Abstract
Septins are members of a highly conserved family of filamentous proteins that are required in many organisms for the completion of cytokinesis. In addition, septins have been implicated in a number of important cellular processes and have been suggested to have roles in regulating membrane traffic. Given the proposed role of septins in cell membrane dynamics, we investigated the function of septins during FcγR-mediated phagocytosis. We show that several septins are expressed in RAW264.7 and J774 mouse macrophage cell lines and that SEPT2 and SEPT11 are colocalized with submembranous actin-rich structures during the early stages of FcγR-mediated phagocytosis. In addition, SEPT2 accumulation is seen in primary human neutrophils and in nonprofessional phagocytes. The time course of septin accumulation mirrors actin accumulation and is inhibited by latrunculin and genistein, but not other inhibitors of phagocytosis. Inhibition of septin function by transient expression of the BD3 domain of BORG3, known to cause septin aggregation, or depletion of SEPT2 or SEPT11 by RNAi, significantly inhibited FcγR-mediated phagocytosis of IgG-coated latex beads. Interestingly, this occurred without affecting the accumulation of actin or the actin-associated protein coronin-1. These observations show that, although not necessary for actin recruitment, septins are required for efficient FcγR-mediated phagocytosis.
INTRODUCTION
Phagocytosis of invading microorganisms is an important immune response orchestrated by neutrophils and cells of the monocyte/macrophage lineage. These specialized cells, termed “professional” phagocytes, recognize foreign microbes using specific cell surface receptors with the aid of host proteins called opsonins. Immunoglobulin G antibodies (IgG) are one of the major opsonins found within serum, and receptors that recognize IgG-opsonized particles (FcγR) do so via a highly conserved extracellular Fc-binding domain (Ravetch and Kinet, 1991). A number of key events take place between the time of initial binding of IgG-coated particles to the cell surface and the eventual degradation of these pathogens within intracellular phagosomes. The first notable event is the clustering and subsequent tyrosine phosphorylation of FcγRs. Although the receptors themselves do not contain any intrinsic tyrosine kinase activity, several protein tyrosine kinases are known to be activated upon the clustering of FcγRs (Greenberg et al., 1994; Indik et al., 1995b). Phosphorylation of tyrosine residues within the conserved immunoreceptor tyrosine activation motif (ITAM) provides important docking sites for SH2-containing molecules, most notably the tyrosine kinase Syk (Greenberg et al., 1994, 1996; Indik et al., 1995a; Crowley et al., 1997; Kiefer et al., 1998). Ultimately these early signaling events lead to local remodeling of the submembranous actin cytoskeleton (Greenberg et al., 1990), whose intracellular force-generating capabilities contribute, along with the targeted delivery of new membrane (Bajno et al., 2000; Niedergang et al., 2003), to the growth of the plasma membrane around the particle. Interestingly, expression of the FcγRIIA receptor in nonphagocytic cells, such as COS or Chinese hamster ovary (CHO) cells, is sufficient to confer on them phagocytic properties (Indik et al., 1995a).
Although FcγR-mediated phagocytosis is an actin-dependent process, the role of other cytoskeletal elements is not particularly well understood. Several members of the septin family have been shown to be colocalized with filamentous actin (Kinoshita et al., 1997, 2002; Xie et al., 1999), possibly by their binding to myosin (Joo et al., 2007). Septins are a highly conserved family of filamentous GTPases that were originally identified in Saccharomyces cerevisiae as factors controlling bud-site selection (for reviews see Gladfelter et al., 2001; Longtine and Bi, 2003). However, since their discovery as factors that control cytokinesis in animal cells (Neufeld and Rubin, 1994; Kinoshita et al., 1997; Surka et al., 2002), numerous observations suggest that septins may also regulate other important cellular functions including cell membrane dynamics and vesicle fusion events (Fares et al., 1995, 1996; DeMarini et al., 1997; Hsu et al., 1998; Beites et al., 1999; Joo et al., 2005).
In this study we investigated the role of mammalian septins during FcγR-mediated phagocytosis. We find that the murine macrophage cell lines J774 and RAW264.7 express a subset of septin gene products. Antibodies to two of these, SEPT2 and SEPT11, reveal that these proteins transiently accumulate at the phagocytic cup in J774, RAW264.7, and human neutrophils and in CHO or HeLa cells engineered to phagocytose. Inhibition of septin function, either by the use of a dominant inhibitor of septins, or by RNA interference (RNAi)-mediated depletion, significantly inhibits FcγR-mediated phagocytosis without affecting the signaling events required for actin accumulation.
MATERIALS AND METHODS
Reagents and Antibodies
Tissue culture supplies were purchased from Wisent (St. Bruno, QC, Canada). Unconjugated latex beads were from Bangs Laboratories (Fisher, IN). Fluorescein isothiocyanate (FITC), Cy3- and Cy5-conjugated donkey anti-rabbit antibodies, and Cy5-conjugated donkey anti-human IgG, were from Jackson ImmunoResearch (West Grove, PA). Human IgG was from Sigma-Aldrich (St-Louis, MO). Mouse anti-phosphotyrosine mAb was from Upstate (Charlotteville, VA). Alexa 488-, Rhodamine-conjugated donkey anti-mouse secondary antibodies, Rhodamine-phalloidin, and Alexa 488-phalloidin were from Molecular Probes (Eugene, OR). Rabbit anti-human septin 2 and 11 antibodies were raised against a synthetic peptides corresponding to the first 12 amino acids of human septin 2 and 11. respectively, and purified on columns containing the covalently bound peptide as described previously (Surka et al., 2002). psiRNA-hH1GFPzeo and psiRNA-hH1gz-Scr (a plasmid containing a control sequence specific for luciferase) vectors were from Invivogen (San Diego, Ca). All restriction enzymes were from New England Biolabs (Beverly, MA). Wortmannin, latrunculin B, and genistein were from EMD Biosciences (San Diego, CA). HeLa cells were obtained from the ATCC (Manassas, VA), and CHO-IIA cells were kindly provided by Dr. Alan Schreiber (University of Pennsylvania, Philadelphia, PA). HP Genome-wide small interfering RNA (siRNA) for human SEPT11 was obtained from Qiagen (Mississauga, ON, Canada) for the following target sequences: hs_SEPT11_2 HP, hereafter called SEPT11 siRNA1, 5′CAAGAGG-AATTGAAGATTAAA3′ and hs_SEPT11_5 HP, hereafter called SEPT11 siRNA2, 5′ACGGCTGAAGTTAACCATTGT3′.
DNA Constructs
The construction and utilization of mammalian expression vectors pEGFP-PLCδPH, pRFP-coronin-1, and pEGFP-PM have been described elsewhere (Botelho et al., 2000; Scott et al., 2005; Yan et al., 2005). GFP3-BD3 plasmid (Joberty et al., 2001) was kindly provided by I. G. Macara (University of Virginia, Charlottesville, VA). pmRFP-PLCδPH was generated by cutting the PH domain from pEGFP-PLCδPH vector using XhoI and BamHI and subcloning the fragment into the corresponding sites of pDsRed-Monomer C1 vector (Clontech, Mountain View, CA). To generate siRNA specific for nucleotides 524–544 relative to the start codon of the human septin 2, the following complementary pair of nucleotides for this region including a hairpin sequence containing a BamHI site were generated to include Acc65I and HindIII at the ends: 5′GTACCTCGAATATTGTGCCTGTCATTGGGATCCCAATGACAGGCACAATATTCTTTTTGGAAA3′ and 5′AGCTTTTCCAAAAAGAATATTGTGCCTGTCATTGGGATCCCAATGACAGGCACAATATTCGAG3′. These were cloned into the corresponding sites of psiRNA-hH1GFPzeo vector, expressing short hairpin RNA (shRNA) and green fluorescent protein (GFP) from H1 and CMV promoters, respectively.
Cell Culture and Transfection with DNA or shRNA
Culture conditions for mouse macrophage cell lines RAW 264.7, J774, and CHO cells stably transfected with FcγRIIA receptor (CHO-IIA) have been described elsewhere (Hackam et al., 1997; Vieira et al., 2003). CHO-IIA cells and RAW 264.7 cells were transiently transfected by using FuGene 6 (Roche, Nutley, NJ) or Lipofectamine 2000 (Invitrogen, Carlsbad, CA) as suggested by the manufacturers. Cells were transfected either with GFP3-BD3 using GFP as a control for 36–48 h or with shRNA-septin 2 using shRNA-control as control for 84–96 h. All other plasmids were transfected for 24 h.
Isolation of Human Neutrophils
Human blood was obtained from healthy volunteers. The neutrophils were purified using Polymorphprep (Accurate Chemical and Scientific, Westbury, NY) gradient separation procedure according to the manufacturer's instructions. Isolated neutrophils were resuspended in PBS containing 1 mM calcium chloride, 1 mM magnesium chloride, and 10 mM glucose at ∼1 × 106 cells/ml and used within 6 h of isolation.
Phagocytosis Assays and Inhibitors Treatment
Latex beads were opsonized with 0.8 mg/ml human IgG, incubated for 1 h at 37°C, and washed with phosphate-buffered saline (PBS) 3 times. When noted, the cells were treated with 250 nM wortmannin or 5 μM latrunculin B for 30 min or 100 μg/ml genistein for 3 h before phagocytosis. Opsonized beads were allowed to attach to the cells for 10 min on ice in cold HEPES-buffered RPMI. Unbound beads were washed away with cold PBS. Phagocytosis was started by replacing cold medium with HEPES-buffered RPMI prewarmed to 37°C and incubated at 37°C for the designated time. When necessary, the beads were stained by incubating with Cy5-labeled donkey anti-human IgG on ice for 15 min. Cells were then fixed with 4% paraformaldehyde and permeabilized as described previously (Yan et al., 2005). Endogenous SEPT2 was detected by immunostaining with rabbit anti-human septin 2 primary antibody and Cy3-, FITC-, or Cy5-labeled donkey anti-rabbit secondary antibodies. In the case of SEPT11, cells were fixed for 10 min in 1% paraformaldehyde at 37°C, as above, stained with Cy5 donkey anti-human antibody to detect external beads, and then permeabilized with MeOH at −20°C for 10 min.
For phagocytic index assays, RAW 264.7 or CHO-IIA cells transfected either with GFP-BD3 or with shRNA-septin 2 were treated as above. HeLa cells were transfected with siRNA for 2 d and then retransfected with a plasmid containing the FcγIIA-GFP receptor, and phagocytosis was performed on the third day. The phagocytic index was calculated by counting the average numbers of internalized beads per cell and normalized to that of the controls performed the same day. The binding index was calculated by counting the average numbers of bound beads on the cell surface without performing phagocytosis and normalized as per the phagocytic index.
Microscopy
Laser-scanning confocal microscopy was performed using an LSA 510 laser scanning confocal microscope (Carl Zeiss MicroImaging, Thornwood, NY) with 63× or 100× oil immersion objective and analyzed using Photoshop 6.0 (Adobe, San Jose, CA). Spinning disk images were captured on a Zeiss Axiovert 200M equipped with a Quorum confocal spinning disk head (Quorum Technologies, Guelph, ON, Canada).
RESULTS
Septin Expression in Phagocytic Cells
To determine if any septin proteins might play a role in phagocytosis, we first performed Western blotting to examine their expression in two different murine macrophage cell lines, RAW264.7 and J774. We have previously found that almost all septin genes are expressed in brain (unpublished observations) so brain tissue was used as a positive control in these experiments. In addition, we examined the septin expression profile in a CHO cell line that has been engineered to phagocytose by stable expression of the FcγIIA receptor. As can be seen in Figure 1, SEPT2, SEPT6, SEPT10, and SEPT11 were abundantly expressed in all four cell types. SEPT9 was detectable in CHO cells but much less so in brain and not at all in RAW 264.7 or J774 cells. SEPT7 and was not studied here because of the lack of a suitable antibody, and SEPT12 is not shown because it is only abundantly expressed in testes and was not present in the brain or any of the cell lines (Steels et al., 2007). Because septins typically associate in coimmunoprecipitating complexes (for example, see Kinoshita et al., 2002) and because our antibodies to SEPT2 and SEPT11 are superior to anti-SEPT6 and SEPT10 for immunocytochemistry, we chose to focus on SEPT2 and SEPT11 for the remaining studies.
Figure 1.
Western blot results of septins in phagocytic cell lines. RAW 264.7, J774, and CHO-IIA cell were harvested, and 10 μg of each cell lysate with 10 μg of rat brain as control were separated by 12% SDS-PAGE before immunoblotting. The blots were probed with rabbit polyclonal antibodies specific to septins 1–6 and 8–11.
Fcγ-Receptor–mediated Phagocytosis: SEPT2 and SEPT11 associate with forming phagosomes
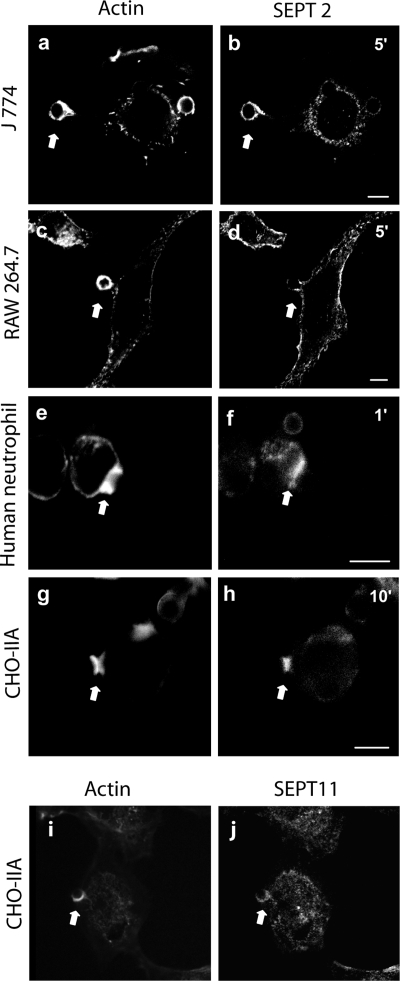
Although the full range of functions of septins remains unknown, these filamentous GTPases are required for cytokinesis and accumulate along with actin at the mitotic cleavage furrow in animal cells (Neufeld and Rubin, 1994; Kinoshita et al., 1997; Xie et al., 1999; Surka et al., 2002). To determine whether septins may play a role in FcγR-mediated phagocytosis, another actin-dependent cellular process, we first examined their distribution in cells phagocytosing latex beads via FcγRIIA receptors (Figure 2). Robust accumulation of SEPT2 was observed in the murine macrophage cell lines J774 and RAW 264.7, although a stronger accumulation was observed in the former. In J774 cells SEPT2 accumulated all around the particle (Figure 2, a and b), whereas in RAW264.7 cells the accumulation was most typically seen at the base of the phagosome pedestal (Figure 2, c and d). Strong accumulation was also observed in primary human neutrophils (Figure 2, e and f) and in CHO-IIA cells stably expressing the FcγIIA receptor (Figure 2, g and h). It should be noted that the time course of phagocytosis in each of these models is quite different, with neutrophils being the fastest and CHO-IIA being the slowest. To determine if this was a specific accumulation of SEPT2, or involved multiple septins, likely in a complex, we also stained for SEPT11 (Figure 2, i and j). Although immunocytochemistry with this antibody is inferior to that of SEPT2, a clear accumulation of SEPT11 could be seen associated with the nascent phagosome in CHO-IIA cells.
Figure 2.
Endogenous SEPT2 and SEPT11 accumulate on phagosomes in different phagocytic cells. Opsonized beads were allowed to attach to the cells for 10 min on ice and then RAW 264.7 (a and b), J 774 (c and d), human neutrophil (e and f), and CHO-IIA cells (g–j) were warmed to 37°C to start phagocytosis for 5, 5, 1, and 10 min, respectively, at 37°C. Cells were then fixed and endogenous SEPT2 was stained with rabbit polyclonal anti-human SEPT2 antibody (b, d, f, and h) or SEPT11 with anti-SEPT11 (j) and detected with Cy3-conjugated donkey anti-rabbit antibody. Actin was stained with Alexa 488-phalloidin (a, c, e, g, and i). The arrows indicate accumulated actin and septin at sites of interaction with opsonized beads in each cell line. Bars, 5 μm.
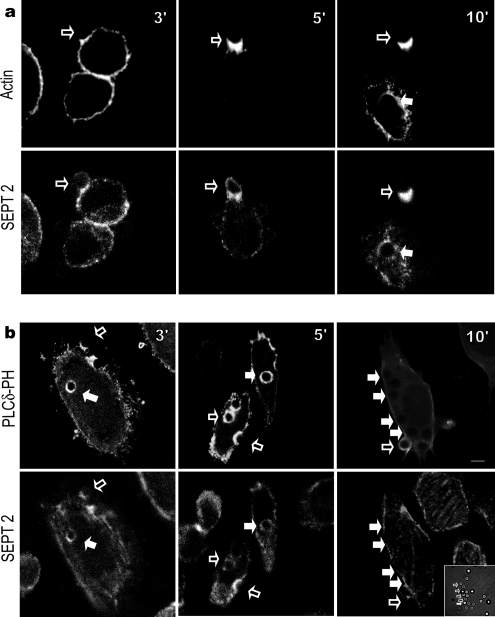
Having established the presence of SEPT2 and SEPT11 in cells capable of FcγR-mediated phagocytosis, we next explored the time course of their appearance on the phagosome. Given the slower kinetics of CHO-IIA phagocytosis, these cells were chosen to monitor the internalization process. CHO-IIA cells were challenged with human IgG-opsonized latex beads for different periods of time at 37°C, fixed, and then stained for filamentous actin using Alexa488-labeled phalloidin and SEPT2. Within 3–5 min, opsonized latex beads are seen engaged in phagocytosis. Unsealed phagosomes were identified by staining for external human IgG after light fixation in the absence of detergent (not shown) and are indicated with open arrows (Figure 3a). The accumulation of filamentous actin in collar-like structures is characteristic of nascent phagosomes (Kolotila and Diamond, 1988; Greenberg et al., 1990). Fully internalized particles not detected by immunostaining for human IgG in nonpermeabilized cells (filled arrows, Figure 3a) appeared to concurrently lose both actin and SEPT2.
Figure 3.
Time course of SEPT2, actin and PLCδ-PH accumulation on phagosomes during phagocytosis in CHO-IIA cells. (a) Opsonized beads were allowed to attach to CHO-IIA cells for 10 min on ice and then warmed up to 37°C to start phagocytosis. At the designated time interval, cells were cooled down on ice to stop phagocytosis. External beads were stained by Cy5-conjugated donkey anti-human IgG antibody for 15 min on ice. Cells were then fixed and stained with rabbit polyclonal anti-human SEPT2 antibody and Cy3-conjugated donkey anti-rabbit secondary antibody for endogenous SEPT2 and Alexa488-phalloidin for actin. (b) CHO-IIA cells were transfected with RFP-PLCδ-PH for 24 h. Phagocytosis was performed and cells were stained as described above. Top panels in a and b are actin and RFP-PLCδ-PH, respectively, and bottom panels are SEPT2. Open arrows point to unsealed phagosomes and solid arrows point to sealed phagosomes. Bars, 5 μm.
We have previously shown that septins possess polybasic sequences that interact with phosphatidylinositol lipids (Zhang et al., 1999), and these lipids are also known to influence actin polymerization. We therefore examined the timing of PIP2 accumulation at the phagosome relative to that of SEPT2. In this case CHO-IIA cells were transiently transfected with pEGFP-PLCδPH (Scott et al., 2005) and examined for 10 min after initiation of phagocytosis. As seen in Figure 3b, SEPT2 accumulates within 3 min to unsealed nascent phagosomes (open arrows) in concert with PIP2 accumulations detected by pEGFP-PLCδPH. As with actin, the SEPT2 and PIP2 levels decrease on sealed phagosomes with a similar time course (solid arrows, 10 min).
SEPT2 Accumulation Depends on Actin
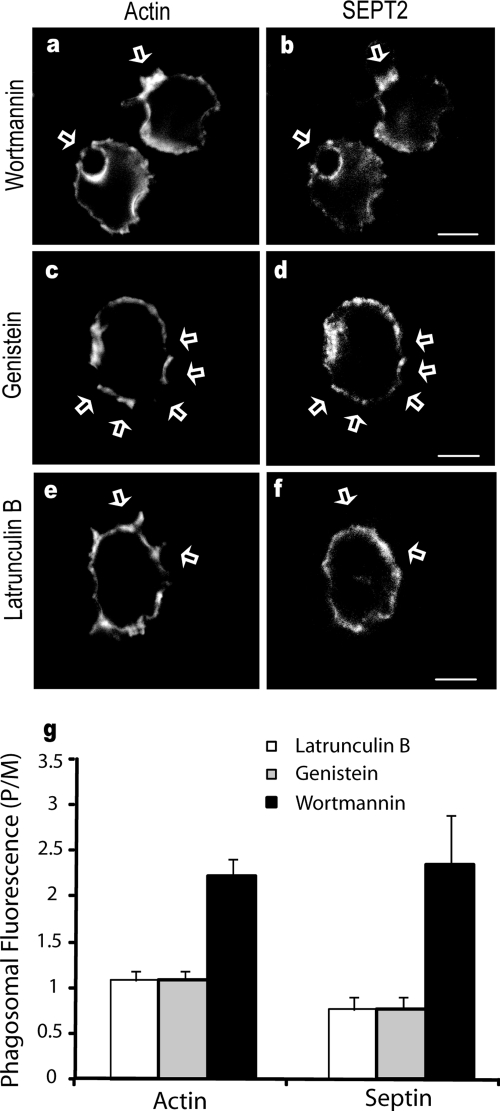
To begin to examine the signals that are responsible for septin accumulation at the nascent phagosome, CHO-IIA cells were treated with inhibitors capable of blocking phagocytosis. Wortmannin, a potent inhibitor of type I and III PI3 kinases, partially inhibits phagocytosis of large particles (Araki et al., 1996; Cox et al., 1999). After confirming that wortmannin had inhibited phagocytosis under the conditions used (not shown), there remains robust accumulation of actin at the base of bound opsonized beads, and SEPT2 can be seen to accumulate at these sites (Figure 4, a and b), indicating that PI3-kinase activity is not required for SEPT2 accumulation. We therefore examined an upstream event in the signaling process, the activation of src-family tyrosine kinases (Greenberg et al., 1993). Using the general inhibitor of tyrosine kinases, genistein, we found that in the majority of the cells actin accumulation at the phagosome was inhibited. In these cells the accumulation of SEPT2 was also significantly reduced (Figure 4, c and d), consistent with the role of tyrosine kinases in early stages of actin remodeling.
Figure 4.
Effects of inhibitors on endogenous SEPT2. CHO-IIA cells were treated with 250 nM wortmannin, or 5 μM latrunculin B for 30 min or 100 μg/ml genistein for 3 h before phagocytosis. Opsonized beads were allowed to attach to the cells for 10 min on ice and then warmed to 37°C to start phagocytosis for 15 min. External beads were stained with Cy5-conjugated donkey anti-human antibody on ice for 15 min. The cells were then fixed and immunostained with Alexa 488- phalloidin for actin and SEPT2 antibody, followed by Cy3-conjugated donkey anti-rabbit antibody for endogenous SEPT2. (a and b) wortmannin, (c and d) genistein, and (e and f) latrunculin treatment. Arrows point to adherent beads. Bars, 5 μm. (g) Quantitation of actin and SEPT2 accumulation after treatment with inhibitors. To quantify the accumulation of actin and SEPT2 at the phagosomal membrane, lines were drawn through the intersections of the phagocytic cup and the contralateral plasma membrane on the colocalized images. The fluorescence intensity of individual pixels was determined using Image J and presented as the ratio of that on the phagosome (P) to that of the normalized plasma membrane (M), hence P/M. In all cases subthreshold intensities were used to ensure that the signals were not saturated. Data are mean ± SE of more than three independent experiments.
Similarly, direct inhibition of actin accumulation at the phagosome by pretreatment of the cells with latrunculin B resulted in an inhibition of both actin and septin accumulation at the phagocytic cup (Figure 4, e and f). Together, these results suggest that the accumulation of septins at the phagosome depends on the accumulation of actin. These results are quantified in Figure 4g.
SEPT2 Function Is Required for Efficient Phagocytosis
The transient accumulation of SEPT2 at the phagosome raised the possibility that it may provide a necessary function for phagocytosis. To determine if septins are required for phagocytosis, we used two approaches to inhibit their function. First, we took advantage of a dominant inhibitor known to inhibit septins by causing their aggregation. Borg proteins are Cdc42 effectors, and one of the Borg isoforms, Borg3, was shown to bind to septins via its third Borg homology domain, BD3. Overexpression of BD3 fused to GFP was shown to result in the coaggregation of GFP-BD3 and septins into cytoplasmic structures in the majority of cells (Joberty et al., 2001). The aggregation depletes the available septins, thereby inhibiting their function. We therefore overexpressed GFP-BD3 in CHO-IIA cells and found cytoplasmic aggregates in which SEPT2 and GFP colocalized in ∼50% of transfected cells (data not shown), similar to that previously reported (Joberty et al., 2001), but for all of these studies we limited our analysis to those cells exhibiting a clear cytoplasmic aggregation of GFP. Transfected cells were exposed to opsonized latex beads, allowed to phagocytose at 37°C for 30 min, and unbound beads were washed away. After fixation without permeabilization, the cells were stained with anti-human IgG antibodies to detect external particles. As can be seen in Figure 5a, cells overexpressing GFP-BD3 (arrowhead) were unable to phagocytose particles, whereas those transfected with GFP alone (Figure 5b, arrowhead) were fully able to internalize numerous beads. To ensure that this was not an artifact of the CHO-IIA model system, we repeated the same experiments in RAW 264.7 cells. As shown in Figure 5c, transfected RAW 264.7 cells (arrowhead) were unable to ingest bound particles (open arrow), whereas neighboring untransfected cells phagocytosed particles completely (filled arrow). Again, control GFP-transfected cells, like their untransfected neighbors, were fully capable of robust phagocytosis (Figure 5d).
Figure 5.
Effects of BD3 and RNAi on endogenous SEPT2 and SEPT11. CHO-IIA cells (a and b) or RAW cells (c and d) were either transfected with GFP-BD3 (a and c) or with GFP as control (b and d) for 36 h. The cells were allowed to ingest IgG opsonized beads for 45 or 30 min at 37°C for CHO-IIA cells or RAW cells, respectively. External beads were stained with Cy5-donkey anti-human IgG for 15 min on ice before immunostaining of the endogenous SEPT2. CHO-IIA cells were transfected with SEPT2 shRNA (e) or with scramble shRNA as control (f) for 84 h. The cells were allowed to ingest IgG opsonized beads for 45 min at 37°C. External beads and endogenous SEPT2 were immunostained as above. Panels from left to right are GFP constructs, endogenous SEPT2, external beads and differential interference contrast (DIC). Open arrows, unsealed phagosomes; solid arrows, sealed phagosomes. Arrowheads point to the transfected cells. (g) As in panel a, CHO-IIA cells were transfected with GFP-BD3 and in this case were stained for SEPT11. (h and i) siRNA specific for SEPT11 (SEPT11 siRNA2) or a scrambled control sequence were transfected into HeLa cells 72 h before the phagocytosis assay, and FcγIIA-GFP was transfected 1 d before the phagocytosis assay. In e and h, transfected cells are also indicated by white dotted lines. Bars, 5 μm.
Although Borg3 is known to bind and influence septin organization in cells, it is not known how it does this or is it certain if it is specific for septins. We therefore sought an additional approach to inhibit septin function and used RNAi to deplete SEPT2 levels in phagocytic cells. To do so, we used a plasmid-based shRNA approach in which a previously characterized sequence was used to deplete SEPT2 (Kinoshita et al., 2002). In cultures transfected with the SEPT2 shRNA vector containing a GFP reporter ∼70% of the cells were effectively transfected, and by Western blotting we see an ∼50% decrease in SEPT2 levels (Figure 6d). In SEPT2-shRNA–transfected cells (in Figure 5e, GFP-positive cells, indicated with a white dotted line) there were few internalized beads and many more that could be detected by antibodies in nonpermeabilized cells (open arrow). In contrast, neighboring GFP-negative cells were capable of ingesting particles efficiently (filled arrows). Transfection of the vector containing a scrambled sequence had no effect on phagocytosis efficiency (Figure 5f). Similar results were obtained when RAW 264.7 cells were used, although the low efficiency of transfection precluded their quantification.
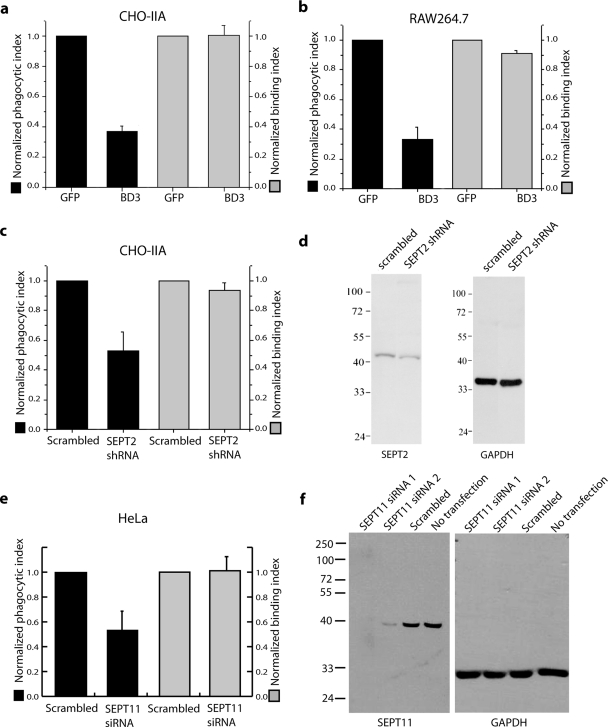
Figure 6.
BD3 or Sept2 shRNA inhibit Fcγ-IIA receptor mediated phagocytosis. (a and b) Quantification of phagocytic and binding properties in cells transfected with either GFP (control) or with GFP-BD3. Phagocytosis was processed at 37°C for 45 min for CHO-IIA cells (a) and 30 min for RAW cells (b). (c) Quantification of phagocytic and binding properties in CHO-IIA cells transfected with either scramble (control) or SEPT2 shRNA. External and internalized beads were discerned by staining external beads with cy5-donkey anti-human IgG staining for 15 min on ice before permeabilization and immunostaining of the endogenous SEPT2 and internal beads. Phagocytic index and binding index were normalized to control. Data are mean ± SE of at least three independent experiments with at least 50 cells being counted in each case; *p < 0.05 and **p < 0.01. (d) Lysates from CHO-IIA cells transfected with scramble DNA or SEPT2 shRNA were run in 12% SDS-PAGE. The blots were probed with either SEPT2 antibody (left) or GAPDH antibody as loading control (right). (e) Quantification of phagocytosis in HeLa cells depleted of SEPT11. Experiments were performed as in c except that SEPT11 siRNA2 was transfected for 3 d before phagocytic assay and FcγIIA-GFP was transfected in the same culture 1 d before assay. GFP-positive cells were counted for presence of internal and total beads. Similar results were obtained with SEPT11 siRNA1. (f) Lysates from HeLa cells transfected with SEPT11 siRNA1 or 2 or a scrambled control were run in 12% SDS-PAGE. The blots were probed with either SEPT11 antibody (left) or GAPDH antibody as loading control (right).
Analogous experiments were also performed for SEPT11. In the first study we transiently transfected the FcγIIA receptor along with GFP-BD3 into CHOIIA cells and found that, like SEPT2, SEPT11 accumulates in the BD3 aggregate and phagocytosis is inhibited in these cells (Figure 5g). This inhibition of phagocytosis could be due solely to the depletion of SEPT2, which is also found in the aggregate, so to determine if SEPT11 is also required for phagocytosis we set out to deplete it by siRNA. Unfortunately, the sequence of SEPT11 in Chinese hamster is not known, and available rodent SEPT11 siRNA sequences did not appear to work in this cell line (data not shown). However, HeLa cells, like CHO cells, become phagocytic when forced to express the FcγIIA receptor. We therefore tested two commercial siRNA reagents specific for distinct target sequences and found that they were efficient at depletion of human SEPT11 protein from HeLa cells (Figure 6f). We therefore transfected HeLa cells with siRNA specific for human SEPT11, subsequently transiently transfected a GFP-tagged version of the FcγIIA receptor, and measured phagocytosis in the GFP-positive cells. As can be seen in Figure 5h, FcγIIA receptor-GFP-positive cells, which were depleted of SEPT11, failed to internalize beads, in contrast to the negative control siRNA. The same results were obtained with both siRNA sequences (not shown).
The results of three independent experiments were quantified and are shown in Figure 6. As can be seen in Figure 6, a–c and e, overexpression of Borg3 GFP-BD3 or shRNA depletion of SEPT2 and siRNA depletion of SEPT11 resulted in a significant decrease of between 50 and 70% of the phagocytosis without affecting the ability of the particles to bind to the phagocytic cells, for both the engineered phagocytic CHO-IIA and HeLa cells and the macrophage cell line RAW 264.7.
SEPT2 Depletion Does Not Affect Actin Accumulation
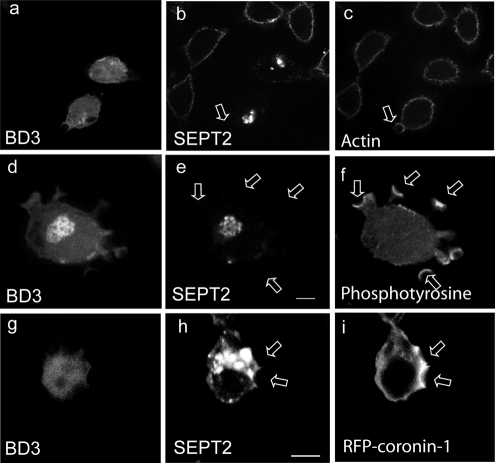
Septins and actin are closely associated in cells (Kinoshita et al., 1997; Xie et al., 1999) and functional interdependence has been suggested to exist between them. Specifically, depletion of septins results in loss of stress fibers, suggesting that they stabilize actin filaments, and actin filaments appear to serve as templates for septin filament assembly (Kinoshita et al., 2002; Schmidt and Nichols, 2004). We therefore examined the effects of septin inhibition on actin accumulation at the nascent phagosome. As shown in Figure 7, a–c, in CHO-IIA cells expressing GFP-BD3 (Figure 7a), when particles bind to these cells there is robust accumulation of actin (arrows, Figure 7c) despite the failure to detect SEPT2 accumulation (Figure 7b). Similar observations are made with phosphotyrosine accumulation (Figure 7, d–f). This indicates that although septins may influence actin stability to some extent, they are not required for the accumulation of F-actin at the phagocytic cup.
Figure 7.
Effects of BD3 on part of the proteins related to Fc receptor signal pathway of CHO-IIA cells. CHO-IIA cells transfected with GFP-BD3 (a, d, and g) or cotransfected with GFP-BD3 and RFP-coronin (i) were allowed to undergo for phagocytosis for 10 min before fixation. Endogenous SEPT2 was immunostained by SEPT2 antibody (b, e, and h), actin by Rhodamine-phalloidin (c) and phosphotyrosine by mouse anti-phosphotyrosine mAb (f). Open arrows, polystyrene beads at unsealed phagocytic cups; Bars, 5 μm.
We have previously shown that coronin-1 is an actin-associated protein necessary for phagosome formation, and one of its functions may be to recruit Arp2/3 to the nascent phagosome (Yan et al., 2005). We therefore examined the accumulation of coronin-1 by transient transfection of RFP-coronin-1 in CHO-IIA cells expressing GFP-BD3. As seen in Figure 7, g–i, cells exhibiting aggregates of SEPT2 (Figure 7h) still had bound particles to which RFP-coronin-1 was recruited (Figure 7i, arrows). Hence, both the accumulation of F-actin and the recruitment of actin assembly factors appear to occur normally in the absence of functional septins.
DISCUSSION
Septins are filamentous GTPases conserved from yeast to man that have been implicated in a variety of cellular processes including cytokinesis, signal transduction, and membrane trafficking. It has also been speculated that they could function as membrane diffusion barriers. Given their many possible functions, we investigated their role in phagocytosis. We have shown here that phagocytic cells express a variety of septins, including septins 2, 6, 10, and 11. Using specific reagents to SEPT2 and SEPT11, we showed that these proteins transiently accumulated on phagosomes produced by murine macrophage cell lines, primary human neutrophils, and even in a CHO cell line engineered to be phagocytic. The time course of septin accumulation at phagosomes was consistent with that of PIP2 and actin. Inhibition of septins, either by causing their aggregation by overexpression of the BD3 domain of Borg3 (Joberty et al., 2001) or by depletion of SEPT2 or SEPT11 by RNAi, decreased the efficiency of phagocytosis between 50 and 70%. This occurred despite the apparently normal recruitment of actin and actin-associated proteins to the nascent phagosome. Moreover, using a phagocytosis-competent cell line, CHO-IIA and transiently transfected HeLa cells, we were able to show that SEPT2 and SEPT11 are involved in the signaling events initiated by a single class of immunoglobulin receptors, FcγRIIA.
The mechanisms recruiting septins to the phagosome are not yet clear, but we and others have previously shown that septins are capable of binding to phosphatidylinositol lipids (Zhang et al., 1999; Casamayor and Snyder, 2003; Rodriguez-Escudero et al., 2005). Septins possess a conserved polybasic region adjacent to the GTPase domain that appears to be responsible for this interaction (Zhang et al., 1999). In light of the importance of PIP2 production (Coppolino et al., 2002; Defacque et al., 2002) and phosphoinositide 3-kinase signaling (Araki et al., 1996) to FcγR-mediated phagocytosis, it is possible that one of roles of these lipids is in recruitment of the septins. Alternatively, others have shown that septins can be recruited to actin bundles by adaptor proteins such as anillin (Kinoshita et al., 2002) and myosin (Joo et al., 2007), raising the possibility that the accumulation of septins at the phagosome may be due to their assembly on actin-rich structures. However, anillin resides in the nucleus of interphase cells and would not be anticipated to be available as a linker. Hence, if septins are recruited to the phagosome by the presence of actin bundles, other scaffolding proteins such as myosin may be necessary to provide the linkage.
The exact role of septins in phagocytosis is unclear, but it does not appear to involve the recruitment or assembly of actin filaments in the vicinity of the nascent phagosome. This is somewhat surprising because mutations in yeast septins have been shown to affect the organization of filamentous actin (Adams and Pringle, 1984). In addition, depletion of septins in mammalian cells results in a reduction of actin stress fibers (Kinoshita et al., 2002; Joo et al., 2007), suggesting that septins may play a role in stabilizing these structures. However, we observed significant actin accumulation at the site of FcγRIIA receptor engagement, even in the absence of SEPT2 accumulation. In the case of SEPT2 or SEPT11 RNAi treatment, this could occur if other septins provided redundant roles in this process, but in cells transfected with GFP-BD3, all of the septins in the cell are aggregated together and therefore unavailable (unpublished observations and Joberty et al., 2001).
Septins have also been implicated in regulating the stability of the microtubule network by binding to the microtubule-stabilizing protein MAP4 (Kremer et al., 2005), and microtubule dynamics have been implicated in phagocytosis (Araki, 2006; Khandani et al., 2007). However, we were unable to see changes in microtubule clustering, MTOC reorientation toward the phagosome, or changes in the levels of acetylated tubulin following SEPT2 knockdown or BD3 transfection (data not shown), as would be expected if septins regulated microtubule dynamics during this process. Another possible role of septins in phagocytosis could stem from their ability to anchor multiprotein complexes, as they do at the yeast bud neck during cytokinesis (Gladfelter et al., 2001). In mammalian cells the recruitment of filamentous septins may aid in recruiting other associated multiprotein complexes (Joo et al., 2007) to forming phagosomes on the plasma membrane and disruption of filaments would diminish the efficiency of the phagosomal machinery of the cell. Evidence suggests that mammalian septins also participate in vesicle trafficking (Hsu et al., 1998; Beites et al., 2001), a process important for efficient phagosome formation (Bajno et al., 2000; Niedergang et al., 2003). It will therefore be interesting to determine whether depletion of septins in phagocytic cells inhibits membrane/vesicle trafficking during FcγR mediated phagocytosis.
ACKNOWLEDGMENTS
The authors thank Dr. Alan Schreiber for kindly providing the CHO-IIA cell line and Dr. Ian Macara for GFP-BD3 plasmid. The authors also thank Dr. Jeffrey Howard for intellectual contributions made early in the project. This work was supported by a grant from the Canadian Institutes of Health Research to W.S.T. and S.G. S.G. holds the Pitblado Chair in Cell Biology at HSC, and W.S.T. is the recipient of a Canada Research Chair in Molecular Cell Biology.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-07-0641) on February 13, 2008.
REFERENCES
- Adams A. E., Pringle J. R. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J. Cell Biol. 1984;98:934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki N., Johnson M. T., Swanson J. A. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki N. Role of microtubules and myosins in Fc gamma receptor-mediated phagocytosis. Front. Biosci. 2006;11:1479–1490. doi: 10.2741/1897. [DOI] [PubMed] [Google Scholar]
- Bajno L., Peng X. R., Schreiber A. D., Moore H. P., Trimble W. S., Grinstein S. Focal exocytosis of VAMP3-containing vesicles at sites of phagosome formation. J. Cell Biol. 2000;149:697–706. doi: 10.1083/jcb.149.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beites C. L., Peng X. R., Trimble W. S. Expression and analysis of properties of septin CDCrel-1 in exocytosis. Methods Enzymol. 2001;329:499–510. doi: 10.1016/s0076-6879(01)29111-3. [DOI] [PubMed] [Google Scholar]
- Beites C. L., Xie H., Bowser R., Trimble W. S. The septin CDCrel-1 binds syntaxin and inhibits exocytosis. Nat. Neurosci. 1999;2:434–439. doi: 10.1038/8100. [DOI] [PubMed] [Google Scholar]
- Botelho R. J., Teruel M., Dierckman R., Anderson R., Wells A., York J. D., Meyer T., Grinstein S. Localized biphasic changes in phosphatidylinositol-4,5-bisphosphate at sites of phagocytosis. J. Cell Biol. 2000;151:1353–1368. doi: 10.1083/jcb.151.7.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamayor A., Snyder M. Molecular dissection of a yeast septin: distinct domains are required for septin interaction, localization, and function. Mol. Cell. Biol. 2003;23:2762–2777. doi: 10.1128/MCB.23.8.2762-2777.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppolino M. G., Dierckman R., Loijens J., Collins R. F., Pouladi M., Jongstra-Bilen J., Schreiber A. D., Trimble W. S., Anderson R., Grinstein S. Inhibition of phosphatidylinositol-4-phosphate 5-kinase Ialpha impairs localized actin remodeling and suppresses phagocytosis. J. Biol. Chem. 2002;277:43849–43857. doi: 10.1074/jbc.M209046200. [DOI] [PubMed] [Google Scholar]
- Cox D., Tseng C. C., Bjekic G., Greenberg S. A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J. Biol. Chem. 1999;274:1240–1247. doi: 10.1074/jbc.274.3.1240. [DOI] [PubMed] [Google Scholar]
- Crowley M. T., Costello P. S., Fitzer-Attas C. J., Turner M., Meng F., Lowell C., Tybulewicz V. L., DeFranco A. L. A critical role for Syk in signal transduction and phagocytosis mediated by Fcgamma receptors on macrophages. J. Exp. Med. 1997;186:1027–1039. doi: 10.1084/jem.186.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defacque H., Bos E., Garvalov B., Barret C., Roy C., Mangeat P., Shin H. W., Rybin V., Griffiths G. Phosphoinositides regulate membrane-dependent actin assembly by latex bead phagosomes. Mol. Biol. Cell. 2002;13:1190–1202. doi: 10.1091/mbc.01-06-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarini D. J., Adams A. E., Fares H., De Virgilio C., Valle G., Chuang J. S., Pringle J. R. A septin-based hierarchy of proteins required for localized deposition of chitin in the Saccharomyces cerevisiae cell wall. J. Cell Biol. 1997;139:75–93. doi: 10.1083/jcb.139.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares H., Goetsch L., Pringle J. R. Identification of a developmentally regulated septin and involvement of the septins in spore formation in Saccharomyces cerevisiae. J. Cell Biol. 1996;132:399–411. doi: 10.1083/jcb.132.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares H., Peifer M., Pringle J. R. Localization and possible functions of Drosophila septins. Mol. Biol. Cell. 1995;6:1843–1859. doi: 10.1091/mbc.6.12.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladfelter A. S., Pringle J. R., Lew D. J. The septin cortex at the yeast mother-bud neck. Curr. Opin. Microbiol. 2001;4:681–689. doi: 10.1016/s1369-5274(01)00269-7. [DOI] [PubMed] [Google Scholar]
- Greenberg S., Burridge K., Silverstein S. C. Colocalization of F-actin and talin during Fc receptor-mediated phagocytosis in mouse macrophages. J. Exp. Med. 1990;172:1853–1856. doi: 10.1084/jem.172.6.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S., Chang P., Silverstein S. C. Tyrosine phosphorylation is required for Fc receptor-mediated phagocytosis in mouse macrophages. J. Exp. Med. 1993;177:529–534. doi: 10.1084/jem.177.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S., Chang P., Silverstein S. C. Tyrosine phosphorylation of the gamma subunit of Fc gamma receptors, p72syk, and paxillin during Fc receptor-mediated phagocytosis in macrophages. J. Biol. Chem. 1994;269:3897–3902. [PubMed] [Google Scholar]
- Greenberg S., Chang P., Wang D. C., Xavier R., Seed B. Clustered syk tyrosine kinase domains trigger phagocytosis. Proc. Natl. Acad. Sci. USA. 1996;93:1103–1107. doi: 10.1073/pnas.93.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackam D. J., Rotstein O. D., Schreiber A., Zhang W., Grinstein S. Rho is required for the initiation of calcium signaling and phagocytosis by Fcgamma receptors in macrophages. J. Exp. Med. 1997;186:955–966. doi: 10.1084/jem.186.6.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. C., Hazuka C. D., Roth R., Foletti D. L., Heuser J., Scheller R. H. Subunit composition, protein interactions, and structures of the mammalian brain sec6/8 complex and septin filaments. Neuron. 1998;20:1111–1122. doi: 10.1016/s0896-6273(00)80493-6. [DOI] [PubMed] [Google Scholar]
- Indik Z. K., Park J. G., Hunter S., Schreiber A. D. The molecular dissection of Fc gamma receptor mediated phagocytosis. Blood. 1995a;86:4389–4399. [PubMed] [Google Scholar]
- Indik Z. K., Park J. G., Pan X. Q., Schreiber A. D. Induction of phagocytosis by a protein tyrosine kinase. Blood. 1995b;85:1175–1180. [PubMed] [Google Scholar]
- Joberty G., Perlungher R. R., Sheffield P. J., Kinoshita M., Noda M., Haystead T., Macara I. G. Borg proteins control septin organization and are negatively regulated by Cdc42. Nat. Cell Biol. 2001;3:861–866. doi: 10.1038/ncb1001-861. [DOI] [PubMed] [Google Scholar]
- Joo E., Surka M. C., Trimble W. S. Mammalian SEPT2 is required for scaffolding nonmuscle myosin II and its kinases. Dev. Cell. 2007;13:677–690. doi: 10.1016/j.devcel.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Joo E., Tsang C. W., Trimble W. S. Septins: traffic control at the cytokinesis intersection. Traffic. 2005;6:626–634. doi: 10.1111/j.1600-0854.2005.00305.x. [DOI] [PubMed] [Google Scholar]
- Khandani A., Eng E., Jongstra-Bilen J., Schreiber A. D., Douda D., Samavarchi-Tehrani P., Harrison R. E. Microtubules regulate PI-3K activity and recruitment to the phagocytic cup during Fcgamma receptor-mediated phagocytosis in nonelicitied macrophages. J. Leukoc. Biol. 2007;82:417–428. doi: 10.1189/jlb.0706469. [DOI] [PubMed] [Google Scholar]
- Kiefer F., Brumell J., Al-Alawi N., Latour S., Cheng A., Veillette A., Grinstein S., Pawson T. The Syk protein tyrosine kinase is essential for Fcgamma receptor signaling in macrophages and neutrophils. Mol. Cell. Biol. 1998;18:4209–4220. doi: 10.1128/mcb.18.7.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M., Field C. M., Coughlin M. L., Straight A. F., Mitchison T. J. Self- and actin-templated assembly of mammalian septins. Dev. Cell. 2002;3:791–802. doi: 10.1016/s1534-5807(02)00366-0. [DOI] [PubMed] [Google Scholar]
- Kinoshita M., Kumar S., Mizoguchi A., Ide C., Kinoshita A., Haraguchi T., Hiraoka Y., Noda M. Nedd5, a mammalian septin, is a novel cytoskeletal component interacting with actin-based structures. Genes Dev. 1997;11:1535–1547. doi: 10.1101/gad.11.12.1535. [DOI] [PubMed] [Google Scholar]
- Kolotila M. P., Diamond R. D. Stimulation of neutrophil actin polymerization and degranulation by opsonized and unopsonized Candida albicans hyphae and zymosan. Infect. Immun. 1988;56:2016–2022. doi: 10.1128/iai.56.8.2016-2022.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer B. E., Haystead T., Macara I. G. Mammalian septins regulate microtubule stability through interaction with the microtubule binding protein MAP4. Mol. Biol. Cell. 2005;16:4648–4659. doi: 10.1091/mbc.E05-03-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine M. S., Bi E. Regulation of septin organization and function in yeast. Trends Cell Biol. 2003;13:403–409. doi: 10.1016/s0962-8924(03)00151-x. [DOI] [PubMed] [Google Scholar]
- Neufeld T. P., Rubin G. M. The Drosophila peanut gene is required for cytokinesis and encodes a protein similar to yeast putative bud neck filament proteins. Cell. 1994;77:371–379. doi: 10.1016/0092-8674(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Niedergang F., Colucci-Guyon E., Dubois T., Raposo G., Chavrier P. ADP ribosylation factor 6 is activated and controls membrane delivery during phagocytosis in macrophages. J. Cell Biol. 2003;161:1143–1150. doi: 10.1083/jcb.200210069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch J. V., Kinet J. P. Fc receptors. Annu. Rev. Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Escudero I., Roelants F. M., Thorner J., Nombela C., Molina M., Cid V. J. Reconstitution of the mammalian PI3K/PTEN/Akt pathway in yeast. Biochem. J. 2005;390:613–623. doi: 10.1042/BJ20050574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K, Nichols B. J. Functional interdependence between septin and actin cytoskeleton. BMC Cell Biol. 2004;5:43. doi: 10.1186/1471-2121-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott C. C., Dobson W., Botelho R. J., Coady-Osberg N., Chavrier P., Knecht D. A., Heath C., Stahl P., Grinstein S. Phosphatidylinositol-4,5-bisphosphate hydrolysis directs actin remodeling during phagocytosis. J. Cell Biol. 2005;169:139–149. doi: 10.1083/jcb.200412162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steels J. D., Estey M. P., Froese C. D., Renaud D., Pace-Asciak C, Trimble W. S. Septin 12 is a component of the sperm annulus. Cell Motil. Cytoskelet. 2007;64:794–807. doi: 10.1002/cm.20224. [DOI] [PubMed] [Google Scholar]
- Surka M. C., Tsang C. W., Trimble W. S. The mammalian septin MSF localizes with microtubules and is required for completion of cytokinesis. Mol. Biol. Cell. 2002;13:3532–3545. doi: 10.1091/mbc.E02-01-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira O. V., Bucci C., Harrison R. E., Trimble W. S., Lanzetti L., Gruenberg J., Schreiber A. D., Stahl P. D., Grinstein S. Modulation of Rab5 and Rab7 recruitment to phagosomes by phosphatidylinositol 3-kinase. Mol. Cell. Biol. 2003;23:2501–2514. doi: 10.1128/MCB.23.7.2501-2514.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H., Howard J., Surka M., Trimble W. Characterization of the mammalian septin H5: distinct patterns of cytoskeletal and membrane association from other mammalian septins. Cell Motil. Cytoskelet. 1999;43:52–62. doi: 10.1002/(SICI)1097-0169(1999)43:1<52::AID-CM6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Yan M., Collins R. F., Grinstein S., Trimble W. S. Coronin-1 function is required for phagosome formation. Mol. Biol. Cell. 2005;16:3077–3087. doi: 10.1091/mbc.E04-11-0989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Kong C., Xie H., McPherson P. S., Grinstein S., Trimble W. S. Phosphatidylinositol polyphosphate binding to the mammalian septin H5 is modulated by GTP. Curr. Biol. 1999;9:1458–1467. doi: 10.1016/s0960-9822(00)80115-3. [DOI] [PubMed] [Google Scholar]