Abstract
Context: Endothelial dysfunction is common in patients with GH deficiency who are at increased risk for premature cardiovascular death. GH regulates vascular tone and reactivity in humans.
Objective: Our objective was to explore the mechanisms underlying the GH’s acute vascular effects.
Design and Study Setting: There were 10 healthy, lean and young, volunteers studied after an overnight fast. GH was infused systemically for 6 h at 0.06 μg/kg·min. Biopsy of the vastus lateralis muscle was done in seven subjects before and after GH infusion. Human aortic endothelial cells (HAECs) were incubated with GH in vitro.
Results: GH infusion increased plasma GH to 32.9 ± 1.5 ng/ml and forearm blood flow by 66% (P < 0.001). GH infusion did not significantly change plasma IGF-I concentrations, muscle IGF-I mRNA expression, and muscle Akt phosphorylation, suggesting a lack of IGF-I action in muscle. Because it was reported that GH exerts an acute vascular effect via a nitric oxide (NO)-dependent mechanism, we performed additional in vitro experiments using HAECs. HAECs express abundant GH receptors. Incubating HAECs with GH at 30 ng/ml for 3 or 6 h did not alter endothelial NO synthase (eNOS) protein content but time dependently increased the phosphorylation and activity of eNOS, thus demonstrating a direct effect of GH on endothelial cells.
Conclusions: GH exerts an acute vascular effect independent of both systemic and local IGF-I production, and this effect is likely via direct action on GH receptors and eNOS in the vascular endothelium.
A biopsy study of the vastus lateralis muscle in 10 lean, young volunteers given a 6-hour GH infusion demonstrates an acute vascular effect independent of both systematic and local IGF-I production. This effect is likely due to the direct action on GH receptors and endothelial nitric oxide synthase in the vascular endothelium.
Patients with GH deficiency have an increased risk of cardiovascular death (1). It appears that endothelial dysfunction may have played a major role in this increased cardiovascular disease risk (1) because previous evidence confirms that endothelial dysfunction, either in a peripheral conduit artery or in the coronary circulation, predicts coronary events (2).
Although the mechanisms underlying endothelial dysfunction in patients with GH deficiency remain unclear, ample evidence suggests that GH plays an important role in regulating peripheral vascular resistance and vascular reactivity, and in maintaining normal vascular function in humans. Patients with GH deficiency have increased peripheral vascular resistance and decreased forearm blood flow response to the endothelium-dependent vasodilator acetylcholine, whereas GH replacement restores the response back to normal (3). In healthy humans, GH infusion acutely lowers peripheral vascular resistance and increases forearm blood flow (4,5,6,7). These effects appear to be mediated by activation of the nitric oxide (NO) pathway because GH deficiency is associated with decreased systemic NO formation (8) and decreased forearm release of nitrite and cyclic GMP (cGMP) during acetylcholine stimulation (3), which revert to normal during GH replacement therapy. GH replacement also restores the peak hyperemic response to 5-min forearm ischemia (3).
GH exerts its multiple biological actions via two major pathways. It stimulates IGF-I production both systemically and locally within the tissue, and it can also directly activate the Janus kinase signal transducer and activator of transcription pathway (9,10). IGF-I is a potent stimulator of the phosphatidylinositol 3 (PI-3)-kinase/protein kinase B (Akt)/endothelial NO synthase (eNOS) pathway. On the other hand, GH can also signal directly through insulin receptor substrate (IRS)-1 to activate PI-3 kinase.
The major purpose of the present study was to explore further the mechanisms underlying the GH’s acute vascular action and whether GH at a high physiological concentration directly stimulates eNOS in cultured human aortic endothelial cells (HAECs). Our findings indicate that GH exerts acute vasodilatory action in healthy humans independent of systemic or local production of IGF-I, and this action is likely secondary to direct activation of eNOS activity in the vascular endothelium.
Subjects and Methods
In vivo human forearm study protocol (Fig. 1)
Figure 1.
Study protocol.
There were 10 healthy volunteers (seven men and three women) studied. Subjects ranged in age from 20–27 yr (24.1 ± 0.8), had an average body mass index of 22.9 ± 0.8 kg/m2, and had no history of endocrine or other major organ system disease. No subject was taking any medication, and all female participants had a negative serum pregnancy test 1–2 d before study. Informed written consent was obtained from each volunteer before the study. The study protocol was approved by the Institutional Review Board and the General Clinical Research Center Advisory Committee at the University of Virginia. After an overnight fast, two forearm venous catheters were placed percutaneously, a peripheral one for GH infusion and a retrograde, antecubital catheter for blood sampling. Each subject received a systemic infusion of GH (Nutropin; Genentech Inc., South San Francisco, CA) for 6 h at a rate of 0.06 μg/kg·min. This infusion rate increases plasma GH concentration to approximately 30 ng/ml, which corresponds to a high physiological, stress concentration (11). Blood samples were obtained at 10-min intervals during the basal period (basal period at −30, −20, −10, and 0 min), and at the end of 3-h (150, 160, 170, and 180 min) and 6-h (330, 340, 350, and 360 min) GH infusion for measurements of insulin, glucose, GH, and IGF-I. Forearm blood flow was measured after each blood sample using capacitance plethysmography. Hand blood flow was excluded by inflating a pediatric sphygmomanometer cuff about the wrist to 200 mm Hg. Biopsy of the vastus lateralis muscle was done in seven subjects before and after GH infusion, using a Bergstrom biopsy needle, as described previously (12,13). Muscle tissues were immediately frozen and stored in liquid nitrogen for later analysis of IGF-I mRNA expression and Akt phosphorylation.
In vitro cell study protocol
HAECs in primary culture (Cambrex Bio-Sciences Walkersville, Inc., Walkersville, MD) were cultured in endothelial basal medium supplemented with 5% fetal bovine serum, bovine brain extract, human epidermal growth factor (10 ng/ml), gentamycin (50 μg/ml), amphotericin-B (50 ng/ml), and hydrocortisone (1 μg/ml). After growing to 80–85% confluence, cells (passages 3–8) were serum starved for 16 h and then treated with or without GH for 3 or 6 h at a final concentration of 30 ng/ml. Control cells were treated with a similar volume of vehicle used to dissolve GH. Cells were then washed twice with ice-cold 1× PBS, lysed by sonication in ice-cold lysis buffer [50 mm Tris-HCl (pH 7.4), 150 mm NaCl, 1% NP40, 0.25% sodium deoxycholate, 1 mm EDTA, 1 mm Na orthovanadate, 1 mm NaF, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin, and 1 mm phenylmethylsulfonylfluoride]. Cell lysates were centrifuged for 5 min at 4 C (13,000 × g), and the supernatants were used for immunoprecipitation and/or Western blotting.
RNA and primers
Total RNA was extracted from the muscle samples using the TRI REAGENT, followed by purification using the RNeasy Mini Kit (QIAGEN, Valencia, CA). RNA concentrations were measured using the RiboGreen Quantitation Kit (Molecular Probes, Eugene, OR). Specific primers for the human IGF-IEa isoform, the isoform most responsive to GH in skeletal muscle, were designed using PRIMER3 (14). IGF-IEa primer sequences are: forward 5′-GTGGATGCTCTTCAGTTCGTGTGTG-3′; reverse 5′-TGCGTTCTTCAAATGTACTTCCTTCTG-3′ (249-bp product). The IGF-IEa forward primer corresponds to exon 3, and the reverse primer to the 5′ end of exon 6 and also encompasses six residues of exon 4 (15). The same exons 3, 6, and 4 are called 2, 5, and 3, respectively, in the sequence used for primer design (GenBank accession no. X57025). 18S rRNA primers are: forward 5′-TCAAGAACGAAAGTCGGAGG-3′; reverse 5′-GGACATCTAAGGGCATCACA-3′ (488-bp product) (16).
Plasmid construction
Plasmids constructed with PCR products generated with the four aforementioned specific primer pairs were cloned in the pGEM-T vector (Promega, Madison, WI) and introduced in Escherichia coli JM109 (Promega). From a selected transformant containing the desired construct, plasmid DNA was isolated using the Qiaprep Spin Miniprep Kit (QIAGEN). The DNA concentration of each resulting plasmid was measured using a Biomate Spectrophotometer (260/280 nm) (Thermo Spectronic, Rochester, NY). A serial dilution of each plasmid was used to make a standard curve for quantification.
RT-PCR
Total RNA was reverse transcribed using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA). The iCycler iQ Real-Time PCR detection system (Bio-Rad Laboratories) was used for cDNA quantification. Each quantitative reaction contained 2 mm MgCl2, 0.2 mm deoxynucleotide triphosphates, 0.35 IU JumpStart Taq DNA Polymerase with supplied buffer (Sigma-Aldrich, St. Louis, MO), 10 nm fluorescein, forward and reverse primers, SYBR Green I (1:75,000 of a 10,000 × stock solution) (Molecular Probes), cDNA (equivalent to 10 ng total RNA), and water up to a volume of 20 μl. The real-time PCR protocol consisted of 3 min at 95 C, followed by 40 cycles of 15 sec at 95 C, 40 sec at 62 C, and 45 sec at 72 C. To assess PCR specificity, melting curves from 55–95 C in 0.5 C steps of 10 sec with measurement of fluorescence were generated at the end of each PCR. A single melting peak and a single band on 2% agarose gel electrophoresis were confirmed. Samples without reverse transcriptase, and samples with cDNA but without specific templates were run as controls, showing no amplification after 40 PCR cycles. Samples were run in triplicate, and the average was interpolated in a standard curve of values generated from the plasmid dilution series. The obtained values (copy number) were normalized to 18S rRNA values (copy number).
Immunoprecipitation of GH receptor
HAECs were cultured and harvested in lysis buffer after being grown in complete endothelial growth medium to 85–90% confluent as described previously. Aliquots of supernatant containing 500 μg protein in 1000 μl lysis buffer were incubated with 2 μg anti-GH receptor polyclonal antibody (H-300; Santa Cruz Biotechnology, Inc.) overnight at 4 C. Protein A/G agarose was then added, and the mixture was kept at 4 C for 1 h with gentle rocking. After washing six times with lysis buffer, the beads were spun down (1000 × g for 30 sec), resuspended in 25 μl 2× sample buffer [375 mm Tris-HCl (pH 6.8), 12% sodium dodecyl sulfate, 60% glycerol, 300 mm dithiothreitol, and 0.06% bromophenol blue], and boiled for 5 min.
Immunoblotting
Pieces (∼10 mg) of frozen vastus lateralis muscle tissue were weighed and powdered in frozen 25 mm Tris-HCl buffer [26 mm KF and 5 mm EDTA (pH 7.5)], then disrupted by sonication. The homogenate was centrifuged at 2000 rpm for 2 min, and the supernatants were used for Western blotting. Cultured HAECs were processed as described previously. Aliquots of tissue homogenate supernatant or cell lysate supernatant containing approximately 20 μg protein or GH receptor immunoprecipitate were diluted with an equal volume of sodium dodecyl sulfate sample buffer and electrophoresed on a 10% polyacrylamide gel, transferred to nitrocellulose membranes, blocked with 5% low-fat milk in Tris-buffered saline plus 0.1% Tween 20. Subsequently, the membranes from GH receptor immunoprecipitates were probed with antibody against GH receptor and membranes from muscle homogenate with antibody against phospho-Akt (Ser473), and membranes from cell lysate with antibody against phospho-eNOS (Ser1177), phospho-Akt (Ser473), or phospho-AMP-activated protein kinase (AMPK)α (Thr172) (Cell Signaling Technology, Danvers, MA) overnight at 4 C. This was followed by a secondary antibody coupled to horseradish peroxidase, and the blots were developed using enhanced chemiluminescence (Amersham Life Sciences, Piscataway, NJ). To ensure equal loading, membranes probed with antibodies against phosphospecific antibodies were stripped and reprobed with primary antibodies against respective proteins. Autoradiographic films were scanned densitometrically (Molecular Dynamics, Piscataway, NJ) and quantitated using Imagequant 3.3. For Akt and eNOS, both the total and phospho-specific densities were quantitated and the ratios of phosphospecific to total density calculated.
eNOS activity assay
The activity of eNOS in cultured HAECs was determined using the nitric oxide synthase kit (Calbiochem, San Diego, CA), as described previously (17). The ratio of 14C-citrulline to 14C-arginine was calculated and used as an indicator of eNOS activity. For each experiment the samples were run in triplicate.
Statistical analysis
All data are presented as mean ± sem. Statistical analyses were performed with SigmaStat 3.1.1 software (Systat Software, Inc., San Jose, CA), using one-way repeated measures ANOVA with post hoc Holm-Sidak testing or two-tailed t test where appropriate. A P value of less than 0.05 was considered statistically significant.
Results
Effects of systemic GH infusion on forearm blood flow and plasma hormonal and glucose concentrations
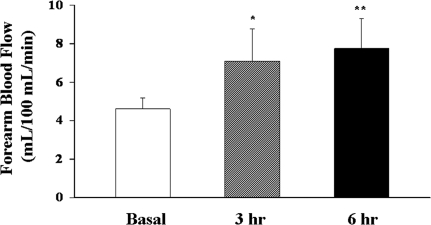
We first examined the effect of systemic GH infusion on human forearm blood flows. Systemic infusion of GH for 6 h significantly increased the forearm blood flow (Fig. 2; P < 0.001) at both 3 h (P < 0.0003) and 6 h (P = 0.0001). The basal values, 4.86 ± 0.68 ml/min·100 ml forearm volume, are similar to our previously reported values of 4.31 ± 0.74 (13) and 4.25 ± 0.5 ml/min·100 ml (12) forearm volume in healthy humans. As shown in Table 1, GH infusion increased plasma GH concentrations by approximately 50-fold to 32 ± 2 ng/ml (P < 0.001), a high physiological, stress concentration (11). Plasma IGF-I concentrations remained stable during the entire infusion period (P = 0.65), suggesting that the GH infusion duration was too short to significantly increase plasma IGF-I concentrations.
Figure 2.
Effect of GH infusion on forearm blood flow in healthy humans. GH infusion at 0.06 μg/kg·min significanty increased forearm blood flow (P < 0.001, ANOVA). Compared with basal: *, P < 0.0003; **, P = 0.0001.
Table 1.
Effect of GH on plasma glucose and hormone concentrations
| Basal | 3 h | 6 h | P value | |
|---|---|---|---|---|
| Plasma glucose (mm) | 4.1 ± 0.1 | 3.74 ± 0.3 | 3.8 ± 0.4 | 0.43 |
| GH (ng/ml) | 0.6 ± 0.3 | 29.2 ± 3.4 | 32.9 ± 1.5 | <0.001 |
| IGF-I (ng/ml) | 143 ± 15 | 128 ± 10 | 149 ± 13 | 0.65 |
| Insulin (pm) | 36 ± 4 | 42 ± 9 | 52 ± 9 | <0.03 |
Values are presented as mean ± sem.
Although systemic infusion of GH for 6 h increased plasma insulin concentrations by approximately 40% (P < 0.03), plasma glucose concentrations remained steady during GH infusion without the need for exogenous glucose infusion. Thus, the increase in plasma insulin concentrations is likely due to acute insulin resistance induced by high physiological concentrations of GH.
Effects of GH infusion on muscle IGF-I mRNA expression and Akt phosphorylation
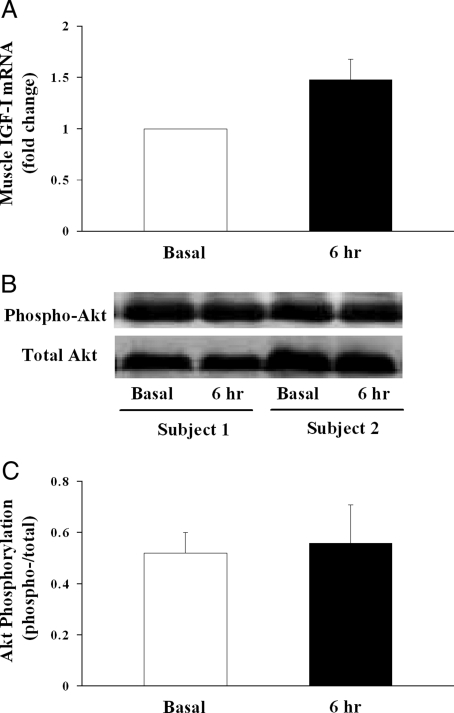
We next examined whether systemic GH infusion modulates muscle IGF-I mRNA expression because GH has been shown to stimulate local production of IGF-I within the skeletal muscle, which may lead to increased blood flow. Although we did observe in biopsied vastus lateralis muscle samples that IGF-I mRNA expression trended up after GH infusion, this increase was not statistically significant (P = 0.535; Mann-Whitney rank sum test; Fig. 3A).
Figure 3.
Effect of GH infusion on muscle IGF-I mRNA expression and Akt phosphorylation in healthy humans. A, Muscle IGF-I mRNA expression (n = 7; P = 0.535). B, Representative Akt gel from two subjects. C, Quantitative Akt phosphorylation (n = 5; P = 0.72).
Both insulin (7,18,19,20,21) and IGF-I (7,22,23,24) have been shown to increase blood flow in humans. As we have observed in the current study that plasma insulin concentrations increased significantly and muscle IGF-I mRNA expression also trended up after GH infusion, we examined the phosphorylation status of Akt, a downstream signaling protein activated by insulin or IGF-I stimulation, in biopsied muscle samples. As shown in Fig. 3, B and C, we did not observe any change in muscle Akt phosphorylation after GH infusion (P = 0.72). This, together with the lack of changes in plasma glucose concentrations, suggests a lack of either insulin or IGF-I action in the muscle after GH infusion.
HAECs express abundant GH receptors
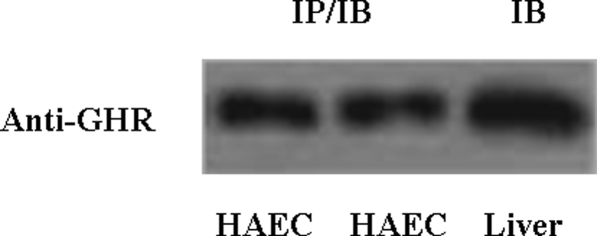
The aforementioned in vivo human data suggest that GH may act directly on the vascular endothelium to increase blood flow. To explore this possibility, we performed additional in vitro experiments using cultured HAECs. We first determined whether endothelial cells express GH receptors by immunoprecipitating and immunoblotting HAEC cell lysates with antibody against GH receptors (Fig. 4). As shown in the left two lanes of Fig. 4, HAECs do express abundant GH receptors.
Figure 4.
HAECs express abundant GH receptor (GHR). Left two lanes, HAECs containing 500 μg proteins were immunoprecipitated (IP) and immunoblotted (IB) using anti-GHR antibody. Right lane, Rat liver proteins (50 μg) were used as a positive control.
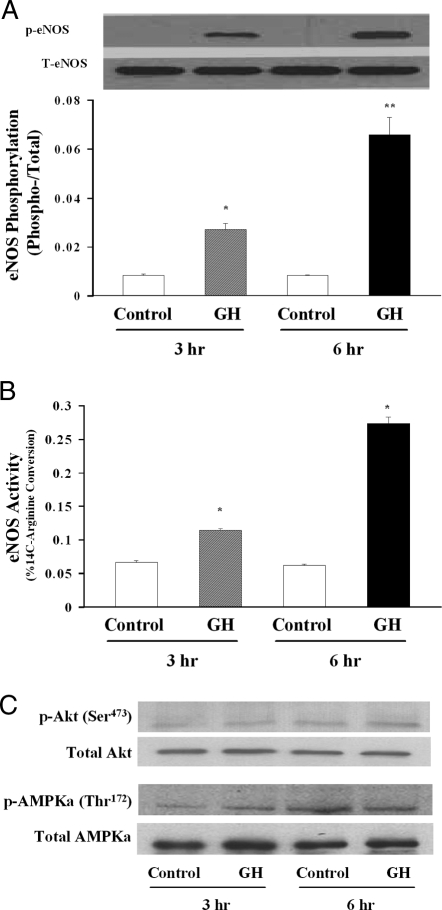
Effects of GH incubation on eNOS phosphorylation and activity in cultured HAECs
We finally examined whether GH directly activates eNOS in cultured HAECs because evidence suggests that GH may exert its acute vascular effect via a NO-dependent mechanism. We incubated HAECs with GH at 30 ng/ml (a concentration achieved during our in vivo human study; Table 1) for 3 or 6 h. GH incubation did not alter eNOS protein content but induced a time-dependent increase of Ser1177 phosphorylation of eNOS by more than 3-fold at 3 h (P < 0.005) and by more than 8-fold at 6 h (P < 0.004) (Fig. 5A). Consistent with this finding, GH also provoked a time-dependent increase of eNOS activity as measured by percentage of 14C-arginine to 14C-citrulline conversion at both 3 and 6 h (Fig. 5B; P < 0.01 for both). Because both Akt and AMPK are able to phosphorylate and activate eNOS, we examined the effect of GH on Akt and AMPK phosphorylation in cultured HAECs. As shown in Fig. 5C, incubation of HAECs with GH for 3 or 6 h did not significantly increase the phosphorylation of either Akt (Ser473) or AMPKα (Thr172).
Figure 5.
Effect of GH on eNOS phosphorylation and activity in cultured HAECs. A, eNOS phosphorylation (n = 4 for each group). Compared with respective controls: *, P < 0.005; **, P < 0.004. B, eNOS activity (n = 3 for each group). Compared with respective control: *, P < 0.01. C, Effect of GH on Akt and AMPK phosphorylation. Representative gel of two independent experiments. p, Phosphorylated; T, total.
Discussion
In the current study, systemic infusion of GH acutely increased forearm blood flow in healthy humans without significantly increasing plasma IGF-I levels, muscle IGF-I mRNA expression, or Akt phosphorylation. GH, at concentrations achieved in vivo, strongly increased eNOS phosphorylation and activity in cultured HAECs. Thus, the current results provide strong evidence that GH acutely exerts its vasodilatory action independent of IGF-I and likely via GH receptor-mediated eNOS activation.
GH has long been known to regulate blood vessel reactivity and endothelial function. GH not only enhances endothelium-dependent vasodilatation but also alters large artery structure. GH-deficient patients have impaired endothelium-dependent vasodilatory responses and increased arterial wall thickness/stiffness, which improves with GH treatment (1,3,25,26). In healthy humans GH acutely lowers peripheral vascular resistance and increases forearm blood flow (4,5,6,7). In animal studies GH enhances regional myocardial blood flow in the aged heart (27). Although the underlying mechanisms remain unclear, strong evidence implicates the involvement of the NO system. GH-deficient patients have substantially lower urinary nitrate and cGMP excretion than in controls, suggesting decreased systemic NO formation, which was normalized during GH replacement therapy (8). Similarly, GH treatment restores the forearm release of nitrite and cGMP during acetylcholine stimulation in patients with GH deficiency (3) and congestive heart failure (28), and induces a significantly increased urinary excretion rate of nitrate and cGMP in patients with dilated cardiomyopathy (29).
We did not perform an additional control infusion study because it has been well described in the past that GH, either given systemically or intraarterially (4,5,6,22), increases blood flow, and previous reports from our institute, using the exact same technique, confirmed that forearm blood flow does not change over a period of 6 h (22). Napoli et al. (4) also confirmed that forearm blood flow does not change during the 6-h period.
NO production in the vascular endothelium is controlled by the activity of eNOS, which is regulated by many factors (30,31,32,33). Previous studies have suggested that GH can regulate eNOS activity. In the cultured human endothelial cell line (EAhy926), GH at 100 ng/ml significantly increased the expression of eNOS mRNA, and protein content at 4 h and at 1000 ng/ml, the release of NO was increased (34). In the current study, we observed a time-dependent increase in eNOS phosphorylation and enzyme activity but did not observe any change in eNOS protein expression after incubating cells with high physiological concentrations of GH (30 ng/ml) for 3–6 h. This difference likely results from the different cell lines as well as GH concentrations used. Whether prolonged GH incubation at physiologically relevant concentrations also alters eNOS protein contents warrants further investigation. In hypophysectomized female rats that have normal levels of eNOS, GH treatment for 7 d resulted in an increased eNOS expression in the intima layer of the aorta (35).
Inasmuch as GH stimulates the production of IGF-I both systemically and locally in tissues (9,10), and we and others have shown that vascular endothelium expresses abundant IGF-I receptors and activation of these receptors activates the PI3-kinase/Akt/eNOS cascade (36,37,38,39), our observation of GH acutely increasing forearm blood flow without significantly increasing systemic IGF-I concentrations, muscle IGF-I mRNA expression, and Akt phosphorylation strongly argues for a direct action of GH on the vascular endothelium, independent of IGF-I, at least in the acute setting. Indeed, GH when infused locally into the brachial artery in healthy humans acutely increases forearm blood flow and NO release, and decreases forearm vascular resistance without increasing forearm IGF-I release (4) or altering systemic GH, IGF-I, insulin, or glucose concentrations (6). That acute intracoronary artery infusion of GH transiently induces coronary vasodilation and reduces coronary perfusion pressure (40) also suggests a direct action of GH on the coronary vasculature. In addition, failure to detect either IGF-Iα or IGF-Iβ transcripts in endothelial cells treated with GH at a concentration as high as 1000 ng/ml for 24 h (34) further argues against the possibility of local production of IGF-I by endothelial cells.
Despite the importance of GH in the regulation of blood vessel reactivity in humans and ample evidence suggesting that GH may have acted directly to increase blood flow independent of IGF-I, there is a paucity of data as to whether GH receptors are expressed in human vascular endothelium. This is important because GH may have a direct role in endothelial cell biology because GH may regulate endothelial proliferation (41), and loss of GH receptor in female mice results in reduced systolic blood pressure (42). GH receptor mRNA is expressed in the endothelial cells of bovine ovarian vessels (43) and in the vascular endothelium of bovine embryos (44). In the current study, we observed that cultured HAECs express abundant GH receptors. Previous studies have confirmed that GH is capable of directly stimulating the phosphorylation of IRS-1, activating PI-3 kinase, and increasing p70S6K phosphorylation in cultured adipocytes, preadipocyte, and fibroblasts (45,46,47). Although we did not examine the effect of GH on GH receptor, IRS-1 phosphorylation, and PI3-kinase activity, we did observe a significant increase in the phosphorylation and activity of eNOS, a signaling protein downstream of IRS-1 and PI3-kinase. Thus, our results suggest the possibility that GH may have acted directly in the endothelium via GH receptors to activate eNOS.
Inasmuch as insulin at physiological concentrations increases eNOS phosphorylation and activity (17,36) and increases forearm blood flow in humans (18,19), and we have in the current study observed a modest but statistically significant increase in serum insulin concentrations, it remains possible that insulin may have contributed to the observed increase in forearm blood flow. However, this appears highly unlikely because the increase in insulin concentrations is very modest, and plasma glucose concentrations remained stable throughout the study. In addition, we did not observe any change in skeletal muscle Akt phosphorylation. This is consistent with a previous study demonstrating GH blunting insulin’s antiproteolytic action in humans when coinfused locally into the brachial artery (48). An additional argument against the potential role of insulin is the fact that after 3-h GH infusion, insulin was marginally affected, whereas blood flow already reached a quasi-maximal response to GH. Indeed, the vasodilatory effect of insulin occurs at much higher concentrations than what we have observed in the current study. We have recently reported a 53% increase in brachial artery blood flow after increasing plasma insulin concentrations by more than 7-fold, to approximately 200 pm (18). In the current study, GH increased forearm blood flow by 46% at 3 h and 60% at 6 h in the presence of only a 17% increase in insulin at 3 h and only a 40% increase at 6 h. Together, the increase in plasma insulin concentrations during GH infusion likely represents mild insulin resistance and did not significantly contribute to the increased blood flow observed.
In summary, our results indicate that: 1) GH exerts an acute vasodilatory effect independent of both systemic and local IGF-I production, 2) HAECs express an abundant amount of GH receptors, and 3) GH causes a time-dependent increase in the phosphorylation and activity of eNOS. These results strongly suggest that GH regulates vascular reactivity likely via a direct action on the GH receptor in the vascular endothelium to increase eNOS phosphorylation and activity.
Acknowledgments
We thank Eugene J. Barrett, M.D., Ph.D., for his thoughtful discussions and critical reading of this manuscript and Dr. Keiji Iida for his help in IGF-I mRNA assay.
Footnotes
This work was supported by an American Diabetes Association Clinical Research Award 7-07-CR-34 (to Z.L.), and National Institutes of Health Grants RR-15540 (to Z.L.) and RR0847 (to the University of Virginia General Clinical Research Center).
Disclosure Statement: G.L., J.-P.d.R., L.A.J., Y.W., B.G., and Z.L. have nothing to declare. M.O.T. received consulting fees from Novo Nordisk and Tercica Inc.
First Published Online January 8, 2008
Abbreviations: AMPK, AMP-activated protein kinase; cGMP, cyclic GMP; eNOS, endothelial NO synthase; HAEC, human aortic endothelial cell; IRS, insulin receptor substrate; NO, nitric oxide; PI-3, phosphatidylinositol 3.
References
- Gola M, Bonadonna S, Doga M, Giustina A 2005 Growth hormone and cardiovascular risk factors. J Clin Endocrinol Metab 90:1864–1870 [DOI] [PubMed] [Google Scholar]
- Lerman A, Zeiher AM 2005 Endothelial function: cardiac events. Circulation 111:363–368 [DOI] [PubMed] [Google Scholar]
- Capaldo B, Guardasole V, Pardo F, Matarazzo M, Di Rella F, Numis F, Merola B, Longobardi S, Sacca L 2001 Abnormal vascular reactivity in growth hormone deficiency. Circulation 103:520–524 [DOI] [PubMed] [Google Scholar]
- Napoli R, Guardasole V, Angelini V, D’Amico F, Zarra E, Matarazzo M, Sacca L 2003 Acute effects of growth hormone on vascular function in human subjects. J Clin Endocrinol Metab 88:2817–2820 [DOI] [PubMed] [Google Scholar]
- Fryburg DA, Barrett EJ 1993 Growth hormone acutely stimulates skeletal muscle but not whole-body protein synthesis in humans. Metabolism 42:1223–1227 [DOI] [PubMed] [Google Scholar]
- Fryburg DA, Gelfand RA, Barrett EJ 1991 Growth hormone acutely stimulates forearm muscle protein synthesis in normal humans. Am J Physiol 260(3 Pt 1):E499–E504 [DOI] [PubMed] [Google Scholar]
- Liu Z, Long W, Fryburg DA, Barrett EJ 2006 The regulation of body and skeletal muscle protein metabolism by hormones and amino acids. J Nutr 136(Suppl):212S–217S [DOI] [PubMed] [Google Scholar]
- Boger RH, Skamira C, Bode-Boger SM, Brabant G, von zur Muhlen A, Frolich JC 1996 Nitric oxide may mediate the hemodynamic effects of recombinant growth hormone in patients with acquired growth hormone deficiency. A double-blind, placebo-controlled study. J Clin Invest 98:2706–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roith D, Bondy C, Yakar S, Liu JL, Butler A 2001 The somatomedin hypothesis: 2001. Endocr Rev 22:53–74 [DOI] [PubMed] [Google Scholar]
- Florini J, Ewton D, Coolican S 1996 Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev 17:481–517 [DOI] [PubMed] [Google Scholar]
- Aimaretti G, Baffoni C, DiVito L, Bellone S, Grottoli S, Maccario M, Arvat E, Camanni F, Ghigo E 2000 Comparisons among old and new provocative tests of GH secretion in 178 normal adults. Eur J Endocrinol 142:347–352 [DOI] [PubMed] [Google Scholar]
- Liu Z, Jahn LA, Long W, Fryburg DA, Wei L, Barrett EJ 2001 Branched chain amino acids activate messenger ribonucleic acid translation regulatory proteins in human skeletal muscle, and glucocorticoids blunt this action. J Clin Endocrinol Metab 86:2136–2143 [DOI] [PubMed] [Google Scholar]
- Liu Z, Jahn LA, Wei L, Long W, Barrett EJ 2002 Amino acids stimulate translation initiation and protein synthesis through an Akt-independent pathway in human skeletal muscle. J Clin Endocrinol Metab 87:5553–5558 [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H 2000 Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132:365–386 [DOI] [PubMed] [Google Scholar]
- Hameed M, Orrell RW, Cobbold M, Goldspink G, Harridge SDR 2003 Expression of IGF-I splice variants in young and old human skeletal muscle after high resistance exercise. J Physiol 547(Pt 1):247–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hughes-Fulford M 2001 Human prostate cancer cells lack feedback regulation of low-density lipoprotein receptor and its regulator, SREBP2. Int J Cancer 91:41–45 [DOI] [PubMed] [Google Scholar]
- Li G, Barrett EJ, Barrett MO, Cao W, Liu Z 2007 Tumor necrosis factor-α induces insulin resistance in endothelial cells via a p38 mitogen-activated protein kinase-dependent pathway. Endocrinology 148:3356–3363 [DOI] [PubMed] [Google Scholar]
- Liu Z 2007 Insulin at physiological concentrations increases microvascular perfusion in human myocardium. Am J Physiol Endocrinol Metab 293:E1250–E1255 [DOI] [PubMed] [Google Scholar]
- Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ 2006 Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes 55:1436–1442 [DOI] [PubMed] [Google Scholar]
- Baron AD 1994 Hemodynamic actions of insulin. Am J Physiol 267(2 Pt 1):E187–E202 [DOI] [PubMed] [Google Scholar]
- Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD 1994 Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest 94:1172–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryburg DA 1994 Insulin-like growth factor I exerts growth hormone- and insulin-like actions on human muscle protein metabolism. Am J Physiol 267(2 Pt 1):E331–E336 [DOI] [PubMed] [Google Scholar]
- Pendergrass M, Fazioni E, Collins D, DeFronzo RA 1998 IGF-I increases forearm blood flow without increasing forearm glucose uptake. Am J Physiol 275(2 Pt 1):E345–E350 [DOI] [PubMed] [Google Scholar]
- Copeland KC, Nair KS 1994 Recombinant human insulin-like growth factor-I increases forearm blood flow. J Clin Endocrinol Metab 79:230–232 [DOI] [PubMed] [Google Scholar]
- Christ ER, Chowienczyk PJ, Sonksen PH, Russel-Jones DL 1999 Growth hormone replacement therapy in adults with growth hormone deficiency improves vascular reactivity. Clin Endocrinol (Oxf) 51:21–25 [DOI] [PubMed] [Google Scholar]
- Irving RJ, Carson MN, Webb DJ, Walker BR 2002 Peripheral vascular structure and function in men with contrasting GH levels. J Clin Endocrinol Metab 87:3309–3314 [DOI] [PubMed] [Google Scholar]
- Khan AS, Lynch CD, Sane DC, Willingham MC, Sonntag WE 2001 Growth hormone increases regional coronary blood flow and capillary density in aged rats. J Gerontol A Biol Sci Med Sci 56:B364–B371 [DOI] [PubMed] [Google Scholar]
- Napoli R, Guardasole V, Matarazzo M, Palmieri EA, Oliviero U, Fazio S, Sacca L 2002 Growth hormone corrects vascular dysfunction in patients with chronic heart failure. J Am Coll Cardiol 39:90–95 [DOI] [PubMed] [Google Scholar]
- Osterziel KJ, Bode-Boger SM, Strohm O, Ellmer AE, Bit-Avragim N, Hanlein D, Ranke MB, Dietz R, Boger RH 2000 Role of nitric oxide in the vasodilator effect of recombinant human growth hormone in patients with dilated cardiomyopathy. Cardiovasc Res 45:447–453 [DOI] [PubMed] [Google Scholar]
- Rabelink TJ, Luscher TF 2006 Endothelial nitric oxide synthase: host defense enzyme of the endothelium? Arterioscler Thromb Vasc Biol 26:267–271 [DOI] [PubMed] [Google Scholar]
- Forstermann U, Munzel T 2006 Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113:1708–1714 [DOI] [PubMed] [Google Scholar]
- Alderton WK, Cooper CE, Knowles RG 2001 Nitric oxide synthases: structure, function and inhibition. Biochem J 357(Pt 3):593–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Poulos TL 2005 Structure-function studies on nitric oxide synthases. J Inorg Biochem 99:293–305 [DOI] [PubMed] [Google Scholar]
- Thum T, Tsikas D, Frolich JC, Borlak J 2003 Growth hormone induces eNOS expression and nitric oxide release in a cultured human endothelial cell line. FEBS Lett 555:567–571 [DOI] [PubMed] [Google Scholar]
- Wickman A, Jonsdottir IH, Bergstrom G, Hedin L 2002 GH and IGF-I regulate the expression of endothelial nitric oxide synthase (eNOS) in cardiovascular tissues of hypophysectomized female rats. Eur J Endocrinol 147:523–533 [DOI] [PubMed] [Google Scholar]
- Li G, Barrett EJ, Wang H, Chai W, Liu Z 2005 Insulin at physiological concentrations selectively activates insulin but not insulin-like growth factor I (IGF-I) or insulin/IGF-I hybrid receptors in endothelial cells. Endocrinology 146:4690–4696 [DOI] [PubMed] [Google Scholar]
- Chisalita SI, Arnqvist HJ 2004 Insulin-like growth factor I receptors are more abundant than insulin receptors in human micro- and macrovascular endothelial cells. Am J Physiol Endocrinol Metab 286:E896–E901 [DOI] [PubMed] [Google Scholar]
- Zeng G, Quon MJ 1996 Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J Clin Invest 98:894–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JA, Montagnani M, Koh KK, Quon MJ 2006 Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 113:1888–1904 [DOI] [PubMed] [Google Scholar]
- Lorusso R, Pasini E, Cargnoni A, Ceconi C, Volterrani M, Burattin A, Valle D, Ferrari R, Giustina A 2003 Preliminary observations on the effects of acute infusion of growth hormone on coronary vasculature and on myocardial function and energetics of an isolated and blood-perfused heart. J Endocrinol Invest 26:RC1–RC4 [DOI] [PubMed] [Google Scholar]
- Rymaszewski Z, Cohen RM, Chomczynski P 1991 Human growth hormone stimulates proliferation of human retinal microvascular endothelial cells in vitro. Proc Natl Acad Sci USA 88:617–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Andersson IJ, Bollano E, Palsdottir V, Gabrielsson BG, Kopchick JJ, Skott O, Bie P, Isgaard J, Bohlooly-Y M, Bergstrom G, Wickman A 2007 Growth hormone receptor deficiency in mice results in reduced systolic blood pressure and plasma renin, increased aortic eNOS expression, and altered cardiovascular structure and function. Am J Physiol Endocrinol Metab 292:E1418–E1425 [DOI] [PubMed] [Google Scholar]
- Kolle S, Sinowatz F, Boie G, Lincoln D 1998 Developmental changes in the expression of the growth hormone receptor messenger ribonucleic acid and protein in the bovine ovary. Biol Reprod 59:836–842 [DOI] [PubMed] [Google Scholar]
- Kölle S, Sinowatz F, Boie G, Lincoln D, Palma G, Stojkovic M, Wolf E 1998 Topography of growth hormone receptor expression in the bovine embryo. Histochem Cell Biol 109:417–419 [DOI] [PubMed] [Google Scholar]
- Ridderstråle M, Degerman E, Tornqvist H 1995 Growth hormone stimulates the tyrosine phosphorylation of the insulin receptor substrate-1 and its association with phosphatidylinositol 3-kinase in primary adipocytes. J Biol Chem 270:3471–3474 [DOI] [PubMed] [Google Scholar]
- Kilgour E, Gout I, Anderson NG 1996 Requirement for phosphoinositide 3-OH kinase in growth hormone signalling to the mitogen-activated protein kinase and p70s6k pathways. Biochem J 315:517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argetsinger LS, Hsu GW, Myers Jr MG, Billestrup N, White MF, Carter-Su C 1995 Growth hormone, interferon-γ, and leukemia inhibitory factor promoted tyrosyl phosphorylation of insulin receptor substrate-1. J Biol Chem 270:14685–14692 [DOI] [PubMed] [Google Scholar]
- Fryburg DA, Louard RJ, Gerow KE, Gelfand RA, Barrett EJ 1992 Growth hormone stimulates skeletal muscle protein synthesis and antagonizes insulin’s antiproteolytic action in humans. Diabetes 41:424–429 [DOI] [PubMed] [Google Scholar]