Abstract
The etiology of esophageal mucosal injury is complex, since it may involve the reflux of gastric acid, bile acid, and pancreatic juice, external factors such as drugs and alcohol, or functional factors such as esophagogastric motility. The mechanism of esophageal mucosal injury has gradually been understood at the molecular biological level. It is particularly important that pro-inflammatory factors, such as inflammatory cytokines (interleukin-6 and -8), leukocytes and oxidative stress, have been demonstrated to be involved in the development of gastroesophageal reflux disease (GERD) including nonerosive reflux disease (NERD). In addition, nociceptors such as acid-sensitive vanilloid receptors, protease-activated receptors and substance P have also been implicated in the pathogenesis of neurogenic inflammation in NERD patients with esophageal hypersensitivity. The development of new therapy with anti-inflammatory and anti-oxidant effects is expected to assist in the treatment of intractable NERD/GERD and the prevention of carcinogenesis.
Keywords: gastroesophageal reflux disease (GERD), nonerosive reflux disease (NERD), inflammation, oxidative stress, substance P
Introduction
Gastroesophageal reflux disease (GERD), which is induced by reflux of the gastric and duodenal contents into the esophagus, has recently come to be recognized as a serious clinical problem and the number of patients has been increasing. GERD significantly affects the quality of life because of associated symptoms, such as pharyngeal pain, chest pain, chronic cough, and asthma, as well as typical reflux symptoms such as heartburn. It has been reported that the psychological burden of patients with GERD is heavier than that of patients with duodenal ulcer or angina pectoris [1]. The occurrence of GERD is closely related to relaxation of the lower esophageal sphincter (LES) and increased gastric acid secretion, both of which are associated with westernization of the lifestyle and diet (a high-fat diet), as well as an increase of geriatric patients with hernia and a decreased prevalence of Helicobacter pylori infection. GERD is classified into two types based on the endoscopic detection of mucosal lesions (such as erosions), which are endoscopically positive GERD and endoscopically negative GERD. The former type of GERD is known as reflux esophagitis and the latter is almost synonymous with nonerosive GERD (NERD) or (narrowly defined) symptomatic GERD (s-GERD). Based on the mechanism of GERD, proton pump inhibitors (PPI), which are strong inhibitors of acid secretion, are the first-line drugs for its treatment. However, several issues remain controversial, such as the pathogenesis of NERD as a functional disorder, the recurrence and poor curability of GERD, the progression and prognosis of Barrett’s epithelium, and the relationship between eradication of Helicobacter pylori infection and GERD.
In the clinical setting, patients with NERD account for 60 to 70% of those with GERD and symptoms such as heartburn have become a major clinical concern. To develop effective treatment strategies for GERD and to estimate the long-term prognosis of this disease, it is very important to understand the mechanism of its development. Although GERD has been examined thoroughly under physiological conditions (e.g., investigation of acid reflux into the esophagus by pH monitoring, measurement of intraesophageal pressure, and observation of peristalsis in the esophagus), leading to the accumulation of important knowledge, biochemical and molecular biological studies of the esophageal mucosa remain far behind in comparison with other digestive organs.
Studies of esophagitis that have focused on factors related to inflammation, such as oxidative stress, chemokines, inflammatory cells, and growth factors, have increasingly drawn attention to a new approach to GERD as an inflammatory disease. With regard to NERD as a functional disorder, investigations are already focusing on factors related to sensory abnormalities, such as neuropeptides, acid sensors, and baroreceptors. In this article, we review the significance of inflammatory factors, such as cytokines, oxidative stress and neuropeptides, in the pathogenesis of GERD, the anti-inflammatory and anti-oxidative actions of PPI, mucosal protective agents and protease inhibitors for the treatment of esophageal mucosal injury in human and experimental animals.
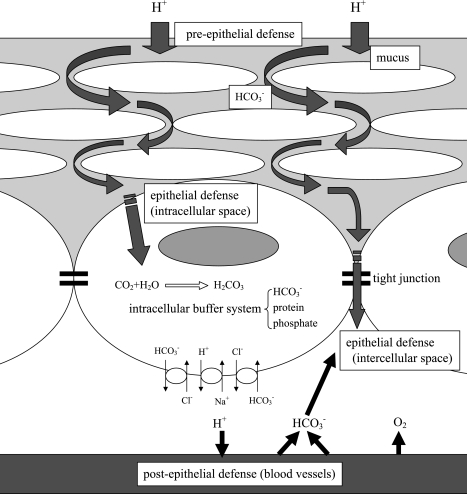
Esophageal Mucosal Resistance (Fig. 1)
Fig. 1.
Esophageal mucosal resistance. The defense mechanism of the esophageal mucosa, which consists of stratified squamous epithelium, is composed of superficial pre-epithelial, epithelial, and deep post-epithelial mechanisms.
The esophageal mucosa is formed by stratified squamous epithelium that consists of 20 to 30 layers of cells. It is composed of three functionally distinct layers-the stratum corneum, the stratum spinosum and the stratum germinativum. Cells divide above the basement membrane and are transformed morphologically and functionally while moving from the stratum germinativum to layers closer to the esophageal lumen, such as stratum spinosum and stratum corneum. The stratum corneum forms a barrier, the stratum spinosum contains cells with a high metabolic activity, and the stratum germinativum is composed of one to two layers of dividing cells located on the basement membrane. Cells move towards the lumen of the esophagus and are eventually sloughed. The half-life of this cycle is reported to be 7 days in rats [2]. Theoretically, the esophageal mucosa has three defense mechanisms, i.e., 1) a pre-epithelial defense mechanism consisting of mucus, bicarbonate ion, and epithelial growth factors, 2) an epithelial defense mechanism consisting of the epithelial cells and intercellular junctional complexes, and 3) a post-epithelial defense mechanism consisting of blood vessels. The pre-epithelial superficial defense mechanism is not very strong, so esophageal epithelial cells are easily exposed to refluxed acid and duodenal juice [3].
Mucosal Inflammation in GERD
Cytokines and oxidative stress in GERD patients
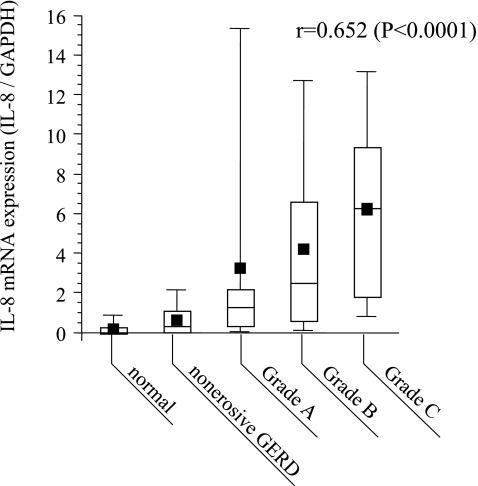
It has recently been demonstrated that inflammatory cytokines, including chemokines, play an important role in inducing early inflammatory changes in patients with GERD. Using esophageal biopsy samples obtained from patients with GERD (including those with NERD), we examined correlations between the expression of various genes (interleukin (IL)-6, IL-8, and monocyte chemoattractant protein 1 (MCP-1)) and the endoscopic findings, histological findings, and symptoms. We found that IL-8 mRNA levels in the esophageal mucosa of patients with GERD were significantly higher than in normal subjects [3, 4]. Regarding the expression of IL-8 mRNA, there was a positive correlation with endoscopic severity (Fig. 2) as well as with the histological neutrophil infiltration score, but there was no clear correlation with the QUEST score (an index of the severity of symptoms). Although an increase of IL-6 (Fig. 3) and MCP-1 was also observed in patients with GERD or NERD, no correlation was detected between the expression of these genes and endoscopic severity. Immunostaining analysis revealed that expression of IL-8 was primarily localized to the basal layer of the esophageal epithelium in patients with GERD. IL-8 is a neutrophil-activating factor that is chiefly produced by leukocytes and vascular endothelial cells. The results of our investigation indicated that esophageal epithelium cells play an important role in the development of mucosal inflammation by producing IL-8. In patients treated with oral PPI therapy for 8 weeks, IL-8 mRNA levels declined quickly as both symptoms and endoscopic findings improved, but there was no change of MCP-1 mRNA levels. These observations indicate that IL-8 is a sensitive marker of esophageal inflammation.
Fig. 2.
Relationship between IL-8 mRNA expression and the endoscopic grade of gastroesophageal reflux disease (GERD) (4). Samples were taken from mucosal breaks in patients with esophagitis. Expression of IL-8 mRNA was quatified by real-time polymerase chain reaction (PCR) using biopsy mucosal materials and was corrected for that of GAPDH mRNA. Endoscopic grading was done according to the Los Angeles (LA) classification. Reprinted with permisson [4]
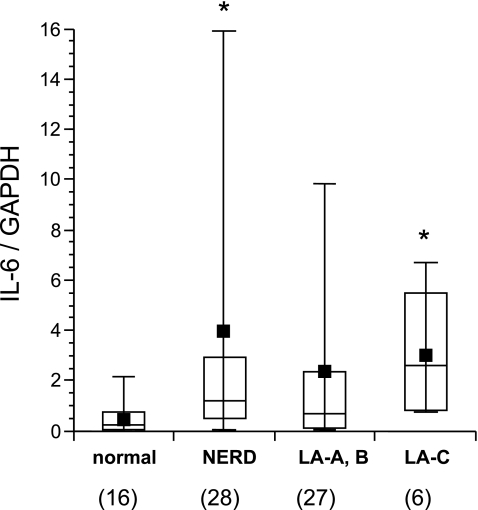
Fig. 3.
IL-6 mRNA expression in esophageal mucosa from patients with GERD. Samples were taken from mucosal breaks in patients with esophagitis. Expression of IL-6 mRNA was quantified and analyzed in the same manner as described in the Fig. 2. *P<0.05 versus normal control.
Interestingly, our study detected IL-8 in all of the patients with NERD and IL-8 gene expression in these patients was significantly increased compared with that in normal subjects, although it was still lower than in patients with endoscopically positive GERD. In addition, slight neutrophil infiltration into the esophageal mucosa was observed in 50% of the patients with NERD. These observations indicate that some patients with NERD may have mild inflammatory changes, which are macroscopically invisible, of the subepithelial region. In fact, detailed analysis of gene expression using a DNA microarray has revealed elevated levels of inflammatory cytokines, such as tumor necrosis factor, interferon α, IL-1, IL-6 and IL-8, in biopsy specimens obtained from NERD patients (Table 1) [5].
Table 1.
Transcriptome analysis of inflammatory cytokines in esophageal mucosa from patients with non-erosive reflux disease (NERD)
| Probe ID | NERD | Normal | Ratio | Gene Title |
|---|---|---|---|---|
| 204470_at | 343.4 | 21.3 | 19.70 | chemokine (C-X-C motif) ligand 2 |
| 205476_at | 1877.3 | 217.9 | 14.93 | chemokine (C-C motif) ligand 20 |
| 207850_at | 1036.9 | 85.2 | 12.13 | chemokine (C-X-C motif) ligand 3 |
| 209774_x_at | 2321.4 | 273.5 | 10.56 | chemokine (C-X-C motif) ligand 2 |
| 211338_at | 18.6 | 5.7 | 4.29 | interferon, alpha 2 /// interferon, alpha 2 |
| 206508_at | 191.5 | 48.7 | 3.73 | tumor necrosis factor (ligand) superfamily, member 7 |
| 210118_s_at | 2570.1 | 555.8 | 3.48 | interleukin 1, alpha |
| 1557908_at | 97.7 | 39.1 | 2.83 | toll-interleukin 1 receptor (TIR) |
| 243977_at | 315.6 | 147.3 | 2.64 | interleukin 6 (interferon, beta 2) |
| 231779_at | 2584.4 | 768.0 | 2.30 | interleukin-1 receptor-associated kinase 2 |
| 202859_x_at | 348.8 | 135.7 | 2.14 | interleukin 8 |
| 221404_at | 1641.2 | 866.7 | 2.00 | interleukin 1 family, member 6 (epsilon) |
| 208771_s_at | 13123.1 | 8115.0 | 1.87 | leukotriene A4 hydrolase |
| 206295_at | 4809.5 | 2367.4 | 1.87 | interleukin 18 (interferon-gamma-inducing factor) |
| 233011_at | 5088.5 | 2337.0 | 1.74 | annexin A1 |
| 207008_at | 927.1 | 515.6 | 1.74 | interleukin 8 receptor, beta |
| 207072_at | 196.1 | 118.2 | 1.74 | interleukin 18 receptor accessory protein |
| 209499_x_at | 81.9 | 57.2 | 1.74 | tumor necrosis factor (ligand) member 13 |
| 203140_at | 3313.6 | 2193.9 | 1.62 | B-cell CLL/lymphoma 6 (zinc finger protein 51) |
| 205291_at | 536.3 | 444.7 | 1.62 | interleukin 2 receptor, beta |
| 202688_at | 3034.4 | 1392.6 | 1.62 | tumor necrosis factor (ligand) superfamily, member 10 |
| 207817_at | 16.7 | 15.9 | 1.62 | interferon, omega 1 |
| 200759_x_at | 2230.3 | 1321.0 | 1.52 | nuclear factor (erythroid-derived 2)-like 1 |
| 205185_at | 46702.0 | 30629.6 | 1.52 | serine protease inhibitor, Kazal type 5 |
| 219403_s_at | 2089.6 | 1591.4 | 1.52 | heparanase |
| 222484_s_at | 5301.6 | 3222.6 | 1.52 | chemokine (C-X-C motif) ligand 14 |
Biopsy specimens were taken from the mucosa at 5 mm above the esophagogastric junction. The GeneChip of human genome U133 Plus 2.0 array (Affymetrix), which contained 54675 probes selected from the UniGene database, was used to analyze gene expression.
There were detected 844 probes that showed a more than 1.5-fold upregulation in expression between normal control (n = 3) and NERD patients (n = 3)
Upregulated genes related to cytokines in NERD patients are shown in this table.
Isomoto et al. [6] reported that chemokines have a role in the pathology of reflux esophagitis. Marked elevation of the levels of IL-8, MCP-1, and RANTES (regulated on activation normal T-cell expressed and presumably secreted) has been observed in the esophageal mucosa of patients with reflux esophagitis. Thickening of the basement membrane is correlated with the expression of IL-8 and MCP-1, while the infiltration of neutrophils and eosinophils into the esophageal mucosa is correlated with the expression of IL-8 and RANTES.
Fitzgerald et al. [7] examined the pathological differences between reflux esophagitis and Barrett’s esophagus by focusing on cytokine expression. They reported that the severity of reflux esophagitis was correlated with the inflammatory cell infiltration score, while IL-1β, IL-8, and interferon-γ (IFN-γ) were expressed more strongly in the esophageal mucosa of patients with reflux esophagitis accompanied by inflammatory cell infiltration than in the mucosa of those without inflammatory cell infiltration or those with Barrett’s esophagus. In patients with Barrett’s esophagus, expression of these acute phase cytokines was weak, but the expression of IL-10 (an anti-inflammatory cytokine) and IL-4 (which is known to increase in Th2-dominant inflammation) was elevated. IL-4 protects the mucosa by inducing intestinal metaplasia with goblet cells [8] and by suppressing neutrophil infiltration [9]. Although it has been assumed that intestinal metaplasia in Barrett’s esophagus is an adaptive response to chronic gastroesophageal reflux [10], the differences of chemokine expression, especially IL-4, between patients with reflux esophagitis and those with Barrett’s esophagus may influence the development of intestinal metaplasia in the esophagus. It seems likely that the expression of various cytokines in the esophageal mucosa causes oxidative stress by inducing the infiltration and activation of inflammatory cells, as well as by increasing the production of reactive oxygen species.
Detection of lipid peroxides, and the expression and activity of antioxidant enzymes have long been used as indexes of oxidative stress.
It has been reported that an increase of chemiluminescence and lipid peroxides can be observed in the esophageal mucosa of patients with reflux esophagitis or Barrett’s esophagus, depending on the severity of their symptoms, indicating that free radicals/active oxygen species are involved in the pathogenesis of reflux esophagitis [11, 12]. It has also been reported that patients with GERD show an increase of superoxide dismutase (SOD), an antioxidant enzyme, in the esophageal mucosa, although the activity of this enzyme is decreased due to nitration of tyrosine residues. This indicates that there are elevated levels of free radicals and peroxynitrite in the esophageal mucosa of these patients [13]. Furthermore, a decreased glutathione concentration and an increase of DNA damage have been observed in the esophageal mucosa of patients with esophagitis, Barrett’s epithelium, dysplasia, or adenocarcinoma, and these two changes show an inverse correlation [14]. This observation indicates that oxidative stress is not only involved in the development of mucosal inflammation, but also has a role in esophageal carcinogenesis.
Antireflux surgery has recently been performed for the prevention and treatment of GERD. Evaluation of 20 patients who underwent antireflux surgery showed that the operation normalized reflux symptoms and 24-hour pH monitoring, but it did not completely abolish intramucosal neutrophil infiltration (MPO activity) or restore the decreased mucosal levels of reduced glutathione [15]. In addition, the authors of this report stated that antireflux surgery is unlikely to prevent the development of adenocarcinoma of the esophagus because it does not improve intramucosal DNA damage [16]. It is impossible to prevent exposure of oxidative stress to the esophageal mucosa by antireflux surgery alone.
Chemokine production by esophageal epithelial cells
Although neutrophils infiltrating into the epithelium and vascular endothelial cells have generally been considered to be the sources of IL-8 in the esophageal mucosa, our immunostaining study with an anti-IL-8 monoclonal antibody revealed that esophageal epithelial cells in the basal layer and the lower spinous layer are also an important source of IL-8 [4]. To investigate the mechanism of IL-8 production, cultured normal human esophageal epithelial cells were stimulated with IL-1β, tumor necrosis factor (TNF)-α, bile acids (unconjugated>conjugated), and trypsin and it was found that the expression of both IL-8 mRNA and protein was increased through the actions of the transcription factors NFκB and AP-1 [17, 18]. Interestingly, our data revealed that conjugated bile acids in acidic media resulted in remarkable increase in IL-8 production compared with those in neural −pH media. As mentioned above, the superficial pre-epithelial defenses of the esophagus, such as mucoprotein, are known to be inadequate to protect the epithelium against various stimulants, and esophageal epithelial cells are directly exposed to refluxed gastric acid and bile acid. Esophageal epithelial cells may play an important role in the initial phase of the development of inflammation. Jenkins et al. [19] examined gene expression using a cDNA array after simulation of human Barrett’s adenocarcinoma cell lines with a bile acid (deoxycholic acid). Among the changes of gene expression observed, the IκB and IL-8 genes were most markedly upregulated, indicating that NFκB-dependent IL-8 production stimulated by deoxycholic acid plays an important role in inflammation.
Inflammatory changes and oxidative stress in animal models of esophagitis
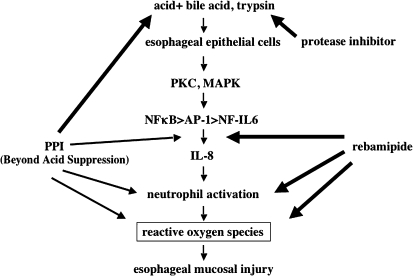
As mentioned above, studies using human esophageal mucosa and cultured esophageal cells have demonstrated that inflammatory cytokines such as IL-8, leukocyte infiltration, and oxidative stress are all involved in the pathogenesis of reflux esophagitis [4, 17, 18] (Fig. 4). To investigate the mechanism in more detail, esophageal mucosal inflammation has been studied in animal models.
Fig. 4.
Esophageal mucosal injury mediated by IL-8/oxidative stress. Production of IL-8 by esophageal epithelial cells is induced by reflux of bile acids with gastric acid or trypsin, and mucosal injury is caused by neutrophil-dependent oxidative stress. Agents that inhibit acid secretion (e.g., proton pump inhibitors: PPIs), mucosal protective agents (e.g., rebamipide), and protease inhibitors are expected to prevent or improve esophageal inflammation by reducing oxidative stress via various mechanisms of action.
We recently reported on the important role of inflammatory cytokines and oxidative stress in the development and progression of esophagitis based on findings obtained in some animal models of acute and chronic esophagitis. In a rat model of acute esophagitis induced by the reflux of gastric acid and duodenal juice, an increase of inflammatory cytokines (cytokine-induced neutrophil chemoattractant-1 (CINC-1, IL-8-like chemokine), TNF-α) in the esophageal mucosa, infiltration of neutrophils and monocytes into the mucosa and submucosa, and mucosal lipid peroxidation were observed before the development of visible lesions. In the same rat model, when the number of circulating neutrophils was reduced to approximately 10% of normal by pretreatment with anti-neutrophil serum, oxidative stress was reduced and significant suppression of esophageal lesions was observed both macroscopically and histologically [20]. In a model of chronic esophagitis due to mixed esophagogastroduodenal reflux, the protease inhibitor, camostat mesilate, significantly reduced trypsin activity in the esophageal lumen, resulting in significant suppression of the increased expression of CINC-1, cyclooxygenase-2 (COX-2), and inducible nitric oxide synthase (iNOS) in the esophageal mucosa, as well as improvement of hyperplastic and inflammatory mucosal changes [21].
It is generally considered that not only reflux of gastric acid but also reflux of duodenal juice, which makes the condition difficult to cure, occurs in GERD. It has been reported that the serum lipid peroxide concentration, protein carbonyl content, and oxidative damage to lymphocyte DNA were significantly greater in an animal model involving the mixed reflux of gastric acid and duodenal juice than in a model of acid reflux alone [22]. These findings suggest that inflammatory cytokines, neutrophils, and oxidative stress induced by the mixed reflux of gastric acid and duodenal juice (containing bile acids and trypsin) play an important role in the development of esophagitis.
To investigate the role of oxidative stress in the development of esophagitis, many studies have been conducted using animal models and the published results include data on changes of antioxidant enzyme activity and the inhibitory effect of antioxidants on mucosal changes. Wetscher et al. [23] reported that reduced glutathione was quickly depleted along with the development of mucosal lesions and lipid peroxidation in rat models of mixed- and acid-type esophagitis, while these responses were inhibited by free radical scavenging enzymes such as SOD and catalase. These observations suggest the important role of reactive oxygen species in the development of esophagitis [23, 24].
In a study using rabbits, SOD markedly prevented the development of lesions due to acid reflux esophagitis, but dimethyl sulfoxide (DMSO, an OH radical-scavenging agent] was ineffective. This observation suggested a significant role of superoxide radicals produced by inflammatory cells in the pathogenesis of esophagitis [25]. On the other hand, in a rabbit model of mild esophagitis due to reflux of acidic pepsin, SOD did not inhibit the development of lesions, but NO synthase was induced in the mucosa and mucosal lesions became worse after the administration of an NO synthesis inhibitor. These observations indicate that superoxide radicals are not directly involved in the development of mucosal lesions, while NO plays an important role in protecting the esophageal mucosa [26].
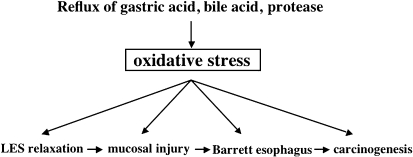
Oh et al. [27] reported that a new antioxidant improved mucosal lesions in a model of mixed esophagitis by significantly inhibiting an increase of mucosal lipid peroxide, activation of NFκB, and decrease of reduced glutathione. Lee et al. [28] also suggested that antioxidants may not only prevent esophageal inflammation but also prevent the development of Barrett’s esophagus by inhibiting excessive cell proliferation. Chen et al. [29] used esophagoduodenal anastomosis to create a rat model of esophagitis in which iron supplementation considerably enhanced oxidative damage to DNA, protein, and lipid, and also induced the development of inflammation and esophageal adenocarcinoma. In a rat model of esophagitis that was created using esophagojejunal anastomosis, administration of SOD decreased mucosal superoxide and peroxynitrite levels, increased the mucosal SOD level, suppressed oxidative damage to DNA, and reduced the risk of esophageal adenocarcinoma or the risk of intestinal metaplasia spreading beyond the anastomotic region [30]. These reports indicate that, as observed in studies using clinical specimens, oxidative stress is involved in the development of esophageal inflammation, Barrett’s epithelium, and esophageal adenocarcinoma in animals (Fig. 5).
Fig. 5.
Oxidative stress-related esophageal disease. Oxidative stress induced by reflux of gastric acid and duodenal fluid (bile acid and pancreatic juice) into the esophagus is associated with the development of inflammation and cancer.
It has long been considered that reflux of gastric contents due to relaxation of the lower esophageal sphincter (LES) plays an important role in the development of reflux esophagitis. Cheng et al. reported that in a cat model of hydrochloric acid reflux, hydrogen peroxide was induced by an increase of IL-1 in the LES, after which it inhibited the release of acetylcholine from cholinergic nerve terminals and caused LES relaxation by stimulating the synthesis of prostaglandin (PG) E2 and platelet-activating factor (PAF) [31, 32]. In addition, hydrogen peroxide seems to be involved in the mechanism of LES relaxation by inhibiting both the influx of calcium into cells and mobilization from calcium stores [33]. In another study on the effects of exposure to acid using esophageal sections including the mucosa and muscle coat [34], IL-1 and IL-6 were found to induce hydrogen peroxide, which was involved in the contraction of the esophageal muscle coat. The authors also performed the same series of studies using human LES tissues. They concluded that mucosal inflammation induced hydrogen peroxide production, which stimulates the synthesis of PAF and PGE2 and is involved in LES relaxation [35]. These reports are interesting in that the authors demonstrated an important role of oxidative stress in reflux due to impaired LES function.
Acid- and Protease-Sensitive Receptors and Neuropeptides in the Esophageal Hypersensitivity: Transcriptome Analysis of Neurogenic Inflammation
Studies of cytokines have revealed that even patients with NERD may have mild mucosal inflammation, but cytokine expression is not necessarily associated with the severity of symptoms such as heartburn [3, 4, 5]. Patient with NERD are known to show a worse response to PPI therapy than those with endoscopically positive GERD in terms of symptomatic improvement. However, the factors associated with the severity of symptoms in NERD patients are unknown. It was recently reported that patients with GERD, especially those with NERD, may have increased esophageal sensitivity.
Trimble et al. [36] reported that when a balloon was inflated at the mid-esophagus of patients with NERD and normal subjects, the patients began to feel pain after less air was added compared with normal subjects. This observation indicates that NERD patients are more sensitive to pressure. Miwa et al. [37] reported that NERD patients felt stronger pain after minimal acid exposure to the esophageal mucosa and it occurred more rapidly than in those with endoscopically positive GERD or normal subjects.
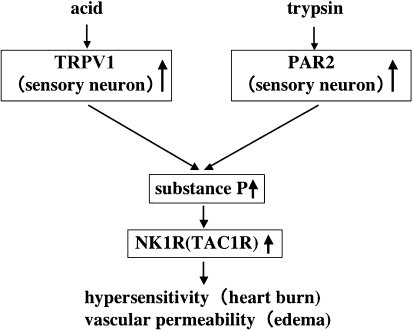
It seemed to us that the greater esophageal sensitivity of NERD patients might be associated with an abnormality of receptors for refluxed substances, such as gastric acid and trypsin, in the esophageal mucosa or an abnormality of neuropeptides involved in the perception of pain, such as substance P. We examined gene and protein expression in endoscopic biopsy specimens by DNA microarray, real-time PCR, and immunostaining. As a result, the mucosal levels of transient receptor potential vanilloid receptor subtype 1 (TRPV1: a capsaicin-, heat-, and acid-sensitive ion channel), protease-activated receptor 2 (PAR2: a receptor for trypsin), substance P, which is secreted by TRPV1- and PAR2-positive nerves, and the NK1 receptor (a receptor for substance P) were significantly higher in NERD patients than in normal subjects [5]. Because NERD patients have higher levels of acid and trypsin receptors in the esophageal mucosa, they may develop edema due to enhanced vascular permeability resulting from a local increase of substance P after the activation of these receptors and pain sensations may be transmitted by substance P-positive nerve fibers arising from the dorsal nerve root ganglion of the spine.
We need to perform further investigations into the important role of receptors/neuropeptides in the development of symptoms in patients with NERD and GERD, e.g., a comparative analysis of pretreatment and post-treatment symptoms (Fig. 6). In addition to analysis of esophageal mucosal changes, it is necessary to investigate the disease from various aspects, such as analysis of sensory abnormalities in the context of the esophagus-brain relationship and analysis of psychological factors.
Fig. 6.
Acid/protease-sensitive receptors and neuropeptides in the hypersensitivity of NERD patients. Substance P associated with increased expression of transient receptor potential vanilloid receptor subtype 1 (TRPV1) and protease-activated receptor 2 (PAR2) may be involved in the development of hypersensitivity of NERD patients.
Treatment of Esophagitis by Targeting Inflammation and Oxidative Stress
PPIs: Beyond the acid suppression
PPIs have been widely used for the treatment of acid-related diseases, such as gastroesophageal reflux disease, as well as for H. pylori eradication therapy, because of a strong inhibitory effect on acid secretion. It has recently been demonstrated that PPIs also have other effects beyond the suppression of acid secretion [38–45].
We have reported that inactive forms of lansoprazole and omeprazole circulating in the blood suppressed important aspects of the acute inflammatory response, such as the adhesion of neutrophils to the endothelium and extravascular migration of neutrophils, by inhibiting the expression of CD11b/CD18 (a neutrophil adhesion molecule) and by inhibiting IL-8 synthesis in gastric mucosal epithelial cells and endothelial cells [39, 45]. These substances are expected to reduce the infiltration of inflammatory cells (lymphocytes and monocytes) related to chronic inflammation by inhibiting the expression of intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) on endothelial cells [39]. An important point is that these effects are observed at serum levels achieved by the clinical dosage (10−5–10−6 M) but not those that would be needed for histamine receptor antagonists, though they are also acid secretion inhibitors. Furthermore, it has been reported that PPIs inhibit free radical production by neutrophils [40, 41] and block neutrophil degranulation [40], though at relatively high concentrations (10−4–10−5 M).
Taken altogether, these evidences suggest that PPIs not only inhibit acid secretion but also reduce inflammation and oxidative stress in the gastric and esophageal mucosa by inhibiting the activation of neutrophils and endothelial cells. Actually, in our study described above [4], PPI therapy rapidly reduced IL-8 levels in the human esophageal mucosa. In an animal model of chronic esophagitis due to acid reflux, PPI therapy markedly inhibited the expression of adhesion molecules and inflammatory cytokines in the esophageal mucosa [42]. In addition, it has been shown that lansoprazole markedly inhibits small intestinal injury associated with the increase in cytokines and neutrophils induced by ischemia/reperfusion or non-steroidal anti-inflammatory drugs (NSAID), which are unrelated to acid secretion [43, 44]. These observations suggest that part of the anti-inflammatory effect of PPIs does not result from the inhibition of acid secretion. In fact, we have demonstrated that the anti-inflammatory and anti-oxidant effects of PPI are partly related to suppression of intracellular calcium metabolism and to blocking the activation of transcription factors [45]. Further non-clinical and clinical investigations into the mechanism of the anti-inflammatory actions of PPI are needed.
Mucosal protective agents
It has long been known that some gastric mucosal protective agents have anti-inflammatory and anti-oxidant effects. In particular, rebamipide shows a variety of anti-inflammatory actions, such as scavenging of free radicals, inhibition of ROS and protease production by neutrophils, inhibition of adhesion molecule expression, and inhibition of cytokine production [46, 47, 48]. We previously reported that in a rat model of mixed reflux esophagitis, intraduodenal rebamipide significantly inhibited the increased expression of mRNA and protein for CINC-1 and TNF-α, as well as neutrophil infiltration and lipid peroxidation in the esophageal mucosa [49], and consequently prevented the development of mucosal lesions both histologically and macroscopically. Further investigations are needed regarding the efficacy of mucosal protective agents for the prevention of esophagitis.
PAR and protease inhibitor
Although the reflux of acid, bile acids, and proteases (such as trypsin) in pancreatic juice seems to play an important role in the development of GERD, especially postgastrectomy damage to the esophageal mucosa, the details of the mechanism involved are unknown.
It has recently been revealed that serine proteases, such as trypsin and thrombin, are involved in a wide range of biological responses, such as inflammation, angiogenesis, cell proliferation, and apoptosis, by activating intracellular signaling cascades through the PAR [50]. PAR is a seven transmembrane trimer-G-protein-coupled receptor that is activated by a specific protease. Of the four family members, PAR1, PAR3, and PAR4 are primarily activated by thrombin while PAR2 is activated by trypsin, mast cell tryptase, and clotting factors VIIa and Xa. We have demonstrated in an animal model of colitis and a model of human chronic ulcerative colitis that PAR activation by mast cell-derived tryptase is involved in the development of colitis and that anti-PAR therapy based on inhibition of tryptase is effective for the treatment of colitis [51, 52].
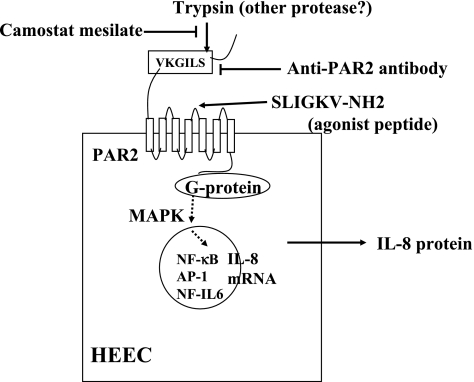
We recently demonstrated that PAR2 is strongly expressed by normal human esophageal epithelial cells and that PAR-mediated NFκB-dependent production of IL-8 is induced by stimulation with trypsin [18]. In addition, camostat mesilate, a protease inhibitor clinically used for the treatment of postgastrectomy reflux esophagitis, dose-dependently suppressed IL-8 production by inhibiting the activity of trypsin (Fig. 7). Because of its inhibitory effect on protease activity, camostat mesilate is expected to improve esophagitis by reducing cytokine production resulting from the PAR-mediated activation of esophageal epithelial cells as well as oxidative stress due to the infiltration of inflammatory cells.
Fig. 7.
Protease-activated receptor 2 (PAR2)-mediated IL-8 production from human esophageal epithelial cells (HEEC). Trypsin activates PAR2 via cleavage of the extracellular N-terminal domain, which then enables the new N terminus (SLIGKV) to bind the receptor itself as a tethered ligand to activate G-protein coupled signal transduction pathways. PAR2 can also be activated without proteolytic cleavage using five to six synthetic peptides (SLIGKV) corresponding to the new amino termini of the cleaved receptors.
PAR2 activation in HEEC by trypsin induces NFκB- and AP-1-dependent IL-8 production in association with activation of p38 MAPK and ERK1/2, suggesting that esophageal inflammation may be induced by PAR2 activation via reflux of trypsin.
Camostat mesilate and anti-PAR2 antibody inhibit IL-8 production via the inhibition of trypsin activity and the blocking against cleavage site, respectively.
References
- 1.Dimenas E. Methodological aspects of evaluation of Quality of Life in upper gastr ointestinal diseases. Scand. J. Gastroenterol. Suppl. 1993;199:18–21. [PubMed] [Google Scholar]
- 2.Orlando R.C. In: Esophageal epithelial resistance. in Gastroesophageal reflux disease: Pathogenesis, Diagnosis, Therapy. Castell D.O., Wu W.C., Ott D.J., editors. Futura; Mount Kisco: 1985. pp. 55–79. [Google Scholar]
- 3.Yoshida N., Yoshikawa T. Defense mechanism of the esophageal mucosa and esophageal inflammation. J. Gastroenterol. 2003;38:31–34. [PubMed] [Google Scholar]
- 4.Yoshida N., Uchiyama K., Kuroda M., Sakuma K., Kokura S., Ichikawa H., Naito Y., Takemura T., Yoshikawa T., Okanoue T. IL-8 expression in the esophageal mucosa of patients with gastroesophageal reflux disease. Scand. J. Gastroenterol. 2004;39:816–822. doi: 10.1080/00365520410006729. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida N., Kuroda M., Uchiyama K., Suzuki T., Okuda T., Tsuboi H., Kajikawa H., Handa O., Kokura S., Ichikawa H., Yoshikawa T., Okanoue T. Microarray analysis of transcriptome in patients with nonerosive reflux disease. Gastroenterology. 2005;128:A-523. [Google Scholar]
- 6.Isomoto H., Wang A., Mizuta Y., Akazawa Y., Ohba K., Omagari K., Miyazaki M., Murase K., Hayashi T., Inoue K., Murata I., Kohno S. Elevated levels of chemokines in esophageal mucosa of patients with reflux esophagitis. Am. J. Gastroenterol. 2003;98:551–556. doi: 10.1111/j.1572-0241.2003.07303.x. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald R.C., Onwuegbusi B.A., Bajaj-Elliott M., Saeed I.T., Burnham W.R., Farthing M.J. Diversity in the oesophageal phenotypic response to gastro-oesophageal reflux: immunological determinants. Gut. 2002;50:451–459. doi: 10.1136/gut.50.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobbagh K., Takeyama K., Lee H., Ueki I.F., Lausier J.A., Nadel J.A. IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J. Immunol. 1999;162:6233–6237. [PubMed] [Google Scholar]
- 9.Colgan S., Resnick M., Parkos C., Delp-Archer C., McGuirk D., Bacarra A.E., Weller P.F., Madara J.L. IL-4 directly modulates function of a model human intestinal epithelium. J. Immunol. 1994;153:2122–2129. [PubMed] [Google Scholar]
- 10.Jankowski J. Gene expression in Barrett’s mucosa: acute and chronic adaptive responses in the oesophagus. Gut. 1993;34:1649–1650. doi: 10.1136/gut.34.12.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wetscher G.J., Hinder R.A., Bagchi D., Hinder P.R., Bagchi M., Perdikis G., McGinn T. Reflux esophagitis in humans is mediated by oxygen-derived free radicals. Am. J. Surg. 1995;170:552–556. doi: 10.1016/s0002-9610(99)80014-2. [DOI] [PubMed] [Google Scholar]
- 12.Olyaee M., Sontag S., Salman W., Schnell T., Mobarhan S., Eiznhamer D., Keshavarzian A. Mucosal reactive oxygen species production in oesophagitis and Barrett’s esophagus. Gut. 1995;37:168–173. doi: 10.1136/gut.37.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jimenez P., Piazuelo E., Sanchez M.T., Ortego J., Soteras F., Lanas A. Free radicals and antioxidant systems in reflux esophagitis and Barrett’s esophagus. World J. Gastroenterol. 2005;11:2697–2703. doi: 10.3748/wjg.v11.i18.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sihvo E.I., Salminen J.T., Rantanen T.K., Ramo O.J., Ahotupa M., Farkkila M., Auvinen M.I., Salo J.A. Oxidative stress has a role in malignant transformation in Barrett’s esophagus. Int. J. Cancer. 2002;20:551–555. doi: 10.1002/ijc.10755. [DOI] [PubMed] [Google Scholar]
- 15.Rantanen T.K., Rasanen J.V., Sihvo E.L., Ahotupa M.O., Farkkila M.A., Salo J.A. The impact of antireflux surgery on oxidative stress of esophageal mucosa caused by gastroesophageal reflux disease: 4-yr follow-up study. Am. J. Gastroenterol. 2006;101:222–228. doi: 10.1111/j.1572-0241.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 16.Oksala N.K., Atalay M., Rantanen T.K. Antireflux surgery and esophageal mucosal DNA damage. Pathophysiology. 2006;21:23–27. doi: 10.1016/j.pathophys.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida N., Imamoto K., Uchiyama K., Kuroda M., Naito Y., Mukaida N., Kawabe A., Shimada Y., Yoshikawa T., Okanoue T. Molecular mechanisms involved in IL-8 production by normal human esophageal epithelial cells. Aliment. Pharmacol. Ther. sympo. ser. 2006;2:219–226. [Google Scholar]
- 18.Yoshida N., Katada K., Handa O., Takagi T., Kokura S., Naito Y., Mukaida N., Soma T., Shimada Y., Yoshikawa T., Okanoue T. Interleukin-8 production via protease-activated receptor 2 in human esophageal epithelial cells. Int. J. Mol. Med. doi: 10.3892/ijmm.19.2.335. In press. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins G.J., Harries K., Doak S.H., Wilmes A., Griffiths A.P., Baxter J.N., Parry J.M. The bile acid deoxycholic acid (DCA) at neutral pH activates NF-kappaB and induces IL-8 expression in oesophageal cells in vitro. Carcinogenesis. 2004;25:317–323. doi: 10.1093/carcin/bgh032. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi T., Yoshida N., Tomatsuri N., Takayama R., Katada K., Takagi T., Ichikawa H., Naito Y., Okanoue T., Yoshikawa T. Cytokine-induced neutrophil accumulation in the pathogenesisi of acute esophagitis in rats. Int. J. Mol. Med. 2005;16:71–77. [PubMed] [Google Scholar]
- 21.Naito Y., Uchiyama K., Kuroda M., Takagi T., Kokura S., Yoshida N., Ichikawa H., Yoshikawa T. Role of pancreatic trypsin in chronic asphagitis induced by gastroduodenal reflux in rats. J. Gastroenterol. 2006;41:198–208. doi: 10.1007/s00535-005-1742-5. [DOI] [PubMed] [Google Scholar]
- 22.Erbil Y., Turkoglu U., Barbaros U., Balik E., Olgac V., Kaya H., Cimsit B. Oxidative damage in an experimentally induced gastric and gastroduodenal reflux model. Surg. Innov. 2005;12:219–225. doi: 10.1177/155335060501200306. [DOI] [PubMed] [Google Scholar]
- 23.Wetscher G.J., Perdikis G., Kretchmar D.H., Stinson R.G., Baguchi D., Redmand E.J., Adrian T.E., Hinder R.A. Esophagitis in Sprague-Dawley rats is mediated by free radicals. Dig. Dis. Sci. 1995;40:1297–1305. doi: 10.1007/BF02065542. [DOI] [PubMed] [Google Scholar]
- 24.Wetscher G.J., Hinder P.R., Bagchi D., Perdikis G., Redmand E.J., Glaser K., Adrian T.E., Hinder R.A. Free radical scavengers prevent reflux esophagitis in rats. Dig. Dis. Sci. 1995;40:1292–1296. doi: 10.1007/BF02065541. [DOI] [PubMed] [Google Scholar]
- 25.Naya M.J., Pereboom D., Ortego J., Alda J.O., Lanas A. Superoxide anions produced by inflammatory cells play an important part in the pathogenesis of acid and pepsin induced oesophagitis in rabbits. Gut. 1997;40:175–181. doi: 10.1136/gut.40.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soteras F., Lanas A., Fiteni I., Royo Y., Jimenez P., Inarrea P., Ortego J., Esteva F. Nitric oxide and superoxide anion in low-grade esophagitis induced by acid and pepsin in rabbits. Dig. Dis. Sci. 2000;45:1802–1809. doi: 10.1023/a:1005521925785. [DOI] [PubMed] [Google Scholar]
- 27.Oh T.Y., Lee J.S., Ahn B.O., Cho H., Kim W.B., Kim Y.B., Surh Y.J., Cho S.W., Hahm K.B. Oxidative damages are critical in pathogenesis of reflux esophagitis: implication of antioxidants in its treatment. Free Radic. Biol. Med. 2001;30:905–915. doi: 10.1016/s0891-5849(01)00472-5. [DOI] [PubMed] [Google Scholar]
- 28.Lee J.S., Oh T.Y., Ahn B.O., Cho H., Kim W.B., Kim Y.B., Surh Y.J., Kim H.J., Hahm K.B. Involvement of oxidative stress in experimentally induced reflux esophagitis and Barrett’s esophagus: cule for the chemoprevention of esophageal carcinoma by antioxidants. Mutat. Res. 2001;480-481:189–200. doi: 10.1016/s0027-5107(01)00199-3. [DOI] [PubMed] [Google Scholar]
- 29.Chen X., Ding Y.W., Yang G., Bondoc F., Lee M.J., Yang C.S. Oxidative damage in an esophageal adenocarcinoma model with rats. Carcinogenesis. 2000;21:257–263. doi: 10.1093/carcin/21.2.257. [DOI] [PubMed] [Google Scholar]
- 30.Piazuelo E., Cebrian C., Escartin A., Jimenez P., Soteras F., Ortego J., Lanas A. Superoxide dismutase prevents development of adenocarcinoma in a rat model of Barrett’s esophagus. World J. Gastroenterol. 2005;11:7436–7443. doi: 10.3748/wjg.v11.i47.7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng L., Cao W., Behar J., Biancani P., Harnett K.M. Inflammation induced changes in arachidonic acid metabolism in cat LES circular muscle. Am. J. Physiol. 2005;288:G787–797. doi: 10.1152/ajpgi.00327.2004. [DOI] [PubMed] [Google Scholar]
- 32.Cheng L., Cao W., Fiocchi C., Behar J., Biancani P., Harnett K.M. Platelet-activating factor and prostaglandin E2 impair esophageal Ach release in experimental esophagitis. Am. J. Physiol. 2005;289:G418–428. doi: 10.1152/ajpgi.00024.2005. [DOI] [PubMed] [Google Scholar]
- 33.Cao W., Harnett K.M., Cheng L., Kirber M.T., Behar J., Biancani P. H2O2: a mediator of esophagitis-induced damage to calcium-release mechanisms in cat lower esophageal sphincter. Am. J. Physiol. 2005;288:G1170–1178. doi: 10.1152/ajpgi.00509.2004. [DOI] [PubMed] [Google Scholar]
- 34.Cheng L., Cao W., Fiocchi C., Behar J., Biancani P., Harmett K.M. In vitro model of acute esophagitis in the cat. Am. J. Physiol. 2005;289:G860–869. doi: 10.1152/ajpgi.00260.2005. [DOI] [PubMed] [Google Scholar]
- 35.Cheng L., Harnett K.M., Cao W., Liu F., Behar J., Fiocchi C., Biancani P. Hydrogen peroxide reduces lower esophageal sphincter tone in human esophagus. Gastroenterology. 2005;129:1675–1685. doi: 10.1053/j.gastro.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Trimble K.C., Pryde A., Heading R.C. Lowered oesophageal sensory thresholds in patients with symptomatic but not excess gastro-oesophageal reflux: evidence for a spectrum of visceral sensitivity in GORD. Gut. 1995;37:7–12. doi: 10.1136/gut.37.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miwa H., Minoo T., Hojo M., Yaginuma R., Nagahara A., Kawabe M., Ohkawa A., Asaoka D., Kurosawa A., Ohkusa T., Sato N. Oesophageal hypersensitivity in Japanese patients with non-erosive gastro-oesophageal reflux diseases. Aliment. Pharmacol. Ther. 2004;20:112–117. doi: 10.1111/j.1365-2036.2004.01990.x. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida N. Progress in study for mechanism of gastric mucosal injury. Nippon Rinsho. 2001;59:1829–1836. [PubMed] [Google Scholar]
- 39.Yoshida N., Yoshikawa T., Tanaka Y. Fujita., Kassai K., Naito Y., Kondo M. A new mechanism for anti-inflammatory actions of proton pump inhibitors-inhibitory effects on neutrophil-endothelial cell interactions. Aliment. Pharmacol. Ther. 2000;14:74–81. doi: 10.1046/j.1365-2036.2000.014s1074.x. [DOI] [PubMed] [Google Scholar]
- 40.Wandall J.H. Effects of omeprazole on neutrophil chemotaxis, superoxide production, degranulation, and translocation cytochrome b-245. Gut. 1992;33:617–621. doi: 10.1136/gut.33.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki M., Mori M., Miura S., Suematsu M., Fukumura D., Kimura H., Ishii H. Omeprazole attenuates oxygen-derived free radical production from human neutrophils. Free Radic. Biol. Med. 1996;21:727–731. doi: 10.1016/0891-5849(96)00180-3. [DOI] [PubMed] [Google Scholar]
- 42.Hamaguchi M., Fujiwara Y., Takashima T., Hayakawa T., Sasaki E., Shiba M., Watanabe T., Tominaga K., Oshitani N., Matsumoto T., Higuchi K., Arakawa T. Increased expression of cytokines and adhesion molecules in rat chronic esophagitis. Digestion. 2003;68:189–197. doi: 10.1159/000075698. [DOI] [PubMed] [Google Scholar]
- 43.Ichikawa H., Yoshida N., Takagi T., Tomatsuri N., Katada K., Isozaki Y., Uchiyama K., Naito Y., Okanoue T., Yoshikawa T. Lansoprazole ameliorates intestinal mucosal damage induced by ischemia-reperfusion in rats. World. J. Gastroenterol. 2004;10:2814–2817. doi: 10.3748/wjg.v10.i19.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuroda M., Yoshida N., Ichikawa H., Takagi T., Okuda T., Naito Y., Okanoue T., Yoshikawa T. Lansoprazole, proton pump inhibitor, reduces the severity of indomethacin-induced rat colitis. Int. J. Mol. Med. 2006;17:89–93. [PubMed] [Google Scholar]
- 45.Handa O., Yoshida N., Fujita N., Tanaka Y., Ueda M., Takagi T., Kokura S., Naito Y., Okanoue T., Yoshikawa T. Molecular mechanisms involved in anti-inflammatory effects of proton pump inhibitors. Inflamm. Res. 2006;55:476–480. doi: 10.1007/s00011-006-6056-4. [DOI] [PubMed] [Google Scholar]
- 46.Naito Y., Yoshikawa T., Tanigawa T., Sakurai K., Yamasaki K., Uchida M., Kondo M. Hydroxyl radical scavenging by rebamipide and related compounds: Electron paramagnetic resonance study. Free Radic. Biol. Med. 1995;18:117–123. doi: 10.1016/0891-5849(94)00110-6. [DOI] [PubMed] [Google Scholar]
- 47.Yoshida N., Yoshikawa T., Iinuma S., Arai M., Takenaka S., Sakamoto K., Miyajima T., Nakamura Y., Yagi N., Naito Y., Mukai F., Kondo M. Rebamipide protects against activation of neutrophils by Helicobacter pylori. Dig. Dis. Sci. 1996;41:1139–1144. doi: 10.1007/BF02088229. [DOI] [PubMed] [Google Scholar]
- 48.Genta R.M. Review article: the role of rebamipide in the management of inflammatory disease of the gastrointestinal tract. Aliment. Pharmacol. Ther. 2003;18:8–13. doi: 10.1046/j.1365-2036.18.s1.5.x. [DOI] [PubMed] [Google Scholar]
- 49.Katada K., Yoshida N., Isozaki Y., Tomatsuri N., Ichikawa H., Naito Y., Okanoue T., Yoshikawa T. Prevention of acute reflux esophagitis in rats with rebamipide. Dig. Dis. Sci. 2005;50:97–103. doi: 10.1007/s10620-005-2813-4. [DOI] [PubMed] [Google Scholar]
- 50.Ossovskaya V.S., Bunnett N.W. Protease-activated receptors: contribution to physiology and disease. Physiol. Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- 51.Isozaki Y., Yoshida N., Kuroda M., Handa O., Takagi T., Kokura S., Ichikawa H., Naito Y., Okanoue T., Yoshikawa T. Therapeutic effect of anti-tryptase treatment using nafamostat mesilate on inflammatory bowel disease. Scand. J. Gastroenterol. 2006;41:944–953. doi: 10.1080/00365520500529470. [DOI] [PubMed] [Google Scholar]
- 52.Yoshida N., Isozaki Y., Takagi T., Takenaka S., Uchikawa R., Arizono N., Yoshiakwa T., Okanoue T. Review article: anti-tryptase therapy in inflammatory bowel disease. Aliment. Pharmacol. Ther. sympo. ser. 2006;2:249–255. [Google Scholar]