Abstract
Varicella Zoster Virus (VZV) is the causative agent for both varicella (chicken pox) and herpes zoster (shingles). As a member of the human herpesvirus family, VZV contains a large DNA genome, encoding 70 unique open reading frames (ORFs). The functions of the majority of these ORFs remain unknown. Recently, the full-length VZV (P-Oka strain) genome was cloned as a VZV bacteria artificial chromosome (BAC) and additionally a firefly luciferase cassette was inserted to generate a novel luciferase VZV BAC. In this study, a highly efficient protocol has been developed exploiting the new luciferase VZV BAC system to identify rapidly and isolate VZV ORF deletion mutants by growth curve analysis in cell culture.
Keywords: Varicella Zoster virus, Bacterial artificial chromosome, Deletion mutagenesis, Bioluminescence
1. Introduction
Varicella Zoster Virus (VZV) is a human alpha-herpesvirus. More than 90% of the US population is infected with VZV (Abendroth and Arvin, 1999). Primary infection of VZV causes varicella (chicken pox) usually during childhood, and reactivation of VZV infection later in life leads to herpes zoster (shingles). VZV has a 125kb-long DNA genome, which encodes at least 70 unique open reading frames (ORFs). However, the functions of a majority of these ORFs remain uncharacterized.
The prevailing method in VZV genetic studies involves a four-cosmid system containing the entire viral genome (Cohen et al., 1993; Niizuma et al., 2003). Using the cosmid system to generate recombinant VZV variants inevitably requires several technically challenging steps, including co-transfection of four large cosmids into permissive mammalian cells and multiple homologous recombination events within a single mammalian cell to create the full-length viral genome. Additionally, VZV has a narrow host range and is highly cell-associated in vitro, which makes it the most difficult to mutagenize of the alpha-herpesviruses (Cohen et al., 2007).
To solve this problem, the full-length VZV (P-Oka strain, a cloned clinical isolate of VZV) genome has been cloned as a VZV bacteria artificial chromosome (BAC) (Nagaike et al., 2004, Zhang et al., 2007). Recently, in our laboratory a firefly luciferase cassette was inserted into the VZV BAC to generate a novel luciferase-expressing VZV (Zhang et al., 2007). A highly efficient protocol is reported in this study for generating VZV ORF deletion mutants and carrying out subsequent growth kinetic studies in cultured cells.
2. Materials and methods
2.1. Cells, VZVluc plasmids and E. coli strain
Human melanoma (MeWo) cells were grown in DMEM supplemented with 10% fetal bovine serum, 100U penicillin-streptomycin/ml and 2.5μg amphotericin B/ml at 37°C in a humidified incubator with 5% CO2. VZVluc was recently developed in the laboratory (Zhang et al., 2007). It contains a full-length VZV P-Oka genome with a firefly luciferase cassette. This cassette, driven by a SV40 early promoter, was inserted between VZV ORF65 and ORF66. The BAC vector was inserted between VZV ORF60 and ORF61, which includes a green fluorescent protein (GFP) expression cassette and a chloramphenicol resistance cassette (cmR). pGEM-oriV/kan was previously constructed (Wang et al., 2004) and used as a PCR template to generate a kanamycin or ampicillin resistance cassette (KanR and AmpR). pGEM-lox-zeo was derived from pGEM-T (Promega, Madison, WI) (Netterwald et al., 2005), and was used to generate VZV ORF deletion rescue clones. E. coli strain DY380 was obtained from Neal Copeland and Craig Stranthdee and used for mutagenesis. A Cre recombinase expression plasmid pGS403 was a gift from L. Enquist.
2.2. BAC methods
2.2.1. Generating ORF deletion mutants
Primers were synthesized by Sigma-Genosys (Woodlands, TX) and stored in TE buffer (100μM). All primers are listed in Table 1. HotStar Taq DNA polymerase (Qiagen, Valencia, CA) was used for general PCR reactions and Platinum Taq DNA polymerase (Invitrogen, Carlsbad, CA) was used for hi-fidelity PCR, both following standard PCR conditions. PCR purification was carried out using a PCR purification kit (Qiagen, Valencia, CA) following the manufacturer’s protocol. The amplified linear DNAs were suspended in sterile ddH2O and were quantified by spectroscopy (NanoDrop Technologies, Wilmington, DE). In order to achieve optimum results, the final concentration of the linear DNA cassette for the subsequent electroporation was adjusted to 100 ng/μl. DpnI (New England Biolabs, Ipswich, MA) treatment following PCR was carried out in order to eliminate circular template DNA. Electroporation was carried out at 1.6kV, 200ohm and 25μF on a Gene Pulser II electroporator (BioRad, Hercules, CA). Two μl of concentrated linear DNA cassette (>200ng) were used for each reaction.
Table 1.
Primers used in this study are listed in this table
| Primer Name | Primer Sequence (5′-3′) | Use |
|---|---|---|
| ORF0KanF | AACCCGCGCCTTTTGCGTCCACCCCTCGTTTACTGCTCGGGCTCTTGTTGGCTAGTGCGTA | Amplify KanR with ORF0 flanking homologies |
| ORF0KanR | GCAAGCGAGAATAAATACCTTCCCCTTCCGGACAGTAGTTTCTGCCAGTGTTACAACCAA | |
| ORF1KanF | ACTCAACTACATGAAACTACTGTCCGGAAGGGGAAGGTATGCTCTTGTTGGCTAGTGCGTA | Amplify KanR with ORF1 flanking homologies |
| ORF1KanR | ATAAGCAAATCCTGTTAATATTATATTTTTGGGATCCGCATCTGCCAGTGTTACAACCAA | |
| ORF2KanF | TAATAGCTATTATCGTAACCCACCCCCGTAAAATCATAAAGCTCTTGTTGGCTAGTGCGTA | Amplify KanR with ORF2 flanking homologies |
| ORF2KanR | AAATACGTACAATCGAAAAAAGGTGTATTTTATTTAGTGATCTGCCAGTGTTACAACCAA | |
| ORF3KanF | CTTTTTTCGATTGTACGTATTTTTATTTAAATGTGTAGTTGCTCTTGTTGGCTAGTGCGTA | Amplify KanR with ORF3 flanking homologies |
| ORF3KanR | GGGTAAAACACACACCAGACGTGTACCGAACGTTTAATTATCTGCCAGTGTTACAACCAA | |
| ORF4KanF | TTAGTATGTTTTGACAAGCATGAAAAAGGTATTTTTTATTGCTCTTGTTGGCTAGTGCGTA | Amplify KanR with ORF4 flanking homologies |
| ORF4KanR | AGGCAACTGCAAACACGCAATTGTCAGATATTTTGCAGCCTCTGCCAGTGTTACAACCAA | |
| ORF5KanF | GGCTCACCCAACCCCGCAATGGGCGTGTTTAGTCACATGAGCTCTTGTTGGCTAGTGCGTA | Amplify KanR with ORF5 flanking homologies |
| ORF5KanR | TGATACTACATCGTGCTTGAATTGCCATCTTCCACGGGTCTCTGCCAGTGTTACAACCAA | |
| ORF6KanF | GATGGCAATTCAAGCACGATGTAGTATCACACGGTTGGTGGCTCTTGTTGGCTAGTGCGTA | Amplify KanR with ORF6 flanking homologies |
| ORF6KanR | ACCGTCTGCATGATTGACTGGCTTTCCAACGTATTGAACTTCTGCCAGTGTTACAACCAA | |
| ORF7KanF | GATTTATCCATAGTTCAATACGTTGGAAAGCCAGTCAATCGCTCTTGTTGGCTAGTGCGTA | Amplify KanR with ORF7 flanking homologies |
| ORF7KanR | AAACATACACCAGAAACGTTTTTAGTTTTTATTTCAATATTCTGCCAGTGTTACAACCAA | |
| ORF8/9AKanF | TATAAAATTAAAACATTGCTGGCTGGCGTGGTTATTACATGCTCTTGTTGGCTAGTGCGTA | Amplify KanR with ORF8/9A flanking homologies |
| ORF8/9AKanR | CCCTCTTATACACGCCTGCCCCTTTTATAGGCAAACGGGTTCTGCCAGTGTTACAACCAA | |
| ORF9KanF | CGTGTTTGGATATTTCACGACCCTATCGTTTATTTACGTAGCTCTTGTTGGCTAGTGCGTA | Amplify KanR with ORF9 flanking homologies |
| ORF9KanR | TACATAATACCGGGTAAACCGTTACTGCGTAATTATATCCTCTGCCAGTGTTACAACCAA | |
| ORF10KanF | GGGAATCGCTTATTTAAACTAAAGATTTTACTCTATAAGTGCTCTTGTTGGCTAGTGCGTA | Amplify KanR with ORF10 flanking homologies |
| ORF10KanR | CGTTTTCGTAATTTATTTACACCCTCTACCCCAATGACGTTCTGCCAGTGTTACAACCAA | |
| ORF11KanF | GGATGTTTTACAGGCGCGTTTGTTTGTCTCGGTTATAAGTGCTCTTGTTGGCTAGTGCGTA | Amplify KanR with ORF11 flanking homologies |
| ORF11KanR | GGAAACGTTCTTTTCATCCTAATGAAAAAAATCACAACCCTCTGCCAGTGTTACAACCAA | |
| ORF0F | ATGGCGACCGTGCACTACTC | Amplify ORF0 |
| ORF0R | TCATGTAGTTGAGTTGGGAGGTTC | |
| ORF1F | TTATTCTCGCTTGCAGCTTGTCG | Amplify ORF1 |
| ORF1R | ATGTCCAGGGTATCGGAGTATG | |
| ORF2F | ATGCATGTAATTTCTGAGACACTTGCATA | Amplify ORF2 |
| ORF2R | TTACATCAATACGCCCTCCGTAG | |
| ORF3F | TCATAGTCCGCCGACAGCC | Amplify ORF3 |
| ORF3R | ATGGATACAACGGGAGCTTCC | |
| ORF4F | TTAGCAGTTAAAGGTACTACACTTAAAATATTTA | Amplify ORF4 |
| ORF4R | ATGGCCTCTGCTTCAATTCCAAC | |
| ORF5F | TTAATGCTTCTGGGAGTTTTCACTTTC | Amplify ORF5 |
| ORF5R | ATGCAGGCTTTAGGAATCAAGACAG | |
| ORF6F | TTAACTCGAAGTTAAATTTGGATAATTAGGTA | Amplify ORF6 |
| ORF6R | ATGGATAAATCCTCCAAACCGACGA | |
| ORF7F | ATGCAGACGGTGTGTGCCAG | Amplify ORF7 |
| ORF7R | TTATACAAGCATAACATGGGATTTCTTGAT | |
| ORF8/9AF | TTAATGTTTTAGTAGAAAATCGACATCGTTTG | Amplify ORF8/9A |
| ORF8/9AR | TTACCACGTGCTGCGTAATACAGAA | |
| ORF9F | ATGGCATCTTCCGACGGTGAC | Amplify ORF9 |
| ORF9R | CTATTTTCGCGTATCAGTTCTTGATG | |
| ORF10F | ATGGAGTGTAATTTAGGAACCGAAC | Amplify ORF10 |
| ORF10R | TTAACGCGTTAAAAACCCACAC | |
| ORF11F | ATGCAGTCGGGTCATTATAACCG | Amplify ORF11 |
| ORF11R | TTAATATTTTCGTAGTAAATGCATGGCTAC | |
| ORF12F | ATGTTTTCTCGGTTTGCGCGTTC | Amplify ORF12 |
| ORF12R | TTAATGATGACTCTTAGGCGTATTTTTCC | |
| ORF4SpeIF | AGTCGAACTAGTTTAGCAGTTAAAGGTACTACACTTAAAATATTTA | Directionally clone ORF4 into pGEM-lox-zeo |
| ORF4NdeIR | AGTCGACATATGATGGCCTCTGCTTCAATTCCAAC | |
| ORF4ZeoF | TTAGTATGTTTTGACAAGCATGAAAAAGGTATTTTTTATTGGATGGATCCATAACTTCGT | Amplify ORF4-zeoR cassette |
| ORF4ZeoR | AGGCAACTGCAAACACGCAATTGTCAGATATTTTGCAGCCATGGCCTCTGCTTCAATTCCAAC |
2.2.2. Induction of lambda recombination system and preparation of electroporation-competent DY380
DY380 cells were grown at 32°C until the OD600nm measurement reached 0.5. The culture was shifted to 42°C by placing the flask into a 42°C water bath with vigorous shaking for 10–15 min. The culture was immediately transferred to ice-water slurry for 30 min, then pelleted, washed with ice-cold sterile ddH2O, and repelleted. Ten percent glycerol (1% of original volume of culture) was used to resuspend cells, and a 40μl aliquot (>1 × 1010 cells) was used for each electroporation reaction.
2.2.3. Antibiotics and selection
The bacteria were transferred to 1ml LB medium immediately after electroporation and recovered at 32°C for 1hr before plating. All antibiotics were obtained from Sigma (St. Louis, MO). LB plates containing the following were used for appropriate selections.
For BACs (single or low copy number):
Chloramphenicol (cm): 12.5μg/ml
Hygromycin B (hyg): 50μg/ml
Kanamycin (kan): 30μg/ml
Ampicillin (amp): 50μg/ml
Zeocin (zeo): 50μg/ml
For plasmids (high copy number):
Kanamycin (kan): 50μg/ml
Ampicillin (amp): 100μg/ml
Zeocin (zeo): 100μg/ml
2.2.4. Mini-BAC DNA preparation
A single DY380 clone containing the putative VZV BAC was inoculated in 5ml LB supplemented with the appropriate antibiotics and cultured at 32°C overnight. BAC DNA was isolated by pelleting the bacteria, resuspending in 1ml resuspension buffer supplemented with RNase A (P1), lysing in 1 ml NaOH/SDS lysis buffer (P2), and neutralizing in 1ml potassium acetate neutralization buffer (P3) for 5 min for each step.(Nucleobond Maxiprep BAC DNA isolation kit from Clontech Laboratories Inc., Palo Alto, CA). The cloudy solution was centrifuged at 4500g for 15min at 4°C. The supernatant was filtered through a small piece of Kimwipe (Kimberly-Clark Global Sales, Inc., Roswell, GA). The filtered solution was extracted with an equal volume of phenol/chloroform and the BAC DNA precipitated with two volumes of ethanol. After the final spin at 4500g for 30min at 4°C, the DNA pellet was resuspended in 20μl sterile ddH2O.
2.2.5. Maxi-BAC DNA preparation
The large-scale BAC DNA preparations using the Nucleobond Maxiprep BAC DNA isolation kit (Clontech Laboratories Inc., Palo Alto, CA) started with 500ml of overnight cultures. The final DNA products were resuspended in 250μl sterile ddH2O and quantified by spectroscopy.
2.2.6. HindIII (New England Biolabs, Ipswich, MA) digestion
Three micrograms of BAC DNA from maxi-preparations were digested with 20U of HindIII in a 20μl reaction at 37°C overnight. HindIII digestion patterns were compared by electrophoresis on ethidium bromide stained 0.5% agarose gels.
2.2.7. Transfection
VZV BAC DNAs from Maxi-preparations were transfected into MeWo cells using the FuGene6 transfection kit (Roche, Indianapolis, IN) according to manufacturer’s standard protocol. One and a half micrograms of BAC DNA and 6μl of transfection reagent were used for a single reaction in one well of 6-well tissue culture plates. In order to prevent the precipitation of BAC in solution, 1.5μg BAC DNA were diluted in serum-free medium, and the volume of DNA solution was adjusted to 50μl. The DNA solution was slowly added to the transfection reagent with gentle stirring using pipettor tips. Because of GFP expression from the BAC vector, VZV plaques were usually visually discernable 3–5 days after transfection. As an option, 0.5 μg of Cre expression plasmid was added during transfection to remove the BAC vector and zeocin marker from the viral genome.
2.3. Virus Assays
2.3.1. Titering
Recombinant viruses were titered by infectious focus assay. MeWo cells were seeded in 6-well tissue culture plates and inoculated with serial dilutions of VZV-infected MeWo cell suspensions. Plaques were counted by fluorescent microscopy at 3 days after inoculation.
2.3.2. Growth curve analyses
Growth curve analyses were carried out by two different methods—a conventional and a new bioluminescent method. The conventional method consisted of infecting MeWo cells with 100 PFU of infected MeWo cells that were seeded in 6-well tissue culture plates. After every 24 hrs, three wells of cells were collected and titered by infectious focus assay described above. The number of plaques from each day was averaged to generate a growth curve (Fig. 4C. black line).
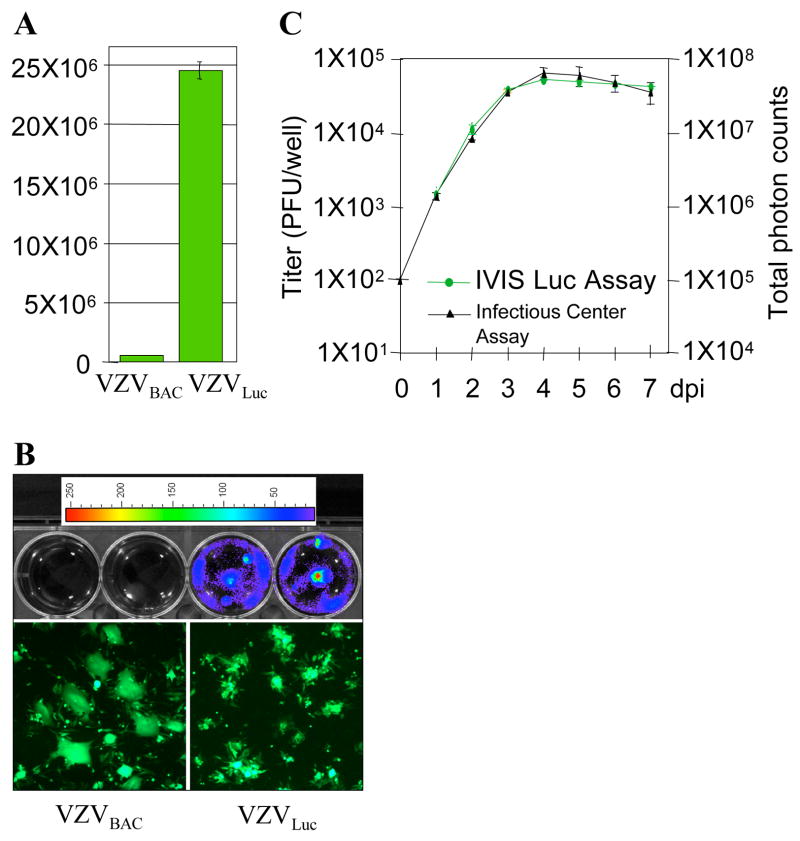
4. Analyzing luciferase VZV growth kinetics based on bioluminescence quantification.
A. Luciferase assay. MeWo cells were infected with VZVLuc and VZVBAC for two days. The cells infected with VZVluc showed a high level of luciferase activity while its parental VZVBAC had no activity. B. Bioluminescence detection. Two six-well MeWo cells were infected with VZVBAC (2 upper left wells) and the other two wells were infected with VZVLuc (2 upper right wells). Two days after infection, D-luciferin was applied to each well and bioluminescence was measured using the IVIS. Bioluminescence signals were detected only from VZVLuc-infected cells. The signal intensities were represented as pseudo colors as shown by an intensity scale bar on the top. Higher intensity is represented by a warmer color. The infection of each well was visualized by fluorescent microscopy due to EGFP expression (bottom panel). C. Correlation of luminescence and plaque numbers. The growth curve generated by infectious center assays (black curve) was compared with the growth curve generated by bioluminescence assays using IVIS (green curve).
The new method is based on live-cell bioluminescence. MeWo cells were infected with 100 PFU of infected MeWo cell suspensions on 6-well tissue culture plates. After every 24 hrs, cell culture media was replaced with media containing 150μg/ml D-luciferin (Xenogen, Alameda, CA). After incubation at 37°C for 10 min, the bioluminescent signals were quantified and recorded using an IVIS Imaging System (Xenogen) following the manufacturer’s instructions. The conditioned cell culture media (harvested before the luciferin addition) was returned to the same plate for further incubation. Measurements from the same plate were repeated every day for 7 days. Bioluminescence signal data from each sample was quantified by manually demarcating regions of interest and analyzed using LivingImage analysis software (Xenogen).
3. Results and discussion
3.1. Generation of VZV ORF deletion mutants
In order to test this new VZVluc system for genetic studies, 12 single ORF deletion mutants from ORF0 to ORF11 were generated. We took advantage of an efficient recombination system for chromosome engineering in E. coli DY380 strain (Yu et al., 2000). A defective lambda prophage supplies the function that protects and recombines linear DNA. This system is highly efficient and allows recombination between homologies as short as 40bp.
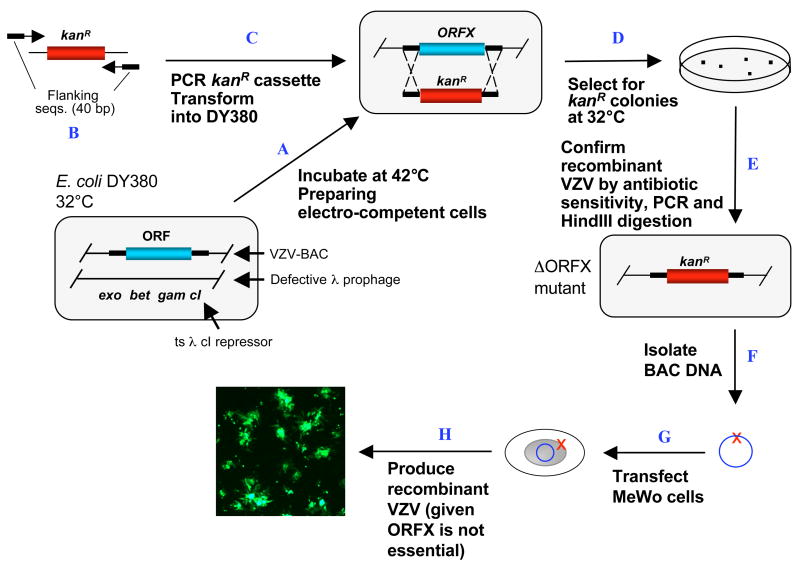
The first step in making each VZV ORF deletion was to amplify the KanR cassette containing 40-bp flanking sequences of the target ORF (Fig. 1). The KanR cassette was amplified from pGEM-oriV/Kan using a HotStar DNA polymerase kit. DpnI treatment was applied to PCR products in order to eliminate the template DNA, which greatly reduced the background in later selections. PCR products were purified and resuspended in water (>100ng/μl).
1. Generating ORF deletion mutants (ORFD).
A. The E. coli DY380 strain provides a highly efficient homologous recombination system, which allows recombination of homologous sequences as short as 40bp. The homologous recombination system is strictly regulated by a temperature sensitive repressor, which permits transient switching on by incubation at 42°C for 15min. VZVluc BAC DNA is introduced into DY380 by electroporation. Electro-competent cells are prepared with homologous recombination system activation. B. Amplification of the KanR expression cassette by PCR using a primer pair adding 40-bp homologies flanking ORFX. C. About 200ng of above PCR product are transformed into DY380 carrying the VZVluc BAC via electroporation. D. Homologous recombination between upstream and downstream homologies of ORFX replaces ORFX with the KanR cassette, creating the ORFX deletion VZV clone. The recombinants are selected on LB agar plates containing kanamycin at 32°C. E. The deletion of ORFX is confirmed by testing antibiotic sensitivity and PCR analysis. The integrity of viral genome after homologous recombination is examined by restriction enzyme digestion. F. VZVluc BAC DNA with ORFX deletion is propagated and isolated from DY380. G. Purified BAC DNA is transfected into MeWo cells. H. 3–5 days after transfection, the ORFX deletion virus is visualized under a fluorescent microscope due to EGFP expression given non-essentiality of ORFX.
VZVluc DNA was transformed into DY380 strain, which contains a homologous recombination system under strict control of a temperature-dependent repressor. Homologous recombination functions were transiently induced by switching the culturing temperature from 32°C to 42°C for 10 to 15 min when electroporation competent cells were made.
Two microliters of KanR cassette DNA (>200ng) were electroporated into competent DY380 cells harboring the VZVluc BAC. Homologous recombination took place between the 40-bp ORF flanking sequences and the targeted ORF was replaced by the linear KanR cassette creating the expected VZV ORF deletion clones. The resultant recombinants were selected on LB agar plates containing kanamycin at 32°C for 16–24 hours.
The successful VZV ORFs/KanR replacements were verified three ways:
Antibiotic sensitivity: It is important to verify that kanamycin resistant colonies are resistant to kanamycin but not ampicillin because the ampicillin resistant circular pGEM-oriV/KanR was used as the PCR template. All 12 VZV ORF deletion clones (VZV ORF0-11) were resistant to chloramphenicol (BAC), hygromycin (BAC) and kanamycin (VZV ORF replacement cassette), but sensitive to ampicillin (potentially from pGEM-oriV/KanR).
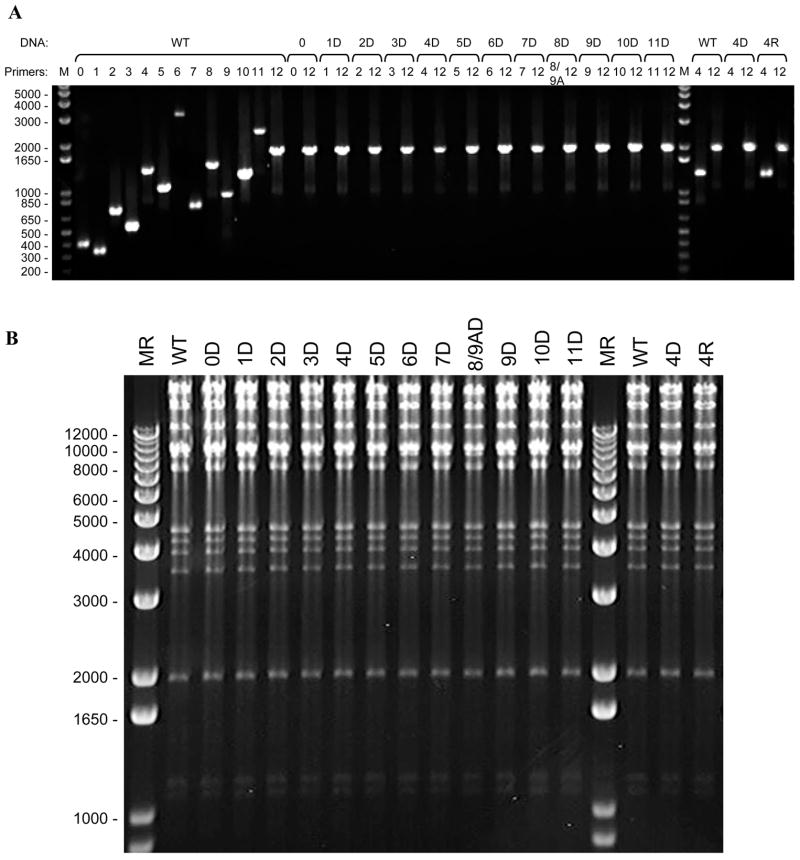
Mini-BAC DNA preparations and PCR verification: Multiple colonies with the correct antibiotic sensitivities were picked for the mini-BAC DNA preparations. The ORF deletions and KanR replacements were confirmed by PCR, as shown in Fig. 2A.
Maxi-BAC DNA preparations and HindIII digestion profiling: PCR verified clones were selected for maxi-BAC DNA preparations. Although it has been shown that VZVluc DNA is highly stable in E. coli (Zhang et al., 2007) under the conditions described in this protocol, large deletions in the BAC clones were observed if the genes for homologous recombination in YD380 were over induced. To confirm that no large VZV genomic DNA segment is deleted, HindIII digestion profiling was routinely carried out. As shown in Fig. 2B, HindIII digestion patterns of each VZV ORF deletion clone were highly comparable with the parental wild type VZVluc clone. Since many large DNA fragments were generated by HindIII digestion, smaller genetic alterations of VZV genome, including replacement of an ORF by a KanR cassette, would be difficult recognized by this assay
2. Confirmation of ORF deletion VZV BAC clones.
A. PCR verification. PCR templates and primer pairs for VZV ORFs are designated above each lane. For each ORF deletion, PCR using such primer pair shows negative result while PCR using ORF12 primer pair serves as a positive control. 5μl of PCR products were analyzed by electrophoresis on ethidium bromide stained 1.0% agarose gels. B. HindIII digestion. Three μg of BAC DNAs from Maxi-preparations were digested with 20U of HindIII in 20μl final volume under the condition of 37°C overnight incubation. HindIII digestion patterns of each ORF deletion clone were compared with parental viral strain by electrophoresis on ethidium bromide stained 0.5% agarose gels.
Verified clones were selected to generate recombinant VZV in MeWo cells using the FuGene transfection reagent. Highly concentrated BAC DNA readily precipitates when added to the transfection reagent. Therefore, we pre-diluted each BAC DNA in media before mixing gently with the transfection reagent. Transfection efficiency was easy to monitor because of the GFP expression from the BACs (Fig. 4B). VZV plaques were detectible after 3–5 days after transfection. If a VZV ORF is essential for viral replication, no plaque will be observed. Since VZV is highly cell-associated in tissue culture, mutant VZV infected MeWo cells were harvested, titered and stored in liquid nitrogen for future studies.
3.2. Generation of VZV ORF deletion revertants
The generation of VZV ORF deletion revertants is necessary to prove the removed DNA is responsible for any growth defect observed in analyses of the deletion mutants. The viral revertants should be able to fully restore the wild type phenotypes. As an example, generating the VZV ORF4 deletion rescue virus is described to demonstrate the approach, below.
VZV ORF4 encodes an immediate-early (IE) regulator protein that transactivates all VZV viral gene expression and enhances the activities of the VZV major transactivator, IE62. It has been shown that VZV ORF4 is essential both for viral replication (Sato et al., 2003) and for establishment of viral latency in a cotton rat model (Cohen et al., 2005). The herpes simplex virus (HSV) homolog of the VZV IE4 protein is ICP27. ICP27 cannot fully complement VZV IE4 function (Cohen et al., 2005). Indeed, VZVluc ORF4 deletion mutants have never been produced after multiple trials of transfection assays in this study.
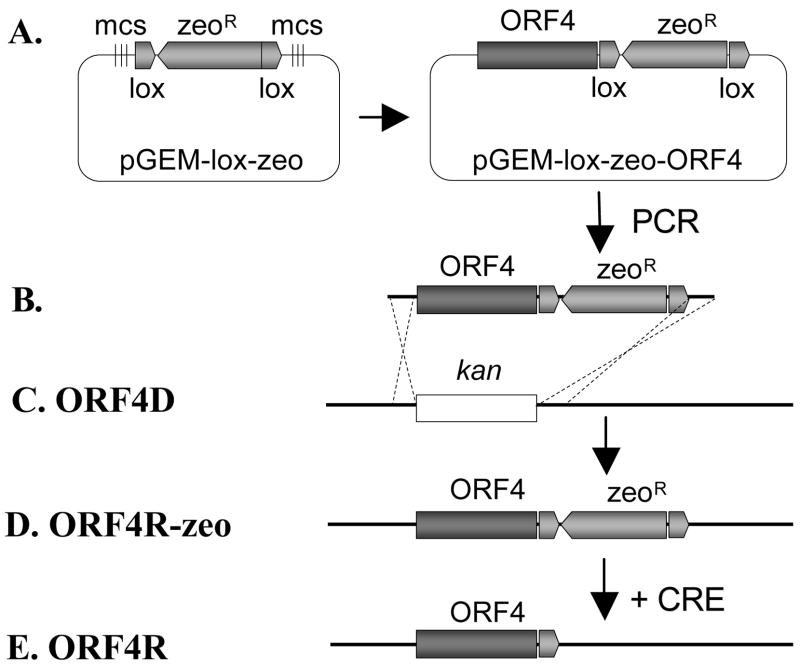
As shown in Fig. 3, a VZV ORF4 deletion rescue virus was generated by using the described homologous recombination system. VZV ORF4 was amplified from wild type VZVluc BAC DNA by PCR. Two unique restriction enzyme sites and two additional 6-bp random sequences were added to the ends of the PCR product. A high fidelity PCR kit was used in order to minimize unwanted mutations during PCRs.
3. Generating ORF4 deletion rescue clone (ORF4R).
A. To generate ORF4R clone, ORF4 was amplified by PCR from the wild type VZV BAC DNA. The ORF4 was directionally cloned into plasmid pGEM-lox-zeo to form pGEM-zeo-ORF4. B. Amplification of the ORF4-ZeoR cassette by PCR using a primer pair adding 40bp homologies flanking ORF4. C. Such PCR product was transformed into DY380 carrying the VZVluc ORF4D BAC via electroporation. D. Homologous recombination between upstream and downstream homologies of ORF4 replaced KanR with the ORF4-ZeoR cassette, creating the ORF4R clone. E. ZeoR was removed while generating virus from BAC DNA by co-transfecting a Cre recombinase expressing plasmid.
The ORF4 gene was cloned directionally into pGEM-zeo to form pGEM-ORF4-zeo. The resulting clone was verified by sequencing analysis. ORF4-zeoR cassette was made by PCR using pGEM-ORF4-zeo as template. The PCR product contained 40-bp homologies of flanking sequences of KanR cassette, which was also used to generate the ORF4 deletion mutant.
The following procedures are similar to producing the ORF deletion mutant. Briefly, the linear ORF4-zeoR cassette was treated with DpnI and electroporated into competent DY380 cells harboring VZVluc ORF4 deletion BAC. Similarly, homologous recombination functions needed to be transiently induced by switching the culture temperature from 32°C to 42°C for 10 min when electroporation-competent cells were grown. The recombinants were selected on LB agar plates containing zeocin. After verification, the ORF4 deletion rescue BAC DNA was isolated from E. coli and transfected into MeWo cells to produce the rescue virus as described above.
The rescue clone was generated by introducing the wild type ORF4 back into the deletion viral genome along with a ZeoR cassette. Since the ZeoR cassette is flanked by two loxP sites, it can be removed from the genome by Cre-mediated recombination. Following the protocol described above, a wild type copy of ORF4 was restored in the same direction and location as the parental wild type strain except a remaining loxP site (34bp) in the 3′ non-coding region of ORF4. The VZVluc ORF4 deletion rescue virus was obtained after 3–5 days of transfection.
3.3. Growth curve analysis based on bioluminescence assay
All recombinant VZV viruses based on the VZVluc BAC system share a common feature of having the firefly luciferase cassette embedded between ORF65 and ORF66 and controlled by the SV40 early promoter. In order to explore the possibility of utilizing bioluminescence signals as an indicator of viral growth, we compared the conventional infectious center assay and bioluminescence assay.
Briefly, for the conventional infectious center assay, 21 wells of 6-well plates were inoculated with wild type VZVluc, and 3 wells were collected every 24 hours post infection. Each sample was titered later by an infectious center assay, and data were used to generate the final growth curve. For the bioluminescence assay, only 3 wells of 6-well plates were inoculated with the same amount of virus as in conventional assay. Bioluminescence signals from the same set of wells were repeatedly measured after every 24 hours post infection during a 7-day period simply by applying reaction substrate D-luciferin to cells. Quantified bioluminescence data were collected and analyzed to generate a new form of viral growth curve. No subsequent titering assays were required.
As shown in Fig. 4C, these two growth curves correlated very well, indicating that bioluminescence signals can be used as an accurate indicator of viral loads in characterizing VZV growth kinetics in vitro. This method has been shown in previous studies (Zhang et al., 2007) to be sensitive and accurate enough to detect subtle growth kinetics changes.
4. Conclusions
Despite the fact that VZV has the smallest genome among human herpesviruses, less than 20% of the VZV genome has been functionally characterized. In recent years, a cosmid-based mutagenesis approach has been developed (Cohen et al., 1993; Niizuma et al., 2003) in order to facilitate studies of VZV genome function. In order to generate recombinant VZV genomes using a 4-cosmid system, four complementary cosmids need to be co-transfected into a single permissive mammalian cell and 4 homologous recombination events between overlapping sequences are also required to obtain a full-length viral genome. We have shown that generating recombinant VZV using the new luciferase VZV BAC simplifies and eliminates co-transfection and recombination steps. Therefore, recombinant VZVs can be generated in a much more efficient manner.
In this study, VZV ORF0–11 deletion mutants and VZV ORF4 deletion revertant have been created efficiently using this VZVluc BAC system. Three additional VZV ORFs (62/71, 63/70, 64/69) have been deleted through the same approach (data not shown). Briefly, one copy of these ORFs was first replaced by a KanR cassette and the other gene was replaced by an additional selection marker, such as AmpR. Similarly, double ORF deletions, which can be any combination of two ORFs throughout the VZV genome, can be easily generated using such a system. Furthermore, genetic manipulation does not need to be limited to deleting ORFs.
Because of VZV’s highly cell-associated nature in cell culture, conventional virology techniques, including plaque purification and plaque assay, become troublesome. By developing and exploiting the new luciferase VZV BAC system, subsequent virus has a removable EGFP expression cassette and a built-in luciferase reporter. In this study, an alternative bioluminescence quantification approach has been developed and validated to monitor viral replication in vitro. Compared to the traditional infectious center assay, the new bioluminescence-based approach not only saves time and labor, but also significantly increases the reproducibility of results. Moreover, the presence of luciferase activity indicates viral replication in cells and not free viral particles, which makes it suitable and preferable for studies of this particular cell-associated virus. This approach has also been successfully used in monitoring VZV growth in other cultured cell systems (data not published) and SCIDhu (thymus/liver and skin) models (Zhang et al, 2007).
Acknowledgments
We are grateful to A. Chu and C. Patterson for critically reading the manuscript. This work was supported by NIH grant AI050709-01 (H.Z.) and American Cancer Association grant RSG-05-076-01-MBC (H.Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abendroth A, Arvin A. Varicella-zoster virus immune evasion. Immunol Rev. 1999;168:143–56. doi: 10.1111/j.1600-065x.1999.tb01289.x. [DOI] [PubMed] [Google Scholar]
- Cohen JI, Krogmann T, Ross JP, Pesnicak L, Prikhod’ko EA. Varicella-zoster virus ORF4 latency-associated protein is important for establishment of latency. J Virol. 2005;79:6969–75. doi: 10.1128/JVI.79.11.6969-6975.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI, Seidel KE. Generation of varicella-zoster virus (VZV) and viral mutants from cosmid DNAs: VZV thymidylate synthetase is not essential for replication in vitro. Proc Natl Acad Sci USA. 1993;90:7376–80. doi: 10.1073/pnas.90.15.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI, Straus SE, Arvin AM. Varicella-Zoster Virus Replication, Pathogenesis, and Management. In: Knipe DM, Howley PM, editors. Fields Virology. Vol. 2. Lippincott Williams & Wilkins; Philadelphia, PA: 2007. pp. 2773–2818. [Google Scholar]
- Nagaike K, Mori Y, Gomi Y, Yoshii H, Takahashi M, Wagner M, Koszinowski U, Yamanishi K. Cloning of the varicella-zoster virus genome as an infectious bacterial artificial chromosome in Escherichia coli. Vaccine. 2004;22:4069–74. doi: 10.1016/j.vaccine.2004.03.062. [DOI] [PubMed] [Google Scholar]
- Netterwald J, Yang S, Wang W, Ghanny S, Cody M, Soteropoulos P, Tian B, Dunn W, Liu F, Zhu H. Two gamma interferon-activated site-like elements in the human cytomegalovirus major immediate-early promoter/enhancer are important for viral replication. J Virol. 2005;79:5035–46. doi: 10.1128/JVI.79.8.5035-5046.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niizuma T, Zerboni L, Sommer MH, Ito H, Hinchliffe S, Arvin AM. Construction of varicella-zoster virus recombinants from P-Oka cosmids and demonstration that ORF65 protein is dispensable for infection of human skin and T cells in the SCID-hu mouse model. J Virol. 2003;77:6062–5. doi: 10.1128/JVI.77.10.6062-6065.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato B, Sommer M, Ito H, Arvin AM. Requirement of varicella-zoster virus immediate-early 4 protein for viral replication. J Virol. 2003;77:12369–72. doi: 10.1128/JVI.77.22.12369-12372.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Patterson CE, Yang S, Zhu H. Coupling generation of cytomegalovirus deletion mutants and amplification of viral BAC clones. J Virol Methods. 2004;121:137–43. doi: 10.1016/j.jviromet.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Yu D, Ellis HM, Lee EC, Jenkins NA, Copeland NG, Court DL. An efficient recombination system for chromosome engineering in Escherichia coli. Proc Natl Acad Sci USA. 2000;97:5978–83. doi: 10.1073/pnas.100127597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Rowe J, Wang W, Sommer M, Arvin A, Moffat J, Zhu H. Genetic Analysis of Varicella Zoster Virus ORF0 to 4 Using A Novel Luciferase Bacterial Artificial Chromosome System. J Virol. 2007;81:9024–33. doi: 10.1128/JVI.02666-06. [DOI] [PMC free article] [PubMed] [Google Scholar]