Abstract
Introduction: A severe burn causes increased levels of urine cortisol and catecholamines. However, little is known about the magnitude of this increase or how and when the levels return to normal. The purpose of this study was to determine in a large clinical prospective trial the acute and long-term pattern of urine cortisol and catecholamine expression in severely burned children.
Methods: Pediatric patients with burns greater than 40% total body surface area (TBSA), admitted to our unit over a 6-yr period, were included into the study. Clinical data including length of stay, number of operations, and duration and number of infections were determined. Patients had regular 24-h urine collections during their acute admission and reconstructive periods. Urine collections were analyzed for cortisol, epinephrine, and norepinephrine. Each urine cortisol was compared with age-adjusted reference ranges. Ninety-five percent confidence intervals and ANOVA analysis were used where appropriate.
Results: Two hundred twelve patients were included in the study (75 females and 137 males), with a mean ± sem TBSA of 58 ± 1% (third-degree 45 ± 2%) and mean age of 9 ± 0.4 yr. Urinary cortisol levels were significantly increased (3- to 5-fold) up to 100 d after the burn and then approached normal levels (P < 0.05). The rise in urine cortisol was significantly higher in male than female patients (P < 0.05). Early hypercortisolemia was associated with increased duration of severe infection (P < 0.05). Persistent hypercortisolemia was associated with increases in both infection rates and duration of severe infection (P < 0.05). Urinary catecholamines showed a significant increase at 11–20 d after the burn (P < 0.05). Urinary norepinephrine levels were significantly increased up to 20 d and then returned to normal (P < 0.05).
Conclusions: Urinary levels of cortisol, epinephrine, and norepinephrine are significantly increased after a major burn. Early hypercortisolemia is associated with increased duration of severe infection. Persistent hypercortisolemia is associated with increases in both infection rates and duration of severe infection.
In pediatric patients with severe burns greater than 40% of their total body surface, early and persistent hypercortisolemia is associated with increased infection rates and duration of severe infection, respectively.
The typical response to stress necessitates the actions of several different biological systems, namely autonomic, endocrine, and immune systems (1). The patient’s response immediately after major thermal injury includes both emotional and physical stress. This rapid response is brought about by sudden increases in sympathetic nervous system activity and endogenous stress hormone levels (2,3). The developmental reason for this response is to allow the body to overcome a transient stressful event or survivable trauma. However, after severe burns, this response continues for a protracted period of time after the initial injury because the stressor remains as a source of stimulation. The concept of acute and chronic stressful responses was described in a classical work by Hans Selye (4) in which he described a short alarm reaction followed by a prolonged resistance phase. It is this persistent stress response that results in the deleterious sequelae including longstanding hypermetabolism, substantial loss of lean body mass, and immune suppression. Severe burns covering more than 40% total body surface area (TBSA) are typically followed by a period of hypermetabolism and catabolism that can last for more than 24 months after injury (5,6,7). The reasons for the changes seen in metabolism are unclear, and the pathways are poorly defined. However, there is a marked and sustained increase in endogenous immunosuppressive stress hormones including glucocorticoids and catecholamines secondary to up-regulation of the hypothalamic-pituitary-adrenal (HPA) axis. Stimulation of this axis may be due, in part, to internal cooling of the patient leading to catecholamine driven increases in resting energy expenditure (REE) to restore normal body temperature (8).
Increases in cortisol levels have been shown to reflect the severity of injury in traumatic insult (9). Significant increases in cortisol (10,11,12,13,14) and catecholamines (15,16) are seen after major burns and thought to initiate the cascade of events leading to the hypermetabolic response with its ensuing catabolic state. This large initial rise in cortisol has been shown to lead to increased REE (17) and short-term bone loss (14) and reduce T helper lymphocyte proliferation with a subsequent reduction in the patient’s ability to fight infection (18). Large increases in cortisol levels have also been shown to increase net protein breakdown of skeletal muscle (19) and increase efflux of intracellular amino acids (17). Little is know about the magnitude of the change in hormone production or how and when the levels return to normal. One study involving 14 pediatric patients showed an 8-fold increase in cortisol levels after severe thermal injury and linked this to a marked decrease in type I collagen and bone formation, together with a lack of detectable surface osteoblasts and a reduction in biochemical markers of osteoblast differentiation (14). The purpose of this study was to determine, in a large clinical prospective trial, the acute and long-term pattern of urine cortisol and catecholamine expression in severely burned children.
Patients and Methods
Two hundred twelve patients admitted to our hospital with burns over 40% TBSA were entered into the study after informed consent was obtained. This study was approved by the Institutional Review Board for Human Studies at the University of Texas Medical Branch, Galveston. Twenty-four-hour urine collections were taken regularly throughout acute hospital stay and during admissions for reconstructive operations. These samples were collected and chilled by the bedside before transport to our clinical lab for processing using a Dynex Technologies (Chantilly, VA) DSX processing system and an ELISA kit from Diagnostic Systems Laboratories (Webster, TX).
Urinary catechloamine levels were measured by HPLC, using commercially available kits from Bio-Rad (Hercules, CA) with manufacturer’s instructions.
Free cortisol levels were measured using an ELISA kit from Diagnostic System Laboratories/Beckman Coulter (Webster, TX) on automatic Dyonex DSX instrument. Total cortisol levels are measured in our lab by liquid-liquid extraction followed by HPLC method. It is important to note that free cortisol levels are affected by the amount of binding protein available, whereas total cortisol levels are an indicator of cortisol secretion. Cortisol binding protein levels are very low during the acute phase post burn and they increase over time during recovery; therefore free cortisol levels decrease faster than total cortisol levels. In this study we chose to measure free cortisol because it was the established clinical laboratory method at the time.
Admission data
On admission, the extent and degree of burn was assessed and recorded on a standard Lund and Browder chart by the attending burns surgeon present. Information also recorded at the time of admission included burn-related (date and mechanism) as well as demographic data (age, gender, and ethnicity). All thermally injured children with burns over 30–40% of their TBSA who consented to an Institutional Review Board-approved experimental protocol between 1998 and 2007 and who were admitted to our burn unit and required at least one surgical intervention were included in this study. Patients were resuscitated according to the Galveston formula with 5000 ml/m2 TBSA burned + 2000 ml/m2 TBSA lactated Ringer’s solution given in increments over the first 24 h. Within 48 h of admission, all patients underwent total burn wound excision, and the wounds were covered with available autograft skin, and any remaining open areas were covered with homograft. After the first operative procedure, it took 5–10 d until the donor site was healed, and patients were then taken back to the operation theater. This procedure was repeated until all open wound areas were covered with autologous skin material.
All patients underwent the same nutritional treatment according to a standardized protocol. The intake is calculated as 1500 kcal/m2 body surface + 1500 kcal/m2 area burn, or we assessed the need by measuring the REE, multiplying by 1.4 with weekly adjustments as previously published (5,6,20). The nutritional route of choice in our patient population was enteral nutrition. Therefore, almost all patients received nutrition via a duodenal (Dobhof) or nasogastric tube. Parenteral nutrition was given only in rare instances if the patient did not tolerate any tube feeds.
Statistical analysis
Elevation of cortisol was evaluated by examining the 95% confidence interval of the mean. Differences between male and female groups were evaluated with two-way ANOVA with factors sex and time followed by Tukey’s test. Expression of epinephrine and norepinephrine were evaluated over time relative to more than 500 d using one-way ANOVA of ranks followed by Dunn’s method.
Results
Two hundred twelve patients, 137 male and 75 female, with burns in excess of 40% TBSA were enrolled into the study. Each patient had regular 24-h urine collections taken both during the acute hospital admission and subsequent stays for reconstructive purposes. Patient characteristics are depicted in Table 1 as means ± sd.
Table 1.
Demographics of patient population
| Males | Females | |
|---|---|---|
| n | 137 (65%) | 75 (35%) |
| Age (yr)a | 9.5 ± 5.1 | 6.7 ± 4.8 |
| TBSA (%)a | 58.7 ± 16.9 | 56.8 ± 14.9 |
| TBSA (% third-degree)a | 45.6 ± 24.1 | 44.4 ± 21.6 |
Mean ± sd.
We divided the results into seven different time points: 0–10 (admission), 11–20 (acute reconstruction), 21–40 (preparation for discharge), 41–100 (discharge), 101–200 (first reconstruction operation appointment), 201–500 (subsequent reconstruction admissions), and more than 500 d (long-term outpatient appointments). The time points chosen reflect the typical time course for pediatric patients through our burn unit.
If any patient had more than one urine collection taken during each time point, the results were averaged to give a single mean result for each patient at each time point. We then compared the results found against standard age-adjusted reference ranges (0–10 yr, 2–27 μg/24 h; 10–20 yr, 5–55 μg/24 h).
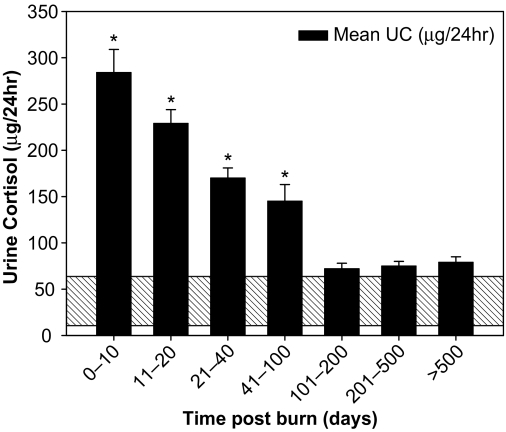
The mean urine cortisol increased initially to 284 ± 26 μg/24 h and then fell steadily over the next 3 months to settle close to the normal range at about 100 d after the burn. This is a 5- to 6-fold increase from normal resting urine cortisol levels (Fig. 1). In addition, we normalized urine cortisol to body surface area, giving us the urine cortisol index for each patient; the resulting data mirror the raw cortisol data shown in Fig. 1.
Figure 1.
Twenty-four-hour urine cortisol (UC) increases by an average of 5- to 6-fold initially followed by a decrease over time to around the normal range at 100 d after the burn (mean ± sem). *, P < 0.05 vs. normal range depicted at base of graph (shaded area).
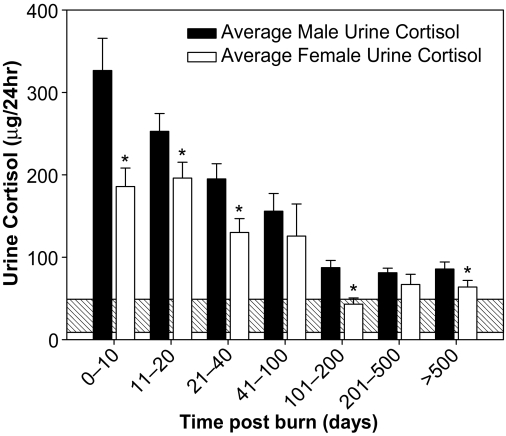
We have previously reported a difference in hypermetabolic response between the groups with higher REE in males than females (6). In light of this, we looked at the differences between males and females as an internal standard control. We found marked differences between the initial urine cortisol values (Fig. 2) (P < 0.05). The male patients had a significantly greater increase in urine cortisol that was sustained to around 40 d after the burn compared with females (P < 0.05). Thereafter, the levels between the groups were very similar, arriving close to the normal range at approximately 3 months after the burn (Fig. 2).
Figure 2.
Difference in male and female urine cortisol expression initially (P < 0.05), although the reduction to normal range is the same for both groups at about 3 months after initial injury (mean ± sem).
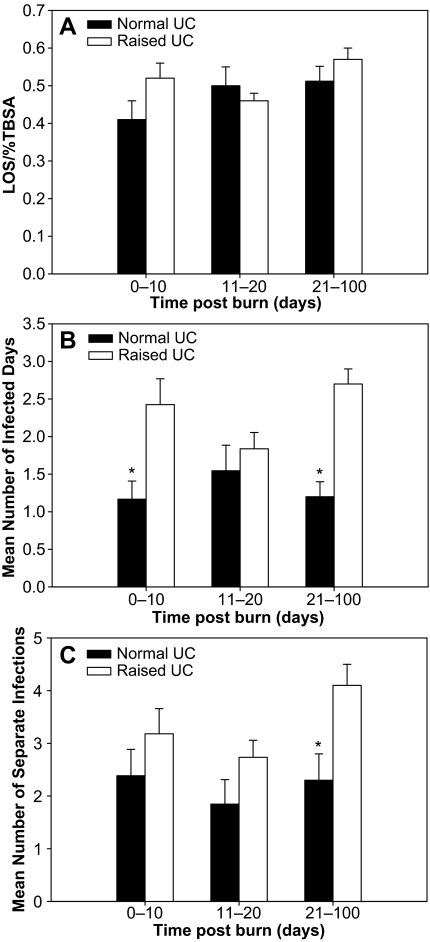
When we correlated the intensity of endocrine activation with outcome, we compared those patients with raised urine cortisol against those within the normal range over the first 100 d after injury. We showed that there were no significant differences in length of stay between the two groups at any time in the first 100 d (Fig. 3A). Hypercortisolemia in patients during the first 10 d after the burn was associated with a significant increase in duration of severe infection (Fig. 3B), whereas persistent hypercortisolemia was associated with a significant increase in both duration and number of separate severe infections (P < 0.05; Fig. 3, B and C). No correlation was found between hypercortisolemia or normocortisolemia and mortality or burn size at any time point.
Figure 3.
A, Normal vs. raised urine cortisol (UC) vs. length of stay (LOS) (normalized for TBSA). No significant differences are seen at any time point (mean ± sem). B, Urine cortisol vs. duration of severe infection. Acute and persistent hypercortisolemia are significantly associated with increased duration of severe infection (mean ± sem) *, P < 0.05. C, Urine cortisol vs. duration of severe infection. Persistent hypercortisolemia is significantly associated with increased number of severe infections (mean ± sem). *, P < 0.05.
No significant association was found between increased stress hormone release and mortality. Neither was there any association between raised endogenous stress hormones and the number of operations needed during the acute admission.
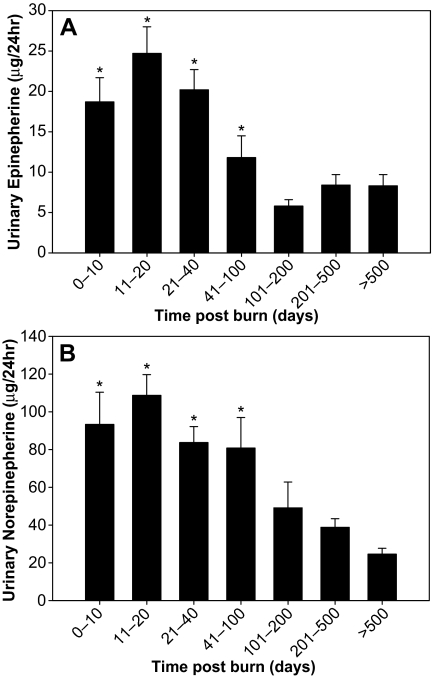
We saw similar patterns of expression in both epinephrine (Fig. 4A) and norepinephrine (Fig. 4B) with a rise initially followed by a fall to around 100 d. Due to the wide disparity in both mean and confidence intervals for reference ranges of epinephrine and norepinephrine, we cannot show that these values are outside the normal range. However, due to the size of the group evaluated, we can state with some confidence that in this case, there was a significant rise followed by decrease in both catecholamines (P < 0.001). The maximal rise on catecholamines was prolonged (11–20 d). This was an unexpected finding because it did not mirror the changes seen in cortisol production.
Figure 4.
A, Urinary epinephrine increases initially after burn injury and then decreases over time to around 100 d (mean ± sem). *, P < 0.001 vs. more than 500 d. B, Urinary norepinephrine increases initially after burn injury and then decreases over time to around 100 d (mean ± sem). *, P < 0.001 vs. more than 500 d.
Discussion
Severe burns result in major metabolic, physiological, and psychological changes in a child’s life. The typical changes in metabolism seen are the development of a hyperdynamic circulation (21), increased body temperature (22), increased protein catabolism with peripheral protein wasting (23), increased lipolysis leading to fatty infiltration of the liver (24), and increased glycolysis and futile substrate cycling (25). These changes are responsible for much of the morbidity and mortality seen with such an injury and as such are important targets for available treatments, including early excision and grafting, aggressive treatment of sepsis, early commencement of high-protein high-carbohydrate enteral feeding, elevation of the immediate environmental temperature to 31.5 C (±0.7 C), and early institution of an aerobic resistive exercise program (26). Over the last 20 yr, great strides have been made in these areas during the initial treatment of massive thermal injuries (26), resulting in decreased morbidity and mortality. The exact cause of the hypermetabolic response is still inadequately understood and the biochemical pathways poorly defined. We know that there is a marked increase in stress hormones including glucocorticoids and catecholamines (14,27). This initial increase is due to stimulation of the HPA axis which should, under normal conditions, be controlled via negative feedback by the products of adrenal stimulation. During the prolonged phase of recovery from severe burns, which typically can be weeks to months, there is a dissociation between high-plasma cortisol and low ACTH levels, which implies non-ACTH-mediated mechanisms for regulation of the adrenal cortex (28,29). Additional circulating factors such as cytokines might suppress ACTH synthesis and secretion. Factors such as endothelin-1 (30) and atrial natriuretic peptides (30,31) are elevated at stages when ACTH is suppressed.
The normal circadian rhythm for serum cortisol is lost after a severe burn (32,33); therefore, for this study, urinary cortisol was taken as a more accurate measure of the total daily production of cortisol by the patient. A limitation of this study is that we were unable to elucidate the time course for the resumption of normal circadian changes in serum cortisol level. This is the focus of ongoing research. A further limitation of this study was that unfortunately, most of the patients were admitted to our unit from abroad, mainly from Mexico and South America. Therefore, we were unable to analyze data from the first few hours after injury.
After a severe injury, cortisol levels increase in proportion to the severity of traumatic insult (9). This increase in cortisol, as well as the increase in circulating catecholamines after a massive burn, initiates a cascade of events leading to the hypermetabolic response with its ensuing catabolic state. This large initial rise in cortisol has been shown to lead to increased REE (17), short-term bone loss (14), and reduced T helper lymphocyte proliferation with a subsequent reduction in the patient’s ability to fight infection (18). Large increases in cortisol levels have also been shown to increase net protein breakdown of skeletal muscle (19) and increase efflux of intracellular amino acids (17). Increases in catecholamines lead to a hyperdynamic circulation (34), loss of lean body mass (35), and increased lipolysis (25), principally in the periphery leading to increases in fatty deposition of the liver (36). However, little is known about the magnitude of increase in either catecholamines or cortisol or indeed when they return to more normal levels. We have shown that urinary catecholamines increase initially and then reduce with time up to around 100 d. However, the significance of these changes is unclear due to the lack of tight reference ranges for pediatric patients of different ages. We have also shown in this study that urine cortisol increases in the initial postburn phase by between 4- and 6-fold. The levels then decrease over time, returning to normal range at around 3 months after injury. Furthermore, we have also shown that there is a marked difference between male and female pediatric patients in response to burn injury. Here, we have shown that although both male and female pediatric patients have raised urine cortisol after burn injury, the increase is significantly greater in male patients. If we can reduce the impact of this initial rise in cortisol, perhaps we can reduce the deleterious effects on the body and therefore improve overall outcome for the patient, restoring them to a normal, functional existence. We are currently undertaking a trial using ketoconazole, an antifungal agent that suppresses the production of cortisol as a side effect. We have had very favorable results so far with reduced infection rates (nonfungal) in those patients with attenuated cortisol production. Side effects have been minimal in our pediatric patients.
Large doses of opiates and opioids are used to reduce nociceptive perception in patients with severe thermal injury. However, the impact of opioids and opiate use in the presence of persistent stressful stimuli has yet to be fully elucidated. Opiate-mediated effects have been hypothesized to result from either direct interaction with opioid receptors on cells of the immune system (37,38) or indirectly through the activation of opioid receptors within the central nervous system and the resulting modulation of HPA axis and the sympathetic nervous system (39,40).
This study provides evidence that severe burn injury in children results in sustained changes in HPA axis dynamics, and we outline the time course for hypercortisolemia in children with severe burns. We also show that measurement of high urine cortisol may indicate an increased susceptibility to infection. Additional studies are needed to determine whether the increases seen are due to a reduced cortisol-binding globulin or increased endogenous production.
Acknowledgments
We gratefully acknowledge the assistance of Gabriella Kulp, M.S., for her invaluable assistance with sample processing.
Footnotes
This project was supported by National Institutes of Health Grants NIH P50 GM 60338, NIH T32 GM 008256, and Shriners Hospital Grants 8480 and 8660.
Disclosure Statement: We have no relationships or conflicts to disclose for any author.
First Published Online January 22, 2008
Abbreviations: HPA, Hypothalamic-pituitary-adrenal; REE, resting energy expenditure; TBSA, total body surface area.
References
- Carrasco GA, Van de Kar LD 2003 Neuroendocrine pharmacology of stress. Eur J Pharmacol 463:235–272 [DOI] [PubMed] [Google Scholar]
- Herndon DN, Barrow RE, Kunkel KR, Broemeling L, Rutan RL 1990 Effects of recombinant human growth hormone on donor-site healing in severely burned children. Ann Surg 212:424–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmore DW 1990 Pathophysiology of the hypermetabolic response to burn injury. J Trauma 30:S4–S6 [DOI] [PubMed] [Google Scholar]
- Selye H 1998 A syndrome produced by diverse nocuous agents. 1936. J Neuropsychiatry Clin Neurosci 10:230–231 [DOI] [PubMed] [Google Scholar]
- Hart DW, Wolf SE, Mlcak R, Chinkes DL, Ramzy PI, Obeng MK, Ferrando AA, Wolfe RR, Herndon DN 2000 Persistence of muscle catabolism after severe burn. Surgery 128:312–319 [DOI] [PubMed] [Google Scholar]
- Mlcak RP, Jeschke MG, Barrow RE, Herndon DN 2006 The influence of age and gender on resting energy expenditure in severely burned children. Ann Surg 244:121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przkora R, Barrow RE, Jeschke MG, Suman OE, Celis M, Sanford AP, Chinkes DL, Mlcak RP, Herndon DN 2006 Body composition changes with time in pediatric burn patients. J Trauma 60:968–971 [DOI] [PubMed] [Google Scholar]
- Harrison HN MJ, Duckett Jr JW, Mason Jr AD 1964 The relationship between energy metabolism and water loss from vaporization in severely burned patients. Surgery 203–211 [PubMed] [Google Scholar]
- Woolf PD 1992 Hormonal responses to trauma. Crit Care Med 20:216–226 [DOI] [PubMed] [Google Scholar]
- Coombes EJ, Batstone GF 1982 Urine cortisol levels after burn injury. Burns Incl Therm Inj 8:333–337 [DOI] [PubMed] [Google Scholar]
- Dolecek R 1989 Endocrine changes after burn trauma: a review. Keio J Med 38:262–276 [DOI] [PubMed] [Google Scholar]
- Hume DM, Nelson DH, Miller DW 1956 Blood and urinary 17-hydroxycorticosteroids in patients with severe burns. Ann Surg 143:316–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffries MK, Vance ML 1992 Growth hormone and cortisol secretion in patients with burn injury. J Burn Care Rehabil 13:391–395 [DOI] [PubMed] [Google Scholar]
- Klein GL, Bi LX, Sherrard DJ, Beavan SR, Ireland D, Compston JE, Williams WG, Herndon DN 2004 Evidence supporting a role of glucocorticoids in short-term bone loss in burned children. Osteoporos Int 15:468–474 [DOI] [PubMed] [Google Scholar]
- Goodall M, Stone C, Haynes Jr BW 1957 Urinary output of adrenaline and noradrenaline in severe thermal burns. Ann Surg 145:479–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan GM, Becker RA, Allen JP, Goodwin Jr CW, Pruitt Jr BA, Mason Jr AD 1982 Cortisol and corticotrophin in burned patients. J Trauma 22:263–273 [DOI] [PubMed] [Google Scholar]
- Brillon DJ, Zheng B, Campbell RG, Matthews DE 1995 Effect of cortisol on energy expenditure and amino acid metabolism in humans. Am J Physiol 268:E501–E513 [DOI] [PubMed] [Google Scholar]
- O’Sullivan ST, Lederer JA, Horgan AF, Chin DH, Mannick JA, Rodrick ML 1995 Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg 222:482–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore DC, Jahoor F, Wolfe RR, Herndon DN 1993 Acute response of human muscle protein to catabolic hormones. Ann Surg 218:679–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart DW, Wolf SE, Chinkes DL, Gore DC, Mlcak RP, Beauford RB, Obeng MK, Lal S, Gold WF, Wolfe RR, Herndon DN 2000 Determinants of skeletal muscle catabolism after severe burn. Ann Surg 232:455–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch MJ, Feldman RJ, Walker HL, Foley FD, Popp RL, Mason Jr AD, Pruitt Jr BA 1973 Systemic and pulmonary hemodynamic changes accompanying thermal injury. Ann Surg 178:218–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YM, Tompkins RG, Ryan CM, Young VR 1999 The metabolic basis of the increase of the increase in energy expenditure in severely burned patients. JPEN J Parenter Enteral Nutr 23:160–168 [DOI] [PubMed] [Google Scholar]
- Herndon DN, Ramzy PI, DebRoy MA, Zheng M, Ferrando AA, Chinkes DL, Barret JP, Wolfe RR, Wolf SE 1999 Muscle protein catabolism after severe burn: effects of IGF-1/IGFBP-3 treatment. Ann Surg 229:713–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barret JP, Jeschke MG, Herndon DN 2001 Fatty infiltration of the liver in severely burned pediatric patients: autopsy findings and clinical implications. J Trauma 51:736–739 [DOI] [PubMed] [Google Scholar]
- Wolfe RR, Herndon DN, Jahoor F, Miyoshi H, Wolfe M 1987 Effect of severe burn injury on substrate cycling by glucose and fatty acids. N Engl J Med 317:403–408 [DOI] [PubMed] [Google Scholar]
- Herndon DN, Tompkins RG 2004 Support of the metabolic response to burn injury. Lancet 363:1895–1902 [DOI] [PubMed] [Google Scholar]
- Wilmore DW, Long JM, Mason Jr AD, Skreen RW, Pruitt Jr BA 1974 Catecholamines: mediator of the hypermetabolic response to thermal injury. Ann Surg 180:653–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JP, Vinson GP, Kapas S, Teja R 1991 The role of endothelin in the control of adrenocortical function: stimulation of endothelin release by ACTH and the effects of endothelin-1 and endothelin-3 on steroidogenesis in rat and human adrenocortical cells. J Endocrinol 128:275–280 [DOI] [PubMed] [Google Scholar]
- Schuetz P, Muller B 2006 The hypothalamic-pituitary-adrenal axis in critical illness. Endocrinol Metab Clin North Am 35:823–838, x [DOI] [PubMed] [Google Scholar]
- Vermes I, Beishuizen A, Hampsink RM, Haanen C 1995 Dissociation of plasma adrenocorticotropin and cortisol levels in critically ill patients: possible role of endothelin and atrial natriuretic hormone. J Clin Endocrinol Metab 80:1238–1242 [DOI] [PubMed] [Google Scholar]
- Morgenthaler NG, Struck J, Christ-Crain M, Bergmann A, Muller B 2005 Pro-atrial natriuretic peptide is a prognostic marker in sepsis, similar to the APACHE II score: an observational study. Crit Care 9:R37–R45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni A, Warpeha RL, Brizio-Molteni L, Albertson DF, Kaurs R 1979 Circadian rhythms of serum aldosterone, cortisol and plasma renin activity in burn injuries. Ann Clin Lab Sci 9:518–523 [PubMed] [Google Scholar]
- Hobson KG, Havel PJ, McMurtry AL, Lawless MB, Palmieri TL, Greenhalgh DD 2004 Circulating leptin and cortisol after burn injury: loss of diurnal pattern. J Burn Care Rehabil 25:491–499 [DOI] [PubMed] [Google Scholar]
- Monk DN, Plank LD, Franch-Arcas G, Finn PJ, Streat SJ, Hill GL 1996 Sequential changes in the metabolic response in critically injured patients during the first 25 days after blunt trauma. Ann Surg 223:395–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessey PQ, Jiang ZM, Johnson DJ, Smith RJ, Wilmore DW 1989 Posttraumatic skeletal muscle proteolysis: the role of the hormonal environment. World J Surg 13:465–471 [DOI] [PubMed] [Google Scholar]
- Barrow RE, Wolfe RR, Dasu MR, Barrow LN, Herndon DN 2006 The use of β-adrenergic blockade in preventing trauma-induced hepatomegaly. Ann Surg 243:115–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DJ, DeCosta BR, Kim CH, Jacobson AE, Guarcello V, Rice KC, Blalock JE 1989 Opioid receptors on cells of the immune system: evidence for δ- and κ-classes. J Endocrinol 122:161–168 [DOI] [PubMed] [Google Scholar]
- Chuang TK, Killam Jr KF, Chuang LF, Kung HF, Sheng WS, Chao CC, Yu L, Chuang RY 1995 μ-Opioid receptor gene expression in immune cells. Biochem Biophys Res Commun 216:922–930 [DOI] [PubMed] [Google Scholar]
- Carr DJ, Rogers TJ, Weber RJ 1996 The relevance of opioids and opioid receptors on immunocompetence and immune homeostasis. Proc Soc Exp Biol Med 213:248–257 [DOI] [PubMed] [Google Scholar]
- Molina PE 2006 Opioids and opiates: analgesia with cardiovascular, haemodynamic and immune implications in critical illness. J Intern Med 259:138–154 [DOI] [PubMed] [Google Scholar]