined by Protein Assay Reagent (Bio-Rad, Hercules, CA).
Immunostaining
Cells were first fixed in 4% paraformaldehyde for 15 min, and then they were permeabilized in 0.2% Triton X-100 for 15 min and blocked in 5% bovine serum albumin/phosphate-buffered saline (PBS) for 1 h. Cells were then incubated with the mouse anti-MHC antibody overnight, washed three times with PBS, and reincubated with the rhodamine-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 h. Then, 100 ng/ml 4,6-diamidino-2-phenylindole (DAPI) was then added for another 10 min. Fluorescence microscopy was performed using an Olympus IX70 microscope linked to a charge-coupled device digital camera (Spot RT; Diagnostic Instruments, Sterling Heights, MI).
Gene Expression Analysis by Microarrays
Duplicate C2C12 cells were transfected separately with siRNAs targeting p38α, p38β, p38γ, or green fluorescent protein (GFP) (control). Twenty-four hours after transfection, cells were switched to DM for another 18 h before harvest. Total RNA was extracted using TRIzol reagent following the manufacturer's suggestions. The GeneChip Mouse Genome 430 2.0 arrays (Affymetrix, Santa Clara, CA) were used for hybridization, which provide 45,000 probe sets to analyze the expression levels of >39,000 transcripts and variants of mouse genes. The raw data were normalized and analyzed using dChip (www.dchip.org) (Li and Hung Wong, 2001). To obtain p38 isoform-specific genes, the combined comparisons were carried out. For example, the combined comparison (control vs. p38α and not control vs. p38β and not control vs. p38γ) was used to identify genes uniquely regulated by p38α with the criteria of group mean fold change >1.5, absolute group mean differences >20, and p ≤ 0.05 for testing equal group means using Student's t test. To identify genes controlled by all three p38 isoforms, we carried out the combined comparison (i.e., control vs. p38α AND control vs. p38β AND control vs. p38γ) by using the same criteria as mentioned above.
Semiquantitative Reverse Transcription (RT)-PCR and SYBR Green-based Quantitative (q)RT-PCR
RT-PCR was performed by a two-step method. Briefly, cDNA was generated from 0.5 μg of total RNA by Improm-II reverse transcription system (Promega, Madison, WI) with oligo(dT)15 as a primer according to the manufacturer's instruction. PCR was performed in a reaction of 25 μl containing 40 ng of cDNA. PCR products were analyzed by 2% agarose gel electrophoresis. For qRT-PCR, 2× SYBR Green Supermix from Bio-Rad (Hercules, CA) was added to 25 μl of PCR reactions according to the manufacturer's instructions. Triplicate samples were subjected to qPCR using a Stratagene Mx3000P real-time PCR system with the maximum cycle number of 40. GAPDH was used as an internal control. The relative abundance of genes of interest was calculated after normalized to GAPDH. Three independent batches of RNA samples were used for qRT-PCR analysis, and data were presented as mean ± SD and analyzed by student t test (p < 0.05 was considered statistically significant). Primer pairs used were as follows: myogenin (forward: 5′-GAC TCC CCA CTC CCC ATT CAC ATA-3′; reverse: 5′-GGC GGC AGC TTT ACA AAC AAC ACA-3′); GAPDH (forward: 5′-CCC ACT CTT CCA CCT TCG-3′; reverse: 5′-TCC TTG GAG GCC ATG TAG GCC AT-3′); p38α (forward: 5′-GCA GGG ACC TTC TCA TAG AT-3′; reverse: 5′-GAG GGA TAG CCT CAG ACC-3′); p38β (forward: 5′-CTG CAA GGA AAG GCC CTC-3′; reverse: 5′-CAG GCA ATG CCT CAC TGC-3′); p38γ (forward: 5′-GAT TAC TGG GAA GAT CCT G-3′; reverse: 5′-CGT CAC AGA GCC GTC TCC-3′); p38δ (forward: 5′-GAC ACT CTT CAA GGG CAA G-3′; reverse: 5′-GCC ATC AAT CAC TGC AGC-3′); E2F2 (forward: 5′-GGT TCC TGT GGT CAG GAG-3′; reverse: 5′-CAG TTC CTG AGG GTG AAC-3′); and WISP1 (forward: 5′-GTC CAG GAC TTC ACA ATT GAG C-3′; reverse: 5′-CCA GGC TTT GCT TCC ATT G-3′).
RESULTS
p38α, β, and γ Are Expressed in Both Mature Muscles and C2C12 Myogenic Cells and Display Distinct Expression Profiles during Myogenic Differentiation
To explore the roles of p38 isoforms in myogenic differentiation, we first examined the mRNA expression patterns of four p38 isoforms in mouse muscle tissues by RT-PCR. The mouse brain, liver, and kidney tissues were used as controls. In agreement with previous reports, p38α and β were abundantly expressed in both muscles and many other tissues, whereas p38γ was preferentially expressed in muscles (Figure 1A) (Lechner et al., 1996). In contrast, p38δ was barely detectable in muscles, even though it was abundantly expressed in kidney tissues (Figure 1A) (Hu et al., 1999). Similarly, we found that p38α, β, and γ, but not δ, were also expressed in C2C12 myogenic cells as judged by RT-PCR (data not shown). To examine the protein levels and expression patterns of p38 isoforms during C2C12 differentiation, we first characterized isoform-specific p38 antibodies. As shown in Figure 1B, each p38 isoform-specific antibody was indeed highly specific for that particular isoform and did not cross-react with other p38 isoforms. Using these isoform-specific antibodies, we found that, during C2C12 differentiation, the levels of p38α remained unchanged and the levels of p38γ gradually increased, whereas the levels of p38β gradually decreased (Figure 1C). Myogenin expression was used here as a marker to monitor the progression of differentiation, whereas β-actin levels were used as a loading control.
Figure 1.
Expression profiles of p38 isoforms in mature muscles and C2C12 cells. (A) Detection of different p38 isoforms in selected mouse tissues by semiquantitative RT-PCR analysis. GAPDH was used as a loading control. (B) 293T cells were separately transfected with plasmids encoding Flag-tagged p38α, β, γ, and δ. Different Flag-p38 isoforms were immunoprecipitated (IP) from cell extracts with the anti-Flag antibody, and the immunoprecipitates were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) and examined by Western blotting (WB) by using isoform-specific p38 antibodies. (C) C2C12 cells were let to grow and differentiate in cell culture. Cells were harvested at different time points as indicated, and WCEs were subjected to SDS-PAGE followed by Western blotting analysis.
Knockdown of Any of the Three p38 Isoforms Inhibits C2C12 Differentiation
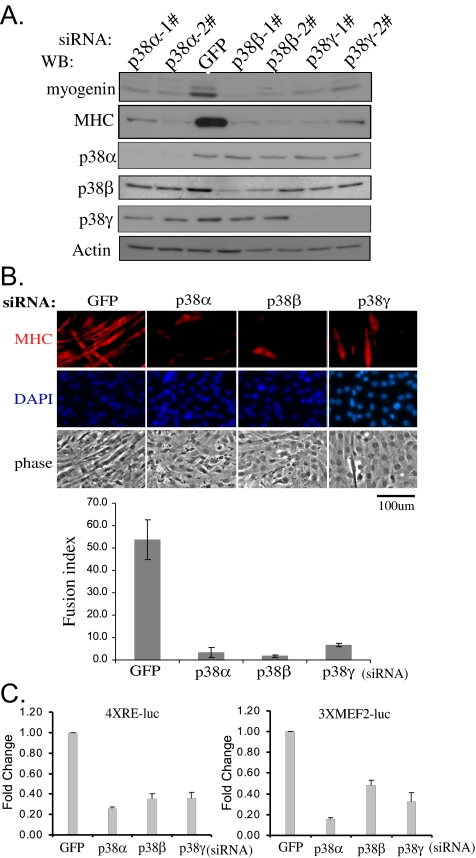
Since early 1990s, pyridinyl imidazole-based p38 inhibitors (e.g., SB203580 or SB202190) have been very popular research tools to implicate p38 MAPK in various biological processes, including myogenic differentiation (Cuenda and Cohen, 1999; Zetser et al., 1999; Wu et al., 2000b). However, there are several obvious drawbacks with these inhibitors: first, they only inhibit p38α and β but not γ and δ (Kumar et al., 1997); and second, they may inhibit cellular targets other than p38 MAPK, especially at higher concentrations (Davies et al., 2000; Lali et al., 2000). To reveal a role for each p38 isoform in C2C12 differentiation without resorting to p38 inhibitors, we used the siRNA-based RNAi approach. To reduce the “off-target” effect, we used different siRNAs for each p38 isoform. After siRNA transfection, cells were then allowed to differentiate for various times. As shown in Figure 2A, our siRNAs were very specific and effective in knocking down specific p38 isoforms. Importantly, compared with cells transfected with GFP-siRNA, cells transfected with two different sets of siRNAs against either p38α, β, or γ were all defective in differentiation as evidenced by reduced expression of myogenin and myosin heavy chain (Figure 2A) and a drastically reduced number of multinucleated myotubes (Figure 2B). Consistently, when C2C12 cells were cotransfected with various siRNAs together with either 4RE-Luc or 3xMEF2-Luc (i.e., the MRF- and MEF2-dependent luciferase reporter genes, respectively), the cells transfected with any of the three p38 isoform-specific siRNAs displayed significantly reduced reporter activities compared with those transfected with the control GFP-siRNA (Figure 2C). Our above-mentioned data showed that p38α, β, and γ are all required for normal myogenic differentiation.
Figure 2.
p38α, β, and γ are all required for C2C12 myogenic differentiation. (A) C2C12 cells were separately transfected with siRNAs targeting either GFP (control) or different p38 isoforms. After growing in GM for 24 h, cells were induced to differentiate in DM for another 24 or 48 h (for detection of MHC only). Cell extracts were harvested and subjected to Western blot analysis. 1# and 2# denote two different siRNAs against each p38 isoform. (B) After differentiating in DM for 48 h, C2C12 cells were fixed and immunostained with an anti-MHC antibody (MF20). Cell nuclei were counterstained with DAPI. The fusion index was calculated as the ratio of the number of MHC-positive cells with two or more nuclei over that of DAPI-positive nuclei in three randomly chosen fields. The results are presented as mean ± SD. Phase, phase-contrast images. (C) C2C12 cells were cotransfected with various siRNAs as indicated together with 4xRE-luc or 3xMEF2-luc. After growing in GM for 24 h, cells were switched to DM for another 24 h before harvest followed by luciferase assays. All experiments were done in triplicate, and the results are shown as mean ± SD. Fold change was the ratio of the luciferase activity in cells transfected with p38-siRNAs over that with GFP-siRNA.
Genome-wide Search for Genes Regulated by Each p38 Isoform during Early Myogenic Differentiation
Because each of the three p38 isoforms (i.e., α, β, and γ) is required for myogenic differentiation, we hypothesized that these p38 isoforms have nonredundant roles in myogenic differentiation. To reveal downstream genes regulated by each p38 isoform, we individually knocked down one particular p38 isoform at a time in C2C12 cells and let cells differentiate for 18 h, which represents a time point during early differentiation when p38 activity is known to be induced (Wu et al., 2000b). Total RNAs from duplicate samples were then extracted and subjected to microarray analysis using the GeneChip Mouse Genome 430 2.0 array (Affymetrix). By comparative and statistical analysis with the dChip software (www.dchip.org) (Li and Hung Wong, 2001), we obtained gene sets that were either induced or repressed by >1.5-fold compared with the control (i.e., cells transfected with GFP-siRNA) when one particular p38 isoform was knocked down. Some of these p38 target genes were further confirmed by RT-PCR and the representative results were shown in Tables 1–4. From these tables, it was obvious that a subset of genes was coregulated by all three p38 isoforms as knockdown of any of the isoforms had a similar effect on these genes (Table 1). In contrast, other subsets of genes were differentially regulated by different p38 isoforms (Tables 2–4). Apparently, many p38 target genes may not play a direct role in myogenic differentiation. To understand why different p38 isoforms are required for early differentiation, we aimed to identify those p38 target genes which could contribute to early myogenic differentiation. To do so, we tried to avoid genes encoding obvious structural proteins (e.g., skeletal muscle α-actin), proteins with unknown function (e.g., RIKEN cDNA E130014H10 gene), and metabolic enzymes (e.g., carbonyl reductase 2). Instead, we mainly focused on genes encoding nuclear factors and signaling molecules. siRNAs against these p38 target genes were designed and their effects on myogenin induction were examined. As a result, three novel p38 target genes including E2F2, cyclin D3 and WISP1 were identified which met the criteria mentioned above. They were selected for further in-depth analysis.
Table 1.
Selected genes coregulated by all three p38 isoforms
| Probe set ID | Gene | GenBank accession no. | Fold change |
||
|---|---|---|---|---|---|
| p38α | p38β | p38γ | |||
| 1450448_at | Stanniocalcin 1 | BQ032752 | −5.57 | −3.32 | −5.85 |
| 1419391_at | Myogenin | NM_031189 | −3.42 | −4.93 | −1.88 |
| 1449178_at | PDZ and LIM domain 3 | NM_016798 | −4.28 | −4.31 | −2.57 |
| 1415927_at | Actin, α, cardiac | NM_009608 | −3.71 | −6.22 | −2.33 |
| 1452651_a_at | Myosin, light polypeptide 1 | AK003182 | −3.57 | −4.62 | −1.81 |
| 1419606_a_at | Rroponin T1, skeletal, slow | NM_011618 | −3.42 | −2.79 | −2.31 |
| 1415999_at | Hairy/enhancer-of-split related with YRPW motif 1 | NM_010423 | −3.24 | −5.25 | −2.18 |
| 1456242_at | Hypothetical LOC433110 | AV101904 | −3.13 | −3.64 | −2.39 |
| 1427735_a_at | Actin, α1, skeletal muscle | M12233 | −2.96 | −3.94 | −1.92 |
| 1417614_at | Creatine kinase, muscle | NM_007710 | −2.61 | −3.06 | −1.98 |
| 1455203_at | RIKEN cDNA A930003A15 gene | BB522820 | −1.96 | −2.79 | −1.89 |
| 1422340_a_at | Actin, γ2, smooth muscle, enteric | NM_009610 | −1.92 | −2.25 | −2.86 |
| 1418509_at | Carbonyl reductase 2 | BC010758 | −1.79 | −1.95 | −2.10 |
| 1455790_at | E2F transcription factor 2 | BB543028 | −1.83 | −1.57 | −1.66 |
The negative numbers in the last three columns denote fold changes in gene repression compared with the reference values of the same set of genes in control cells (i.e., cells transfected with GFP-siRNA).
Table 2.
Selected genes uniquely regulated by p38α
| Probe set ID | Gene | GenBank accession no. | Fold change |
|---|---|---|---|
| 1458813_at | Sodium channel, voltage-gated, type V, α | BB516098 | −1.86 |
| 1418578_at | Diacylglycerol kinase, α | BC006713 | −1.69 |
| 1428623_at | Plexin A1 | AK011193 | −1.68 |
| 1438661_a_at | ADP-ribosylation factor 2 | AV023312 | 1.66 |
| 1457827_at | RIKEN cDNA 9330196J05 gene | BB084936 | 1.75 |
| 1418417_at | Musculin | NM_010827 | 2.44 |
| 1418105_at | Stathmin-like 4 | NM_019675 | 4.18 |
The negative and positive numbers in the last column denote fold changes in gene repression and induction respectively compared with the reference values of the same set of genes in control cells.
Table 3.
Selected genes uniquely regulated by p38β
| Probe set ID | Gene | GenBank accession no. | Fold change |
|---|---|---|---|
| 1426852_x_at | Nephroblastoma overexpressed gene | X96585 | −4.26 |
| 1416164_at | Fibulin 5 | NM_011812 | −2.47 |
| 1415907_at | Cyclin D3 | NM_007632 | −2.45 |
| 1436458_at | Sema domain, transmembrane domain, and cytoplasmic domain, (semaphorin) 6A | AI606937 | −2.23 |
| 1448737_at | Tetraspanin 7 | AF052492 | −2.12 |
| 1449315_at | Odd Oz/ten-m homologue 3 (Drosophila) | NM_011857 | −2.1 |
| 1446554_at | Glycoprotein 6 (platelet) | BB139810 | −2.1 |
| 1418012_at | Src homology (SH)3-domain GRB2-like B1 (endophilin) | BB221842 | −2.02 |
| 1434944_at | Dystrophia myotonica-protein kinase | AW108486 | −1.93 |
| 1448788_at | Cd200 antigen | AF004023 | −1.91 |
| 1433924_at | Paternally expressed 3 | BM200248 | −1.91 |
| 1419668_at | Sarcoglycan, β (dystrophin-associated glycoprotein) | AK014381 | −1.87 |
| 1448398_s_at | Ribosomal protein L22 | NM_009079 | −1.86 |
| 1433477_at | Active BCR-related gene | AV325116 | −1.81 |
| 1429459_at | Sema domain, immunoglobulin domain (Ig), short basic domain, secreted, (semaphorin) 3D | BB499147 | −1.8 |
| 1428785_at | Angiomotin-like 1 | BG917015 | −1.79 |
| 1457952_at | CD80 antigen | BM121947 | −1.79 |
| 1448259_at | Follistatin-like 1 | BI452727 | −1.77 |
| 1418393_a_at | Integrin α7 | NM_008398 | −1.73 |
| 1418011_a_at | SH3-domain GRB2-like B1 (endophilin) | BB221842 | −1.68 |
| 1418482_at | Cytochrome b-561 domain containing 2 | NM_019720 | 1.71 |
| 1430780_a_at | Phosphomannomutase 1 | BI739353 | 1.72 |
| 1424211_at | RIKEN cDNA 5730438N18 gene | BC011293 | 1.86 |
| 1450004_at | Thymic stromal lymphopoietin | NM_021367 | 2.16 |
| 1418741_at | Integrin β7 | NM_013566 | 2.24 |
| 1438405_at | Fibroblast growth factor 7 | BB791906 | 2.7 |
The negative and positive numbers in the last column denote fold changes in gene repression and induction, respectively, compared with the reference values of the same set of genes in control cells.
Table 4.
Selected genes uniquely regulated by p38γ
| Probe set ID | Gene | GenBank accession no. | Fold change |
|---|---|---|---|
| 1438556_a_at | Tropomodulin 3 | BB224629 | −3.79 |
| 1416342_at | Tenascin C | NM_011607 | −3.33 |
| 1423089_at | Tropomodulin 3 | AK017725 | −2.57 |
| 1419149_at | Serine (or cysteine) proteinase inhibitor, clade E, member 1 | NM_008871 | −2.55 |
| 1437385_at | Transcribed locus | AV264768 | −2.49 |
| 1451407_at | Junction adhesion molecule 4 | BC004806 | −2.34 |
| 1435083_at | Cortexin | BI155559 | −2.27 |
| 1449133_at | Small proline-rich protein 1A | NM_009264 | −2.16 |
| 1423088_at | Tropomodulin 3 | AK017725 | −2.1 |
| 1450171_x_at | Granzyme E | NM_010373 | −2.08 |
| 1422520_at | Neurofilament 3, medium | NM_008691 | −1.99 |
| 1438769_a_at | Thymocyte protein thy28 | BF719766 | −1.82 |
| 1416022_at | Fatty acid binding protein 5, epidermal | BC002008 | −1.8 |
| 1435077_at | Additional sex combs like 1 (Drosophila) | BE956516 | −1.76 |
| 1448593_at | WISP-1 | NM_018865 | −1.74 |
| 1428942_at | Metallothionein 2 | AA796766 | 1.69 |
| 1443977_at | RIKEN cDNA E130014H10 gene | BB538930 | 1.94 |
| 1435264_at | Elastin microfibril interfacer 2 | BB811788 | 1.98 |
| 1451191_at | Cellular retinoic acid binding protein II | BC018397 | 2.37 |
| 1435504_at | Restin-like 2 | BM217861 | 2.38 |
| 1428662_a_at | Homeobox only domain | AK009007 | 2.59 |
| 1439630_x_at | Suprabasin | AI844734 | 2.62 |
| 1417932_at | Interleukin 18 | NM_008360 | 2.94 |
| 1452031_at | Solute carrier family 1 (glial high-affinity glutamate transporter), member 3 | BB357585 | 3.08 |
The negative and positive numbers in the last column denote fold changes in gene repression and induction respectively compared with the reference values of the same set of genes in control cells.
E2F2 Is Coregulated by p38α, β, and γ and Essential for Myogenin Expression
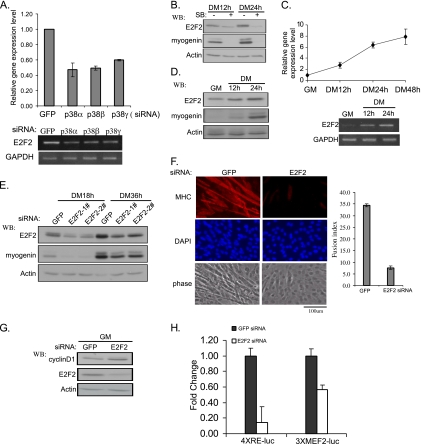
As revealed by microarray analysis (Table 1) and confirmed by both RT-PCR and qRT-PCR, E2F2, a member of the E2F family (Trimarchi and Lees, 2002; Dimova and Dyson, 2005), was coregulated by p38α, β, and γ, because individual knockdown of either p38α, β, or γ all led to reduced expression levels of E2F2 (Figure 3A). As SB202190 inhibits both p38α and β, as expected, the addition of SB202190 to C2C12 cells reduced the expression levels of both E2F2 and myogenin (Figure 3B). It was interesting to note that E2F2 was already present at low levels before differentiation; however, upon differentiation, both mRNA and protein levels of E2F2 further increased (Figure 3, C and D). Based on qRT-PCR analysis, by 48 h after differentiation, the amount of E2F2 mRNA was nearly eightfold higher than that in proliferating myoblasts (Figure 3C). Importantly, knockdown of E2F2 by two different sets of siRNAs significantly inhibited myogenic differentiation as judged by reduced myogenin expression (Figure 3E), and a drastically reduced number of multinucleated myotubes (Figure 3F). To understand why E2F2 is required for myogenin expression, we examined the effect of E2F2 knockdown on cell proliferation as well as expression and activity of several key cell cycle regulators and myogenic factors. We found that knockdown of E2F2 did not significantly affect cell cycle distribution or cell proliferation rate (Supplemental Figure). However, it increased the cyclin D1 level without affecting the expression levels of other cell cycle regulators (e.g., CDK4, Rb, p27, and p21) (Figure 3G; data not shown). In addition, knockdown of E2F2 did not affect the expression levels of MyoD and MEF2, but it significantly reduced both MRF- and MEF2-dependent gene transcription as judged by reporter assays (Figure 3H; data not shown). Our above-mentioned data indicated that E2F2 is a novel target of p38α, β, and γ and that it mediates p38 MAPK-induced myogenin expression.
Figure 3.
E2F2 is coregulated by p38α, β, and γ and required for myogenic differentiation. (A) C2C12 cells were separately transfected with various p38-siRNAs and induced to differentiate in DM for 24 h. Total RNA was extracted and the E2F2 mRNA level was analyzed by both RT-PCR (bottom) and qRT-PCR (top). (B) Near-confluent C2C12 cells were induced to differentiate for 12 and 24 h with or without SB202190 (10 μM) (added at the time of medium change). WCEs were subjected to Western blotting analysis. + and − signs denote the presence and absence of SB202190, respectively. (C and D) Total RNA (C) and WCEs (D) were harvested from C2C12 cells at different times as indicated. The mRNA and protein levels of E2F2 were analyzed by RT-PCR, qRT-PCR, and Western blotting, respectively. (E–G) C2C12 cells were separately transfected with E2F2-siRNA and GFP-siRNA, and they were induced to differentiate for various times as indicated. In E and G, WCEs were prepared and analyzed by SDS-PAGE and Western blotting. In F, cells were fixed after growing in DM for 48 h followed by immunostaining with an anti-MHC antibody (MF20). Cell nuclei were counterstained with DAPI. The calculation of fusion index and data presentation were essentially the same as described in the legend of Figure 2B. (H) C2C12 cells were cotransfected with various siRNAs as indicated together with 4xRE-luc or 3xMEF2-luc. After growing in GM for 24 h, cells were switched to DM for another 24 h before harvest followed by luciferase assays. All experiments were done in duplicate, and the results are shown as mean ± SD. Fold change was the ratio of the luciferase activity in cells transfected with E2F2-siRNAs over that with GFP-siRNA.
Cyclin D3 Is a Unique Target of p38β and Contributes to Early Myogenic Differentiation
Our microarray data revealed that cyclin D3 was specifically repressed by p38β-siRNA (Table 3), which was independently confirmed by Western blotting (Figure 4A). Like E2F2, cyclin D3 was already present in proliferating myoblasts (Figure 4B). However, upon differentiation, the expression levels of cyclin D3 further increased, which correlated with increased expression of myogenin (Figure 4B). To evaluate the impact of cyclin D3 on myogenic differentiation, we designed two different cyclin D3-siRNAs. Both were effective and inhibited the expression of myogenin and MHC (Figure 4C). Similar to what was seen with E2F2, knockdown of cyclin D3 did not affect cell cycle distribution and cell proliferation (Supplementary Figure), yet it significantly enhanced the expression levels of cyclin D1 (Figure 4D). Unlike E2F2 which affected both MRF- and MEF2-dependent gene transcription (Figure 3H), knockdown of cyclin D3 only affected MRF-dependent gene transcription as judged by reporter assays (Figure 4E).
Figure 4.
Cyclin D3 is uniquely regulated by p38β and required for myogenic differentiation. (A) C2C12 cells were transfected with various siRNAs and induced to differentiate for various times. (B) C2C12 cells were induced to differentiate for various times. (C and D) C2C12 cells were separately transfected with either GFP-siRNA or two different cyclin D3-siRNAs, then induced to differentiate for various times. WCEs from A–D were subjected to SDS-PAGE and Western blotting. (E) C2C12 cells were cotransfected with siRNAs and reporter plasmids as indicated. The measurement of the luciferase activity, calculation of the fold change and data presentation were the same as described in the legend of Figure 3H.
WISP1 Is Specifically Regulated by p38γ and Required for Myogenic Differentiation
Based on our microarray data, the gene encoding WISP1 was specifically repressed by p38γ-siRNA (Table 4), which was confirmed by both RT-PCR and qRT-PCR (Figure 5A). In C2C12 cells, we found that WISP1 mRNA was already expressed in proliferating myoblasts and that its levels gradually increased during differentiation (Figure 5B). To assess the contribution of WISP1 to differentiation, we designed two different siRNAs against WISP1. When transfected into C2C12 cells, both siRNAs were able to inhibit the expression of myogenin and MHC (Figure 5C). To understand why WISP1 is required for myogenin induction, we examined the effect of WISP1 knockdown on cell proliferation and expression and activity of key cell cycle regulators and myogenic factors. No obvious effect was seen on cell proliferation or expression levels of several cell cycle regulators (e.g., cyclin D1, p21, p27, CDK4, and Rb). However, a significant inhibition on MRF- and MEF2-dependent gene transcription was observed (Figure 5D). Because WISP1 is a secreted protein (Brigstock, 2003), our data suggested that WISP1 could affect myogenic differentiation in an autocrine manner. To test this hypothesis, Flag-WISP1 and an empty vector (control) were separately overexpressed in C2C12 cells and the conditioned medium were collected and added to C2C12 cells transfected with either GFP-siRNA or WISP1-siRNA. As shown in Figure 5E, in cells transfected with GFP-siRNA, addition of the conditioned medium from WISP1-overexpressing cells only slightly elevated myogenin expression levels (compare lanes 1 and 2). This could be due to the presence of the endogenous WISP1 secreted in the conditioned medium from the vector-transfected cells. In cells transfected with WISP1-siRNA, the myogenin expression was inhibited compared with cells transfected with GFP-siRNA (compare lanes 1 and 3), which was in agreement with the results in Figure 5C. However, addition of the conditioned medium from WISP1-overexpressing cells efficiently rescued the inhibitory effect of WISP1-siRNA on myogenin expression (compare lanes 3 and 4). Thus, our above-mentioned results indicate that WISP1 positively regulate myogenic differentiation in an autocrine manner.
Figure 5.
WISP1 is uniquely regulated by p38γ and required for myogenic differentiation. (A and B) Total RNA was isolated from C2C12 cells either transfected with various siRNAs (A) or left untreated and induced to differentiate for various times (B). The expression of WISP1 mRNA was analyzed by qRT-PCR (top) and RT-PCR (bottom). (C) C2C12 cells were separately transfected with various siRNAs as indicated. 24 h after transfection, cells were induced to differentiate in DM for another 18 or 36 h (for detection of MHC) before harvest. (D) C2C12 cells were cotransfected with various siRNAs together with reporter plasmids as indicated. After growing in GM for 24 h, cells were switched to DM for another 24 h before harvest followed by luciferase assays. All experiments were done in duplicate, and the results are shown as mean ± SD. (E) C2C12 cells were transfected with GFP-siRNA or WISP1-siRNA. Twenty-four hours after transfection, the culture media were replaced with conditioned media collected from C2C12 cells transfected with either an empty vector or Flag-WISP1. Cells were induced to differentiate in conditioned media for another 24 h before harvest. WCEs from C and E were subjected to SDS-PAGE and Western blotting.
DISCUSSION
All Three p38 Isoforms Are Involved in Normal Myogenic Differentiation
Since the initial findings about the indispensable role of the p38 MAPK pathway in myogenic differentiation (Cuenda and Cohen, 1999; Zetser et al., 1999; Wu et al., 2000b), many research groups have carried out in-depth studies to elucidate the underlying molecular mechanisms. Because four p38 isoforms exist in the human and mouse genomes, a pertinent question to address is whether all four p38 isoforms are involved in myogenic differentiation. Using C2C12 cells as a model, we show here that three p38 isoforms (i.e., α, β, and γ) are expressed in C2C12 cells and that all three are required for C2C12 differentiation. A recent report by Perdiguero et al. (2007) also addressed the same question using primary myoblasts isolated from mice deficient in one of the four p38 isoforms. Although both their studies and ours agree that p38α is required for myogenic differentiation, their findings differ from ours in that they found that myoblasts deficient in either p38β or γ proliferate and differentiate normally compared with the wild-type counterparts (Perdiguero et al., 2007). It is likely that there is an active compensatory mechanism in animals deficient in either p38β or γ, which may mask the normal physiological effects of p38β and γ. In this regard, it would be very informative to reveal the levels and activities of the remaining p38 isoforms in primary myoblasts deficient in a particular p38 isoform. Apparently, such a compensatory mechanism does not efficiently operate in C2C12 cells, because the expression levels of the remaining p38 isoforms in C2C12 cells did not change significantly when one particular p38 isoform was transiently knocked down (Figure 2A). We do not think that the results from our siRNA approach are artifacts, because different siRNAs targeting distinct regions of either p38β or γ displayed similar effects. In addition, our results about the involvement of p38γ in myogenic differentiation were consistent with a previous report (Lechner et al., 1996). Furthermore, our whole-genome gene expression profiling clearly revealed that there are indeed subsets of genes uniquely controlled by either p38β or γ. Importantly, some of these p38β- or γ-controlled genes (e.g., cyclin D3 and WISP1) are required for myogenic differentiation. Thus, we think that the difference between our study and that by Perdiguero et al. (2007) is due to different approaches used and that the two approaches are complementary to each other.
Identification of Novel p38 Target Genes Involved in Myogenic Differentiation
Many novel p38 target genes have been identified through our genome-wide gene expression profiling, and some of them are found to be required for early myogenic differentiation, which provides a mechanism to explain why different p38 isoforms are required. E2F2, a member of the E2F family, is coregulated by p38α, β, and γ. Although E2F2 is normally involved in cell proliferation in many cellular systems (Lukas et al., 1996; Wu et al., 2001), unexpectedly, we find that it also plays an important role during early myogenic differentiation (Figure 3, E and F). Because E2F2 is already present in proliferating myoblasts (Figure 3, C and D), and it is required for myogenin induction upon differentiation, it suggests that, in addition to MEF2, MyoD, and Six proteins (Xu et al., 2002), E2F2 is another key mediator involved in p38 MAPK-mediated myogenin expression. However, it is noteworthy that these mediators are differentially regulated by p38 MAPK: whereas MEF2 serves as a direct substrate of p38 MAPK (Han et al., 1997; Wu et al., 2000b), E2F2 is regulated by p38 MAPK at either the transcriptional or posttranscriptional level. For MyoD and Six, it remains unclear how exactly p38 MAPK activates them. At present, we do not know whether E2F2 regulates myogenin expression through direct binding to the myogenin promoter or through other intermediate molecules that in turn bind to the myogenin promoter. Our initial analysis suggests that E2F2 is required for MRF- and MEF2-dependent gene transcription. In addition, E2F2 may facilitate cell cycle exit by promoting cyclin D1 down-regulation, because knockdown of E2F2 enhances cyclin D1 levels.
Unlike E2F2, cyclin D3 is uniquely regulated by p38β during myogenic differentiation. Consistently, the involvement of cyclin D3 in myogenic differentiation has been recognized in several previous reports (Kiess et al., 1995; Rao and Kohtz, 1995; Cenciarelli et al., 1999). Unlike cyclin D1, which is rapidly down-regulated upon differentiation, cyclin D3 levels gradually increase and peak in postmitotic myotubes (Figure 4B). Unphosphorylated Rb is found to interact with and stabilize cyclin D3 protein, which in turn forms a multiprotein complex containing inactive CDK2 and CDK4, p21, and proliferating cell nuclear antigen (Kiess et al., 1995; Cenciarelli et al., 1999). Such a complex is thought to contribute to irreversible cell cycle exit and maintenance of the differentiated state. In addition to these previous findings, our current study shows for the first time that cyclin D3 is required for myogenin induction. We suggest that cyclin D3 may facilitate cell cycle exit by promoting cyclin D1 down-regulation, because knockdown of cyclin D3 prominently up-regulates cyclin D1 levels (Figure 4D). Furthermore, we show that cyclin D3 is required for MRF-dependent gene transcription. Because cyclin D3 is a transcription target of MyoD (Cenciarelli et al., 1999), this suggests the existence of a positive regulatory loop between MyoD and cylin D3. It remains unclear how exactly cyclin D3 exerts its effect on cyclin D1- and MRF-dependent gene transcription.
WISP1, a known target gene induced by Wnt-1 (Pennica et al., 1998; Xu et al., 2000), is a member of the CCN family proteins that consists of Cysteine-rich 61 (CCN1), connective tissue growth factors (CTGF or CCN2), nephroblastoma overexpressed (Nov or CCN3), WISP1 (CCN4), WISP2 (CCN5), and WISP3 (CCN6) (Brigstock, 2003). The receptors for members of the CCN family remain poorly characterized. In several cases, certain integrins are thought to be the receptors for CCN1, CCN2, and CCN3 (Brigstock, 2003). We show here that WISP1 is specifically induced by p38γ during myogenic differentiation (Figure 5). As a secreted molecule, we show that WISP1 can regulate myogenin expression in an autocrine manner (Figure 6). Furthermore, WISP1 enhances both MRF- and MEF2-dependent gene transcription. Interestingly, CCN3, another member of the CCN family, is found to be specifically induced by p38β (Table 3). However, the functional significance of CCN3 induction by p38 β remains unclear, because knockdown of CCN3 does not affect myogenin expression (data not shown). It also remains unclear how WISP1 affects myogenin expression. Because WISP1 is also induced by Wnt-1, it may serve as a converging point to integrate signals from both the p38γ pathway and the Wnt pathway.
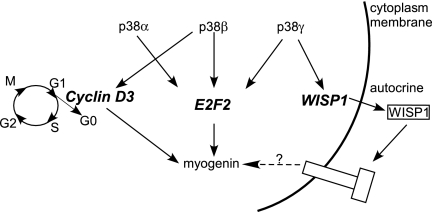
Figure 6.
Schematic on how different p38 isoforms regulate myogenin induction through distinct target genes. Three p38 isoforms can regulate myogenin expression through both common target genes (e.g., E2F2) and unique isoform-specific target genes (e.g., cyclin D3 and WISP1). Unlike E2F2 and cyclin D3, WISP1 participates in myogenin regulation in an autocrine manner. G0, G1, G2, S, and M denote different phases of the cell cycle.
In summary, our current study demonstrates that different p38 isoforms can regulate myogenic differentiation through both common and unique target genes. It is less likely that perturbation of any one of these target genes would completely recapitulate the effect caused by that of a particular p38 isoform. However, an important point we try to make here is that different p38 isoforms may exert certain nonredundant effects on myogenic differentiation through distinct target genes. To understand precisely how different p38 isoforms contribute to myogenic differentiation, it is essential that we first understand how these different p38 target genes function during myogenic differentiation.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. J. Han for p38 expression vectors, Dr. J. R. Nevins (Duke University) for E2F2 expression vector, Dr. M. A. Bogoyevitch for p38γ antibody, and Carol Wong for technical assistance. This project is supported by Hong Kong Research Grant Council grants HKUST6412/05M, HKUST6496/06M, and CA06/07.SC02 (to Z.W.); Area of Excellence Scheme AoE/B-15/01; and a 973 project from the Ministry of Science and Technology of China (2002 CB513005) (to Z.W. and Y.J.).
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/10.1091/mbc.mbc.E07-08-0817) on February 6, 2008.
REFERENCES
- Black B. L., Olson E. N. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu. Rev. Cell Dev. Biol. 1998;14:167–196. doi: 10.1146/annurev.cellbio.14.1.167. [DOI] [PubMed] [Google Scholar]
- Brigstock D. R. The CCN family: a new stimulus package. J. Endocrinol. 2003;178:169–175. doi: 10.1677/joe.0.1780169. [DOI] [PubMed] [Google Scholar]
- Cenciarelli C., De Santa F., Puri P. L., Mattei E., Ricci L., Bucci F., Felsani A., Caruso M. Critical role played by cyclin D3 in the MyoD-mediated arrest of cell cycle during myoblast differentiation. Mol. Cell. Biol. 1999;19:5203–5217. doi: 10.1128/mcb.19.7.5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenda A., Cohen P. Stress-activated protein kinase-2/p38 and a rapamycin-sensitive pathway are required for C2C12 myogenesis. J. Biol. Chem. 1999;274:4341–4346. doi: 10.1074/jbc.274.7.4341. [DOI] [PubMed] [Google Scholar]
- Davies S. P., Reddy H., Caivano M., Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimova D. K., Dyson N. J. The E2F transcriptional network: old acquaintances with new faces. Oncogene. 2005;24:2810–2826. doi: 10.1038/sj.onc.1208612. [DOI] [PubMed] [Google Scholar]
- Han J., Jiang Y., Li Z., Kravchenko V. V., Ulevitch R. J. Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature. 1997;386:296–299. doi: 10.1038/386296a0. [DOI] [PubMed] [Google Scholar]
- Hu M. C., Wang Y. P., Mikhail A., Qiu W. R., Tan T. H. Murine p38-delta mitogen-activated protein kinase, a developmentally regulated protein kinase that is activated by stress and proinflammatory cytokines. J. Biol. Chem. 1999;274:7095–7102. doi: 10.1074/jbc.274.11.7095. [DOI] [PubMed] [Google Scholar]
- Jones N. C., Tyner K. J., Nibarger L., Stanley H. M., Cornelison D. D., Fedorov Y. V., Olwin B. B. The p38alpha/beta MAPK functions as a molecular switch to activate the quiescent satellite cell. J. Cell Biol. 2005;169:105–116. doi: 10.1083/jcb.200408066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren A., Tamir Y., Bengal E. The p38 MAPK signaling pathway: a major regulator of skeletal muscle development. Mol. Cell. Endocrinol. 2006;252:224–230. doi: 10.1016/j.mce.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Kiess M., Gill R. M., Hamel P. A. Expression of the positive regulator of cell cycle progression, cyclin D3, is induced during differentiation of myoblasts into quiescent myotubes. Oncogene. 1995;10:159–166. [PubMed] [Google Scholar]
- Kumar S., McDonnell P. C., Gum R. J., Hand A. T., Lee J. C., Young P. R. Novel homologues of CSBP/p38 MAP kinase: activation, substrate specificity and sensitivity to inhibition by pyridinyl imidazoles. Biochem. Biophys. Res. Commun. 1997;235:533–538. doi: 10.1006/bbrc.1997.6849. [DOI] [PubMed] [Google Scholar]
- Lali F. V., Hunt A. E., Turner S. J., Foxwell B. M. The pyridinyl imidazole inhibitor SB203580 blocks phosphoinositide-dependent protein kinase activity, protein kinase B phosphorylation, and retinoblastoma hyperphosphorylation in interleukin-2-stimulated T cells independently of p38 mitogen-activated protein kinase. J. Biol. Chem. 2000;275:7395–7402. doi: 10.1074/jbc.275.10.7395. [DOI] [PubMed] [Google Scholar]
- Lechner C., Zahalka M. A., Giot J. F., Moller N. P., Ullrich A. ERK6, a mitogen-activated protein kinase involved in C2C12 myoblast differentiation. Proc. Natl. Acad. Sci. USA. 1996;93:4355–4359. doi: 10.1073/pnas.93.9.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Hung Wong W. Model-based analysis of oligonucleotide arrays: model validation, design issues and standard error application. Genome Biol. 2001;2:RESEARCH0032. doi: 10.1186/gb-2001-2-8-research0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lluis F., Ballestar E., Suelves M., Esteller M., Munoz-Canoves P. E47 phosphorylation by p38 MAPK promotes MyoD/E47 association and muscle-specific gene transcription. EMBO J. 2005;24:974–984. doi: 10.1038/sj.emboj.7600528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas J., Petersen B. O., Holm K., Bartek J., Helin K. Deregulated expression of E2F family members induces S-phase entry and overcomes p16INK4A-mediated growth suppression. Mol. Cell. Biol. 1996;16:1047–1057. doi: 10.1128/mcb.16.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkentin J. D., Olson E. N. Defining the regulatory networks for muscle development. Curr. Opin. Genet. Dev. 1996;6:445–453. doi: 10.1016/s0959-437x(96)80066-9. [DOI] [PubMed] [Google Scholar]
- Penn B. H., Bergstrom D. A., Dilworth F. J., Bengal E., Tapscott S. J. A MyoD-generated feed-forward circuit temporally patterns gene expression during skeletal muscle differentiation. Genes Dev. 2004;18:2348–2353. doi: 10.1101/gad.1234304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennica D., et al. WISP genes are members of the connective tissue growth factor family that are up-regulated in wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc. Natl. Acad. Sci. USA. 1998;95:14717–14722. doi: 10.1073/pnas.95.25.14717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdiguero E., et al. Genetic analysis of p38 MAP kinases in myogenesis: fundamental role of p38alpha in abrogating myoblast proliferation. EMBO J. 2007;26:1245–1256. doi: 10.1038/sj.emboj.7601587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri P. L., Sartorelli V. Regulation of muscle regulatory factors by DNA-binding, interacting proteins, and post-transcriptional modifications. J. Cell. Physiol. 2000;185:155–173. doi: 10.1002/1097-4652(200011)185:2<155::AID-JCP1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Rao S. S., Kohtz D. S. Positive and negative regulation of D-type cyclin expression in skeletal myoblasts by basic fibroblast growth factor and transforming growth factor beta. A role for cyclin D1 in control of myoblast differentiation. J. Biol. Chem. 1995;270:4093–4100. doi: 10.1074/jbc.270.8.4093. [DOI] [PubMed] [Google Scholar]
- Simone C., Forcales S. V., Hill D. A., Imbalzano A. N., Latella L., Puri P. L. p38 pathway targets SWI-SNF chromatin-remodeling complex to muscle-specific loci. Nat. Genet. 2004;36:738–743. doi: 10.1038/ng1378. [DOI] [PubMed] [Google Scholar]
- Tapscott S. J. The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development. 2005;132:2685–2695. doi: 10.1242/dev.01874. [DOI] [PubMed] [Google Scholar]
- Trimarchi J. M., Lees J. A. Sibling rivalry in the E2F family. Nat. Rev. Mol. Cell Biol. 2002;3:11–20. doi: 10.1038/nrm714. [DOI] [PubMed] [Google Scholar]
- Wu H., et al. MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fiber type. EMBO J. 2000a;19:1963–1973. doi: 10.1093/emboj/19.9.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., et al. The E2F1–3 transcription factors are essential for cellular proliferation. Nature. 2001;414:457–462. doi: 10.1038/35106593. [DOI] [PubMed] [Google Scholar]
- Wu Z., Woodring P. J., Bhakta K. S., Tamura K., Wen F., Feramisco J. R., Karin M., Wang J. Y., Puri P. L. p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Mol. Cell. Biol. 2000b;20:3951–3964. doi: 10.1128/mcb.20.11.3951-3964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Corcoran R. B., Welsh J. W., Pennica D., Levine A. J. WISP-1 is a Wnt-1- and beta-catenin-responsive oncogene. Genes Dev. 2000;14:585–595. [PMC free article] [PubMed] [Google Scholar]
- Xu Q., Yu L., Liu L., Cheung C. F., Li X., Yee S. P., Yang X. J., Wu Z. p38 Mitogen-activated protein kinase-, calcium-calmodulin-dependent protein kinase-, and calcineurin-mediated signaling pathways transcriptionally regulate myogenin expression. Mol. Biol. Cell. 2002;13:1940–1952. doi: 10.1091/mbc.02-02-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetser A., Gredinger E., Bengal E. p38 mitogen-activated protein kinase pathway promotes skeletal muscle differentiation. Participation of the Mef2c transcription factor. J. Biol. Chem. 1999;274:5193–5200. doi: 10.1074/jbc.274.8.5193. [DOI] [PubMed] [Google Scholar]
- Zhao M., New L., Kravchenko V. V., Kato Y., Gram H., di Padova F., Olson E. N., Ulevitch R. J., Han J. Regulation of the MEF2 family of transcription factors by p38. Mol. Cell. Biol. 1999;19:21–30. doi: 10.1128/mcb.19.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.