Abstract
Context: Mechanisms underlying the brain response to hypoglycemia are not well understood.
Objective: Our objective was to determine the blood glucose level at which the hypothalamus and other brain regions are activated in response to hypoglycemia in type 1 diabetic patients and control subjects.
Design: This was a cross-sectional study evaluating brain activity using functional magnetic resonance imaging in conjunction with a hyperinsulinemic hypoglycemic clamp to lower glucose from euglycemia (90 mg/dl) to hypoglycemia (50 mg/dl).
Setting: The study was performed at the Brain Imaging Center in the McLean Hospital.
Study Participants: Seven type 1 diabetic patients between 18 and 50 yr old and six matched control subjects were included in the study.
Intervention: Hyperinsulinemic hypoglycemic clamp was performed.
Main Outcome Measures: Blood glucose level at peak hypothalamic activation, amount of regional brain activity during hypoglycemia in both groups, and difference in regional brain activation between groups were calculated.
Results: The hypothalamic region activates at 68 ± 9 mg/dl in control subjects and 76 ± 8 mg/dl in diabetic patients during hypoglycemia induction. Brainstem, anterior cingulate cortex, uncus, and putamen were activated in both groups (P < 0.001). Each group also activated unique brain areas not active in the other group.
Conclusions: This application of functional magnetic resonance imaging can be used to identify the glucose level at which the hypothalamus is triggered in response to hypoglycemia and whether this threshold differs across patient populations. This study suggests that a core network of brain regions is recruited during hypoglycemia in both diabetic patients and control subjects.
Using functional magnetic resonance imaging, hypothalamic activation is detected during declining blood glucose in both type 1 diabetic patients and non-diabetic controls at glucose levels similar to those required to trigger counterregulatory hormone release.
Hypoglycemia is a limiting factor in maintaining optimal glycemic control among type 1 diabetic patients. Recurrent hypoglycemia shifts the glycemic threshold (i.e. the plasma glucose level) at which autonomic and neuroglycopenic signals are triggered (1,2,3), leading to hypoglycemia unawareness, a potentially reversible condition in which the patient cannot sense decreasing blood glucose (BG) levels (4,5,6). This syndrome is associated with a reduction in both the magnitude of the counter-regulatory hormone response and the BG level at which these hormones are released. The combination of these factors impairs the mechanisms required to restore glucose levels toward normal (7). In addition, recurrent severe hypoglycemic events may cause structural damage to the brain (8,9,10).
An essential issue is to establish the relationship between impaired counter-regulatory responses and the impact on key brain areas during hypoglycemia. Thus far, few studies have investigated regional brain activity under hypoglycemic conditions in humans (11,12,13,14,15,16). Only one study reported glucose uptake differences in brain regions known to contain glucose sensing neurons, but because of the small size of the hypothalamus, this region was not identified, and the study was not designed to determine the time or BG level at which this change in glucose uptake occurred (13). By combining functional magnetic resonance imaging (fMRI) techniques with the hypoglycemic clamp technique (17,18), localized brain activation can be measured dynamically across a range of BG levels. Establishing a technique to measure hypothalamic response to hypoglycemia can improve our understanding of the brain’s response to hypoglycemia and can be useful in designing possible treatment interventions for patients with hypoglycemia unawareness.
Our study addressed the following three research questions: 1) Can changes in hypothalamic activity during gradual reductions in BG levels be detected by fMRI? 2) If so, does the amount of activation and BG threshold for activation differ between type 1 diabetic patients and nondiabetic control subjects? 3) Do diabetic patients and control subjects recruit the same brain regions in response to acute hypoglycemia?
Subjects and Methods
Subjects
The study sample consisted of 13 participants. Seven individuals had type 1 diabetes, and six were nondiabetic control subjects. Three additional control subjects were excluded from fMRI analysis due to head movement, leading to motion artifacts in the echo planar imaging (EPI) fMRI series (19). Hormone samples were available for four of the six control subjects and all the diabetic patients. The patients had disease duration of 10–25 yr (mean ± sd = 18.4 ± 3.5). Age of diabetes onset was 20.6 ± 8.2 yr. Body mass index measurements and fasting BG levels were obtained for all participants before testing (Table 1). Patients were excluded from this study if they had painful neuropathy, clinically significant nephropathy as evidenced by urinary albumin levels more than 300 mg/d, or proliferative retinopathy indicated through review of medical records, physical examination, or self-report. We also excluded participants with a history of psychosis, schizophrenia, cocaine, heroin, or alcohol dependence as assessed by phone screen. Any contraindications to magnetic resonance imaging (MRI), such as gunshot wound, pacemaker, pregnancy, and claustrophobia, were also exclusionary factors. In addition, three different nondiabetic participants (two male, mean age 32 ± 7.8 yr) participated in a euglycemic control study.
Table 1.
Demographical and clinical characteristics of type 1 diabetic patients and control subjects
| Clinical and demographical factors | Type 1 diabetic patients (n = 7) | Nondiabetic control subjects (n = 6) |
|---|---|---|
| Age (yr) | 39.9 ± 8.7 | 32.0 ± 6.5 |
| Gender | 5 M, 2 F | 6 M |
| Education (yr) | 17.0 ± 1.0 | 18.3 ± 2.8 |
| BMI (kg/m2) | 25.9 ± 2.4 | 25.6 ± 3.7 |
| Fasting BG (mg/dl) | 104 ± 16 | 90 ± 7 |
| HbA1c | 7.6 ± 1.2 | 5.2 ± 0.3a |
Results are reported as means ± sd. BMI, Body mass index; F, females; M, males.
P < 0.05.
Following approval from the Institutional Review Boards of both the Joslin Diabetes Center and McLean Hospital (where the MRI was performed), patients and nondiabetic control subjects provided the following information during screening: psychiatric history, handedness, medical history, current medications, height, and weight. In addition, diabetic patients provided self-reports of the number of severe hypoglycemic events leading to unconsciousness (20).
General experimental protocol
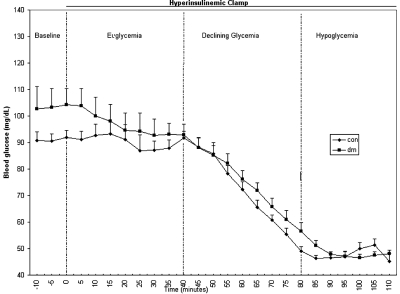
The night before the study, diabetic patients were instructed to check BG levels before and 2 h after dinner, at bedtime, and if any symptoms of hypoglycemia occurred. If glucose levels less than 70 mg/dl or more than 180 mg/dl were noted during these times, the study was rescheduled for another day. The experiment was composed of four successive time periods corresponding to different BG levels: baseline, euglycemia, declining glycemia, and hypoglycemia (Fig. 1). In the euglycemic study, the protocol was identical except that BG was maintained at 90 mg/dl during declining glycemia and hypoglycemia. The method for the insulin clamp technique is described below.
Figure 1.
Study protocol indicating levels of glycemia and BG levels for each patient group during the course of the study. con, Control; dm, diabetic patient.
Before entering the scanner, the catheters for the insulin clamp were inserted. The subjects then lay supine on the scanner bed, and were positioned within the head coil and advanced into the magnetic resonance (MR) scanner until their head coincided with the magnet isocenter. During the 20-min baseline period, the MR system calibrations, adjustments, and anatomical MRI were performed. At time zero, the insulin clamp began, and euglycemia was maintained for the next 40 min. This was followed by 40-min of declining glycemia during which the BG level was steadily lowered to a target level of 50 mg/dl. During this period, blood oxygenation level dependent (BOLD) fMRI whole brain images were acquired every 6 sec to localize brain activation related to the glycemic decline. After the declining glycemia period, the BG level was maintained at a constant hypoglycemic level of 50 mg/dl for the final 30 min.
fMRI acquisition methods
MR images were acquired using 3.0 Tesla Siemens Trio scanner (Siemens, Erlangen, Germany) using a circularly polarized birdcage radiofrequency head coil tuned to the proton frequency. Global field uniformity was adjusted at the beginning of each scanning session using Siemens’ automated shimming routines. A three-dimensional (3D), T1-weighted anatomical image was acquired using a magnetization-prepared-rapid gradient echo sequence (repetition time/echo time = 2100/2.74 msec, spatial resolution = 1 × 1 × 1.3 mm3, matrix size = 256 × 256 × 128) and later used for functional image registration. BOLD fMRIs covering the whole brain were acquired in the coronal plane using an EPI sequence (repetition time/echo time = 6000/30 msec, 41 slices, slice thickness = 4 mm, field of view = 200 × 200 mm2, matrix size = 64 × 64) with an in-plane resolution of 3.1 × 3.1 mm2. A total of 400 EPI multi-slice volumes were acquired to cover the 40-min declining glycemia period. One multi-slice volume covering the whole brain was acquired every 6 sec.
In this study, increases in the BOLD EPI signal are interpreted as increases in neural activity (or brain activation) in accordance with a widely accepted basic physiological model for BOLD fMRI (21,22). The BOLD signal depends mainly on the tissue ratio of diamagnetic oxyhemoglobin to paramagnetic deoxyhemoglobin, which is affected by regional cerebral perfusion, blood volume, and oxygen metabolic rate (22). When neural activity is increased, neurovascular coupling results in regional increases in perfusion, oxy- to deoxy-hemoglobin ratio and BOLD signal due to the difference in magnetic properties of the two forms of hemoglobin (21).
Insulin clamp technique
An iv catheter was inserted into the antecubital vein for administration of insulin and glucose, and a second catheter was inserted into a distal forearm or hand vein for withdrawal of blood samples. A heated gel pack was used to warm the hand to arterialize the venous blood (23,24). After a period of 20 min (baseline period), during which a series of structural MR images were obtained, an infusion of regular human insulin was begun at 12 pmol/kg·min (2 mU/kg·min) for 110 min. During the initial euglycemic period, the BG level was maintained at 90 mg/dl by infusion of 20% dextrose using a negative feedback algorithm as previously described (18,25,26). After 40-min of euglycemia, the glucose infusion rate was reduced to allow the BG to decline by 40 mg/dl over the next 40 min (declining glycemia). This was followed by a final 30-min period of hypoglycemia, during which the BG was maintained at 50 mg/dl. During the entire protocol, BG levels were measured every 5 min, and levels of epinephrine, glucagon, GH, cortisol, and insulin were measured every 10 min. At the end of the protocol, the insulin infusion was discontinued, the glucose infusion was increased to restore euglycemia, and the subjects were taken out of the scanner and given a meal.
Data analyses
Image processing and display were performed using Brain Voyager QX v 1.7.9 (Brain Innovation, Maastricht, The Netherlands) software running on a Pentium 4 personal computer (Intel Corp., Santa Clara, CA). Preprocessing of all functional EPI time series was performed by successively applying the following preprocessing routines available in the Brain Voyager QX software: slice scan time correction, 3D motion correction, spatial smoothing with a 6-mm full-width at half-maximum 3D Gaussian filter, and temporal smoothing with a 24-sec full-width at half-maximum Gaussian filter. Linear trends were removed from voxel time courses.
All brain images were registered to Talairach space to allow identification of all regions of interest (ROIs), in particular the hypothalamus, in Talairach coordinates (27). A cubic spline interpolation was applied to the initial EPI multi-slice image of the fMRI series to create an isotropic EPI volume with 1 × 1 × 1 mm3 voxels and distortions identical to those of the fMRI volume series. A mask was constructed from the resampled EPI volume for each person to restrict analyses to voxels within the brain.
The hypothalamus was identified on the isometric EPI volume for each participant using manual drawing guided by Talairach coordinates, the coregistered T1-weighted image volume, and landmarks (28). With the third ventricle taken as the medial limit, the right and left hypothalamic ROIs were drawn in three adjacent 1-mm thick sagittal planes to the right and left sides of the third ventricle by taking the thalamus and lateral ventricles as the superior limit, the tissue void as the inferior and anterior limits, and an anterior-posterior dimension upper limit of approximately 15 mm (27,28). The inferior wall of the third ventricle was not included. The hypothalamic ROIs defined this way may include parts of the optic chiasm, fornix, and mammillothalamic tract as small fractions of the total hypothalamic ROI volume. The time course for the hypothalamic region was examined to identify the time and the BG level at which the hypothalamus showed initial BOLD activation. The baseline BOLD signal level for the hypothalamic ROI was defined as the average BOLD signal during the first 15 min (before any hypothalamic activation) from the start of the declining glycemia period. The time of the peak in BOLD signal relative to baseline was then matched with the corresponding BG level to determine when the hypothalamus first responded to declining glycemic level.
To uncover the brain regions with activity correlated to hypothalamic activity, we performed a functional connectivity analysis. The time course of the hypothalamic region for each participant was used as the subject-specific predictor model for a multiple-subject linear correlation analysis using the general linear model (GLM) method. Other GLM predictors were used to consider variance in image intensity correlating with confounds not related to brain activation. The six motion correction time courses (values of x, y, and z translations, and rotations about the x, y, and z axes applied to align the image volume series) were used as predictors to account for variance correlating with head movement (29). The mean global image intensity time course was used to account for variance correlating with image intensity drifts due to instrumental causes. All predictors were normalized in intensity before application of the GLM. Voxels were considered significantly activated if they surpassed a threshold of P < 0.001 corrected for multiple comparisons. Regions showing significant connectivity with the hypothalamus were identified using the Talairach atlas (27) and the Talairach coordinates of significantly correlated clusters.
Standard statistical tests, including t tests for paired and unpaired data as appropriate, were used to compare glucose and hormone levels between diabetic and nondiabetic subjects during baseline, euglycemia, and hypoglycemia. All tests were conducted using a two-sided α- level of 0.05. Patient characteristics are presented in Table 1. Demographic data are presented as mean ± sd. BG and hormone data are presented as mean ± sem.
Laboratory analyses
Glucose was measured by the glucose oxidase method using a HemoCue Glucose 201 Analyzer (HemoCue, Inc., Lake Forest, CA). Glucagon (LINCO Research, Inc., St. Charles, MO), GH (immunoradiometric assay; Diagnostic System Laboratories, Inc., Webster, TX), cortisol (DiaSorin Inc., Stillwater, MN), and insulin (Diagnostic System Laboratories) were measured by RIA. Epinephrine was measured by ELISA (ALPCO Diagnostics, Salem, NH).
Results
Brain activation during induction of hypoglycemia
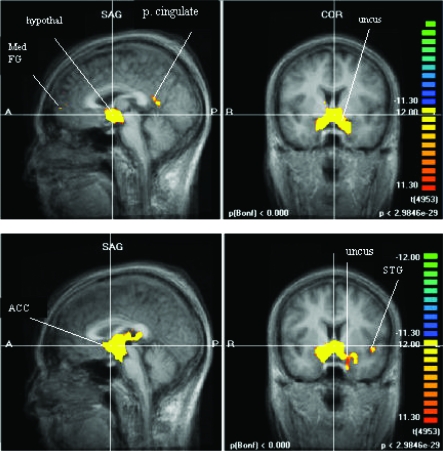
During hypoglycemic induction we evaluated the hypothalamic time course and identified other brain regions with correlated time courses (Fig. 2). This activation was consistently observed at a mean ± sd glucose level of 68 ± 9 mg/dl (range 60–76) in control subjects and 76 ± 8 mg/dl (range 68–92) in diabetic patients. This difference in BG levels across groups was not significant. Both groups showed activation in the hypothalamus, brainstem, anterior cingulate cortex, uncus, and putamen. In the control group, additional active regions included medial frontal gyrus and posterior cingulate. Regions active in only the diabetic patients were superior temporal gyrus and insula (Fig. 2 and Table 2). When we compared the relative amount of activation in brain regions that were stimulated in both groups, the hypothalamus was more active in the diabetic group, and the left uncus, left anterior cingulate, and left putamen were more active in the control group (Table 2).
Figure 2.
Network of activated regions in control subjects (top) and diabetic patients (bottom). Yellow regions indicate areas significantly activated during hypoglycemia relative to baseline. ACC, Anterior cingulate cortex; hypothal, hypothalamus; Med FG, medial frontal gyrus; p cingulate, posterior cingulate; STG, superior temporal gyrus; COR, coronal; SAG, sagittal.
Table 2.
Network of activated regions for control subjects and type 1 diabetic patients (P < 0.001)
| Region | Cluster size | Talairach coordinates (27)
|
Direction of statistical differences between regions common to both groups | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Nondiabetic control subjects (n = 6) | |||||
| L hypothalamus | 939 | 0 | −4 | −7 | DM > Cont subj |
| L uncus | 750 | −12 | −4 | −20 | Cont subj > DM |
| L putamen | 559 | −19 | 19 | −7 | Cont subj > DM |
| R brainstem | 81 | 3 | −16 | 15 | Cont subj = DM |
| L ACC | 568 | −2 | 8 | −5 | Cont subj > DM |
| R ACC | 58 | 3 | 36 | −2 | |
| L medial frontal gyrus | 18 | −4 | 60 | 4 | |
| R medial frontal gyrus | 36 | 2 | 64 | 3 | |
| L posterior cingulate | 237 | −2 | −46 | 15 | |
| Type 1 diabetic patients (n = 7) | |||||
| L hypothalamus | 985 | 0 | −4 | −7 | |
| L uncus | 79 | −20 | −2 | −26 | |
| L putamen | 244 | −43 | −4 | −6 | |
| R brainstem | 74 | 8 | −26 | −15 | |
| L ACC | 455 | −2 | 8 | −5 | |
| L brainstem (pons) | 281 | −9 | −34 | −30 | |
| L superior temporal gyrus | 234 | −46 | 4 | −7 | |
| L insula | 480 | −45 | 4 | 2 | |
Activated regions common to both groups appear in bold. ACC, Anterior cingulate cortex; Cont subj, control subjects; DM, diabetic patients; L, left; R, right.
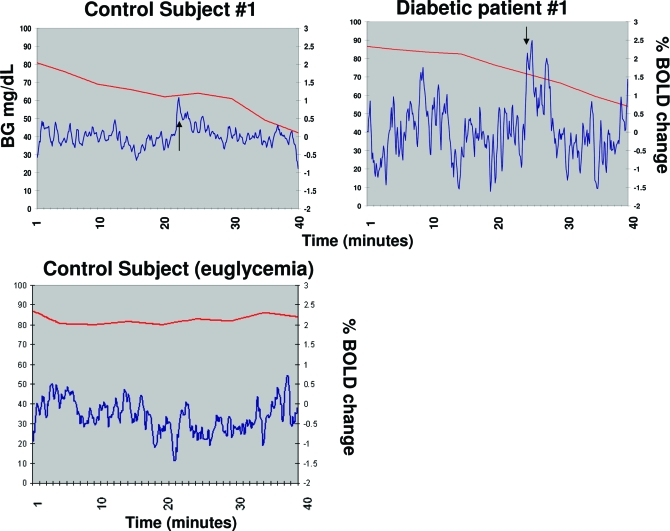
Because of the role of the hypothalamus in glucose sensing, we also specifically examined the time course of activation in the hypothalamus and determined the BG level at which the initial peak hypothalamic activation occurred for each patient. The BOLD signal intensity time course for each participant had a flat baseline during the initial 15-min declining glycemia, followed by a peak in intensity occurring between 18 and 30 min. Figure 3 shows the hypothalamic time course for a representative control subject (Fig. 3A) and a representative diabetic patient (Fig. 3B). In the euglycemic control condition (Fig. 3C), peaks of hypothalamic activation were not consistently observed as they were in the hypoglycemic condition.
Figure 3.
Hypothalamic functional activation. Percent BOLD signal change from baseline during declining glycemia in a control subject (top left), diabetic patient (top right), and control subject clamped at euglycemia (bottom). The red line shows the decrease in BG during the declining glycemia period, and the blue line indicates the percent BOLD signal change over time. Arrows indicate peak hypothalamic response. Declining glycemia was replaced by euglycemia for the patient whose data are depicted in the bottom panel.
We evaluated the rates of hypoglycemic onset to determine whether differences in fMRI results across groups could be accounted for by the time to reach a hypoglycemic state. The rates of hypoglycemia onset were the same in both groups (1.1 ± 0.04 mg/dl·min in control subjects and 0.9 ± 0.07 mg/dl·min in diabetic patients; P = not significant). The absolute glucose nadir was lower (P < 0.05) in control subjects (49 ± 2 mg/dl) than in diabetic patients (58 ± 4 mg/dl).
We also compared patients with a glycosylated hemoglobin (HbA1c) less than 7% to those with a HbA1c more than or equal to 7% to determine whether glycemic control was associated with the amount of hypothalamic activation and found that patients with lower HbA1c levels showed less activation than those with higher HbA1c levels (P < 0.001). The rate of hypoglycemic onset was similar in patients with HbA1c less than 7% (1.1 ± 0.07 mg/dl·min) and HbA1c more than or equal to 7% (0.7 ± 0.07 mg/dl·min), and the absolute glucose nadir reached between these groups did not differ.
No significant correlations were found between age and extent of brain activation or between age and peak glucose levels [age × cluster size (rho = 0.23, −0.12, and −0.21) and age × peak glucose (rho = 0.18, 0.44, and −0.42)] for the entire group, control subjects, and diabetic patients, respectively.
Glucose and hormone analyses
During the clamp, the mean ± sd BG levels at baseline, euglycemia, and the end of the hypoglycemic period were 92 ± 2, 90 ± 5, and 52 ± 7 mg/dl for the control subjects, and 104 ± 5, 97 ± 5, and 54 ± 9 mg/dl for the diabetic patients, respectively. Basal insulin levels in control subjects were 3 ± 1 μU/ml and increased to 84 ± 8 μU/ml during the clamp. The corresponding values in diabetic patients were 14 ± 6 and 78 ± 30 μU/ml. The differences between groups were not statistically significant.
The data for the counter-regulatory hormones are given in Table 3. In control subjects the concentration of all counter-regulatory hormones increased significantly during hypoglycemia as previously demonstrated (3). In type 1 diabetic patients, all hormones except glucagon also increased during hypoglycemia, and the glucagon and epinephrine responses were significantly reduced compared with the control subjects.
Table 3.
Plasma levels of counter-regulatory hormones (mean ± se) during the baseline and euglycemia periods and at the end of the hypoglycemic period in control subjects and patients with type 1 diabetes
| Baseline | Euglycemia | Hypoglycemia | |
|---|---|---|---|
| Nondiabetic control subjects (n = 4) | |||
| Epinephrine (pg/ml) | 28 ± 10 | 46 ± 8 | 555 ± 129a,b |
| Glucagon (pg/ml) | 61 ± 11 | 48 ± 7a | 175 ± 8c,d |
| GH (ng/ml) | <0.1 | 0.5 ± 0.4 | 9.7 ± 3.3a,b |
| Cortisol (μg/dl) | 11 ± 0.9 | 10 ± 1 | 20 ± 2a,b |
| Type 1 diabetic patients (n = 7) | |||
| Epinephrine (pg/ml) | 39 ± 8 | 43 ± 9 | 171 ± 102a,e |
| Glucagon (pg/ml) | 45 ± 7 | 37 ± 4 | 42 ± 4f |
| GH (ng/ml) | 0.7 ± 0.5 | 0.9 ± 0.5 | 9.0 ± 2.5a,b |
| Cortisol (μg/dl) | 16 ± 2 | 15 ± 1 | 19 ± 2b |
P < 0.05 vs. baseline.
P < 0.05 vs. euglycemia.
P < 0.01 vs. baseline.
P < 0.01 vs. euglycemia.
P < 0.05 vs. control subjects.
P < 0.001 vs. control subjects.
Discussion
The method introduced in this study can be used to determine the BG level at which specific brain regions become activated. We showed that the BG levels at which the hypothalamus activates coincide with the BG level typically associated with counter-regulatory hormone release (30,31,32). Although other neuroimaging studies have detected regional brain activation when hypoglycemia has already been achieved (12,13,14), only one has investigated brain activation in the hypothalamic region (13), and none has measured brain activation continuously during hypoglycemia or pinpointed the BG level at which hypothalamic activation is initiated. In the current study, we identified the BG level at which one of the major central glucose sensors, the hypothalamus, is activated in response to hypoglycemia in both diabetic patients and control subjects. The glucose threshold for hypothalamic activation did not differ between groups, although this may be due to the increased range of peak glucose thresholds in the diabetic group or to the small sample size.
We found that patients with higher HbA1c levels showed significantly more hypothalamic activation than those with lower HbA1c levels. This finding cannot be attributed to differences in the rate of hypoglycemia onset between these two patient groups. However, there was no difference between the BG levels at which peak hypothalamic activation occurred. The small sample size and variation in HbA1c levels (6.5–9.7%) make generalization difficult. Future studies can investigate different response patterns in clinically meaningful diabetes subgroups.
The hypothalamic region, in both patients and nondiabetic control subjects, was functionally connected to the anterior cingulate, putamen, uncus, and brainstem in response to hypoglycemia. These regions may comprise a core network that is recruited to respond to hypoglycemia. Some of these active regions (e.g. anterior cingulate, uncus) may play an important role in cognitive functioning during hypoglycemia, and it may be noteworthy that patients do not activate these regions as much as nondiabetic control subjects. The brainstem is believed to contain glucose sensing neurons and to direct glucose counter-regulatory responses to hypoglycemia (33). Thus, activation in the brainstem is consistent with the presence of glucose sensors (33) and likely corresponds to either glucose sensing or some other function related to the brain’s response to hypoglycemia. Recent positron emission tomography (14) and cerebral blood flow (34) studies involving nondiabetic subjects support our finding that the anterior cingulate is recruited during hypoglycemia (54 mg/dl). Our study evaluates brain activation at a higher BG level than Teves et al. (14), thus the results may be comparable, yet not identical (e.g. they reported thalamic activation, and we did not).
We found a network of common activated brain regions in both groups as well as several regions that responded in one group only. These findings suggest that the two groups may respond differently to hypoglycemia, and experience with prior hypoglycemia may modulate the network. The dissimilar patterns of activity between groups reflect functional differences, but it is not clear whether these are related to underlying structural changes. In our previous research in diabetic patients, we found gray matter density loss in a variety of brain regions, including the prefrontal regions that the present study demonstrated are responsive to hypoglycemia (10). Thus, the difference in functional activity between the diabetic patients and control subjects may be associated with regional gray matter loss in the prefrontal region that precludes its participation in the brain’s response to hypoglycemia.
In addition, the diabetic patients showed increased activation relative to control subjects in the left superior temporal gyrus. This brain area also showed reduced gray matter density in our previous study (10). Thus, it is possible that the reduced gray matter in this brain region may be related to the increased brain response observed in diabetic patients.
Finally, the final BG level attained during hypoglycemia was lower in control subjects than diabetic patients. Although this would not affect the stimulation of the hypothalamus during descent to hypoglycemia, it could be partly responsible for the higher epinephrine levels obtained in controls.
One of the most important contributions of our study is that it identifies the BG level at which hypothalamic activation occurs during declining glycemia, and includes both diabetic patients and control subjects. However, it is limited in the following ways:
The study requires patients to be scanned in a MR scanner in conjunction with a hyperinsulinemic clamp procedure. Both of these methods can be stressful for the patient. It is known that MRI scanning can cause anxiety (35), which can influence epinephrine levels (36). Although the epinephrine levels at baseline and during the clamp are somewhat high, we still observed a difference between groups.
Although the age difference between the diabetic patients and control subjects did not reach significance, the difference in ages was notable. However, we found that age did not contribute to extent of brain activation or to peak glucose levels.
There was a wide variation in age and duration of diabetes in our patient group.
The two groups were not gender matched. Future studies will aim to gender match the groups because women respond to hypoglycemia with both less cognitive impairment and a reduced counter-regulatory hormone response (37).
In summary, we demonstrated a method that can be applied to assess hypoglycemia and its effects on brain function. We identified the BG level at which hypothalamic activation occurred, and observed both similarities and differences in brain regions that respond to declining glycemia. This research helps elucidate the brain’s response to hypoglycemia in type 1 diabetes as a first step toward understanding how metabolic abnormalities affect brain regions responsible for glucose sensing. Finally, this study outlines a useful methodology that could have broad applications for the development of interventions and treatment approaches to the common clinical syndrome of hypoglycemia unawareness.
Acknowledgments
We thank Judi Lauerman and Karen Branch for nursing assistance.
Footnotes
This study was supported in part by National Institutes of Health Grants DK60754, DK62218, DK073843, and P30 DK36836 (Joslin Diabetes and Endocrinology Research Center), and the Herbert Graetz Fund. Hormone analyses were conducted by the Specialized Assay Core at the Joslin Diabetes Center.
Disclosure Information: A.M.J. received consulting fees from Pfizer-Exubera and lecture fees from Sunstar. P.F.R. received consulting fees from Novartis, GSK, and Kyowa Hakko; owns stock in Tetragenex, NPS Pharma, Sonus Pharma, and PHR Laboratories; and received lecture fees and past grant support from Eli Lilly. A.R. received lecture fees from Merck, and K.W. received lecture fees from Roche/Accucheck. None are related to this work. No other authors have anything to disclose.
First Published Online January 15, 2008
Abbreviations: BG, Blood glucose; BOLD, blood oxygenation level dependent; EPI, echo planar imaging; fMRI, functional MRI; GLM, general linear model; HbA1c, glycosylated hemoglobin; MR, magnetic resonance; MRI, MR imaging; ROI, region of interest; 3D, three-dimensional.
References
- Widom B, Simonson DC 1992 Intermittent hypoglycemia impairs glucose counterregulation. Diabetes 41:1597–1602 [DOI] [PubMed] [Google Scholar]
- Dagogo-Jack SE, Craft S, Cryer PE 1993 Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus. Recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia. J Clin Invest 91:819–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiel SA, Tamborlane WV, Simonson DC, Sherwin RS 1987 Defective glucose counterregulation after strict glycemic control of insulin-dependent diabetes mellitus. N Engl J Med 316:1376–1383 [DOI] [PubMed] [Google Scholar]
- Bolli GB 2003 Treatment and prevention of hypoglycemia and its unawareness in type 1 diabetes mellitus. Rev Endocr Metab Disord 4:335–341 [DOI] [PubMed] [Google Scholar]
- Heller SR, Cryer PE 1991 Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic humans. Diabetes 40:223–226 [DOI] [PubMed] [Google Scholar]
- Ovalle F, Fanelli CG, Paramore DS, Hershey T, Craft S, Cryer PE 1998 Brief twice-weekly episodes of hypoglycemia reduce detection of clinical hypoglycemia in type 1 diabetes mellitus. Diabetes 47:1472–1479 [DOI] [PubMed] [Google Scholar]
- Cryer PE 2002 Hypoglycaemia: the limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia 45:937–948 [DOI] [PubMed] [Google Scholar]
- Perros P, Deary IJ, Sellar RJ, Best JJ, Frier BM 1997 Brain abnormalities demonstrated by magnetic resonance imaging in adult IDDM patients with and without a history of recurrent severe hypoglycemia. Diabetes Care 20:1013–1018 [DOI] [PubMed] [Google Scholar]
- Ferguson SC, Blane A, Perros P, McCrimmon RJ, Best JJ, Wardlaw J, Deary IJ, Frier BM 2003 Cognitive ability and brain structure in type 1 diabetes: relation to microangiopathy and preceding severe hypoglycemia. Diabetes 52:149–156 [DOI] [PubMed] [Google Scholar]
- Musen G, Lyoo IK, Sparks CR, Weinger K, Hwang J, Ryan CM, Jimerson DC, Hennen J, Renshaw PF, Jacobson AM 2006 Effects of type 1 diabetes on gray matter density as measured by voxel-based morphometry. Diabetes 55:326–333 [DOI] [PubMed] [Google Scholar]
- Criego AB, Tkac I, Kumar A, Thomas W, Gruetter R, Seaquist ER 2005 Brain glucose concentrations in patients with type 1 diabetes and hypoglycemia unawareness. J Neurosci Res 79:42–47 [DOI] [PubMed] [Google Scholar]
- Bingham EM, Dunn JT, Smith D, Sutcliffe-Goulden J, Reed LJ, Marsden PK, Amiel SA 2005 Differential changes in brain glucose metabolism during hypoglycaemia accompany loss of hypoglycaemia awareness in men with type 1 diabetes mellitus. An [11C]-3-O-methyl-D-glucose PET study. Diabetologia 48:2080–2089 [DOI] [PubMed] [Google Scholar]
- Cranston I, Reed LJ, Marsden PK, Amiel SA 2001 Changes in regional brain (18)F-fluorodeoxyglucose uptake at hypoglycemia in type 1 diabetic men associated with hypoglycemia unawareness and counter-regulatory failure. Diabetes 50:2329–2336 [DOI] [PubMed] [Google Scholar]
- Teves D, Videen TO, Cryer PE, Powers WJ 2004 Activation of human medial prefrontal cortex during autonomic responses to hypoglycemia. Proc Natl Acad Sci USA 101:6217–6221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal JM, Amiel SA, Yaguez L, Bullmore E, Hopkins D, Evans M, Pernet A, Reid H, Giampietro V, Andrew CM, Suckling J, Simmons A, Williams SC 2001 The effect of acute hypoglycemia on brain function and activation: a functional magnetic resonance imaging study. Diabetes 50:1618–1626 [DOI] [PubMed] [Google Scholar]
- MacLeod KM, Gold AE, Ebmeier KP, Hepburn DA, Deary IJ, Goodwin GM, Frier BM 1996 The effects of acute hypoglycemia on relative cerebral blood flow distribution in patients with type I (insulin-dependent) diabetes and impaired hypoglycemia awareness. Metabolism 45:974–980 [DOI] [PubMed] [Google Scholar]
- Simonson DC, Tamborlane WV, DeFronzo RA, Sherwin RS 1985 Intensive insulin therapy reduces counterregulatory hormone responses to hypoglycemia in patients with type I diabetes. Ann Intern Med 103:184–190 [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Tobin JD, Andres R 1979 Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237(3):E214–E223 [DOI] [PubMed] [Google Scholar]
- Turner R, Howseman A, Rees GE, Josephs O, Friston K 1998 Functional magnetic resonance imaging of the human brain: data acquisition and analysis. Exp Brain Res 123:5–12 [DOI] [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial Research Group1997 Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes 46:271–286 [PubMed] [Google Scholar]
- McIntyre M, Richter W, Morden D, Wennerberg A, Frankenstein U 2003 Blood oxygen level dependent functional magnetic resonance imaging. Concepts in Magnetic Resonance, Part A; 16A:5–15 [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW 1990 Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA 87:9868–9872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MD, Heiling VJ 1991 Heated hand vein blood is satisfactory for measurements during free fatty acid kinetic studies. Metabolism 40:406–409 [DOI] [PubMed] [Google Scholar]
- Liu D, Andreasson K, Lins PE, Adamson UC, Macdonald IA 1993 Adrenaline and noradrenaline responses during insulin-induced hypoglycaemia in man: should the hormone levels be measured in arterialized venous blood? Acta Endocrinol (Copenh) 128:95–98 [DOI] [PubMed] [Google Scholar]
- Widom B, Simonson DC 1990 Glycemic control and neuropsychologic function during hypoglycemia in patients with insulin-dependent diabetes mellitus. Ann Intern Med 112:904–912 [DOI] [PubMed] [Google Scholar]
- Amiel SA, Sherwin RS, Simonson DC, Tamborlane WV 1988 Effect of intensive insulin therapy on glycemic thresholds for counterregulatory hormone release. Diabetes 37:901–907 [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P 1988 Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. Stuttgart: Beorg Thieme Verlag [Google Scholar]
- Carpenter MB, Sutin J 1983 Human neuroanatomy. 8th ed. Baltimore: Williams and Wilkins [Google Scholar]
- Johnstone T, Ores Walsh KS, Greischar LL, Alexander AL, Fox AS, Davidson RJ, Oakes TR 2006 Motion correction and the use of motion covariates in multiple-subject fMRI analysis. Hum Brain Mapp 27:779–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz NS, Clutter WE, Shah SD, Cryer PE 1987 Glycemic thresholds for activation of glucose counterregulatory systems are higher than the threshold for symptoms. J Clin Invest 79:777–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrakou A, Ryan C, Veneman T, Mokan M, Jenssen T, Kiss I, Durrant J, Cryer P, Gerich J 1991 Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cerebral dysfunction. Am J Physiol 260(1 Pt 1):E67–E74 [DOI] [PubMed] [Google Scholar]
- Fanelli C, Pampanelli S, Epifano L, Rambotti AM, Ciofetta M, Modarelli F, Di Vincenzo A, Annibale B, Lepore M, Lalli C, Del Sindaco P, Brunetti P, Bolli GB 1994 Relative roles of insulin and hypoglycaemia on induction of neuroendocrine responses to, symptoms of, and deterioration of cognitive function in hypoglycaemia in male and female humans. Diabetologia 37:797–807 [DOI] [PubMed] [Google Scholar]
- Frizzell RT, Jones EM, Davis SN, Biggers DW, Myers SR, Connolly CC, Neal DW, Jaspan JB, Cherrington AD 1993 Counterregulation during hypoglycemia is directed by widespread brain regions. Diabetes 42:1253–1261 [DOI] [PubMed] [Google Scholar]
- Rao J, Auerbach E, Ugurbil K, Seaquist E 2006 Detection of regional cerebral blood flow changes during hypoglycemia using continuous arterial spin labeling. Diabetes 55(Suppl 1):A47 [Google Scholar]
- Brennan SC, Redd WH, Jacobsen PB, Schorr O, Heelan RT, Sze GK, Krol G, Peters BE, Morrissey JK 1988 Anxiety and panic during magnetic resonance scans. Lancet 2:512 [DOI] [PubMed] [Google Scholar]
- Wortsman J, Frank S, Cryer PE 1984 Adrenomedullary response to maximal stress in humans. Am J Med 77:779–784 [DOI] [PubMed] [Google Scholar]
- Amiel SA, Maran A, Powrie JK, Umpleby AM, Macdonald IA 1993 Gender differences in counterregulation to hypoglycaemia. Diabetologia 36:460–464 [DOI] [PubMed] [Google Scholar]