Abstract
Context: Dihydrotestosterone (DHT), the primary active androgen in peripheral target tissues, is metabolized by 3α-hydroxysteroid dehydrogenase type III (3α-HSD), encoded by the AKR1C2 gene, forming 5α-androstane-3α,17β-diol (3α-diol). 3α-HSD may play a role in the pathogenesis of hirsutism.
Objectives: Our objective was to evaluate the role of 3α-HSD in hirsutism by comparing 1) tissue levels of active androgens, 2) relative gene expression of AKR1C2, and 3) activity of 3α-HSD in genital skin from normal and hirsute women.
Design: Genital skin was obtained from normal and hirsute women. After homogenization, testosterone (T) and DHT levels were quantified by conventional RIA. From isolated RNA, relative expression of AKR1C2 was determined by real-time PCR. In addition, minced genital skin was incubated with [3H]DHT, and the product, [3H]3α-diol, was quantified by radio-HPLC.
Setting: The study took place at an inner-city hospital.
Patients: Patients included women undergoing posterior colporrhaphy.
Main Outcome Measures: We assessed 1) tissue levels of T, DHT, and 3α-diol; 2) relative expression of AKR1C2; and 3) conversion ratio of [3H]3α-diol to [3H]DHT.
Results: In genital skin, tissue DHT and T concentrations in hirsute women were 1.90-fold and 1.84-fold higher than in normal women (P =0 .002 and 0.03), and relative expression of AKR1C2 mRNA was reduced approximately 7-fold (P = 0.04). Genital skin from hirsute women showed less metabolism of [3H]DHT to [3H]3α-diol (conversion ratio, 0.24 ± 0.19 vs. 0.85 ± 0.55, P = 0.01).
Conclusions: In genital skin of hirsute women, reduced AKR1C2 gene expression and 3α-HSD activity results in decreased DHT metabolism and elevated tissue levels of DHT. Diminished DHT metabolism may play an important role in the pathogenesis of hirsutism.
In hirsute women, a seven-fold reduction in expression of the 3α-hydroxysteroid dehydrogenase gene, AKR1C2, is seen, resulting in decreased metabolism and higher serum levels of dihydroxytestosterone.
Hirsutism, defined as male-pattern terminal hair growth in females, afflicts 5–10% of all women (1,2,3). Androgens play a pivotal role in determining the type and distribution of hair growth over the human body. In vivo experiments have demonstrated that androgens can stimulate vellus-type hair follicles to produce terminal hair and prolong the anagen phase of body hair (4,5), thereby promoting male-pattern terminal hair growth in women.
Although hirsutism is believed to be secondary to androgen excess, 39% of hirsute women have normal circulating androgen levels (6). Because skin and its appendages (including hair follicles, sebaceous glands, and eccrine glands) contain the enzymes necessary for androgen biosynthesis and metabolism, tissue concentrations of androgens may not necessarily correlate with their respective serum concentrations. Rather, local concentrations of these sex steroids may be determined by the rate at which they are produced and metabolized in target tissues such as skin. This local or intracrine effect may play a significant role in the development of disorders of androgen excess, including hirsutism, in women with otherwise normal serum androgen concentrations.
Dihydrotestosterone (DHT) is the primary active androgen in target tissues such as skin (7). This highly potent androgen is produced by reduction of testosterone (T) or androstenedione at the Δ4,5 position through the action of the enzymes, 5α-reductase type 1 and 5α-reductase type 2, encoded by the SRD5A1 and SRD5A2 genes, respectively (8). Once DHT is produced in peripheral tissues, it is readily metabolized to the relatively less potent androgen, 5α-androstane-3α,17β-diol (3α-diol). This reaction is catalyzed in tissues (including skin) by the enzyme 3α-hydroxysteroid dehydrogenase (3α-HSD). Therefore, this enzyme may be an important regulator of DHT levels in skin.
There are three known cytosolic isozymes of 3α-HSD (9,10). They are encoded by genes belonging to the aldo keto reductase (AKR) gene superfamily. The members of this family encode for a group of (reduced) nicotinamide adenine dinucleotide phosphate [NADP(H)]-dependent oxido-reductases that share significant sequence homology (>84%), yet catalyze stereospecific reactions with distinct steroids. 3α-HSD type I is encoded by AKR1C4, which is expressed exclusively in the liver. In contrast, 3α-HSD type II has weak 3α-HSD activity and predominantly 17β-HSD type V activity. It converts androstenedione to T and is encoded by AKR1C3. Of importance in the present study is 3α-HSD type III (3α-HSD III), which is encoded by AKR1C2 and is the major cutaneous 3α-HSD responsible for conversion of DHT to 3α-diol.
Serum concentrations of T, DHT, and 3α-diol, expression of SRD5A1/SRD5A2, and activity of 5α-reductase have been studied extensively in hirsute women. However, very little is known about the concentration of androgens in genital skin. Also, no studies have investigated the expression of AKR1C2 and activity of 3α-HSD III, which metabolizes DHT to its less active metabolite, 3α-diol, in skin.
The objective of this study was to determine the role of 3α-HSD III in the development of clinical hirsutism. To establish that role, we sought to compare 1) the tissue concentrations of the relevant androgens, 2) expression of AKR1C2, and 3) DHT metabolism to 3α-diol, as a reflection of 3α-HSD III activity, in genital skin from hirsute and normal women. We hypothesized that in genital skin from hirsute women AKR1C2 expression would be reduced, leading to less 3α-HSD III activity and conversion of DHT to 3α-diol, resulting in higher DHT tissue levels.
Subjects and Methods
Subjects
Premenopausal and postmenopausal subjects scheduled for elective posterior colporrhaphy were approached for enrollment in the study. Subjects were excluded if they had thyroid disease, Cushing’s syndrome, virilization, cancer, or precancerous lesions involving the genital skin or had used hormones within 6 wk of surgery or medications that alter androgen production or metabolism. After consent, height, weight, medical, surgical, and medication history were obtained from each subject. Menopausal status and ethnicity were not determined. After examination of the subject, a modified Ferriman-Gallwey score was calculated (11). Subjects with a Ferriman-Gallwey score of 7 or higher were defined as hirsute, and subjects with a score less than 7 were considered normal. Blood was obtained perioperatively to measure serum androgen levels. The subject’s primary gynecologist performed the posterior colporrhaphy.
Institutional Review Board approval was obtained for this study.
Determination of tissue androgen levels
Genital skin was obtained from 23 normal and nine hirsute women during posterior colporrhaphy. Skin samples were obtained from the posterior fourchette and distal to the hymen and usually involved non-hair-bearing skin. They were collected in the operating room and rapidly frozen using liquid nitrogen. Specimens were then stored 1–6 months in a −70 C freezer before processing. At the time of processing, the tissues were first thawed at room temperature, dissected free of sc fat and hair follicles, and weighed immediately. The tissue was then immersed in liquid nitrogen and was pulverized, and the resulting powder was mixed with 0.1 m PBS (pH 7.4). Approximately 800 dpm of high-specific-activity [3H]T, [3H]DHT, and [3H]3α-diol were added to monitor procedural losses. After a 30-min incubation at 37 C, steroids were extracted using ethyl acetate-hexane (3:2 vol/vol) and separated using Celite column partition chromatography, with ethylene glycol as the stationary phase. DHT, T, and 3α-diol were eluted off the column using 10, 40, and 60% toluene in isooctane, respectively. Each compound was then quantified using a sensitive and specific RIA (12,13). Intra- and interassay coefficients of variation were in the range of 4–8% and 9.5–12%, respectively. The assay sensitivities were 20, 20, and 40 pg/ml for the T, DHT, and 3α-diol RIAs. Androgen levels were expressed as nanogram of androgen per gram of tissue.
Quantification of AKR1C2 expression
A subset of the original samples including genital skin from 16 normal and seven hirsute women was stored and subsequently processed, as described previously for determination of androgen levels. Relative expression of AKR1C2, AKR1C3, SRD5A1, and SRD5A2 was determined using a previously described gene-specific real-time PCR (14,15), in which the expression in paired tissues was compared with RNase P. Total RNA was isolated using the RNeasy Midi Kit (QIAGEN, Valencia, CA). cDNA libraries for TaqMan quantitative real-time PCR were made using the Omniscript Kit (QIAGEN) primed with random hexamers (Applied Biosystems, Foster City, CA).
Measurement of DHT metabolism: a marker of 3α-HSD III activity
Additional samples of genital skin were obtained from 10 normal and four hirsute women during posterior colporrhaphy. Specimens were obtained directly from the operating room and immediately weighed. Skin samples were dissected free of sc fat and hair follicles and were minced while on ice. Minces, averaging 227 ± 81 mg, were placed in vials containing 2 ml RPMI-1640 medium and 100 mm NADPH. Approximately 100,000 dpm of [3H]DHT (40–60 Ci/mmol) along with 250 ng purified DHT were added to each vial. Control vials, containing the same amounts of medium, NADPH, [3H]DHT, and purified DHT, but no skin sample, were also prepared and run in parallel with each batch of skin samples to assess any nonenzymatic conversion of [3H]DHT to [3H]3α-diol. The control vials and those containing skin samples were then incubated for 3 h at 37C with mechanical agitation in a Dubnoff metabolic incubator. This duration of incubation was based on a pilot study conducted with skin from an additional subject, divided into three equal portions and incubated (as described above) for 60, 120, and 180 min (see Results).
At the end of the incubations, 10 ml ethyl acetate-hexane (3:2 vol/vol) was added to each vial to extract the steroids. After a second extraction and evaporation of the organic solvents, the steroids were suspended in ethanol, stored, and later reconstituted in methanol before HPLC. Radiolabeled T, DHT, and 3α-diol were separated and quantified by radio-HPLC, using the reverse-phase method of O’Donnell et al. (16), with minor modifications, which included the use of a Waters Spherisorb ODS-2 column (5 μm, 250 mm × 4.6 mm). A Shimadzu LC-10AT HPLC system equipped with an in-line IN/US βRAM 2B radioactivity detector was used to quantify the radioactivity associated with each peak in the column effluent. The 1 ml/min effluent was continuously mixed with 3 ml/min of ScintiVerse E scintillation cocktail (Fisher Scientific, Pittsburgh, PA) in the radio-detector. Pure [3H]DHT and [3H]3α-diol (Perkin-Elmer, Shelton, CT) standards were used to identify their respective peaks on the chromatograms. Unlabeled DHT (coeluting with [3H]DHT) and androsterone standards were also used and were identified by UV detection. Because no nonenzymatic conversion of [3H]DHT occurred, chromatograms from control vials showed only a single peak consistent with [3H]DHT. The total radioactivity from the control vial was therefore used to represent the total radioactivity for the corresponding tissue incubation. Thus, [3H]DHT and [3H]3α-diol radioactivity was calculated and expressed as a percentage of the control total radioactivity per unit weight of tissue. From these, a conversion ratio (13) of [3H]3α-diol to unmetabolized [3H]DHT was calculated for each sample as an estimate of metabolism of [3H]DHT to [3H]3α-diol.
Statistical analysis
All data are presented as mean ± sd. Differences between groups were compared using Student’s t test. The paired t test was used to make comparisons within groups. Ordinal variables (Ferriman-Gallwey scores) were compared using the Mann-Whitney U test. A two-sided P value less than 0.05 was considered significant.
Results
Tissue androgen levels
Genital skin samples from nine hirsute and 23 normal women were used to quantify androgen levels. Hirsute subjects did not differ significantly from the controls in age (45.5 ± 4.1 vs. 48.6 ± 2.6 yr, respectively) or BMI (31.6 ± 2.7 vs. 31.4 ± 2.1 kg/m2). However, they did differ significantly by Ferriman-Gallwey score (9.0 ± 1.1 vs. 2.0 ± 0.9). In genital skin from normal and hirsute women, mean DHT levels were 2.27-fold and 2.35-fold higher, respectively, than T levels. The mean tissue DHT and T concentrations in hirsute women were 1.90-fold and 1.84-fold higher than in normal women, respectively (Table 1). Tissue levels of 3α-diol were below assay sensitivity.
Table 1.
Androgen levels (mean ± sd) in genital skin from normal and hirsute women
| Hirsute | Normal | P value | |
|---|---|---|---|
| DHT (ng/g tissue) | 0.55 ± 0.21 | 0.29 ± 0.19 | 0.002 |
| T (ng/g tissue) | 0.24 ± 0.21 | 0.13 ± 0.07 | 0.03 |
| P value | 0.01 | 0.0002 |
Expression of AKR1C2
Genital skin samples from seven hirsute women and 16 normal women were used to quantify mRNA. The subjects were comparable with respect to age and BMI: 46 ± 4.9 vs. 49 ± 2.2 yr and 30.5 ± 2.5 vs. 30.8 ± 1.35 kg/m2, respectively. The Ferriman-Gallwey scores of the hirsute women ranged from 8–10 (mean, 8.6 ± 0.4), and those of the normal women ranged from 0–6 (mean, 2.0 ± 0.6).
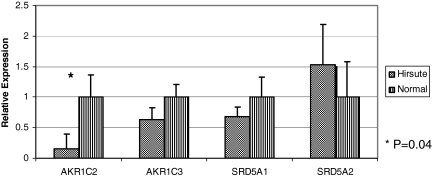
Figure 1 shows the relative expression of AKR1C2, AKR1C3, SRD5A1, and SRD5A2. Expression of the AKR1C2 gene, which encodes 3α-HSD III, was reduced almost 7-fold in hirsute compared with normal subjects (P = 0.04). Expression of AKR1C3, which encodes 17β-HSD type 5, did not differ significantly between the two groups. Similarly, expression of SRD5A1 and SRD5A2 genes, which code for 5α-reductase types 1 and 2, respectively, did not differ between the hirsute and normal groups.
Figure 1.
Relative expression of AKR1C and SRD5A genes in hirsute vs. normal genital skin. Average values for skin from normal subjects were set to 1, and levels in skin from hirsute subjects were expressed relative to skin from normal subjects.
3α-HSD III activity
A pilot study was conducted by incubating three equal sections of genital skin from a normal subject for 60, 120, and 180 min, respectively, with [3H]DHT. The results showed continuous disappearance of [3H]DHT and formation of [3H]3α-diol (data not shown). Thus, the 3-h point was selected for conducting the incubations of skin samples from all subjects to ensure that there would be sufficient accumulation of radioactivity associated with the 3α-diol peaks for accurate and precise quantification by radio-HPLC.
A total of 14 genital skin specimens, four from hirsute and 10 from control subjects, were incubated for 3 h after addition of labeled and unlabeled DHT at zero time, as described in Subjects and Methods. The subjects were comparable with respect to age (46.3 ± 6.0 vs. 46.5 ± 4.1 yr) but not BMI (39.8 ± 11.2 vs. 28.5 ± 5.0 kg/m2, respectively, P = 0.02). The Ferriman-Gallwey scores of the hirsute women ranged from 7–13 (median, 10.5), and those of the normal women ranged from 0–4 (median, 1.5) (P = 0.004).
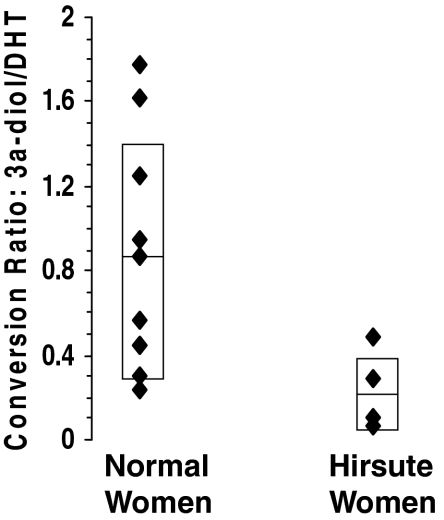
At the end of 3 h, the radioactivity was associated predominantly with DHT, 3α-diol, and androsterone. The radioactivity accounted for 75–86% of the total, with the balance appearing in minor unidentified peaks. Table 2 shows the distribution of radioactivity associated with unmetabolized DHT and the two identified DHT metabolites. No differences were observed between the hirsute and nonhirsute groups in the radioactivity appearing under the androsterone peak. However, DHT was metabolized to a much lesser extent in genital skin from hirsute women compared with the normal subjects, as evident by the higher retention of [3H]DHT and lower formation of [3H]3α-diol. In addition, skin from hirsute women showed on average a lower conversion ratio of [3H]3α-diol to [3H]DHT compared with skin from normal women (0.24 ± 0.19 vs. 0.85 ± 0.55, respectively, P = 0.01, Fig. 2). The Ferriman-Gallwey score correlated negatively with the conversion ratio (r = 0.58; P = 0.03).
Table 2.
Distribution of radioactivity associated with unmetabolized DHT and two metabolites of DHT, expressed as percent per 200 mg of tissue (mean ± sd), after in vitro incubation of genital skin from normal and hirsute women with [3H]DHT
| Hirsute | Normal | P value | |
|---|---|---|---|
| [3H]3α-diol | 8.9 ± 7.2 | 15.3 ± 7.7 | 0.18 |
| [3H]DHT | 46.0 ± 33.8 | 21.5 ± 8.0 | 0.04 |
| [3H]androsterone | 7.1 ± 3.8 | 6.9 ± 6.3 | 0.94 |
Figure 2.
Conversion ratio of 3α-diol to DHT in normal women and hirsute women. Individual values are depicted together with the mean ± sd in the two groups.
Discussion
We have shown that, in genital skin, DHT levels are higher than T levels. In serum, T levels are on average four times higher than DHT levels (17). Circulating T provides the precursor for peripheral DHT formation through the action of the enzyme, 5α-reductase type 1, which converts T to DHT. Studies have confirmed that genital skin is a sensitive marker of androgen action in humans (18,19) and can be used to differentiate hirsute and normal women (13).
DHT levels were higher in genital skin from hirsute women compared with genital skin from normal women. Despite this finding, 3α-diol, the metabolite of DHT, could not be detected in the tissue. Very low concentrations of 3α-diol are found in the circulation (20), which is most likely due to its rapid conjugation by glucuronyltransferase, forming 3α-diol glucuronide, in peripheral tissues (21,22). Serum 3α-diol glucuronide levels have been shown in some studies to correlate highly with Ferriman-Gallwey scores (23) and predict hirsutism (21). The 3α-diol RIA used in the present study lacked sensitivity. We used [3H]3α-diol as the radioligand in the RIA. An iodinated radioligand should provide greater sensitivity in the assay; however, it is not available commercially.
Other studies have shown that AKR1C2, the gene that codes for the enzyme 3α-HSD III, is expressed in multiple hormone-responsive tissues, such as breast and prostate (9,24), and that its expression is reduced in cancers of these tissues (14,15). Our study shows that AKR1C2 is also expressed in genital skin and that its expression is reduced in women with hirsutism.
In the third part of our study, we determined that the metabolism of DHT is reduced in genital skin from hirsute women compared with controls by conducting in vitro studies using radioactive DHT. 3α-HSD III, originally identified as the human bile acid binder (25), has a high affinity (low Km) for DHT (10). In our studies, we added relatively large amounts of unlabeled DHT to the incubation medium. The addition of 250 ng purified DHT to the [3H]DHT dose used (∼0.3 ng equivalent to 100,000 dpm) in each incubation provided an overriding amount of the substrate compared with its existing intracellular pool sizes in our specimens, which averaged 0.069 and 0.11 pg, respectively, in our normal and hirsute tissue samples. This ensured that no isotope dilution effects would interfere and artifactually account for any of the observed differences between the normal and hirsute tissue samples.
Although 3α-HSD III can function either as an oxidase or a reductase in vitro in the presence of NADPH, it has been shown to preferentially reduce DHT to 3α-diol in prostate cells (26). We hypothesize that the reduced activity of 3α-HSD III in the hirsute genital skin is due to decreased gene expression, in agreement with our prior findings in the prostate and breast (14,15). The mechanisms responsible for differential suppression of AKR1C2 transcription in genital skin of hirsute women have yet to be determined.
In addition to decreased metabolism of DHT, 5α-reductase activity appears to be increased in women with hirsutism (13,27). However, in the present study, expression of SRD5A1 or SRD5A2, which codes for 5α-reductase, was not altered significantly. Thus, increased 5α-reductase activity may not be due to increased gene expression. Previous studies of SRD5A gene expression in women with hirsutism have not shown a definitive relationship between the level of SRD5A expression and degree of hirsutism (28). Instead, genetic variations in SRD5A1 may explain altered 5α-reductase activity (29).
Significant and essentially equivalent amounts of androsterone were formed in the incubations of both control and hirsute genital skin. No other steroids were detected in significant or consistent amounts. Androsterone can be formed from DHT by two different pathways, either by prior conversion to 3α-diol by 3α-HSD III and its subsequent transformation to androsterone by 17β-HSD or by prior conversion to 5α-androstanedione by 17β-HSD and subsequent transformation to androsterone by 3α-HSD. It is not known which of these routes is more important. However, it is known that androsterone is formed predominantly from androstenedione via 5α-androstanedione. Androsterone is converted to androsterone glucuronide, which has been shown to be a good marker of clinical manifestations of acne in hyperandrogenic women (30).
It is possible that the low conversion of DHT to 3α-diol in hirsute women could have been due to preferential conversion to androsterone. We do not believe that this was the case because, first, androsterone formation was not significantly higher in the hirsute group. Second, formation of androsterone is still 3α-HSD dependent, and we have shown that AKR1C2 expression is reduced in women with hirsutism.
Because skin samples were obtained from women undergoing routine posterior colporrhaphy, all women tended to be older than women that usually present with complaints of hirsutism. The impact of age on 3α-HSD production and activity is unknown. However, hirsute women and control women did not differ in age. Although information on race and ethnicity was not collected, the small number of subjects in this study would have limited any subgroup analysis to determine effects of ethnicity on enzyme production and activity.
In summary, from our series of experiments, we have shown that in genital skin, DHT levels are higher than T levels. Both DHT and T levels are higher in skin from hirsute women compared with skin from normal subjects. Expression of AKR1C2, as measured by mRNA production, was dramatically reduced in hirsute women. The conversion of DHT to 3α-diol, which reflects 3α-HSD III activity was also reduced in hirsute women. From these series of experiments, we conclude that reduced expression of AKR1C2 in skin of hirsute women results in lower 3α-HSD type III enzyme production, leading to decreased conversion of DHT to the metabolically less active 3α-diol, higher tissue DHT levels, and the clinical manifestation of hirsutism.
In conclusion, women may develop hirsutism due to elevated levels of androgenic precursors of DHT in the skin, increased production of DHT due to increased 5α-reductase activity, and/or decreased metabolism of DHT due to decreased 3α-HSD III activity. Current therapies either reduce androgen levels or inhibit 5α-reductase. Future therapies should consider targeting DHT metabolism to reduce androgen-dependent hair growth in hirsute women.
Footnotes
The radio-HPLC analyses were conducted by the Cell Biology Core of the USC Research Center for Liver Diseases (NIDDK P30-DK48522).
This work was conducted while A.Z.S. was at the Department of Obstetrics and Gynecology, University of Southern California Keck School of Medicine, Los Angeles, California. She has since moved to the University of North Carolina.
Disclosure Statement: A.Z.S., Q.I., M.O., L.C., and A.S. have nothing to disclose. R.J.P. consults for and receives lecture fees from Ferring Pharmaceuticals. F.Z.S. consults for Agile Therapeutics and Novo Nordisk.
First Published Online February 5, 2008
Abbreviations: DHT, Dihydrotestosterone; 3α-diol, 5α-androstane-3α,17β-diol; 3α-HSD, 3α-hydroxysteroid dehydrogenase; 3α-HSD III, 3α-HSD type III; NADP(H), (reduced) nicotinamide adenine dinucleotide phosphate; T, testosterone.
References
- Ferriman D, Gallwey JD 1961 Clinical assessment of body hair growth in women. J Clin Endocrinol Metab 21:1440–1447 [DOI] [PubMed] [Google Scholar]
- McKnight E 1964 The prevalence of “hirsutism” in young women. Lancet 1:410–413 [DOI] [PubMed] [Google Scholar]
- Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R 1998 Prevalence of the polycystic ovarian syndrome in unselected black and white women in the Southeastern United States: a prospective Study. J Clin Endocrinol Metab 83:3078–3082 [DOI] [PubMed] [Google Scholar]
- Ebling JF 1986 Hair follicles and associated glands as androgen targets. J Clin Endocrinol Metab 15:319–339 [DOI] [PubMed] [Google Scholar]
- Randall VA 1994 Androgens and human hair growth. Clin Endocrinol (Oxf) 40:439–457 [DOI] [PubMed] [Google Scholar]
- Mehta A, Matwijiw I, Taylor PJ, Salamon EA, Kredentser JV, Faiman C 1992 Should androgen levels be measured in hirsute women with normal menstrual cycles? Int J Fertil 37:354–357 [PubMed] [Google Scholar]
- Mauvis-Jarvis P 1986 Regulation of androgen receptor and 5α-reductase in skin of normal and hirsute women. J Clin Endocrinol Metab 15:307–317 [DOI] [PubMed] [Google Scholar]
- Andersson S, Bishop RW, Russell DW 1989 Expression cloning and regulation of steroid 5α-reductase, an enzyme essential for male sexual differentiation. J Biol Chem 264:16249–16255 [PMC free article] [PubMed] [Google Scholar]
- Penning TM, Burczynski ME, Jez MF, Hung CF, Lin HK, Ma H, Moore M, Palackal N, Ratnam K 2000 Human 3α-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto-reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem J 351:67–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning TM, Jin Y, Steckelbroeck S, Rizner TL, Lewis M 2004 Structure-function of human 3α-hydroxysteroid dehydogenases: genes and proteins. Mol Cell Endocrinol 215:63–72 [DOI] [PubMed] [Google Scholar]
- Hatch R, Rosenfield R, Kim MH, Tredway D 1981 Hirsutism: Implications, etiology, and management. Am J Obstet Gynecol 140:815–830 [DOI] [PubMed] [Google Scholar]
- Goebelsmann U, Bernstein GS, Gale JA, Kletzky OA, Nakamura RM, Coulson AH, Korelitz JJ 1979 Serum gonadotropin testosterone estradiol and estrone levels prior to and following bilateral vasectomy. In: Lepow, IH, Crozier R, eds. Vasectomy: immunologic and pathophysiologic effects in animals and man. New York: Academic Press; 165 [Google Scholar]
- Serafini P, Ablan F, Lobo RA 1985 5α-Reductase activity in the genital skin of hirsute women. J Clin Endocrinol Metab 60:349–355 [DOI] [PubMed] [Google Scholar]
- Ji Q, Chang L, VanDenBerg D, Stanczyk FZ, Stolz A 2003 Selective reduction of AKR1C2 in prostate cancer and its role in DHT metabolism. Prostate 54:275–289 [DOI] [PubMed] [Google Scholar]
- Ji Q, Aoyama C, Nien YD, Liu PI, Chen PK, Chang L, Stanczyk FZ, Stolz A 2004 Selective loss of AKR1C1 and AKR1C2 in breast cancer and their potential effect on progesterone signaling. Cancer Res 64:7610–7617 [DOI] [PubMed] [Google Scholar]
- O’Donnell L, Stanton PG, Wreford NG, Robertson DM, McLachlan RI 1996 Inhibition of 5α-reductase activity impairs the testosterone-dependent restoration of spermiogenesis in adult rats. Endocrinology 137:2703–2710 [DOI] [PubMed] [Google Scholar]
- White PC 2001 Synthesis and metabolism of corticosteroids. In: Becker KL, ed. Principles and practice of endocrinology and metabolism. 3rd ed. Philadelphia: Lippincott Williams, Wilkins; 708–710 [Google Scholar]
- Griffin JE, Punyashthiti K, Wilson JD 1976 Dihydrotestosterone binding by cultured human fibroblasts. Comparison of cells from control subjects and from patients with hereditary male pseudohermaphroditism due to androgen resistance. J Clin Invest 57:1342–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TR, Rothwell SW, Migeon CJ 1981 Comparison of methyltrienolone and dihydrotestosterone binding and metabolism in human genital skin fibroblasts. J Steroid Biochem 14:1013–1022 [DOI] [PubMed] [Google Scholar]
- Horton R, Hawks D, Lobo R 1982 3α,17β-Androstanediol glucronide in plasma. J Clin Invest 69:1203–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo RA, Paul WL, Gentzschein E, Serafini PC, Catalino JA, Paulson RJ, Horton R 1987 Production of 3α-androstanediol glucuronide in human genital skin. J Clin Endocrinol Metab 65:711–714 [DOI] [PubMed] [Google Scholar]
- Duffy DM, Legro RS, Chang L, Stanczyk FZ, Lobo RA 1995 Metabolism of dihydrotestosterone to 5α-androstane-3α,17β-diol glucuronide is greater in the peripheral compartment than in the splachnic compartment. Fertil Steril 64:736–739 [DOI] [PubMed] [Google Scholar]
- Carmina E, FZ Stanczyk, Gentzchein E, Lobo RA 1995 Time-dependent changes in serum 3α-androstanediol glucuronide correlate with hirsutism scores after ovarian suppression. Gynecol Endocrinol 9:215–220 [DOI] [PubMed] [Google Scholar]
- Shiraishi H, Ishikura S, Matsuura K, Deyashiki Y, Ninomiya M, Sakai S, Hara A 1998 Sequence of the cDNA of a human dihydrodiol dehydrogenase isoform (AKR1C2) and tissue distribution of its mRNA. Biochem J 334:399–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz A, Sugiyama Y, Kuhlenkamp J, Kaplowitz N 1984 Identification and purification of a 36 kDa bile acid binder in human hepatic cytosol. FEBS Lett 177:31–35 [DOI] [PubMed] [Google Scholar]
- Rizner T, Lin HK, Peehl DM, Steckelbroeck S, Bauman DR, Penning TM 2003 Human type 3 3α-hydroxysteroid dehydrogenase (AKR1C2) and androgen metabolism in prostate cells. Endocrinology 144:2922–2932 [DOI] [PubMed] [Google Scholar]
- Serafini P, Lobo RA 1985 Increased 5α-reductase activity in idiopathic hirsutism. Fertil Steril 43:74–78 [PubMed] [Google Scholar]
- Skalba P, Dabkowska-Huc A, Kazimierczak W, Samojedny A, Samojedny MP, Chelmicki Z 2005 Content of 5-α-redutase (type 1 and type 2) mRNA in dermal papillae from the lower abdominal region in women with hirsutism. Exp Dermatol 31:564–570 [DOI] [PubMed] [Google Scholar]
- Goodarzi MO, Shah NA, Antoine HJ, Pall M, Guo X, Azziz R 2006 Variants in the 5α-reductase type 1 and type 2 genes are associated with polycystic ovary syndrome and the severity of hirsutism in affected women. J Clin Endocrinol Metab 91:4085–4091 [DOI] [PubMed] [Google Scholar]
- Carmina E, Stanczyk FZ, Matteri RK, Lobo RA 1991 Serum androsterone conjugates differentiate between acne and hirsutism in hyperandrogenic women. Fertil Steril 55:872–876 [PubMed] [Google Scholar]