Abstract
Various radical-scavenging activities of thiamin and thiamin diphosphate (TDP) were found in some in vitro experiments. Thiamin and TDP caused considerable suppressive effects on superoxide generation in hypoxanthine and xanthine oxidase system which was measured by a sensitive chemiluminescence method using 2-methyl-6-[p-methylphenyl]-3,7-dihydroimidazo[1,2-alpha]pyrazin-3-one (MCLA), and their 50% inhibition (IC50) values were estimated to be 158 and 56 µM, respectively. They also showed the significant suppression against hydroperoxide generation derived from oxidized linoleic acid which was estimated by aluminum chloride method and their IC50 values were calculated to be 260 and 46 µM. They further prevented the oxygen radical generation in opsonized zymosan-stimulated human blood neutrophils which was shown by chemiluminescence method using luminol, and their IC50 values were calculated to be 169 and 38 µM. In contrast, they caused weak but significantly suppressive effects on the hydroxyl radical generation by Fenton reaction which was measured by electric spin resonance (ESR) method, their IC50 values were calculated to be 8.45 and 1.46 mM respectively. These results strongly suggest a possibility that thiamin and TDP play as radical scavengers in cell-free and cellular systems.
Keywords: vitamin B1, thiamin, thiamin diphosphate, radical-scavenging activity
Introduction
Since the discovery of thiamin (vitamin B1) as antifactor against “Beriberi” disease, various biochemical and pharmacologic activities of thiamin and its derivatives have been reported. For example, a phosphate ester form of thiamin, thiamin diphosphate (TDP) possesses coenzyme activity for oxidative decarboxylation of alpha-keto acids such as pyruvate, alpha-ketoglutalate and branched chain amino acids [1]. TDP also plays as a coenzyme for transketolase reaction which catalyzes the transfer of glycoaldehyde from keto-sugars to aldo-sugars [2]. These enzyme reactions are associated with cellular bioenergetics involving adenine 5'-triphosphate (ATP) synthesis. As another important pharmacologic activity, thiamin and its derivatives play important roles in nerve functions. For example, thiamin and thiamin triphosphate are responsible for nerve transmission involving sodium and potassium ion chanelling [3]. Furthermore, thiamin improved the metabolic state of the brain of Alzheimer’s disease patients, and it was analogous effect compared with the effect on thiamin-deficient brain disease Wernikke-Korsacoff syndrome [4–6]. On the other hand, other researchers found that thiamin and TDP inhibited the glycation of protein using high concentrations of glucose in a diabetic model experiment [7] and other studies indicated that the glycation and other events in diabetic complications are responsible for various oxidative processes such as oxygen radical generation [8, 9]. However, the antioxidant or radical-scavenging activities of thiamin and its derivatives have not been systematically analyzed until present.
On the other hand, we recently found the considerable antioxidant and radical-sacavenging activities in the water extract of rice bran and the active principles for these activities were analyzed. As an active protein molecule, we identified the radical-scavenging enzyme such as peroxidase which exhibited the strong antioxidant and antigenotoxic activities [10]. Furthermore, we found another antioxidant and radical-scavenging activities in the low-molecular-weight fraction of the rice bran extract (unpublished data). Since this fraction contained various B group vitamins, we investigated the antioxidant and radical-scavenging activities of B group vitamins by some in vitro experiments.
In the present study, we assumed a possibility that thiamin and TDP have potent antioxidant or radical-scavenging activities and detected their preventive effects on lipid peroxidation, hydroxyl radical generation in Fenton reaction, superoxide generation in hypoxanthine and xanthine oxidase system and oxygen radical generation in human blood neutrophils.
Materials and Methods
Chemicals
Thiamin hydrochloride, TDP, luminol sodium salt were purchased from Wako Pure Chemicals Co. (Osaka, Japan). 2-methyl-6-[p-methylphenyl]-3,7-dihydroimidazo[1,2-alpha] pyrazin-3-one (MCLA) was purchased from Tokyo Kasei Co. (Tokyo, Japan). Hypoxanthine (HPX), xanthine oxidase (XOD), bovine erythrocyte superoxide dismutase (SOD), and yeast cell wall-derived zymosan were purchased from Sigma-Aldorich Japan (Tokyo, Japan). Linoleic acid was purchased from Kanto Chemical Co. (Tokyo, Japan). 5,5-dimethyl-1-pyrroline N-oxide (DMPO) was purchased from Labotec co. (Tokyo, Japan). Other chemicals for experiments were purchased from Sigma-Aldorich Chemicals Co (St. Louis, MO). except for specific chemicals which are described in text.
Assay for superoxide generation in hypoxanthine and xanthine oxidase system
Superoxide generation was measured by a slight modification of the previous method [11]. This method shows about 100-fold more sensitivity than usual cytochrome C method. Initially, a reaction mixture contains 25 µl of 0.3 M Tris-HCl buffer (pH 7.8) with 1 mM etylenediaminetetraacetic acid (EDTA), 5 µl of 20 µM MCLA, 5 µl of 3 mM HPX and 1.4 ml of distilled water, phosphate buffered saline (PBS) or vitamin solution, which was preincubated at 25°C for 3 min. The superoxide-dependent chemiluminescence (CHL) generation was initiated by the addition of 5 µl of XOD solution (60 µg/ml) to the reaction mixture mentioned above and continued for another 5 min incubation at 25°C. The CHL generations in the presence and absence of SOD (500 U/ml) were compared. The CHL measurement was carried out by a CHL reader (Type BLR-102; Aloka Co., Tokyo, Japan). The experimental result was expressed as the integral CHL intensity for 5 min reaction.
Assay for hydroxyl radical-scavenging activity using ESR method
The hydroxyl radical-scavenging activity was assayed by the modification of the previous Electron Spin Resonance (ESR) method [12]. Distilled water, PBS or vitamin solution (50 µl), 0.18 M DMPO in distilled water (50 µl) and 2 mM hydrogen peroxide (50 µl) were mixed in a glass test tube and finally added with 50 µl of 2 mM ferrous sulphate in water prepared daily. The reaction mixture was immediately transferred into a quartz ESR flat cell and measured ESR spectra exactly 40 sec after the addition of ferrous sulphate solution using an ESR analyzing apparatus (Type JES-TE200; Nihon Denshi Co., Hamamatsu, Japan). The settings of ESR instrument are as follows: magnetic field; 335.2 mT/power; 8.0 mW/modulation frequency; 9.42 GHz/modulation width; 0.1 mT/sweeping time; 2 min/response time; 0.1 sec/amplitude; 50. The intensity of DMPO-OH spin adduct that is generated from the reaction between DMPO and hydroxyl radical is expressed as a ratio of the signal intensity at the lowest magnetic field to that of manganese ion in water (MnO) used as an internal standard marker.
Assay for the hydroperoxide generation from linoleic acid peroxidation
The amount of hydroperoxide generated from oxidized linoleic acid was measured by the modification of the previous method [13]. One ml of the test solution of thiamin and TDP was mixed with 1 ml of linoleic acid in ethanol and 2 ml of 50 mM phosphate buffer (pH 7.5) in a glass test tube and stood for 4 weeks at 33°C. As a negative control experiment, 1 ml of distilled water or PBS was used in place of test solution. After four weeks reaction, the test solution mixture mentioned above (200 µl), 250 µl of 2% Potassium Iodine (KI) solution in ethanol, 2% aluminum chloride solution and 1 ml of hexane were mixed in a glass test tube and incubated for 5 min at 37°C under dark condition, then 250 µl of starch solution and 7.5 ml of 10 mM HCl were mixed and shaken vigorously. The solution was centrifuged at 1,500 × g for 5 min, and the absorbance of the lower layer was measured at 560 nm using Shimadzu UV-265 spectrophotometer.
Assay for oxygen radical generation in human blood neutrophils
Isolation of the neutrophils was carried out as follows. Human venous bloods obtained from healthy non-smoking male donors were recovered in the presence of 3.8% citrate and centrifuged using Mono-poly Resolving Medium (Dai-nihon Pharmatheutical Co., Osaka, Japan) and Krebs-Ringer phosphate solution (KRP, pH 7.4). The neutrophils in the cell layer were recovered and washed two times with KRP by centrifugations and kept in ice until using for the experiment. The viabilities of neutrophils using in reactive oxygen species (ROS) generation experiments were more than 95%. Assay for ROS generation was carried out according to the modification of the previous method [14]. Human neutrophils (1 × 105 cells) were incubated with 50 mM phosphate buffered saline (PBS, pH 7.4) in the presence of luminol sodium salt (250 µM) at a final volume of 500 µl. After the preincubation of the cell suspension mixture at 37°C for 3 min, the reaction was started by adding opsonized zymosan (1 µg/ml) and further incubated for another 30 min at 37°C. Zymosan (5 mg/ml) was opsonized with 50 mM PBS (pH 7.4) including 25% human serum for 30 min at 37°C and washed with 50 mM PBS extensively. Opsonized zymosan (Opz, 1 mg/ml PBS) was kept at −80°C until the use of experiment. For the analysis of effects of thiamin and TDP, they were added to the neutrophils mixture before the exposure of Opz to the cells. The CHL intensity of the cell mixture was recorded continuously during the incubation for 30 min at 37°C using a CHL reader (BLR-201; Aloka Co., Tokyo, Japan).
Expression of experimental results and statistical analysis of data
Experimental result was expressed as the mean and standard error of triplicate assays. Relative inhibitory activity (% inhibition) was expressed as a following equation: (positive control value – vitamin-treated value/positive control value) × 100%. These values were plotted as a dose-response curve on a semilogarithmic graph in which the concentration required for 50% inhibition (IC50) was determined. The statistical comparison of the data between control and vitamin-treated experiments was carried out using Student’s t test. A p value less than 0.05 was considered to be significantly different.
Results
Suppressive effects of thiamin and TDP on superoxide generation in hypoxanthine and xanthine oxidase system
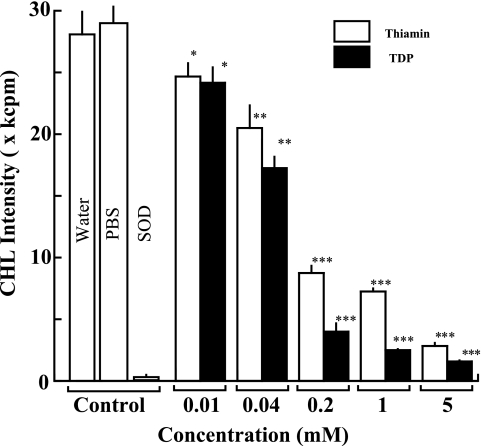
First, we examined effects of thiamin and TDP on superoxide generation in HPX and XOD system by a sensitive CHL method using MCLA. As shown in Fig. 1, a considerable amount of superoxide was generated in HPX and XOD system as control experiments (distilled water or PBS) which showed the strong CHL intensity. This CHL generation was almost completely scavenged by the exogenous addition of SOD (500 U/ml). When various concentrations of thiamin and TDP were added to the reaction mixture before the start of the superoxide-generating enzyme reaction, the superoxide-dependent CHL generation was suppressed in a dose-dependent fashion from 5 mM to 10 µM (Fig. 1). In addition, the highest concentration (10 mM) of thiamin or TDP caused almost complete suppression (96.3 or 99.9 % inhibition compared with the control CHL generation). Furthermore, TDP exhibited much stronger activity than thiamin at all the concentrations tested, and IC50 values of thiamin and TDP were calculated to be 158 and 56 µM, respectively. In addition, PBS did not show any significant effects on this experimental system (Fig. 2).
Fig. 1.
Effects of thiamin and TDP on superoxide generation in HPX and XOD system. The column and bar show the mean and SD of CHL intensities of triplicate assays. The white and hatched columns represent CHL intensity of thiamin and TDP, respectively. The statistical difference of the results between control and vitamin-treated experiments is analyzed by Student’s t test. The p value is expressed by each asterisk as follows. *p<0.05, **p<0.01, ***p<0.001.
Fig. 2.
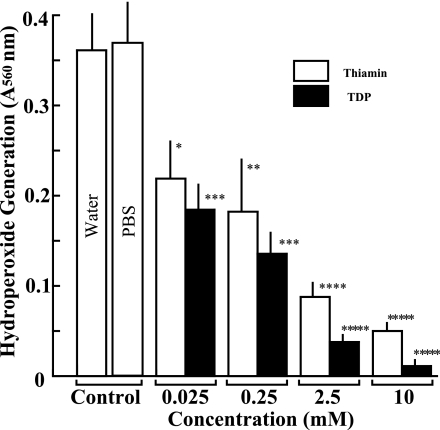
Effects of thiamin and TDP on hydroperoxide generation in lipid peroxidation. The column and bar show the mean and SD of absorbance by hydroperoxide generation in triplicate assays. The white and hatched columns represent absorbance of thiamin and TDP, respectively. The statistical difference between control and vitamin-treated experiments is analyzed by Student’s t test. The p value is expressed by each asterisk as follows. *p<0.05, **p<0.02, ***p<0.005, ****p<0.001, *****p<0.0001.
Suppressive effects of thiamin and TDP on hydroperoxide generation in lipid peroxidation
We then analyzed the effects of thiamin and TDP on hydroperoxide generation in auto-oxidation of linoleic acid by alminum chloride method. As shown in Fig. 2, when freshly prepared linoleic acid solution was incubated at 33°C for four weeks, the considerable amount of hydroperoxide was generated by the auto-oxidation of linoleic acid. Thiamin and TDP from 10 mM to 25 µM showed significant suppressive effects on hydroperoxide generation in a dose-dependent manner. TDP exhibited much stronger antioxidant activity than thiamin at all the concentrations tested, and IC50 values of thiamin and TDP were calculated to be 260 and 46 µM, respectively. In addition, PBS did not show any significant effects on this experimental system (Fig. 2).
Hydroxyl radical-scavenging activity of thiamin and TDP in Fenton reaction
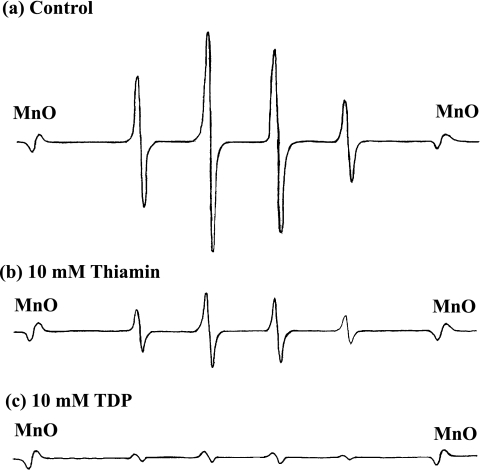
Hydroxyl radical generated by Fenton reaction was measured by ESR method using electron signal-trapping reagent DMPO. As a positive control experiment, the strong signals of typical ESR spectra for hydroxyl radical were observed in the presence of ferrous ion and hydrogen peroxide compared with the signal of manganese ion as an internal standard marker and 10 mM thiamin and TDP showed the strong suppression on the expression of ESR signals (Fig. 3). As a typical data, 10 mM thiamin and TDP showed the strong suppression on the expression of ESR signals (Fig. 3). As shown in Table 1, when pretreated with different concentrations of thiamin or TDP before the generation of hydroxyl radical, a dose-dependent suppressive effect on hydroxyl radical generation in Fenton reaction was observed from 10 mM to 1 mM, which was shown as the relative signal intensity of hydroxyl radical. However, lower concentrations (20 and 100 µM) of these vitamins caused apparently suppressive effects, but not statistically significant effects. TDP showed much stronger suppressive effect than thiamin, and IC50 values of thiamin and TDP were calculated to be 8.45 and 1.46 mM, respectively. This result indicates that hydroxyl radical-scavenging effects of thiamin and TDP are relatively weak compared with their effects in other assays of our present study.
Fig. 3.
Typical ESR data of effects of thiamin and TDP on Fenton reaction. (a) positive control experiment of Fenton reaction using ferrous ion and hydrogen peroxide as explained in Materials and Methods. (b) Effect of 10 mM thiamin on Fenton reaction. (c) Effect of 10 mM TDP on Fenton reaction. MnO shows the position of manganese oxide as an internal standard marker.
Table 1.
Suppressive effects of thiamin and TDP on hydroxyl radical generation in Fenton reaction
| Relative signal intensity | % Inhibition | ||
|---|---|---|---|
| Control | (distilled water) | 13.92 ± 1.68 | – |
| Control | (PBS) | 13.56 ± 1.05 | – |
| Thiamin | 10 mM | 6.12 ± 0.45 (P<0.01) | 56.0 |
| 5 mM | 8.85 ± 0.69 (P<0.01) | 36.4 | |
| 1 mM | 9.91 ± 1.70 (P<0.05) | 28.8 | |
| 100 µM | 12.04 ± 1.27 (P<0.20) | 13.5 | |
| 20 µM | 12.32 ± 1.08 (P<0.30) | 11.5 | |
| TDP | 10 mM | 0.89 ± 0.12 (P<0.001) | 93.6 |
| 5 mM | 1.89 ± 0.18 (P<0.001) | 86.4 | |
| 2.5 mM | 2.88 ± 0.42 (P<0.001) | 79.3 | |
| 1 mM | 8.63 ± 0.67 (P<0.01) | 38.0 | |
| 100 µM | 10.98 ± 1.53 (P<0.10) | 21.1 | |
| 20 µM | 12.15 ± 1.92 (P<0.30) | 12.7 |
The signal intensity of hydroxyl radical generation is expressed as a ratio of the signal intensity at the lowest magnetic field to that of manganese ion in MnO used as an internal standard. Results are shown as the mean and SD of triplicate assays. The statistical difference of the results between control and vitamin-treated experiments is estimated by Student’s t test.
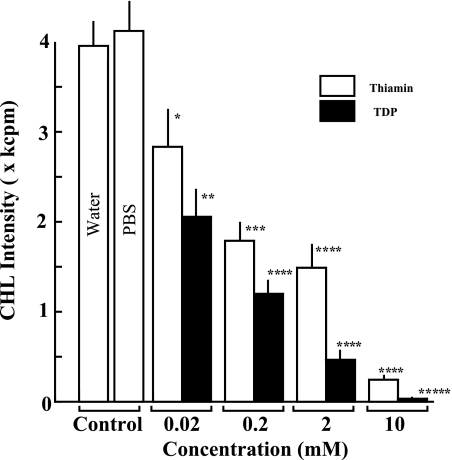
Preventive effects of thiamin and TDP on oxygen radical generation in opsonized zymosan (Opz)-stimulated human blood neutrophils
In addition to the cell-free experiments mentioned above, we analyzed the effects of thiamin and TDP on the oxygen radical generation in Opz-stimulated human blood neutrophils as a typical oxygen radical-producing cell system. As shown in Fig. 4, Opz considerably enhanced oxygen radical generation in human blood neutrophils which showed the strong luminol-dependent CHL generation. Another control solution PBS did not cause any significant effects on Opz-stimulated oxygen radical generation. When various concentrations (10 mM–20 µM) of thiamin and TDP were added to the cell mixture system before the exposure of Opz to neutrophils, Opz-induced oxygen radical generation was significantly suppressed in a dose-dependent fashion. Especially, at the highest concentration (10 mM), thiamin and TDP showed remarkable suppressive effects (93.7 and 99.2% inhibition). In this experimental system, TDP showed much stronger activity than thiamin, and IC50 values of thiamin and TDP were calculated to be 169 and 38 µM, respectively.
Fig. 4.
Effects of thiamin and TDP on oxygen radical generation in Opz-stimulated human blood nutrophils. The column and bar show the mean and SD of CHL intensities generated by oxygen radicals in triplicate assays. The statistical difference of the results between control and vitamin-treated experiments is analyzed by Student’s t test. The p value is expressed by each asterisk as follows. *p<0.05, **p<0.01, ***p<0.005, ****p<0.001, *****p<0.0001.
Discussion
Previously, B group vitamins were considered to be non-antioxidant vitamins. However, recent studies showed that vitamin B6 family such as pyridoxine and pyridoxamine caused suppressive effects on glucose-induced lipid peroxidation and superoxide generation in diabetic model experiments [15, 16]. These results indicate a possibility that some of B group vitamin possess potent antioxidant or radical-scavenging activity.
On the other hand, other investigators reported that thiamin and TDP caused the significant inhibitory effects on protein glycation in a diabetic model experiment [7]. Although the inhibitory mechanism of thiamin and TDP was not analyzed in their report, the other studies indicate that the glycation process of proteins in diabetic complications involves not only Schiff-base formation and Amadori reaction but also another oxidation process such as oxygen radical generation [8, 9]. These observations suggest a possibility that thiamin and TDP have potent antioxidant or radical-scavenging activities. Possibly, thiazole structure in thiamin and TDP may have potent inhibitory activity against the protein glycation because a thiazole derivative (ALT-711) showed the preventive effects on glucose-derived protein cross-linking [17] and improved large artery properties in diabetic rats [18]. Furthermore, another thiazole derivative showed radical-scavenging activity in ethanol-induced oxidative stress in rat alveolar cells [19]. These findings may be associated with the potent radical-scavenging activities of thiamin and TDP. As shown in our present study, thiamin and TDP exhibited the radical-scavenging activities in some in vitro experiments.
Previously, other researchers reported a controversial result which showed antioxidant and prooxidant activities of thiamin in some in vitro experiments [20]. In their report, thiamin did not cause significant effects at 0.5–2 mM, but exhibited 32% inhibition at 5 mM against superoxide generation by pyrogallol auto-oxidation. In contrast, our experimental result indicated that thiamin and TDP showed much stronger activities, and their IC50 values were 168 and 56 µM against superoxide generation in HPX and XOD system. Furthermore, in their report, thiamin reversely enhanced the prooxidant activity against microsomal lipid peroxidation at 2.5–10 mM. However, we showed the inhibitory effects of thiamin and TDP on the hydroperoxide generation in lipid peroxidation at 25 µM–10 mM (Fig. 2). Although the reason of the controversy between our present and their previous results is not clear at present, these different results may reflect the difference of experimental conditions or experimental systems. Possibly, more detailed comparative analysis will be required to estimate exactly these controversial results.
As another interesting finding in our study, the effective concentrations of thiamin and TDP in superoxide generation in HPX and XOD system were relatively low compared with those in the generation of hydroxyl radical in Fenton reaction. As calculated from the experimental result, IC50 values of thiamin and TDP for superoxide generation were 158 and 56 µM, but IC50 values for hydroxyl radical were 8.45 and 1.46 mM. These observations seem to be consistent with their effects on oxygen radical generation in Opz-stimulated human neutrophils, in which their IC50 values were 169 and 38 µM, respectively. Opz-dependent CHL generation in human neutrophils using luminol reflects primarily superoxide-generating activity of NADPH-dependent oxidase in the cell membrane [21]. Possibly, thiamin and TDP caused more specific inhibitory effects on superoxide generation than on hydroxyl radical generation. Furthermore, the present finding is consistent with the following observation in the previous biochemical study [22]. A TDP-dependent enzyme transketolase catalyzes the transfer of glycoaldehyde from keto-sugars to aldo-sugars to produce glycolate. This enzyme reaction absolutely requires TDP that is localized at the active site of the enzyme. Interestingly, it was shown that this enzyme reaction was preferably proceeded by superoxide, but not by hydroxyl radical according to the experiments using different radical scavengers [22]. Although the direct interaction between TDP and superoxide was not analyzed in this report, our present finding suggests that TDP localizes in the active site of transketolase and possesses much higher affinity for superoxide than hydroxyl radical.
In addition, thiamin and TDP also showed suppressive effects on hydroperoxide generation in the auto-oxidation of linoleic acid (Fig. 2). Generally, lipid peroxidation consists of different steps such as initiation, propagation and termination steps. Especially, the propagation step contains hydroperoxide generation which is associated with peroxyl radical generation in the initiation step and deprivation of hydrogen atom from linoleic acid [23]. The suppressive effect of thiamin and TDP on hydroperoxide generation from oxidized linoleic acid may be responsible for their inhibitory effects on these reactions in the auto-oxidation of linoleic acid.
Interestingly, our present study indicated that TDP showed much stronger radical-scavenging activity than that of thiamin in all assays (Fig. 1, 2, and 3, Table 1). Although the role of diphosphate in radical-scavenging activity of TDP is not elucidated completely, the metal-chelating property of diphosphate may strengthen the radical-scavenging activity of this compound. For example, since hydroxyl radical generation in Fenton reaction absolutely requires metal ions such as ferrous or copper ion, the metal-chelating property of diphosphate in TDP may contribute to the effective radical-scavenging of TDP. Furthermore, in some cases, lipid peroxidation involves the metal-dependent reaction associated with various oxygen radical generation [24, 25]. The metal-chelating activity of TDP may cause the inhibitory effects on the generation of peroxyl radical and hydroperoxide radical.
Somehow, although the more detailed study for radical-scavenging activities of thiamin and TDP should be required, the findings in our present study may give a new biochemical or pharmacologic aspect of these vitamins.
Acknowledgements
The authors would like to thank Dr. Imada, I. (Department of Biochemistry and Molecular Pathology, Osaka City University Medical School) and Dr. Haraguchi H. (Department of Life Science Engineering, Fukuyama University) for useful discussion during the present study.
Abbreviations
- CHL
Chemiluminescence
- DMPO
5,5-diethyl-1-pyroline-N-oxide
- ESR
Electric spin resonance
- HPX
Hypoxanthine
- MCLA
2-methyl-6-[p-methylphenyl]-3,7-dihydroiidaz[1,2-alpha]pyran-3-one
- Opz
Opsonized zymosan
- PBS
10 mM Phosphate-buffered saline (pH 7.4)
- SOD
Superoxide dismutase
- TDP
Thiamin diphosphate
- XOD
Xanthine oxidase
References
- 1.Cathcart A.E., Thurnham D.I. In: Thiamin-Physiology, in Encyclopedia of Human Nutrition. Sadler M.J., Strain J.J., Caballero B., editors. Academic Press; New York: 1998. pp. 1858–1863. [Google Scholar]
- 2.Bailey A.L., Finglas P.M., Wright A.J., Southon S. Thiamin intake, erythrocyte transketolase (EC 2.2.1.1) activity and total erythrocyte thiamin in adolescents. Br. J. Nutr. 1994;72:111–125. doi: 10.1079/bjn19940014. [DOI] [PubMed] [Google Scholar]
- 3.Cooper J.R., Pincus J.H. The role of thiamine in nervous tissue. Neurochem. Res. 1979;4:223–239. doi: 10.1007/BF00964146. [DOI] [PubMed] [Google Scholar]
- 4.Blass J.P., Gleason P., Brush D., DiPonte P., Thaler H. Thiamine and Alzheimer’s disease. A pilot study. Arch. Neurol. 1988;45:833–835. doi: 10.1001/archneur.1988.00520320019008. [DOI] [PubMed] [Google Scholar]
- 5.Mastrogiacoma F., Bettendorff L., Grisar T., Kish S.J. Brain thiamine, its phosphate esters, and its metabolizing enzymes in Alzheimer’s disease. Ann. Neurol. 1996;39:585–591. doi: 10.1002/ana.410390507. [DOI] [PubMed] [Google Scholar]
- 6.Kanofsky J.D. Thiamin status and cognitive impairment in the elderly. J. Am. Coll. Nutr. 1996;15:197–198. doi: 10.1080/07315724.1996.10718589. [DOI] [PubMed] [Google Scholar]
- 7.Booth A.A., Khalifah R.G., Todd P., Hudson B.G. In vitro kinetic studies of formation of antigenic advanced glycation end products (AGEs). Novel inhibition of post-Amadori glycation pathways. J. Biol. Chem. 1997;272:5430–5437. doi: 10.1074/jbc.272.9.5430. [DOI] [PubMed] [Google Scholar]
- 8.Wolff S.P., Jiang Z.Y., Hunt J.V. Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radic. Biol. Med. 1991;10:339–352. doi: 10.1016/0891-5849(91)90040-a. [DOI] [PubMed] [Google Scholar]
- 9.Jain S.K., Kannan K., Lim G. Ketosis (acetoacetate) can generate oxygen radicals and cause increased lipid peroxidation and growth inhibition in human endothelial cells. Free Radic. Biol. Med. 1998;25:1083–1088. doi: 10.1016/s0891-5849(98)00140-3. [DOI] [PubMed] [Google Scholar]
- 10.Higashi-Okai K., Kanbara K., Amano K., Hagiwara A., Sugita C., Matsumoto N., Okai Y. Potent antioxidative and antigenotoxic activity in aqueous extract of Japanese rice bran-association with peroxidase activity. Phytother. Res. 2004;18:628–633. doi: 10.1002/ptr.1576. [DOI] [PubMed] [Google Scholar]
- 11.Nakano M. Assay for superoxide dismutase based on chemiluminescence of luciferin analog. Methods Enzymol. 1990;186:227–232. doi: 10.1016/0076-6879(90)86112-9. [DOI] [PubMed] [Google Scholar]
- 12.Noda Y., Kohno M., Mori A., Packer L. Automated electron spin resonance free radical detector assays for antioxidant activity in natural extracts. Methods Enzymol. 1999;299:28–34. doi: 10.1016/s0076-6879(99)99006-7. [DOI] [PubMed] [Google Scholar]
- 13.Asakawa T., Matsushita S. A colorimetric microdetermination of peroxide values utilizing aluminum chloride as the catalyst. Lipids. 1980;15:965–967. [Google Scholar]
- 14.Imada I., Sato E.F., Miyamoto M., Ichimori Y., Minamiyama Y., Konaka R., Inoue M. Analysis of reactive oxygen species generated by neutrophils using a chemiluminescence probe L-012. Anal. Biochem. 1999;271:53–58. doi: 10.1006/abio.1999.4107. [DOI] [PubMed] [Google Scholar]
- 15.Onorato J.M., Jenkins A.J., Thorpe S.R., Baynes J.W. Pyridoxamine, an inhibitor of advanced glycation reactions, also inhibits advanced lipoxidation reactions. Mechanism of action of pyridoxamine. J. Biol. Chem. 2000;275:21177–21184. doi: 10.1074/jbc.M003263200. [DOI] [PubMed] [Google Scholar]
- 16.Jain S.K., Lim G. Pyridoxine and pyridoxamine inhibits superoxide radicals and prevents lipid peroxidation, protein glycosylation, and (Na+ + K+)-ATPase activity reduction in high glucose-treated human erythrocytes. Free Radic. Biol. Med. 2001;30:232–237. doi: 10.1016/s0891-5849(00)00462-7. [DOI] [PubMed] [Google Scholar]
- 17.Vasan S., Zhang X., Zhang X., Kapurniotu A., Bernhagen J., Teichberg S., Basgen J., Wagle D., Shih D., Terlecky I., Bucala R., Cerami A., Egan J., Ulrich P. An agent cleaving glucose-derived protein crosslinks in vitro and in vivo. Nature. 1996;382:275–278. doi: 10.1038/382275a0. [DOI] [PubMed] [Google Scholar]
- 18.Wolffenbuttel B.H., Boulanger C.M., Crijns F.R., Huijberts M.S., Poitevin P., Swennen G.N., Vasan S., Egan J.J., Ulrich P., Cerami A., Levy B.I. Breakers of advanced glycation end products restore large artery properties in experimental diabetes. Proc. Natl. Acad. Sci. U. S. A. 1998;95:4630–4634. doi: 10.1073/pnas.95.8.4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown L.A., Harris F.L., Guidot D.M. Chronic ethanol ingestion potentiates TNF-alpha-mediated oxidative stress and apoptosis in rat type II cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;281:L377–386. doi: 10.1152/ajplung.2001.281.2.L377. [DOI] [PubMed] [Google Scholar]
- 20.Hu M.L., Chen Y.K., Lin Y.F. The antioxidant and prooxidant activity of some B vitamins and vitamin-like compounds. Chem. Biol. Interact. 1995;97:63–73. doi: 10.1016/0009-2797(95)03608-8. [DOI] [PubMed] [Google Scholar]
- 21.McPhail L.C., DeChatelet L.R., Johnston R.B., Jr. Generation of chemiluminescence by a particulate fraction isolated from human neutrophils. Analysis of molecular events. J. Clin. Invest. 1979;63:648–655. doi: 10.1172/JCI109347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takabe T., Asami S., Akazawa T. Glycolate formation catalyzed by spinach leaf transketolase utilizing the superoxide radical. Biochemistry. 1980;19:3985–3989. doi: 10.1021/bi00558a015. [DOI] [PubMed] [Google Scholar]
- 23.Porter N., Wujek D.G. Autoxidation of polyunsaturated fatty acids, an expanded mechanistic study. J. Am. Chem. Soc. 1984;106:2626–2629. [Google Scholar]
- 24.Noguchi T., Nakano M. Effect of ferrous ions on microsomal phospholipid peroxidation and related light emission. Biochim. Biophys. Acta. 1974;368:446–455. doi: 10.1016/0005-2728(74)90189-3. [DOI] [PubMed] [Google Scholar]
- 25.Thomas M.J., Mehl K.S., Pryor W.A. The role of the superoxide anion in the xanthine oxidase-induced autoxidation of linoleic acid. Biochem. Biophys. Res. Commun. 1978;83:927–932. doi: 10.1016/0006-291x(78)91484-5. [DOI] [PubMed] [Google Scholar]