Abstract
Context: The insulinoma syndrome is marked by fasting hypoglycemia and inappropriate elevations of insulin. The gastrinoma syndrome is characterized by hypergastrinemia, ulcer disease, and/or diarrhea. Rarely, insulinoma and gastrinoma coexist in the same patient simultaneously.
Objective: Our objective was to determine the cause of a patient’s hypoglycemic episodes and peptic ulcer disease.
Design and Setting: This is a clinical case report from the Clinical Research Center of the National Institutes of Health.
Patient and Intervention: One patient with hypoglycemic episodes and peptic ulcer disease had a surgical resection of neuroendocrine tumor.
Results: The patient was found to have a single tumor cosecreting both insulin and gastrin. Resection of this single tumor was curative.
Conclusions: A single pancreatic neuroendocrine tumor may lead to the expression of both the hyperinsulinemic and hypergastrinemic syndromes.
A rare patient with both insulinoma and gastrinoma due to a single pancreatic neuroendocrine tumor shows that both syndromes can be caused by a single neoplasm.
The insulinoma syndrome is characterized by fasting hypoglycemia and inappropriately elevated insulin and proinsulin. Over 90% of the cases are caused by a single, usually benign, neuroendocrine tumor of the pancreas (1). The gastrinoma or Zollinger-Ellison syndrome (ZES) is characterized by hypergastrinemia, ulcer disease, and/or diarrhea. This syndrome is caused by both intra- and extrapancreatic neuroendocrine tumors that are frequently malignant (1). These two conditions may occur in the same patient either sequentially or occasionally simultaneously in the syndrome multiple endocrine neoplasia type 1 (MEN1). However, when they occur together in MEN1, each hormone, i.e. insulin and gastrin, is secreted by a different, distinct tumor.
The present case is unique to the extent we can determine from long experience in both syndromes and from our review of the literature in that a single pancreatic neuroendocrine tumor led to the expression of both the hyperinsulinemic and hypergastrinemic syndromes.
Patient and Methods
Lab procedures
During the patient’s initial hospitalization, routine analytical tests were performed at Exempla Saint Joseph Hospital laboratory in Denver, Colorado. Insulin, C-peptide, proinsulin, gastrin, and chromogranin A were measured by ARUP laboratories in Salt Lake City, Utah.
All subsequent routine analytical tests, immunohistochemistry, and immunoelectron microscopy were performed at the National Institutes of Health (NIH) Clinical Center Laboratory in Bethesda, Maryland, with the exception of proinsulin and gastrin which were performed by Mayo Medical Labs in Rochester, Minnesota.
Sequence analysis of exons 2–10 of the MEN1 gene was performed by Gene Dx in Gaithersburg, Maryland.
Immunohistochemistry
Immunohistochemistry stains were performed on sections obtained from formalin-fixed, paraffin-embedded tissue blocks. For all antibodies, except the anti-somatostatin antibody, pretreatment with citrate buffer was performed for antigen retrieval. The following antibodies were used in the concentrations indicated: chromogranin (mouse monoclonal antihuman chromogranin A, clone LK2H10, 1:3000; Biocare Medical, Concord, CA); synaptophysin (rabbit polyclonal antihuman synaptophysin, 1:100; Zymed, Carlsbad, CA); gastrin (polyclonal rabbit antihuman gastrin, 1:5000; DakoCytomation, Carpinteria, CA); insulin (mouse monoclonal antihuman insulin, clone HB125, 1:100; BioGenex, San Ramon, CA); and somatostatin (polyclonal rabbit antihuman somatostatin, 1:1000; DakoCytomation).
Immunoelectron microscopy
Tissue pieces were removed from a paraffin block, deparaffinized in xylene, placed in absolute ethanol, and embedded in LR White (SPI, West Chester, PA). Ultrathin sections were mounted on 150-mesh uncoated nickel grids. Grids were floated on blocking solution [PBS, 0.1% Tween 20, and 0.5% cold-water fish gelatin (Ted Pella, Inc., Redding, CA)] for 20 min, incubated for 1 h with the primary antibody (against gastrin or insulin), rinsed in blocking buffer for 5 min, blocked with 2% goat serum, rinsed with blocking buffer, and then incubated with gold-conjugated secondary goat antibody [20 nm for the anti-gastrin antibody and 10 nm for the anti-insulin antibody (Ted Pella)], rinsed in PBS, and air dried. The first and second antibody labeling were separated by 24 h. Sections were stained with aqueous uranyl acetate and examined with a Phillips CM10 electron microscope.
Results
Clinical course and investigation
In January of 2007, an 18-yr-old white female was hospitalized for abdominal pain, nausea, and vomiting. For the 18 months before admission, the patient had experienced intermittent episodes of symptomatic hypoglycemia. She had several episodes of amnesia while driving or performing normal daily activities. Three of these episodes were associated with documented hypoglycemia, and symptoms resolved with administration of glucose. Initially, the patient was able to control her hypoglycemia by frequent snacking; however, she began to have additional symptoms of nausea, vomiting, and weight loss secondary to a duodenal ulcer. The patient denied using hypoglycemic agents or insulin injections and had no access to them. She was previously healthy with no surgeries. She had no family history of gastrinomas, insulinomas, parathyroid adenomas, or pituitary tumors to suggest MEN1.
On presentation to the emergency room, she was found to have a blood glucose of 30 mg/dl (60–110 mg/dl). She had an elevated fasting insulin of 16 μIU/ml, C-peptide 3 ng/ml, proinsulin 95 pmol/liter (≤22 pmol/liter), and a corresponding glucose of 39 mg/dl (60–110 mg/dl). Insulinoma was suspected on the basis of her fasting hypoglycemia and inappropriately elevated insulin levels. She also underwent evaluation of her abdominal discomfort.
Esophagogastroduodenoscopy revealed a duodenal ulcer. After being on iv pantoprazole for 6 d, her gastrin was elevated at 358 pg/ml (0–100 pg/ml). In addition, chromogranin A, a marker of neuroendocrine tumors, was elevated at 534 ng/ml (0–375 ng/ml). Helicobacter pylori IgG was negative. During her hospitalization, she was switched to omeprazole 40 mg by mouth twice daily and was referred to the NIH Clinical Center for further evaluation.
Upon presentation to the NIH, her physical examination was unremarkable and did not reveal skin findings associated with MEN1, such as collagenomas or angiofibromas. A 48-h fast was initiated and was terminated at 23 h when she exhibited neuroglycopenic symptoms (2). At this point, the patient’s fasting plasma glucose was 43 mg/dl (70–115 mg/dl), and corresponding serum insulin was inappropriately elevated at 13.6 μU/ml (Table 1). Proinsulin was elevated at 170 pmol/liter (≤22 pmol/liter), and C-peptide was 2.4 ng/ml. Anti-insulin antibodies and sulfonylurea screen were negative.
Table 1.
Comparison of 24-h fast before vs. after resection of insulinoma
| Time | Insulin (μIU/ml) | Glucose, serum (mg/dl) | |
|---|---|---|---|
| Preoperative (February 2007) | Baseline, time 0 | 28.1 | 91 |
| End of fast, +23 h | 13.6 | 43 | |
| Postoperative (July 2007) | Baseline, time 0 | 19.4 | 119 |
| End of fast, +24 h | 4 | 79 |
Multiple values were obtained throughout the fast.
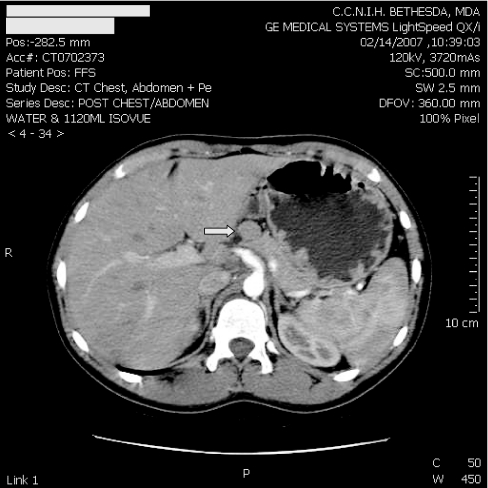
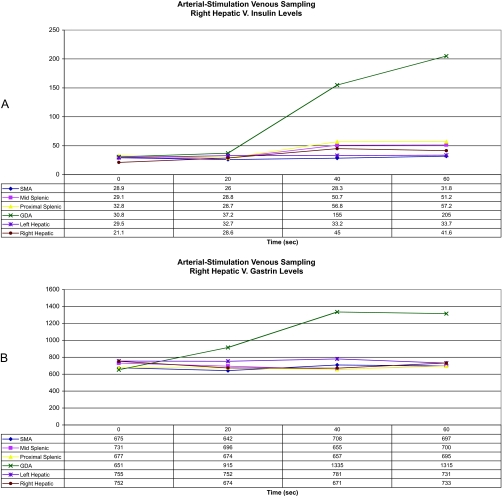
An ultrasound of the pancreas was performed that revealed a less than 2-cm solid nodule arising from the anterior portion of the mid-body of the pancreas. A computed tomography (CT) scan of the abdomen with contrast revealed a 1.5-cm nonenhancing mass arising from the superior aspect of the body of the pancreas and no evidence of metastatic disease (Fig. 1). The patient underwent both arteriography with calcium gluconate stimulation and then hepatic venous sampling for insulinoma localization (3). Both gastrin and insulin levels showed a step up in the territory of the gastroduodenal artery, corresponding to the anatomic localization of the insulinoma seen on previous imaging studies (Fig. 2).
Figure 1.
CT scan of abdomen. Arrow points to 1.5-cm nonenhancing rounded mass arising from the superior aspect of the body of the pancreas.
Figure 2.
Intraarterial calcium stimulation. Graphs show insulin (A) and gastrin (B) levels from our patient with a neuroendocrine tumor in the head of the pancreas. After infusion of calcium gluconate into selected arteries [superior mesenteric artery (SMA), mid splenic artery, proximal splenic artery, gastroduodenal artery (GDA), left hepatic artery, and right hepatic artery], insulin levels were assayed from blood samples taken at various time intervals from the right hepatic vein.
Additional evaluation for a potential gastrinoma was performed. The patient’s gastrin levels were consistently elevated at 510–960 pg/ml (0–99 pg/ml). Basal acid output was elevated at 11.5 mEq/h. A secretin test was performed with an elevated baseline gastrin level of 526 pg/ml and a minimal incremental rise with a peak gastrin level of 556 pg/ml. Both tests were performed while the patient was taking omeprazole 40 mg twice daily. An octreotide scan showed no focal localization of radionucleotide. An endoscopic ultrasound was performed that identified a round mass in the pancreatic neck measuring 14 × 12 mm, and three abnormal lymph nodes were visualized in the peripancreatic head region. The duodenal wall was diffusely thickened, and the duodenal mucosa was congested and nodular, both consistent with a gastrinoma.
The patient was screened for other MEN1-associated endocrinopathies. She did not have evidence of a parathyroid adenoma or prolactinoma with normal calcium of 2.39 mmol/liter (2.05–2.50 mmol/liter), PTH 44.3 pg/ml (16–87 pg/ml), and prolactin 9 μg/dl (1–25 μg/dl). The patient’s genetic testing was negative for disease-associated mutations in exons 2–10 of the MEN1 gene.
Surgical procedure
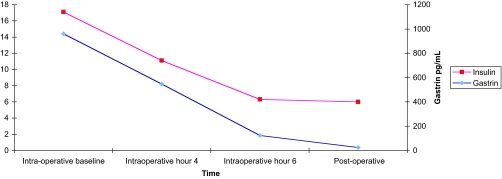
The patient underwent an exploratory laparotomy, guided by intraoperative pancreatic ultrasound. At operation, a pedunculated mass of the pancreatic head was enucleated. It correlated with the CT scan findings and was consistent with a neuroendocrine tumor on frozen section. Intraoperative ultrasound revealed three mildly enlarged lymph nodes in the periportal and periduodenal areas. These nodes were removed and were negative for tumor on frozen section. Gastroscopy revealed gastric fold hypertrophy. Transduodenal illumination of the entire duodenum was performed. A mild amount of scarring consistent with peptic ulcer disease was noted in the duodenal bulb. No other lesions were noted. A longitudinal duodenotomy was performed in the second portion of the duodenum, and bimanual palpation of the first, second, and third portions, including the ampulla, was performed. No lesions were found. Serum gastrin and insulin levels fell by 87 and 63%, respectively, after enucleation of the lesion as determined by intraoperative assays (Fig. 3).
Figure 3.
Change in serum insulin and gastrin levels as a function of time, intraoperatively and 6 d postoperatively. Red squares, insulin; blue diamonds, gastrin.
Pathology and ultrastructural studies
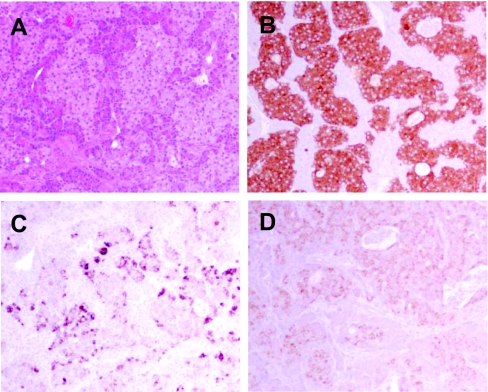
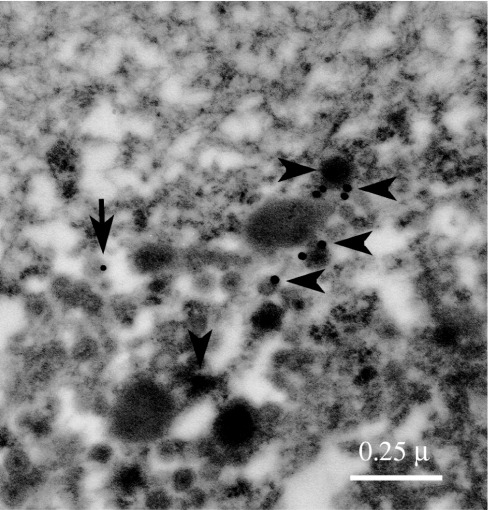
On gross examination, the pancreatic head mass received in Surgical Pathology consisted of a single white to yellow soft tissue nodule measuring 1.8 × 1.8 × 1.5 cm. Multiple sections of tumor showed nests of polygonal pale cells with eosinophilic cytoplasm and nuclei that exhibit a finely dispersed salt-and-pepper chromatin pattern (Fig. 4A). Margins of resections were free of tumor cells. Immunohistochemistry studies showed the neoplastic cells to be positive for synaptophysin (Fig. 4B), chromogranin (not shown), gastrin (Fig. 4C), and insulin (Fig. 4D). Somatostatin staining was positive in occasional tumor cells, and pancreatic polypeptide was negative (not shown). Additionally, four tissue fragments, taken from the medial aspect of the second portion of the duodenum, the periduodenal area, and the superior portion of the pancreatic head, showed reactive lymphoid follicles with no evidence of tumor cells. Immunogold electron microscopy was performed with anti-gastrin and anti-insulin antibodies, indicating the presence of both gastrin and insulin within the same pancreatic cell but not within the same granule (Fig. 5).
Figure 4.
A, Hematoxylin/eosin-stained sections show a neuroendocrine tumor; B–D, immunohistochemistry stains of neuroendocrine tumor are positive for synaptophysin (B), gastrin (C), and insulin (D).
Figure 5.
An electron microscopy micrograph showing labeling of gastrin (arrowheads, 20-nm gold beads) and insulin (arrow, 10-nm gold bead) within the same pancreatic cell.
Postoperative course
Postoperatively, the patient’s serum gastrin normalized to 24 pg/ml (0–99 pg/ml), fasting blood sugars normalized to 88 mg/dl (70–115 mg/dl), and fasting insulin levels decreased to normal at 6 μU/ml (6–27 μU/ml). She was discharged home on omeprazole 40 mg once daily.
In July of 2007, the patient was readmitted to the NIH Clinical Center for follow-up. She had discontinued her omeprazole 2 wk before admission. She underwent a 24-h fast (Table 1). At 24 h of fasting, serum glucose was normal at 79 mg/dl (70–115 mg/dl) and serum insulin level was appropriately suppressed at 4.0 μU/ml, with corresponding proinsulin of 11 pmol/liter (≤22 pmol/liter) and C-peptide level of 1.0 ng/ml. Her insulin/glucose ratio after an overnight fast was normal at 0.05, compared with her elevated preoperative ratio of 0.32. During the secretin test, her gastrin levels remained low throughout, between 17 and 26 pg/ml (0–99 pg/ml).
Discussion
At age 18 yr, the patient presented simultaneously with both an insulinoma and gastrinoma, found to arise from a single tumor. Immunohistochemistry confirmed that the tumor was composed of cells producing both insulin and gastrin. Furthermore, the double-labeled immunogold electron microscopy demonstrated that subpopulations of granules expressing insulin and gastrin colocalized within the same cell.
Several studies have used immunohistochemistry to illustrate more than one peptide-containing cell type within the same pancreatic tumor (4,5,6,7,8,9,10). Although a single tumor may show immunohistochemical presence of more than one hormone/peptide, this has not been shown to correlate with simultaneous clinical manifestations of multiple hormone oversecretion. Occasional examples exist of sequential transition from one type of syndrome to another. Rarely, patients with a malignant tumor, particularly gastrinoma, later show evidence of a second clinical syndrome such as insulinoma or Cushing’s syndrome (6,7,9) (Jensen, R. T., personal communication). To our knowledge, this is the first case demonstrating insulin and gastrin immunoreactivity in the same cell in a patient with concurrent, symptomatic insulinoma and gastrinoma.
This case raises interesting questions regarding the mechanism of cell differentiation in islet cells. One possibility is that the tumor cells are derived from pluripotent stem cells that retain the capacity to produce multiple hormones (11). In this theory, common precursor cells originating in the neural crest eventually mature into peptide-secreting endocrine cells. Neoplastic transformation of these precursor cells is one explanation for multi-hormone-producing neuroendocrine cells (12).
This case presented both diagnostic and surgical management questions. Biochemical assays confirmed a diagnosis of insulinoma, and the lesion was localized by both intraarterial calcium stimulation and endoscopic ultrasound. Her symptoms dictated resection of the insulinoma. Although the insulinoma resection appeared straightforward, the presence of a possible gastrinoma complicated the operative decision making. It was necessary to interpret the hypergastrinemia in light of concurrent therapy with omeprazole, because proton pump inhibitors can complicate the diagnosis of ZES. Repeat gastrin levels ranged from 510–960 pg/ml (0–99 pg/ml). It is possible that treatment with omeprazole can be associated with this degree of hypergastrinemia (13); however, this occurs in the setting of low basal acid output and not with the gastric acid hypersecretion as exhibited in this patient.
Although the calcium gluconate stimulation did localize the gastrinoma to the territory of the gastroduodenal artery, the octreotide scan failed to identify the gastrinoma. It is not unusual for gastrinomas to be difficult to localize. In a study of 176 sporadic and MEN1 patients who underwent laparotomy for ZES, the majority of gastrinomas were found in the duodenum and pancreas, but a significant number of primary tumors were also found in lymph nodes (26%) or other locations (6%) or were never found (6%) (14). In general, caution before proceeding to resection of gastrinoma is warranted. In a study of 128 patients undergoing exploration with curative intent, 30% of patients with gastrinoma not associated with MEN1 were cured, whereas only 5% of patients with MEN1 were cured (15). This case was made more difficult by the uncertainty of the patient’s MEN1 status at the time of surgery.
If the entity of a single tumor cosecreting both insulin and gastrin had been known to exist before the surgery, a different surgical approach may have been used, and a duodenotomy may not have been performed. Gastrinomas often originate in the duodenum and are locally invasive or have already metastasized at the time of diagnosis. Because of this finding, a duodenotomy to search for gastrinoma lesions is usually performed if surgery for gastrinoma is undertaken. Often, surgical resection of gastrinomas is not feasible in MEN1 patients because of high recurrence risk, and treatment is with proton-pump inhibitors alone (16).
The decision was made to proceed with both resection of the insulinoma and intraoperative localization and resection of the presumed gastrinoma during the same operation. Factors important in the decision were the severity of the duodenal disease in an otherwise healthy young woman, the absence of H. pylori and nonsteroidal antiinflammatory usage, and the need for exploration to resect the insulinoma.
In patients known to have MEN1, the estimated penetrance at age 40 yr of enteropancreatic tumors are as high as 40% for gastrinoma and 10% for insulinoma (17). In a patient as young as 18 yr, the percentages would be expected to be lower. In addition, it is extremely unusual to have both syndromes simultaneously in a single patient. Our patient does not have an identifiable germline mutation associated with MEN1. In addition, she does not fit the consensus criterion for MEN1: a case with two of the three main MEN1-related endocrine tumors, i.e. parathyroid adenomas, enteropancreatic endocrine tumors, and pituitary tumors (17).
This case represents a unique presentation of the sporadic occurrence of two distinct clinical syndromes with a single neuroendocrine tumor cosecreting two functionally significant hormones. Resection of this single tumor was curative.
Acknowledgments
We thank Dr. Robert T. Jensen and Dr. Stephen J. Marx for reviewing the manuscript. We thank Dr. David J. DePaolo for referring the patient to our care.
Footnotes
This work was supported by intramural research funding of the National Institute of Diabetes and Digestive and Kidney Diseases of the NIH.
M.L. is a Commissioned Officer in the U.S. Public Health Service, Department of Health and Human Services.
Disclosure Statement: The authors have nothing to disclose.
First Published Online February 5, 2008
Abbreviations: CT, Computed tomography; MEN1, multiple endocrine neoplasia type 1; ZES, Zollinger-Ellison syndrome.
References
- Jensen RT 2005 Endocrine tumors of the gastrointestinal tract and pancreas. In: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, Isselbacher KJ, eds. Harrison’s principles of internal medicine. 16th ed. New York: McGraw Hill; 2220–2230 [Google Scholar]
- Hirshberg B, Livi A, Bartlett DL, Libutti SK, Alexander HR, Doppman JL, Skarulis MC, Gorden P 2000 Forty-eight-hour fast: the diagnostic test for insulinoma. J Clin Endocrinol Metab 85:3222–3226 [DOI] [PubMed] [Google Scholar]
- Brown CK, Bartlett DL, Doppman JL, Gorden P, Libutti SK, Fraker DL, Shawker TH, Skarulis MC, Alexander HR 1997 Intraarterial calcium stimulation and intraoperative ultrasonography in the localization and resection of insulinomas. Surgery 122:1189–1193 [DOI] [PubMed] [Google Scholar]
- Heitz PU, Kasper M, Polak JM, Kloppel G 1982 Pancreatic endocrine tumors. Hum Pathol 13:263–271 [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Enjoji M 1992 Endocrine neoplasms of the pancreas: a clinicopathologic study of 24 cases and immunohistochemical remarks. Surg Today 22:305–312 [DOI] [PubMed] [Google Scholar]
- Broor SL, Soergel KH, Garancis JC, Wilson SD 1979 Hormone producing pancreatic islet cell carcinoma: changing clinical presentation. Am J Med Sci 278:229–233 [DOI] [PubMed] [Google Scholar]
- Liaw CC, Lin JT, Chen TJ 1989 Multiple-hormone-producing islet cell carcinoma: report of a case. J Formosan Med Assoc 88:722–725 [PubMed] [Google Scholar]
- Larsson LI, Grimelius L, Håkanson R, Rehfeld JF, Stadil F, Holst J, Angervall L, Sundler F 1975 Mixed endocrine pancreatic tumors producing several peptide hormones. Am J Pathol 79:271–284 [PMC free article] [PubMed] [Google Scholar]
- Asa SL, Kovacs K, Killinger DW, Marcon N, Platts M 1980 Pancreatic islet cell carcinoma producing gastrin, ACTH, α-endorphin, somatostatin and calcitonin. Am J Gastroenterol 74:30–35 [PubMed] [Google Scholar]
- Takahashi M, Hoshii Y, Kawano H, Setoguchi M, Gondo T, Yamashita Y, Nakayasu K, Kamei T, Ishihara T 1998 Multihormone-producing islet cell tumor of the pancreas associated with somatostatin-immunoreactive amyloid: immunohistochemical and immunoelectron microscopic studies. Am J Surg Pathol 22:360–367 [DOI] [PubMed] [Google Scholar]
- Baylin SB, Mendelsohn G 1980 Ectopic (inappropriate) hormone production by tumors: mechanisms involved and the biological and clinical implications. Endocr Rev 1:45–77 [DOI] [PubMed] [Google Scholar]
- Dayal Y 1991 Endocrine pathology of the gut and pancreas. Boca Raton, FL: CRC Press; 195–225 [Google Scholar]
- Berna MJ, Hoffmann KM, Serrano J, Gibril F, Jensen RT 2006 Serum gastrin in Zollinger-Ellison syndrome. I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Medicine (Baltimore) 85:295–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton JA, Fraker DL, Alexander HR, Venzon DJ, Doppman JL, Serrano J, Goebel SU, Peghini PL, Roy PK, Gibril F, Jensen RT 1999 Surgery to cure the Zollinger-Ellison syndrome. N Engl J Med 341:635–644 [DOI] [PubMed] [Google Scholar]
- Alexander HR, Bartlett DL, Venzon DJ, Libutti SK, Doppman JL, Fraker DL, Norton JA, Gibril F, Jensen RT 1998 Analysis of factors associated with long-term (five or more years) cure in patients undergoing operation for Zollinger-Ellison syndrome. Surgery 124:1160–1166 [DOI] [PubMed] [Google Scholar]
- Norton JA, Fang TD, Jensen RT 2006 Surgery for gastrinoma and insulinoma in multiple endocrine neoplasia type 1. J Natl Compr Canc Netw 4:148–153 [DOI] [PubMed] [Google Scholar]
- Brandi ML, Gagel RF, Angeli A, Bilezikian JP, Beck-Peccoz P, Bordi C, Conte-Devolx B, Falchetti A, Gheri RG, Libroia A, Lips CJ, Lombardi G, Mannelli M, Pacini F, Ponder BA, Raue F, Skogseid B, Tamburrano G, Thakker RV, Thompson NW, Tomassetti P, Tonelli F, Wells Jr SA, Marx SJ 2001 Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab 86:5658–5671 [DOI] [PubMed] [Google Scholar]