Abstract
Previously metal-ion sites have been used as structural and functional probes in seven transmembrane receptors (7TM), but as yet all the engineered sites have been inactivating. Based on presumed agonist interaction points in transmembrane III (TM-III) and -VII of the β2-adrenergic receptor, in this paper we construct an activating metal-ion site between the amine-binding Asp-113 in TM-III—or a His residue introduced at this position—and a Cys residue substituted for Asn-312 in TM-VII. No increase in constitutive activity was observed in the mutant receptors. Signal transduction was activated in the mutant receptors not by normal catecholamine ligands but instead either by free zinc ions or by zinc or copper ions in complex with small hydrophobic metal-ion chelators. Chelation of the metal ions by small hydrophobic chelators such as phenanthroline or bipyridine protected the cells from the toxic effect of, for example Cu2+, and in several cases increased the affinity of the ions for the agonistic site. Wash-out experiments and structure–activity analysis indicated, that the high-affinity chelators and the metal ions bind and activate the mutant receptor as metal ion guided ligand complexes. Because of the well-understood binding geometry of the small metal ions, an important distance constraint has here been imposed between TM-III and -VII in the active, signaling conformation of 7TM receptors. It is suggested that atoxic metal-ion chelator complexes could possibly in the future be used as generic, pharmacologic tools to switch 7TM receptors with engineered metal-ion sites on or off at will.
Rhodopsin-like G protein-coupled receptors, which constitute the largest superfamily of proteins in our organism, are believed to have a common seven helical transmembrane (7TM) fold and conceivably a common, still elusive molecular mechanism of activation (1). Cryoelectron microscopy of rhodopsin has provided the basis for a generally accepted model for the overall structure of 7TM receptors (2–5). However, their detailed three-dimensional structure and the conformational changes leading to their activation is still rather ill-defined (6–8).
Previously metal-ion site engineering has been employed for structural and functional analysis of 7TM receptors (9–12). Originally, the binding site for a nonpeptide antagonist was exchanged with a high affinity, tridentate Zn2+ binding site located between two helices in the NK1 tachykinin receptor (9). This site could be transferred as a zinc switch without loss of metal-ion affinity to the distantly related κ-opioid receptor, indicating that the overall structure within the family of rhodopsin-like 7TM receptors is well conserved (10). Interhelical bidentate zinc sites subsequently have been used to probe helix–helix interactions and, for example to prove that the seven helical bundle is arranged in an anti-clock-wise arrangement as viewed from outside the cell (11). However, as yet all metal-ion sites created both by us and others have been antagonistic (9–13). Such sites are interesting, from a pure structural point of view, but in fact they provide rather limited information about the actual mechanism of receptor activation. The problem is, that probably an indefinite number of different, inactive conformations can be stabilized by artificial metal-ion sites, which all more or less indirectly can prevent the receptor from obtaining an active conformation. To address the active conformation of a receptor, actual activating metal-ion sites need to be constructed.
The appropriate starting point for designing such agonistic metal-ion sites should conceivably be the natural binding site for an agonist. The most solid evidence concerning ligand binding is derived from rhodopsin, in which retinal is known to be covalently attached to the ɛ-amino group of Lys-296 in transmembrane-VII (TM-VII) (LysVII:10) through a Schiff base, which in the nonsignaling ground state of the molecule is protonated and stabilized through interaction with the charged Glu-113 in TM-III (8, 14, 15) (GluIII:04).† In monoamine receptors a well-characterized interaction site for the amine group of the ligands is AspIII:08 being located one helical turn below GluIII:04 of rhodopsin (17). In TM-VII of the catecholamine and 5-hydroxytryptamine (5HT) receptors residue VII:06 has consistently been identified as an interaction site for partial agonists as well as antagonists as based on mutational studies giving even up to 1,000-fold gain of affinity (16, 18–20). Residue VII:06 is located one helical turn above LysVII:10 of rhodopsin and is conceivably facing rather directly toward TM-III (Fig. 1). In the present study we introduce metal-ion binding residues, i.e., His or Cys residues, at positions III:08 and VII:06 in the β2-adrenergic receptor and thereby enable Zn2+ or Cu2+ instead of catecholamines to activate the receptor either as free metal ions or in complex with small hydrophobic metal-ion chelators such as phenanthroline or bipyridine.
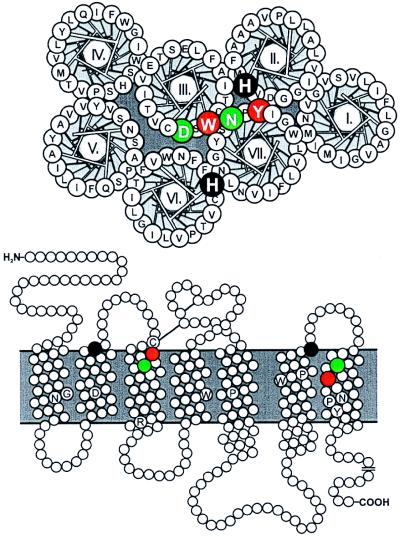
Figure 1.
Helical wheel and serpentine diagram of the β2-adrenergic receptor. Amino acid residues probed by metal-ion site engineering are indicated in white on green or white on black, and the residues corresponding to the retinal interaction site in TM-III and -VII in rhodopsin are indicated in white on red. The helical wheel diagram has been constructed for the supposedly most extracelluarly located 18 residues of each helix in accordance with the general model of Baldwin (4, 5) and with the helices arranged counter-clockwise as viewed from the outside-in in accordance with previous studies by using interhelical, inhibitory bis-His sites (11). In the serpentine model, a few highly conserved “finger-print” residues in each TM segments are indicated in black on white (16). The generic numbering system of Baldwin (4) for 7TM receptor residues is used in parallel with the specific numbering throughout the paper (4, 16).
Materials and Methods
Ligands.
The catecholamine analogs L-159,084 and L-158,870 were generously provided by A. A. Patchett (Merck Research Laboratories, Rahway, NJ). These compounds are used here as control agonists in β2-adrenergic receptors where the important Asp-113 (AspIII:08) was mutated. These analogs were specifically designed not to depend on the presence of an acidic residue at this position (17). Originally it was believed that the aspartate had to be substituted with a serine residue for these compounds to work. In the present study we confirm that they do not work as agonists in the wild-type receptor but surprisingly that they do stimulate mutant forms of the β2-adrenergic receptor where Asp-113 has been substituted not only with Ser (17) but also in mutants where it had been substituted with either Ala, Cys, or His (see Table 1); although, in the case of His, only L-158,870 but not L-159,084 were active.
Table 1.
cAMP production in response to 100 μM compound or 1 μM pindolol
| Generic numbering | Basal level | Pindolol | L-159.084 | Zn2+ | Zn2+ (Phen)3 | Cu2+(Phen)3 | Phenanthroline | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mock-transfected cells | 2.6 ± 0.4 | (5) | 3.4 ± 0.5 | (5) | 4.4 ± 0.6 | (3) | 3.0 ± 0.5 | (4) | 1.8 ± 0.4 | (3) | 2.1 ± 0.3 | (4) | 3.3 ± 0.4 | (3) | |
| hβ2-AR wild type | 6.9 ± 0.7 | (15) | 42 ± 3 | (15) | 9.4 ± 1.1 | (4) | 8.0 ± 0.8 | (7) | 5.2 ± 0.5 | (5) | 5.4 ± 0.6 | (8) | 8.0 ± 0.8 | (3) | |
| [N312H] | VII:06 | 9.4 ± 1.7 | (5) | 9.1 ± 1.9 | (5) | ND | 8.3 ± 1.7 | (5) | 20 ± 3 | (5) | 9.0 ± 1.3 | (4) | 8.0 ± 1.6 | (3) | |
| [N312C] | VII:06 | 7.0 ± 0.7 | (12) | 18 ± 1 | (12) | 33 ± 3 | (5) | 12 ± 1† | (4) | 9.4 ± 1.0 | (4) | 33 ± 7 | (6) | 7.9 ± 0.9 | (3) |
| [H93A;N312C] | II:24;VII:06 | 7.8 ± 1.3 | (8) | 12 ± 2 | (6) | ND | 11 ± 1 | (5) | 12 ± 2 | (5) | 26 ± 2 | (5) | 10 ± 1 | (4) | |
| [H296A;N312C] | VI:23;VII:06 | 12 ± 1 | (4) | 30 ± 2 | (4) | ND | 20 ± 1 | (3) | 21 ± 2 | (4) | 37 ± 3 | (4) | 14 ± 2 | (3) | |
| [D113A] | III:08 | 5.6 ± 0.7 | (5) | 6.0 ± 0.4 | (5) | 79 ± 15 | (5) | 6.2 ± 0.6 | (5) | 5.8 ± 0.7 | (4) | 5.5 ± 0.3 | (5) | 5.6 ± 0.5 | (3) |
| [D113C] | III:08 | 5.6 ± 1.1 | (4) | 6.4 ± 0.7 | (4) | 47 ± 15 | (4) | 6.2 ± 1.2 | (4) | 5.8 ± 0.9 | (4) | 7.0 ± 1.8 | (4) | 5.4 ± 0.6 | (3) |
| [D113H] | III:08 | 5.9 ± 1.0 | (4) | 6.1 ± 0.8 | (4) | 5.9 ± 1.3* | (4) | 6.8 ± 1.1 | (4) | 6.2 ± 0.6 | (4) | 6.4 ± 0.9 | (4) | 6.7 ± 1.1 | (3) |
| [D113A;N312C] | III:08;VII:06 | 4.7 ± 0.5 | (8) | 5.7 ± 0.6 | (8) | 79 ± 20 | (6) | 5.8 ± 0.6 | (6) | 5.0 ± 0.7 | (5) | 5.7 ± 0.6 | (6) | 4.9 ± 0.3 | (4) |
| [D113C;N312C] | III:08;VII:06 | 7.3 ± 0.9 | (5) | 7.8 ± 1.1 | (5) | 82 ± 20 | (4) | 8.5 ± 1.6 | (4) | 6.7 ± 1.2 | (3) | 12 ± 2 | (5) | 7.2 ± 1.0 | (3) |
| [D113H;N312C] | III:08;VII:06 | 7.5 ± 0.5 | (7) | 8.5 ± 0.5 | (7) | 9.5 ± 0.8 | (6) | 27 ± 4 | (7) | 8.7 ± 0.1 | (4) | 21 ± 2 | (6) | 8.5 ± 1.1 | (5) |
Data are presented as mean ± SEM. (n), in fmol/105 cells. ND, not determined.
*The [D113H] construct was activated by the catecholamine analog L-158,870 (37 ± 10 fmol/105 cells, n = 4), hence it is expressed in a functional form.
†Activation of [N312C] with 1 mM ZnCl2 yields 17 ± 2 fmol/105 cells.
Pindolol, 1,10-phenanthroline, 2,2′-bipyridine, and 4,7-diphenyl-1,10-phenanthroline disulfonic acid were obtained from Sigma. 4,7-Phenanthroline and 4,7-dihydroxy-1,10-phenanthroline were obtained from Aldrich. Zn2+(phenanthroline)3 and Cu2+(phenanthroline)3 were prepared by dissolving phenanthroline in ethanol and mixing with aqueous solutions of ZnCl2 or CuSO4 to a final molar ratio of 3:1.
Molecular Biology.
The point mutations were constructed by using oligonucleotide-directed mutagenesis and recombinant PCR. cDNAs encoding wild-type and mutant receptors were cloned into the eukaryotic expression vector pTEJ-8; all mutations were verified by restriction endonuclease mapping and DNA sequencing (ALFexpress DNA Sequencer, Amersham Pharmacia).
Cell Biology.
Cloned receptors were transiently expressed in COS-7 cells transfected 2 days before analysis. COS-7 cells were grown in DMEM 1885 supplemented with 10% FCS, 2 mM glutamine, and 10 μg/ml gentamicin.
Determination of intracellular cAMP accumulation.
COS-7 cells were seeded in 6-well culture dishes 1 day after transfection at a density of 400,000 cells/well and supplemented with 2 μCi [3H]adenine/ml (Amersham Pharmacia Biotech). Two days after transfection, cells were washed twice with HBS buffer [25 mM Hepes, 0.75 mM NaH2PO4, 140 mM NaCl (pH 7.2)] and incubated in HBS buffer supplemented with 1 mM 3-isobutyl-1-methylxanthine. Pindolol, catecholamine analogs, free metal ion, or metal-ion complex was added and the cells were incubated for 30 min at 37°C. The assay was terminated by aspirating the buffer followed by addition of ice-cold 5% trichloroacetic acid containing 0.1 mM unlabeled cAMP and ATP. cAMP was isolated by applying the supernatant to a 50W-X4 resin (Bio-Rad) followed by an alumina resin (A-9003; Sigma). Determinations were made in duplicate. Because a small population of endogenous catecholamine receptors is present in COS-7 cells, which are stimulated by isoproterenol but not by pindolol, the latter was used as agonist in the present study.
Binding experiments.
Monoiodinated 125I-labeled pindolol was obtained from New England Nuclear. Transfected COS-7 cells were transferred to culture plates 1 day after transfection. The number of cells per well was determined by the apparent expression efficiency of the individual clones aiming at 5–10% binding of the added radioligand. Two days after transfection cells were assayed by competition binding for 3 h at 4°C by using 15 pM 125I-labeled pindolol plus variable amounts of unlabeled ligand in 0.5 ml of a 25 mM Tris⋅HCl buffer (pH 7.4) supplemented with 5 mM MgCl2, 0.1% (wt/vol) BSA, and 40 μg/ml bacitracin. Nonspecific binding was determined as the binding in the presence of 1 μM pindolol. Determinations were made in duplicate.
Molecular Graphics.
The coordinates for models of the prototypic 7TM receptor rhodopsin were generously provided by R. E. Hubbard and P. Herzyk (21) and J. M. Baldwin (5). In both models Ala-117 and Ala-292 corresponding to Asp-113 and Asn-312 of the β2-adrenergic receptor were substituted with a His and a Cys residue, respectively. Molecular graphics was done by using WebLab ViewerPro (Molecular Simulations, Waltham, MA).
Data Analysis.
The cAMP curves were analyzed and EC50 values were determined by computerized nonlinear regression using GraphPad prizm (GraphPad Software, San Diego, CA).
Results
Asn-312 (AsnVII:06) was chosen as a starting point for metal-ion site engineering in the β2-adrenergic receptor because it previously has been characterized as an interaction site for partial agonists such as pindolol (16, 18–20). Substitution of AsnVII:06 with either His or Cys did not affect the basal signaling activity of the receptor. It should be noted that the basal, constitutive activity of the β2-receptor was in fact not increased significantly by any of the mutations of the present study except in one of the control constructs, [H296A;N312C] (Table 1).
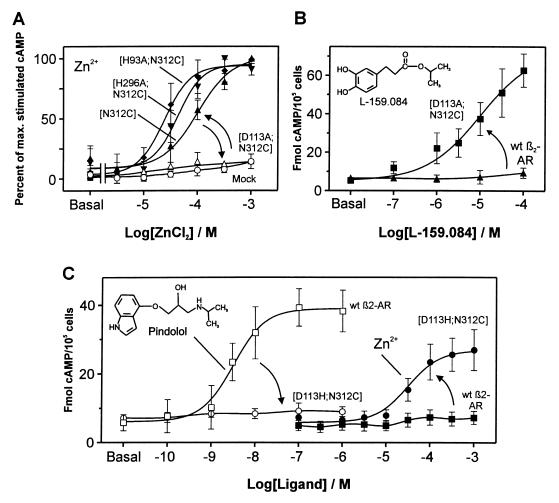
As expected based on the literature (16, 18–20), substitution of AsnVII:06 with either His or Cys impaired the affinity for pindolol and thereby eliminated the binding of 125I-labeled pindolol (data not shown) and also impaired the ability of pindolol to stimulate cAMP production (Table 1). Despite the fact that only a single potential metal-ion binding residue had been introduced in the CysVII:06 mutant, Zn2+ in the form of ZnCl2 was able to stimulate cAMP production with an EC50 of 91 μM (Fig. 2A). Zn2+ had no effect on mock-transfected cells, on cells transfected with the wild-type receptor, or on cells transfected with the β2-adrenergic receptor having a His residue introduced instead of the Cys at position VII:06 (Fig. 2 A and C, Table 1). Based on the relatively high affinity for Zn2+ we would expect that CysVII:06 was able to form a bidentate metal-ion site with another residue in the β2-adrenergic receptor. In the molecular model the three most likely partners for CysVII:06 in the binding of the activating zinc ion would be His-93 (HisII:24), Asp-113 (AspIII:08), and His-296 (HisVI:23) (Fig. 1) (5, 21). Further exchange of His-93 or His-296 with Ala in the CysVII:06 substituted β2-adrenergic receptor did not eliminate the Zn2+ stimulation of cAMP production; if anything it shifted the dose-response curves slightly to the left (Fig. 2A, Table 1). But Zn2+ was unable to induce signaling when the CysVII:06 substitution was combined with Ala substitution of Asp-113 (AspIII:08) (Fig. 2A, Table 1). AspIII:08 is an essential residue for agonist binding and action in basically all monoamine receptors (17, 22). Thus the negative result for Zn2+ in the double mutation involving substitution of AspIII:08 could very well be misleading, because this residue could possibly be structurally important for the expression of the active conformation as such. Importantly, this construct was well expressed and in a functional form as the catecholamine analog L-159,084, which in contrast to ordinary catecholamines is known not to depend on an Asp residue in position III:08 (17), could stimulate cAMP production through the receptor with the [D113A;N312C] double mutation to a similar degree as pindolol did in the wild-type receptor (Fig. 2B, Table 1).‡ Thus, it is concluded that the inability of Zn2+ to stimulate this mutant receptor is caused by the elimination of Asp-113 (AspIII:08) as the partner for the introduced CysVII:06 in the coordination of Zn2+ in an activating metal-ion site.
Figure 2.
Substitution of agonist function of catecholamines with agonist function of Zn2+ after metal-ion site engineering in the β2-adrenergic receptor. (A) Effect of ZnCl2 on cAMP accumulation in the construct with the Asn-312 (AsnVII:06) to Cys substitution, [N312C] β2-adrenergic receptor (β2-AR) (▴); and in constructs where residues were substituted to find the metal-ion site partner for Cys-312 by further substitution of His-93 (HisII:24), [H93A;N312C] β2-AR (♦), His-296 (HisVI:23), [H296A;N312C] β2-AR (▾), and Asp-113 (AspIII:08) [D113A;N312C] β2-AR (▵), the effect of ZnCl2 on mock-transfected cells is also shown (○). The EC50 values for Zn2+ in stimulating cAMP production were as follows: [N312C] β2-AR (▴) = 91 ± 12 μM, [H93A;N312C] β2-AR (♦) = 30 ± 8 μM, [H296A;N312C] β2-AR (▾) = 41 ± 5 μM, and [D113A;N312C] β2-AR ([tri]) > 1000 μM (n = 3–5). (B) Positive control demonstrating the signaling potential of the zinc-unresponsive construct, [D113A;N312C] β2-AR (■) by using the catecholamine analog L-159.084 designed to bind β2-AR constructs substituted at the amine-binding Asp-113 (AspIII:08) (17), wild-type β2-AR (▴) (n = 3). (C) Total exchange of the catecholamine agonist binding site with a metal-ion site in the [D113H;N312C] β2-AR construct. Stimulation of cAMP production by pindolol (open symbols) and by Zn2+ (filled symbols) in wild-type β2-AR (squares) and the [D113H;N312C] β2-AR construct (circles). The EC50 for Zn2+ in [D113H;N312C] β2-AR was 38 ± 2 μM. Data are presented as mean ± SEM (n = 3–5).
Total substitution of the catecholamine agonist site with an activating metal-ion site was achieved by introducing a His residue in stead of the important Asp at position III:08 in the CysVII:06-substituted receptor. In this [D113H;N312C] construct, the stimulating activity of not only pindolol but also that of the L-159,084 catecholamine analog was eliminated (Table 1). Nevertheless, free Zn2+ now functioned as an even more efficacious agonist (i.e., giving a larger response) than in the receptor with only CysVII:06 (Fig. 2C, Table 1).
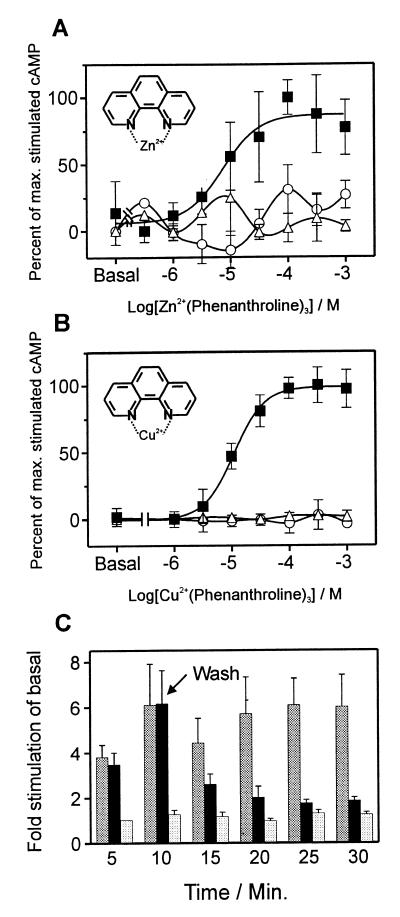
Not only free zinc ions but also Zn2+ and Cu2+ in complex with the chelator 1,10-phenanthroline could bind and activate the β2-adrenergic receptors with engineered metal-ion sites. As observed for the free Zn2+, the metal-ion complex stimulated neither mock-transfected cells nor cells transfected with the wild-type receptor (Fig. 3A). The affinity of Zn2+ for the CysVII:06 substituted β2-adrenergic receptor was increased approximately 6-fold by the formation of the complex with phenanthroline, i.e., the EC50 for cAMP stimulation decreased from 91 to 16 μM (Figs. 2A and 3A), whereas the efficacy of Zn-phenanthroline was similar to that of the free zinc ion (Table 1). Because of the general harmful effect on cells of free copper-ions at concentrations above ≈10 μM, we were unable to determine the affinity and efficacy of free Cu2+ as such in these receptors. However, in the CysVII:06-substituted β2-adrenergic receptor Cu2+ in complex with phenanthroline was equally potent as Zn-phenanthroline and gave an almost four times as large incremental response as that induced by the zinc-complex (Fig. 3B, Table 1). In contrast to Zn2+, Cu2+ is redox-active and it is therefore important to determine whether the effect of Cu-phenanthroline could be a result of oxidation of amino acid side chains or formation of disulfide bridges within the receptor. Importantly, the increased cAMP production induced by Cu-phenanthroline at low micromolar concentrations, returned rapidly toward basal levels after removal of the Cu-phenanthroline complex from the transfected cells by simple washing (Fig. 3C). In contrast, the high level of signal transduction continued after a short lag period in cells where the Cu-phenanthroline was readded after the washing. Phenanthroline alone stimulated neither the wild-type β2-adrenergic receptor nor any mutant form of this (Fig. 3B, Table 1).
Figure 3.
Effect of metal-ion chelator complexes on signaling in the [N312C] β2-adrenergic receptor (β2-AR). Stimulation with Zn2+ (A) and Cu2+ (B) in complex with the metal-ion chelator 1,10-phenanthroline on the [N312C] β2-AR (■) as compared with wild-type β2-AR (○) and mock-transfected cells (▵). The EC50 values on the [N312C] β2-AR were as follows: Zn2+(phenanthroline)3 = 16 ± 10 μM, Cu2+(phenanthroline)3 = 11 ± 3 μM. For comparison, the EC50 on the [D113H;N312C] β2-AR construct (not shown on the figure) was for Cu2+(phenanthroline)3 = 4.1 ± 0.7 μM (n = 3–6). (C) Elimination of the effect of Cu2+(phenanthroline)3 by washing. The [N312C] β2-AR construct was stimulated with 100 μM copper-phenanthroline complex (dark gray and filled bars) at time zero; after 10 min cells were washed two times and then half of the cells were either rechallenged with 100 μM Cu2+(phenanthroline)3 (dark gray bars) or with buffer (filled bars). The basal cAMP accumulation was monitored in parallel (light gray bars). The level of cAMP production was sampled for each 5-min interval (n = 3).
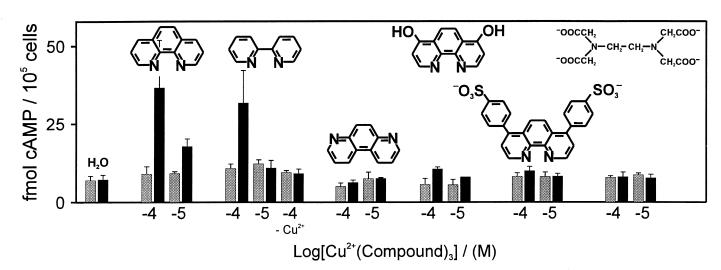
Just as 1,10-phenanthroline, the structurally similar metal-ion chelating compound 2,2′-bipyridine could also in complex with Cu2+ stimulate cAMP production through the CysVII:06 substituted β2-adrenergic receptors (Fig. 4). In contrast, 4,7-phenanthroline, which because of the unfavorable position of the nitrogens cannot chelate metal-ions, was inactive. The 4,7-dihydroxy-1,10-phenanthroline and 4,7-diphenyl-1,10-phenanthroline disulfonic acid, which both are able to chelate Cu2+, but which are larger and more hydrophilic than 1,10-phenanthroline, were in complex with Cu2+ both inactive on the mutant receptor (Fig. 4). Also, the highly polar but efficient metal-ion chelator EDTA was unable to activate the metal-ion site engineered receptors (Fig. 4). But EDTA could compete away the stimulating effect of, for example, Cu-phenanthroline (data not shown). This albeit rather limited analysis of the structure activity relationship for metal-ion chelator complexes indicates, that their activity depends on the size and/or the hydrophobicity of the chelator as well as on the metal ion itself.
Figure 4.
Structure–function analysis of 1,10-phenanthroline. The effect of 10−4 and 10−5 M of 1,10-phenanthroline and analogs of this in complex with Cu2+ on cAMP production in COS-7 cells transfected with the [N312C] β2-adrenengic receptor (β2-AR) construct (black bars) as compared with wild-type β2-AR (gray bars). The compounds are from left: 1,10-phenanthroline; 2,2′-bipyridine; 4,7-phenanthroline; 4,7-dihydroxy-1,10-phenanthroline; 4,7-diphenyl-1,10-phenanthroline disulfonic acid; EDTA (n = 2–4). Data are presented as mean ± SEM.
Discussion
The present study, in which the binding site for catecholamines in the β2-adrenergic receptor was structurally and functionally exchanged with a metal-ion site, expands the usefulness of metal-ion site engineering in 7TM receptors significantly—i.e., both in respect of structural analysis and in respect of biological analysis of the receptors. Although the previously constructed inactivating metal-ion sites also supplied interesting structural information, they did however, at best, only indirectly tell about the conformational changes which occur on receptor activation. Here, the actual active conformation is stabilized by either a metal ion or a metal ion in complex with a small hydrophobic chelator molecule. The latter point is important, because it could possibly enable future exploitation of metal-ion site engineered receptors not only in vitro, but possibly also in vivo.
A Distance Constraint in the Active 7TM Conformation.
The present study imposes an important distance constraint in the active conformation of the β2-adrenergic receptor and thereby conceivably in 7TM receptors in general. From numerous x-ray structures it is well known that the distance from a zinc or copper ion to each of the coordinating atoms is close to 2 Å (23, 24). When we built the side chains of Asp or His into position III:08 and Cys into position VII:06 of the rhodopsin-based 7TM models of either Baldwin et al. (5) or Herzyk and Hubbard (21), there was no need to change the Cβ distance, being ≈7.6 Å in both cases, to rotate the remaining part of the two side chains into a proper position for them to bind a metal ion, for example, in a tetrahedral coordination geometry (Fig. 5). Thus our data support the prevailing molecular models of 7TM receptors in general. However, because of a lack of detailed structural knowledge about either the inactive or the active conformation, we cannot as yet say whether the distance constraint determined in the present study favors a particular conformational change to occur during activation. It should in this context be noted that although the structural basis for the current molecular models originally was the cryoelectron microscopical structures of dark or inactive states of rhodopsin (2, 3), these structures have in fact only provided a basic scaffold for the supposed arrangement of the helices (4, 5, 21). The suggested identity of the individual helices in the density map and importantly the relative vertical position and rotation of the helices in the molecular scaffold of the molecular models is based mainly on a multitude of nonexperimental, structural data such as analysis of hydrophobicity, conservation, effect of mutations, etc. (4, 5, 21). Thus, in respect of helical rotation, etc., the molecular models are not particularly biased toward the inactive versus the active state of the receptor. Nevertheless, the most well-established distance constraint in rhodopsin, i.e., the salt bridge between Glu-113 and Lys-296 (8, 14, 15), is fulfilled in the current molecular models albeit with an intercalated water molecule (25). Because this salt bridge is found mainly in the dark, inactive state of the molecule, our study does not at present impose a need for a major conformational change to occur between TM-III and -VII in the interconversion from the inactive to the active state.
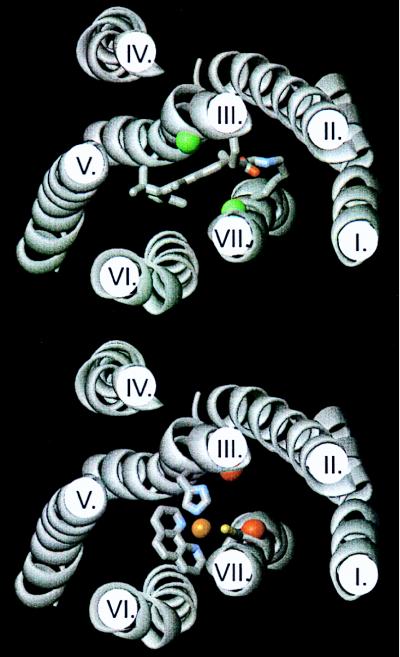
Figure 5.
Molecular models of rhodopsin (Upper) and the [His-113,Cys-312] β2-adrenergic receptor (β2-AR) (Lower) built over the rhodopsin model of Herzyk and Hubbard (21). In rhodopsin the retinal ligand in the all-trans, ground state attached to Lys-296 through the Schiff base as well as Glu-113 (GluIII:04) and Lys-296 (LysVII:10) are shown in stick models. The Cαs of residues III:08 and VII:06 (constituting the activating metal-ion site in the β2-AR) are indicated by large green spheres. In the [His-113,Cys-312] β2-AR His-113 (HisIII:08) and Cys-312 (VII:06) are indicated in stick models and the proposed coordination of a metal-ion between these two residues and the two nitrogens of 1,10-phenanthroline is also indicated. The Cαs of residues III:04 and VII:10 (the two main residues involved in retinal binding) are indicated by large red spheres.
Previously, studies employing electron paramagnetic resonance (EPR) and fluorescence spectroscopy as well as mutational analysis with inhibitory disulfide bridges or inhibitory metal-ion sites have focused on conformational changes occurring between TM-III and -VI during activation of 7TM receptors (13, 26, 27). This conformational change is believed to consist of a relative movement of the cytosolic part of TM-VI away from TM-III accompanied by a relative rotation of TM-VI in the counter-clockwise direction as viewed from the extracellular side (26). In rhodopsin, the main body of the retinal ligand, which is covalently attached at the Shiff base connection between TM-III and -VII, is located between TM-III and -VI, reaching toward TM-V and toward the center of the receptor molecule. Interestingly, this is directly through the position where the metal ions and the metal-ion chelators are believed to bind (Fig. 5). Whether the ligands at this position of the active receptor conformation bring the helices closer together or in fact keep them further apart than in the inactive state remains to be demonstrated. Importantly, the present study demonstrates that a paramagnetic copper ion can be bound here, i.e., in the middle of the active form of a 7TM receptor, which offers a special opportunity to study in detail by EPR the conformational changes that occur during the activation process (28–31). It is, for example, possible to measure distances from the fixed paramagnetic copper ion to spin labels introduced by site-directed mutagenesis at various positions in the receptor.
Activation of Metal-Ion Site Engineered Receptors by Metal-Chelator Complexes.
In serine proteases it has recently been demonstrated by x-ray crystallography that metal ions can function as bridges between ligands and proteins providing high affinity and selectivity of the inhibitory complexes (32). In the enzyme, Zn2+ is bound between the His and Ser residues of the active site triade and two nitrogens on the inhibitor. This binding mode is closely analogous to what here is suggested for the phenanthroline complex in the mutated β2-adrenergic receptor (Fig. 5). Because phenanthroline in contrast to the chelators used as enzyme inhibitor binds Cu2+ with a very high equilibrium constant of ≈1020 M (33), which results in a very low concentration of free ions, we suggest that the phenanthroline or bipyridine metal-ion complexes as such act as metal-ion guided, agonistic ligands.
It is difficult to assess how good agonists (i.e., in respect of efficacy) the metal ions and their chelator complexes are in the various receptor constructs because the stimulatory effect of the normal catecholamine ligands was impaired strongly or even eliminated by the crucial mutations. However, as shown in Fig. 2C, the maximally achievable response for Zn2+ in the [D113H;N312C] β2-adrenergic receptor was only slightly smaller than the response for pindolol in the wild-type receptor under similar conditions; and, the efficacy of pindolol is generally considered to correspond to 30–50% of a full agonist depending on the assay conditions (34). The reason why Cu2+-phenanthroline is more efficacious than Zn2+-phenanthroline could possibly be related to a more favorable interaction of Cu2+ because of its preference for octahedral coordination of ligands as opposed to the tetrahedral coordination generally preferred by Zn2+, but this is obviously still only speculation.
The observation that Zn2+ and Cu2+ in complex with small hydrophobic chelators can bind with high affinity in activating as well as in inactivating metal-ion sites (unpublished observations) could possibly open for the opportunity to use relatively atoxic chelator complexes as generic agonistic or antagonistic “drugs” in, for example, transgenic animals expressing receptors with engineered metal-ion sites. It is important to note that metal-ion sites in many cases can be constructed as silent “switches,” i.e., in a way that allows the natural ligand (for example a peptide) to bind normally in the absence of the metal ion (9, 10). If the wild-type receptor in an animal is replaced with a receptor holding such a silent metal-ion switch, the animal should develop normally and no compensatory mechanisms would be up-regulated. At any given time during development or in adult life, it should the be possible to turn the receptor on or off, depending on the engineered site, with suitable chelator complexes. Such pharmacologically controlled, conditioned knockout models could be useful, for example, in the evaluation of the pharmacological potential of 7TM drug targets as well as in the characterization of the physiological role of orphan receptors. However, compared with ordinary drug targets, the metal-ion sites are of relatively low affinity and it therefore remains to be shown whether metal-ion chelators having appropriate, pharmacokinetic properties can be developed to obtain full occupancy of such metal-ion switches in vivo.
Acknowledgments
We thank Dr. Art Patchett for his generous supply of catecholamine analogs and Drs. Wayne Hubbell, Gebhard Schertler, and Henry Bourne for critical reading of the manuscript. We also thank Helle Iversen and Mette Simons for expert technical assistance. This study was supported by grants from the Biotechnology Center for Molecular Recognition through the Danish Medical Research Council as well as grants from the Carlsberg and Lundbeck Foundations.
Abbreviations
- 7TM receptor
seven transmembrane receptor
- TM
transmembrane
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
A generic numbering and nomenclature system for residues in the TM segments of 7TM receptors is used in the present paper to help identify residues located at corresponding positions in different receptors but having different specific numbers in each receptor (4, 16).
L-159,084 here functions as a positive control; however, the details of the molecular mechanism by which the compound is able to efficiently stimulate the mutant receptor in which Asp-113 has been substituted with an Ala residue is unclear as it originally was designed to interact with a Ser-substituted receptor (17).
References
- 1.Bourne H R. Curr Opin Cell Biol. 1997;9:134–142. doi: 10.1016/s0955-0674(97)80054-3. [DOI] [PubMed] [Google Scholar]
- 2.Schertler G F, Villa C, Henderson R. Nature (London) 1993;362:770–772. doi: 10.1038/362770a0. [DOI] [PubMed] [Google Scholar]
- 3.Unger V M, Hargrave P A, Baldwin J M, Schertler G F. Nature (London) 1997;389:203–206. doi: 10.1038/38316. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin J M. EMBO J. 1993;12:1693–1703. doi: 10.1002/j.1460-2075.1993.tb05814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldwin J M, Schertler G F, Unger V M. J Mol Biol. 1997;272:144–164. doi: 10.1006/jmbi.1997.1240. [DOI] [PubMed] [Google Scholar]
- 6.Liri T, Farfel Z, Bourne H R. Nature (London) 1998;394:35–38. doi: 10.1038/27831. [DOI] [PubMed] [Google Scholar]
- 7.Kim J M, Altenbach C, Turmond R L, Khorana H G, Hubbell W L. Proc Natl Acad Sci USA. 1997;94:14273–14278. doi: 10.1073/pnas.94.26.14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakmar T P. Prog Nucleic Acid Res Mol Biol. 1998;59:1–34. doi: 10.1016/s0079-6603(08)61027-2. [DOI] [PubMed] [Google Scholar]
- 9.Elling C E, Nielsen S M, Schwartz T W. Nature (London) 1995;374:74–77. doi: 10.1038/374074a0. [DOI] [PubMed] [Google Scholar]
- 10.Thirstrup K, Elling C E, Hjorth S A, Schwartz T W. J Biol Chem. 1996;271:7875–7878. doi: 10.1074/jbc.271.14.7875. [DOI] [PubMed] [Google Scholar]
- 11.Elling C E, Schwartz T W. EMBO J. 1996;15:6213–6219. [PMC free article] [PubMed] [Google Scholar]
- 12.Elling C E, Thirstrup K, Nielsen S M, Hjorth S A, Schwartz T W. Folding Des. 1997;2:S76–S80. doi: 10.1016/s1359-0278(97)00068-0. [DOI] [PubMed] [Google Scholar]
- 13.Sheikh S P, Zvyaga T A, Lichtarge O, Sakmar T P, Bourne H R. Nature (London) 1996;383:347–350. doi: 10.1038/383347a0. [DOI] [PubMed] [Google Scholar]
- 14.Zhukovsky E A, Oprian D D. Science. 1989;246:928–930. doi: 10.1126/science.2573154. [DOI] [PubMed] [Google Scholar]
- 15.Sakmar T P, Franke R R, Khorana H G. Proc Natl Acad Sci USA. 1989;86:8309–8313. doi: 10.1073/pnas.86.21.8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz T W. Curr Opin Biotechnol. 1994;5:434–444. doi: 10.1016/0958-1669(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 17.Strader C D, Gaffney T, Sugg E E, Candelore M R, Keys R, Patchett A A, Dixon R A F. J Biol Chem. 1991;266:5–8. [PubMed] [Google Scholar]
- 18.Suryanarayana S, Daunt D A, Zastrow M v, Kobilka B K. J Biol Chem. 1991;266:15488–15492. [PubMed] [Google Scholar]
- 19.Guan X-M, Peroutka S J, Kobilka B K. Mol Pharmacol. 1992;41:695–698. [PubMed] [Google Scholar]
- 20.Metcalf M A, McGuffin R W, Hamblin M W. Biochem Pharmacol. 1992;44:1917–1920. doi: 10.1016/0006-2952(92)90092-w. [DOI] [PubMed] [Google Scholar]
- 21.Herzyk P, Hubbard R E. J Mol Biol. 1998;281:741–754. doi: 10.1006/jmbi.1998.1981. [DOI] [PubMed] [Google Scholar]
- 22.Strader C D, Fong T M, Tota M R, Underwood D, Dixon R A. Annu Rev Biochem. 1994;63:101–132. doi: 10.1146/annurev.bi.63.070194.000533. [DOI] [PubMed] [Google Scholar]
- 23.Glusker J P. Adv Protein Chem. 1991;42:1–76. doi: 10.1016/s0065-3233(08)60534-3. [DOI] [PubMed] [Google Scholar]
- 24.Gregory D S, Martin A C R, Cheetham J C, Rees A R. Protein Eng. 1993;6:29–35. doi: 10.1093/protein/6.1.29. [DOI] [PubMed] [Google Scholar]
- 25.Nagata T, Terakita A, Kandori H, Kojima D, Shichida Y, Maeda A. Biochemistry. 1997;36:6164–6170. doi: 10.1021/bi962920t. [DOI] [PubMed] [Google Scholar]
- 26.Farrens D L, Altenbach C, Yang K, Hubbell W L, Khorana H G. Science. 1996;274:768–770. doi: 10.1126/science.274.5288.768. [DOI] [PubMed] [Google Scholar]
- 27.Gether U, Lin S, Ghanouni P, Ballesteros J A, Weinstein H, Kobilka B K. EMBO J. 1997;16:6737–6747. doi: 10.1093/emboj/16.22.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Voss J, Salwinski L, Kaback H R, Hubbell W L. Proc Natl Acad Sci USA. 1995;92:12295–12299. doi: 10.1073/pnas.92.26.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voss J, Hubbell W L, Kaback H R. Proc Natl Acad Sci USA. 1995;92:12300–12303. doi: 10.1073/pnas.92.26.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He M M, Voss J, Hubbell W L, Kaback H R. Biochemistry. 1995;34:15661–15666. doi: 10.1021/bi00048a009. [DOI] [PubMed] [Google Scholar]
- 31.He M M, Voss J, Hubbell W L, Kaback H R. Biochemistry. 1995;34:15667–15670. doi: 10.1021/bi00048a010. [DOI] [PubMed] [Google Scholar]
- 32.Katz B A, Clark J M, Finer-More J S, Jenkins T E, Johnson C R, Ross M J, Luong C, Moore W R, Stroud R M. Nature (London) 1998;391:608–612. doi: 10.1038/35422. [DOI] [PubMed] [Google Scholar]
- 33.Holyer R H, Hubbard C D, Kettle S F A, Wilkins R G. Inorg Chem. 1965;4:923–935. [Google Scholar]
- 34.Clark B J, Menninger K, Bertholet A. Br J Clin Pharmacol. 1982;13:149S–158S. doi: 10.1111/j.1365-2125.1982.tb01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]