Summary
Following exposure to trauma, a vulnerable sub-population of individuals develop post-traumatic stress disorder (PTSD) with characteristic persistent autonomic hyper-responsivity, associated increased startle response, and commonly altered hypothalamo-pituitary-adrenal regulation. A goal of this investigation was to identify a predictive marker for this vulnerability. Previous investigators have developed a model for PTSD in which male mice were exposed to a single brief episode of inescapable footshock followed by one-minute contextual reminders of this trauma at weekly intervals for six weeks. Exposure to these reminders induced a progressive and persistent increase in the amplitude of acoustic startle consistent with the persistently increased acoustic startle of individuals exhibiting PTSD. We adapted this model to adult male Wistar rats, with added characterization of initial (pre-trauma) startle response. After one episode of inescapable footshock (10 second, 2 mA) or control treatment followed by six weekly one-minute contextual reminders, acoustic startle was re-tested. Data were analyzed after dividing rats within each treatment into LOW vs MID vs HIGH (33% in each group) pre-treatment startle responders. Rats which exhibited pre-treatment LOW- and MID-range acoustic startle responses did not develop increased acoustic startle responses following subsequent traumatic stress + reminders ([TS+R]) treatment. However, rats which exhibited HIGH pre-treatment startle responses exhibited further significant (p<0.01) [TS+R]-induced persistent enhancement of this already elevated startle response. Furthermore, rats exhibiting HIGH pre-treatment startle responses were also the only subgroup which exhibited increased basal plasma corticosterone levels following [TS+R] treatment. These results suggest that initial pre-stress acoustic startle response can identify subgroups of rats which are predisposed to, or resistant to, developing a PTSD-like syndrome following subsequent trauma.
Keywords: PTSD, trauma, startle, corticosterone, vulnerability, risk factors
Introduction
It is estimated that 20-30% of individuals who experience severe uncontrollable stress or trauma as a result of combat, rape, etc., subsequently exhibit symptoms of post-traumatic stress disorder (PTSD) (Kulka et al., 1990; Resnick et al., 1993). PTSD is commonly characterized by long-lasting: 1) hyperarousal (such as increased startle response, hypervigilance, and insomnia); 2) avoidance of reminders of the traumatic event; and 3) involuntary re-experiencing of the trauma (such as in recurrent nightmares, flashbacks and/or intrusive recollections) (American Psychiatric Association, 1994). It is currently not clear what factors or characteristics determine who is most likely to be included in the subset of individuals who develop PTSD after traumatic stress; one goal of our investigations is to identify a marker for this vulnerability. The ability to prospectively identify this subset would greatly facilitate the search for mechanisms, and thus bases for therapy. Ability to prospectively identify especially vulnerable individuals could potentially also facilitate effective targeting of appropriate preventive and interventional measures to those individuals most likely to benefit during anticipated subsequent trauma - such as that associated with combat, police or disaster operations.
Traumatized individuals are commonly reminded of the traumatic event, and it is thought that re-exposure to distinctive cues which had been present during a traumatic event can play an important role in development of chronic PTSD (Pitman, 1988). Previous investigators developed an animal model for PTSD in which male mice were exposed to a single 10-second episode of inescapable footshock followed by one-minute contextual reminders of this trauma at weekly intervals for six weeks (Pynoos et al., 1996). Exposure to these weekly reminders induced a progressive increase in acoustic startle response - i.e., reflex muscle contractions which occur in response to a sharp noise. This is notable because increased acoustic startle response is a recognized symptom of human PTSD (Butler et al., 1990; Grillon and Baas, 2003). We have now used a modification of this traumatic stress + reminders [TS+R] model with adult male rats in a first step toward identifying effective predictive indices for vulnerability to develop PTSD.
In one animal model of PTSD in which rats were exposed to either a cat (Cohen et al., 2003) or soiled cat litter (Cohen et al., 2006), only approximately 25% of Sprague-Dawley rats developed increased sympathetic activity, anxiety-like behavior and acoustic startle response consistent with PTSD. This heterogeneity in outbred rat responses to traumatic stress was thus similar to the heterogeneity in human vulnerability to develop PTSD after traumatic stress. Clinical findings have demonstrated that acoustic startle reactivity correlates with anxiety levels (Morgan et al., 1993) and is high in individuals at high familial risk for anxiety disorders (Grillon et al., 1998; Grillon et al., 2005), and that anxiety is a familial risk factor for PTSD (Connor and Davidson, 1997). This suggests that acoustic startle response may constitute a vulnerability marker for development of PTSD. Together, the findings from these rat and human studies suggested that: 1) appropriate modeling could produce a subset of rats similar in characteristics and proportionate in incidence to trauma-induced PTSD in humans; and 2) this subset may be prospectively identifiable on the basis of pre-existing acoustic startle response. To address this hypothesis, we investigated the effect of administering [TS+R] treatment to rats which had been characterized as exhibiting pre-treatment low, medium or high acoustic startle responses. We hypothesized that rats exhibiting high pre-treatment acoustic startle and receiving subsequent [TS+R] treatment would develop a PTSD-like syndrome characterized by enhanced startle (relative to control rats exhibiting high pre-treatment startle response but not receiving [TS+R] treatment), and rats with low pre-treatment acoustic startle response would not.
There is clinical evidence that some individuals with PTSD exhibit increased acoustic startle only under environmentally stressful conditions which induce increased state anxiety (reviewed in (Grillon and Baas, 2003)). It has also been reported that males at high family history risk for anxiety disorders exhibited enhanced (compared to low-risk subjects) acoustic startle when tested in a context which induced anxious apprehension but not when tested under non-anxiogenic basal conditions (Grillon et al., 1998; Grillon et al., 2005). Consequently, we included analyses of potential contributions of environmentally-induced anxiety stress by investigating the [TS+R]-induced changes in startle under both basal (dark) and bright light conditions since, for rats, bright light is an unconditioned anxiogenic stimulus (Walker and Davis, 1997a).
Finally, it has been reported that only rats with low initial plasma corticosterone levels exhibited increased acoustic startle responses 19 days after footshock stress (Milde et al., 2003), suggesting that low glucocorticoid secretion may also be a predictive index for vulnerability to develop PTSD. This hypothesis is consistent with extensive evidence that PTSD is associated with altered regulation of circulating cortisol, the predominant circulating glucocorticoid in humans. However, the relationship remains unclear, since levels are reported to be increased in some studies and decreased in others (reviewed in (Cohen et al., 2006)). Nonetheless, it has recently been reported that administration of corticosterone to rats one hour before a traumatic stress reduced enhanced acoustic startle response and anxiety-like behaviors one week later (Cohen et al., 2006). These results suggest that pre-existing altered regulation of the hypothalamo-pituitary-adrenal axis could potentially predispose individuals to development of PTSD after a traumatic stress. Consequently, we also investigated plasma corticosterone levels to determine if a subset of rats which would respond to [TS+R] treatment with development of PTSD-like startle responses would also be characterized by altered basal glucocorticoid levels.
Methods
Animals
Sixty-nine adult (270-290 g) male Wistar rats (Simonsen Laboratories, Gilroy, CA) were individually housed with lights on from 0600-1800 h each day, with ad libitum access to chow and water. All procedures were approved by the University of Washington Institutional Animal Care and Use Committee and conducted in accord with the NIH Guide for Care and Use of Laboratory Animals.
Protocol
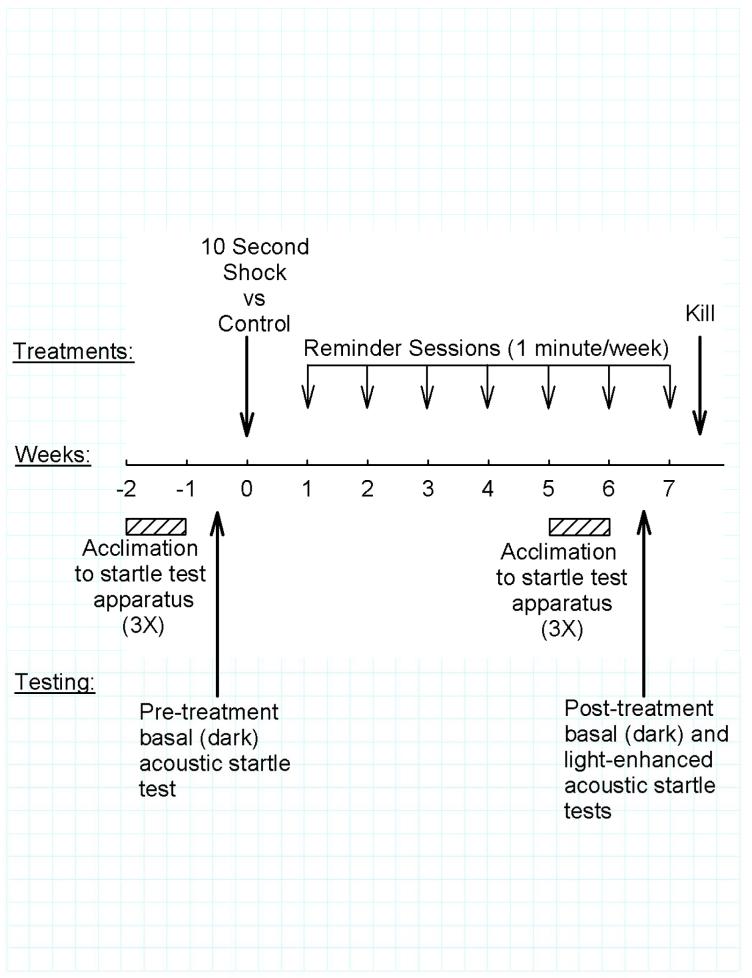
The overall protocol is summarized in Figure 1.
Fig. 1.
Experiment protocol.
Each rat was initially tested for pre-treatment acoustic startle response as described under “Startle Testing”, below. The rats were then ranked on the basis of this pre-treatment startle response amplitude and assigned in alternating sequence to Shock (n=36) vs Control (n=33) treatment to achieve equivalent pre-treatment acoustic startle response distribution between the two treatment groups.
Shock and Control treatments were administered with a Gemini Avoidance System (San Diego Instruments, San Diego, CA) in a procedure adapted from Pynoos et al. (Pynoos et al., 1996). The Gemini Avoidance System consists of two chambers separated by a wall with guillotine door; the floors are stainless steel grids used to administer shock. Both chambers were dark at the start of each trial. A trial was initiated by placing the rat in one of the chambers which, after 10 seconds, was then brightly illuminated (approximately 3,000 lux at the center of the chamber). The guillotine door was opened one minute after illuminating the chamber, allowing access to the adjoining chamber, which remained dark. When the rat entered the dark chamber the door was closed and 2 mA shock was administered continuously for 10 seconds. Control rats experienced the same procedure, but without administration of shock after entering the dark chamber. All treatments were administered between 0700 and 1000 h.
Each rat was briefly reminded of this experience on one occasion during each of the next six weeks. This was accomplished by placing the rat in the darkened starting chamber for 10 seconds and then illuminating the chamber for one minute; the rat was returned to its home cage without entering the second chamber and without being shocked.
After six weekly one-minute reminders, startle responses were quantitated. Rats then received an additional one-minute reminder during week seven and on the next day were killed by decapitation at between 0800 and 0900 h. Plasma prepared from trunk blood collected at the time of decapitation was stored at -80°C until radioimmunoassay of corticosterone.
Startle Testing
General procedure
Acoustic startle testing was performed with an SR-LAB system (San Diego Instruments, San Diego, CA) which includes a ventilated, sound-attenuated cabinet containing a plexiglas cylindrical rat enclosure mounted on a piezoelectric accelerometer which detected movement. The force exerted on the accelerometer during movement was digitized and expressed by the SR-LAB system in millivolt units. An integrated tweeter provided background white noise and startle stimuli. Startle response signals generated by the accelerometer were recorded as 65 consecutive 1-msec recordings, starting at the onset of each startle stimulus. Results were analyzed as maximum peak amplitude. A 40 msec 100 db white noise startle stimulus was determined in preliminary trials to provide robust but sub-maximal startle responses when administered during background 60 db white noise. Sound levels were calibrated with a Radio Shack Digital Sound Level Meter (33-2055, RadioShack Corp., Fort Worth, TX) placed in the center of the cylindrical rat enclosure. The overall startle protocol was modified from procedures described by Geyer and Swerdlow (Geyer and Swerdlow, 1998).
Pre-test acclimation
The rats were handled daily during the week prior to testing. In addition, on each of the three days immediately prior to testing, each rat was acclimated to the startle system without presentation of startle stimulus. This acclimation consisted of placing the rat in the cylindrical enclosure for 5 minutes with the chamber illumination off and with constant 60 db background white noise. The rat enclosure was cleaned with 0.5 % Liquinox (Alconox, Inc., New York, NY) between trials during these pre-test acclimations as well as during subsequent test trials.
Testing
Testing in semi-randomized order was conducted one to four hours after the start of the daily light period. Each rat was allowed a five-minute acclimation period in the SR-Lab with background white noise at 60 db before quantitation of startle responses to 10 consecutive presentations of a 40 msec 100 db white noise stimulus. To minimize habituation and anticipatory changes, the intervals between sequential presentations of the acoustic stimulus were varied as follows: 21, 7, 20, 9, 14, 21, 11, 8, 23 seconds (average interval: 15 seconds). This interval sequence was used in all trials for all rats. In trials testing light-enhancement of the acoustic startle response, this entire process was then immediately (i.e., without removing the rat from the test chamber) repeated with the chamber illuminated to approximately 2000 lux, including a five-minute acclimation period. Illumination was provided by a 13 watt fluorescent light mounted near the top of the side wall of the cabinet, parallel to and extending the entire length of the cylindrical rat enclosure. This full length fluorescent bulb lighting configuration was used so illumination of the rat’s eyes would be consistent regardless of position in the enclosure. However, fluorescent lights can emit ultrasonic noise; no light noise was detectable by sound meter, and it was assumed that any ultrasonic signal generated by the low wattage light was masked by the 60 db white background noise generated by the mixed-frequency tweeter and the ventilation fan. Nonetheless, it must be assumed that an auditory cue associated with the bright light may have still been perceptible by the rat. Illumination level was measured with a luminometer (Model 401025 Foot Candle/Lux Meter, Extech Instruments Corp., Waltham, MA) placed at the center of the rat enclosure, directed upward.
Corticosterone Radioimmunoassay
Plasma total corticosterone levels were determined with an ImmunoChem™ Double Antibody Corticosterone 125I RIA Kit for Rats and Mice (MP Biomedicals, LLC; Orangeburg, NY). All samples were analyzed in a single assay, with 4.5% coefficient of variation.
Data Analyses
Data were analyzed using the SigmaStat 3.0 program (SPSS Inc., Chicago, IL); specific statistical tests used to analyze individual data sets are noted in the Results section. Sigmastat utilizes a general linear model for analyses of variance (ANOVA). When necessary to achieve normal distribution and equal variance, data were transformed before analysis. Transformation as square root was appropriate for some analyses, log10 or reciprocal for others; the specific transformations are noted in the Results section. p<0.05 was considered significant. Data are presented as mean±SEM.
Results
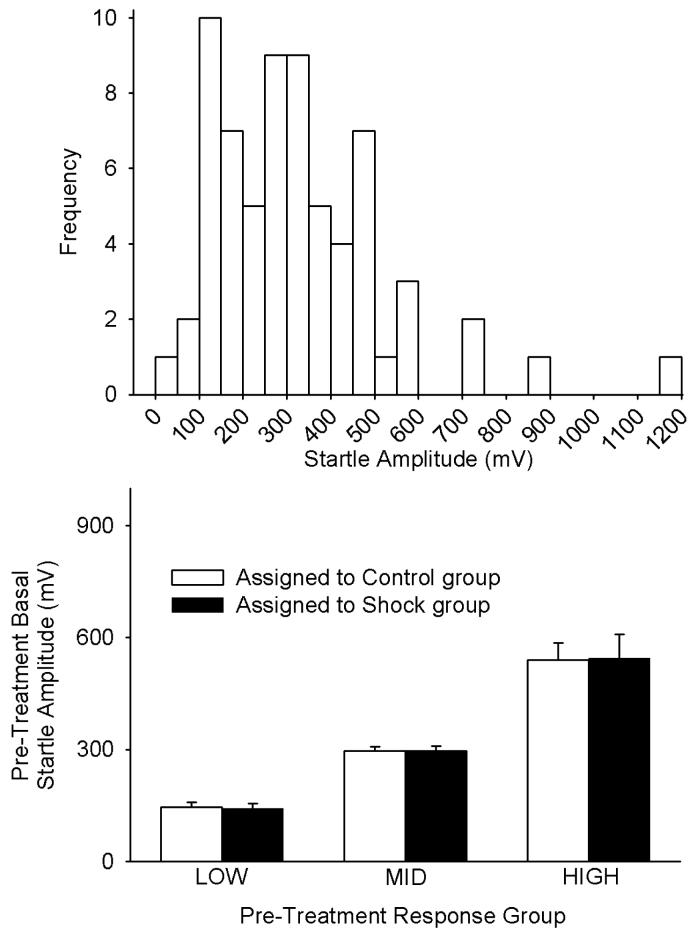
As illustrated in the upper panel of Fig. 2, there was a relatively wide range of pre-treatment basal acoustic startle responses. For subsequent analyses, the rats were divided into LOW, MID and HIGH pre-treatment responder groups on the basis of pre-treatment acoustic startle amplitude (i.e., 1/3 of the sequentially-ranked rats assigned to each group). The LOW, MID and HIGH responder groups included rats with pre-treatment acoustic startle amplitudes spanning the ranges of 46-217, 230-356, and 366-1171 mV, respectively. Within each of these pre-treatment responder groups, further alternating assignment of sequentially-ranked rats yielded equivalent average pre-treatment startle amplitudes in each sub-group which would subsequently receive either Shock or Control treatment (Fig. 2, lower panel); lack of significant differences between rats assigned to subsequently receive either Shock or Control treatment within the individual LOW, MID or HIGH responder groups was confirmed by t-tests (all p>0.8).
Fig. 2.
Frequency histogram of pre-treatment basal startle amplitudes of all rats in the study (UPPER PANEL), and mean±SE pre-treatment basal startle amplitudes after grouping the rats as LOW (lowest 1/3), MID (middle 1/3) and HIGH (upper 1/3) initial responders (LOWER PANEL). Within each of the LOW, MID and HIGH initial responder groups, the rats were assigned in alternating order to Shock versus Control treatment groups. The lower panel illustrates that this assignment protocol yielded equivalent startle amplitudes in the Shock and Control treatment groups prior to administration of each of these treatments. The term “basal” reflects the fact that these tests were conducted under basal - i.e., not light-enhanced - conditions. Each bar represents the mean±SE of 11-12 rats.
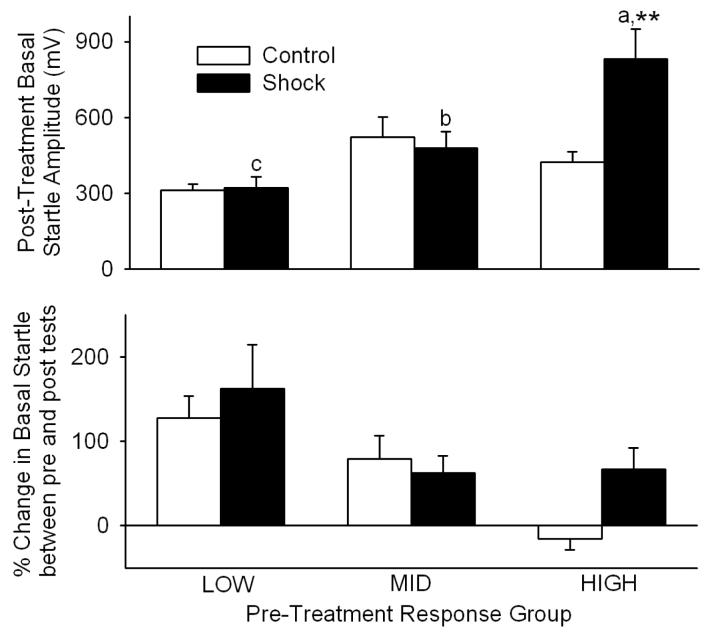
Analysis of startle amplitudes following [TS+R] Shock vs Control treatment, shown in the upper panel of Fig. 3, was conducted after log10 transformation of the data. Two-way ANOVA (response group × treatment) revealed that, after 10-second Shock vs Control treatment followed by six one-minute weekly reminders, startle amplitudes differed significantly among LOW vs MID vs HIGH pre-treatment startle groups [F(2,61)=11.2, p<0.001] after allowing for differences in Shock vs Control treatment. Startle amplitudes did not differ significantly between Shock vs Control treatments [F(1,61)=2.4] after allowing for effects of differences in startle group. However, there was significant interaction [F(2,61)=3.8, p=0.027] between response group and treatment, suggesting that the effect of Shock treatment was dependent upon startle group. Further analysis with Student-Newman-Keuls multiple comparisons then revealed that [TS+R] Shock treatment increased (p<0.01) acoustic startle in the HIGH pre-treatment response group 96% relative to pre-treatment HIGH responders receiving Control treatment (Fig. 3, upper panel). In contrast, average startle responses of pre-treatment LOW and MID responders were not altered following [TS+R] treatment, relative to pre-treatment LOW and MID responders receiving Control treatment (Fig. 3, upper panel; p>0.75 for each).
Fig. 3.
Traumatic shock + reminders ([TS+R]) - induced changes in basal startle amplitudes of LOW, MID and HIGH initial startle groups (UPPER PANEL), and % change in this startle amplitude relative to initial startle amplitude (LOWER PANEL). **p<0.01 vs Control. a vs b and a vs c, p<0.01. b vs c, p<0.05. Each bar represents the mean±SE of 11-12 rats.
Inspection of the startle response levels used to assign rats to pre-treatment response groups (Fig. 2, lower panel) vs corresponding startle response levels exhibited following the [TS+R] paradigm (Fig. 3, upper panel) suggested that basal startle may have changed between the initial characterizations and the post-treatment tests, and that this change may have been dependent upon pre-treatment response grouping. As shown in the lower panel of Fig. 3, this was evaluated as % change in basal startle between pre- and post-treatment tests. The data were first converted to all positive numbers by adding 100, and then analyzed as log10 transforms. Two-way ANOVA (response group × treatment) revealed that the % change in basal startle amplitude between initial characterizations and post-[TS+R] tests differed significantly among LOW vs MID vs HIGH pre-treatment startle groups [F(2,61)=10.0, p<0.001] after allowing for differences in Shock vs Control treatment. Further analysis with Student-Newman-Keuls multiple comparisons then revealed that the % increase in basal startle in the LOW response group (145 ± 28%, overall) was greater (p<0.05) than the corresponding increase in the MID group (71±17%, overall), which was greater (p<0.05) than the increase in the HIGH group (29±17%, overall). The % change in startle amplitudes did not differ significantly between Shock vs Control treatments [F(1,61)=2.2] after allowing for effects of differences in startle group. However, there was a trend suggesting likely interaction between response group and treatment [F(2,61)=2.6, p=0.08], i.e., that % changes in basal startle from initial characterization to post-[TS+R] may have depended upon startle group. Although this interaction was not quite statistically significant (p=0.08), inspection of the results revealed that whereas the basal startle in HIGH response rats declined 15±13% lower after Control treatment, basal startle in pre-treatment HIGH response rats following Shock treatment was increased 67±25%. Consequently, Bonferroni t-tests were used to compare Control vs Shock differences in % changes within the LOW, MID and HIGH response groups. Shock-induced % changes in basal startle were increased (p<0.05) relative to Control treatment only in the HIGH responder group.
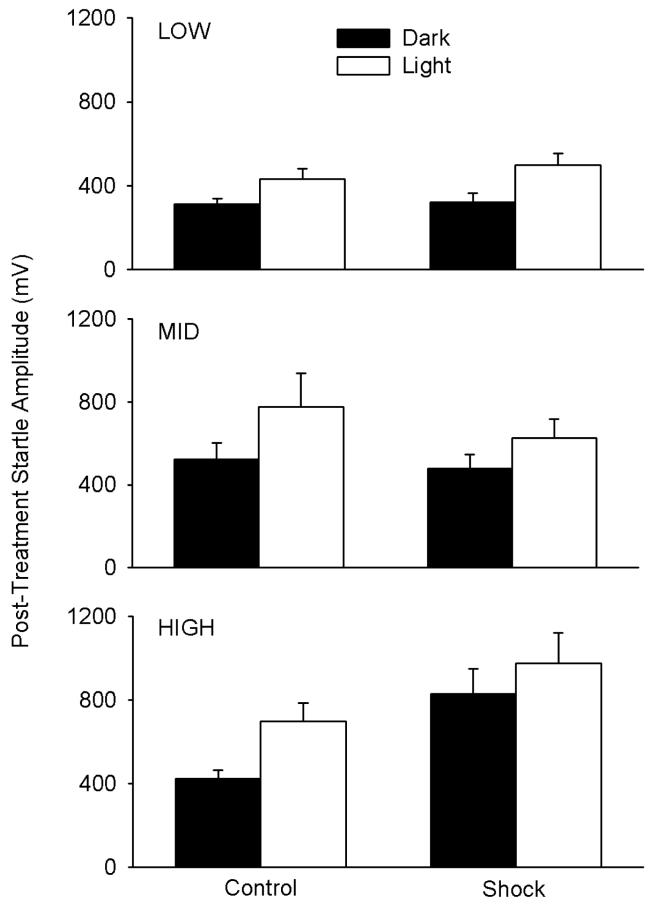
In order to analyze the effect of bright light on post-treatment startle amplitude, the data within the individual response groups required different transformations to achieve normal distribution and equal variance, thus requiring separate analyses. Reciprocal transformation was used for the LOW group, square root transformation for the MID group, and no transformation for the HIGH group. Two way (treatment × light) repeated measures ANOVA with repetition during dark vs subsequent bright light conditions were then separately conducted for the independently-transformed data of the LOW, MID and HIGH groups. In the LOW group, illustrated in the upper panel of Fig. 4, there was a significant main effect of light level after allowing for effects in differences in treatment [F(1,21)=14.7, p<0.001], but no significant main effect of Control vs Shock treatment [F(1,21)=0.02] and no significant interaction between light level and treatment [F(1,21)= 2.19]. In the MID group (center panel of Fig. 4), there was also a significant main effect of light level [F(1,21)=9.1, p<0.01], no significant main effect of Control vs Shock treatment [F(1,21)=0.31], and no significant interaction between light level and Control vs Shock treatment [F(1,21)=0.34]. In the HIGH group (bottom panel of Fig. 4), there was a significant main effect of light level [F(1,21)=4.9, p<0.05] but also a main effect of Control vs Shock treatment after allowing for effects of the change in light level [F(1,21)=8.5, P<0.01]; the effects of Control vs Shock treatment did not significantly depend on dark vs light conditions [interaction: F(1,21)=0.463]. However, it should be noted that the powers of the performed tests for interactions were low for the analyses of each of the response (LOW, MID, HIGH) groups, ranging from 0.05-0.17 with alpha=0.05, so ability to resolve potential interactions was limited. It should also be noted that if correction for multiple comparisons is applied in response to the necessary separate analyses of the three response groups, increasing stringency for consideration as significant by a factor of 3, LOW and MID group main effects of light treatment remain significant; in the HIGH group, the main effect of light would become non-significant, but the main effect of Control vs Shock treatment after allowing for effects of the change in light level remains significant.
Fig. 4.
Basal (Dark) versus Light-Enhanced (Light) post-treatment startle amplitude in the LOW, MID and HIGH responder groups with (Shock) and without (Control) shock treatment followed by six weekly reminders. The basal (Dark) responses are the same results as shown in the upper panel of Fig. 3, and the associated Light bars show responses of the same rats under subsequent bright light conditions. Each bar represents the mean±SE of 11-12 rats.
When light-enhancement of post-treatment startle responses was analyzed as % change between basal and light-enhanced conditions for individual rats, results were variable and there were not significant differences between Control vs Shock treatment within LOW, MID or HIGH pre-treatment response groups, or between LOW vs MID vs HIGH pre-treatment response groups for either Control or Shock treatments (p>0.15 for all, results not shown).
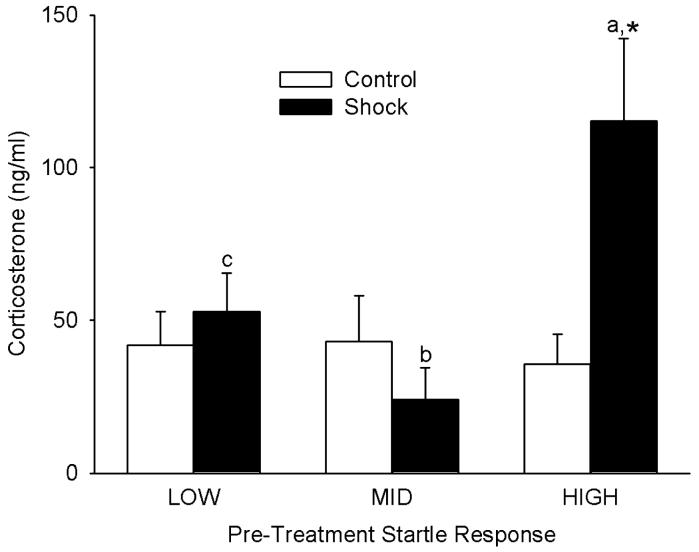
Analysis of corticosterone levels in trunk blood collected in the morning after the seventh weekly one-minute reminder session was conducted after log10 transformation of the data. Two-way ANOVA (response group × treatment) revealed that plasma corticosterone levels differed significantly among LOW vs MID vs HIGH pre-treatment startle groups [F(2,58)=3.3, p<0.05] after allowing for differences in Shock vs Control treatment. Corticosterone levels did not differ significantly between Shock vs Control treatments [F(1,58)=1.0] after allowing for effects of differences in startle group. However, there was significant interaction [F(2,58)=3.5, p<0.05] between startle group and treatment, suggesting that the effect of Shock treatment differed between startle groups. Further analysis with Student-Newman-Keuls multiple comparisons revealed that within the HIGH response group - but not the LOW or MID groups - Shock treatment significantly (p<0.05) increased plasma corticosterone levels, by 213% (Fig. 5). Among rats receiving Control treatment, there were no significant differences due to LOW vs MID vs HIGH response grouping (all comparisons: p>0.8). However, among rats receiving Shock treatment, rats in the HIGH response group exhibited significantly higher plasma corticosterone levels than rats in the MID (p<0.01) and LOW (p<0.05) response groups, with no significant differences between LOW and MID groups (Fig. 5).
Fig. 5.
Plasma corticosterone levels in trunk blood from pre-treatment LOW, MID and HIGH responders, collected one day after the seventh weekly reminder session. Each bar represents the mean±SE of 11-12 rats. * p<0.05 vs Control. a vs b, p<0.01. a vs c, p<0.05.
Discussion
Rats which exhibited pre-treatment LOW- and MID-range acoustic startle did not develop increased acoustic startle in response to a single 10-second episode of inescapable shock followed by six weekly one-minute contextual reminders of the experience (i.e., [TS+R] treatment). In contrast, rats which exhibited HIGH pre-treatment acoustic startle (i.e., the 33% of rats with greatest initial responsivity) exhibited further significant [TS+R]-induced persistent enhancement of their already elevated acoustic startle responses. This selective [TS+R]-induced enhancement of startle sensitivity in the subset of rats which exhibited HIGH pre-existing startle, achieving persistent markedly elevated startle responses, and the fact that this subset of prospectively identifiable vulnerable rats was also the only subset which exhibited [TS+R]-induced increased corticosterone levels, are the key findings of this study.
Startle response consists of a brief reflex twitch of somatic muscles in response to a sudden, intense stimulus such as a sharp noise, and is dependent upon brainstem cochlear nuclei, the caudal pontine nucleus, and spinal motor neurons (Lee et al., 1996; Yeomans and Frankland, 1996; Koch and Schnitzler, 1997). Because acoustic startle amplitude has been demonstrated to correlate with anxiety levels (Morgan et al., 1993), it is thought that this reflex response provides a useful model for investigating neurobiological bases of anxiety and fear (Sandbak et al., 2000). Pynoos et al. (Pynoos et al., 1996) investigated changes in acoustic startle following acute traumatic stress using a time-dependent sensitization model which featured repeated exposures to contextual reminders of the traumatic stress. Mice which were shocked and received contextual reminders of the experience (i.e., same as the current [TS+R] treatment) developed increased acoustic startle response, increased aggressiveness, and increased variability in anxiety-like behavior when compared to mice which were either not shocked or were shocked but not exposed to weekly reminders. These abnormal behaviors did not diminish during the course of the study. Indeed, startle amplitude in the shocked mice which received reminders was progressively increased with repetitions of the reminders. These original studies have recently been extended to rats by Louvart et al. (Louvart et al., 2005; Louvart et al., 2006), who modified the model by using fewer (three) contextual reminders but nonetheless again demonstrated that a single exposure to inescapable footshock followed by brief weekly contextual reminders persistently increased anxiety-like behavior, altered social behavior, and increased avoidance, further demonstrating consistency between responses in this rodent [TS+R] paradigm and PTSD. In the Pynoos et al. time-dependent sensitization model the repeated contextual reminders were demonstrated to play a necessary role in the progressive development of persistently increased startle and other parameters characteristic of PTSD, consistent with evidence that re-exposure to distinctive cues which had been present during a traumatic event can play an important role in development of chronic PTSD (Pitman, 1988) and that the subset of individuals who develop PTSD following trauma exhibit a progressive increase in startle reactivity over the months following the traumatic event (Shalev et al., 2000). An important role of contextual reminders is also consistent with results from a study (Maier, 2001) investigating inescapable shock effects on rat escape learning, suggested to be a model of depression and anxiety-related disorders such as PTSD. Reminding the rats of the original trauma by re-exposing them to the environment in which inescapable shock had occurred (i.e., similar to the contextual reminders of the original Pynoos et al. model and the [TS+R] model used in the current study) prolonged the duration of behavioral change, and repeated exposures prolonged it indefinitely (Maier, 2001). These effects of contextual reminders are in contrast to studies of footshock alone, in which it has been demonstrated that although increasing the intensity or number of foot shocks can prolong duration of startle sensitization, the effect is not permanent and, for example, has been reported to last 4 but not 10 days (Servatius et al., 1994). Nonetheless, there are also reports of rat and mouse models in which episodes of traumatic stress without subsequent presentation of contextual reminders produced sensitization of acoustic startle responses and also increased fear/anxiety-related behaviors, sympathetic activity and/or plasma corticosterone levels 7-19 days after the stress (Milde et al., 2003; Cohen et al., 2003; Adamec et al., 2004; Cohen et al., 2006). Consequently, the requirement of the presentations of contextual reminders for inducing or maintaining the long-term (at least 6-7 week) sensitization of acoustic startle response and plasma corticosterone levels in the HIGH pre-existing startle response subset of rats in the current study remains to be confirmed.
In contrast to results of the study by Pynoos et al. (Pynoos et al., 1996), in the current study only approximately a third of the rats developed increased basal acoustic startle in response to [TS+R] treatment. This difference in results between the two studies could be in part due to differences between mouse and rat responses to, and recovery from, stressors - including potential differences in habituation to reminders of the original traumatic stress. It may also be significant that Pynoos et al. used an acoustic startle stimulus of 120 db whereas we used 100 db. We chose to use 100 db because preliminary studies demonstrated that it was an intensity which produced consistent but sub-maximal startle responses in rats, whereas 120 db commonly produced maximal responses which we were concerned could potentially, due to ceiling effects, compromise the ability to resolve [TS+R]-induced changes. Similar variability in startle responses to similarly differing relative stimulus intensities have previously been demonstrated. For example, the magnitude of human startle amplitudes elicited by 90 db - but not 114 db - acoustic stimuli correlated with number of previous alcohol detoxifications (Krystal et al., 1997). Finally, inherent greater variability in behavioral responses of an outbred strain of rodents (i.e., the Wistar rats used in the current study) compared to an inbred strain (i.e., the C57BL6J mice used in the Pynoos et al. study) could also explain why more variation in startle reactivity in response to treatment was seen in the present study. In another rat model of PTSD, inescapable exposure to a cat or soiled cat litter likewise induced persistent fear-related behaviors (including increased sympathetic activity and anxiety-like behavior) in only about 25% of outbred Sprague-Dawley rats tested (Cohen et al., 2003; Cohen and Zohar, 2004; Cohen et al., 2006) - again consistent with the proportional incidence of PTSD in humans subjected to traumatic stress (Kulka et al., 1990; Resnick et al., 1993). It has thus been suggested that focusing on the comparable subset of outbred rats which tend to develop a PTSD-like syndrome following traumatic stress is equivalent to focusing on the subset of human subjects who, after experiencing traumatic stress, meet diagnostic criteria for PTSD (Cohen et al., 2003; Cohen and Zohar, 2004). This targeting of the vulnerable subset of outbred rats may thus facilitate resolution of mechanisms specifically relevant to PTSD. For example, it remains to be determined whether the subset of outbred rats (or humans) developing a persistent PTSD-like syndrome do so because of differing mechanisms in responding to traumatic stress versus differing efficacies in recalling and re-living the traumatic stress, i.e., stimulus-dependent memory.
As illustrated in the lower panel of Fig. 3, there was a significant overall increase in startle reactivity from the pre-treatment characterization to the post-treatment testing, and this increase in startle was most predominant in the LOW and MID pre-treatment startle groups. We hypothesize that these overall increases may have been due to the moderate repeated stress associated with the weekly reminder sessions, since there is evidence that baseline startle reflex is gradually elevated in response to repeated stress (Gewirtz et al., 1998).
For rats, bright light is an unconditioned anxiogenic stimulus which increases acoustic startle response, does not depend on learning or memory, and is reduced by anxiolytic drugs (Walker and Davis, 1997a; de Jongh et al., 2002; Walker and Davis, 2006). Intracerebroventricular (icv) administration of corticotropin-releasing factor (CRF) is anxiogenic and potentiates the acoustic startle (Swerdlow et al., 1986), and icv administration of CRF receptor antagonist can suppress both light-enhanced startle (de Jongh et al., 2003) and indices of anxiety (Korte et al., 1994; Koob and Heinrichs, 1999), suggesting that CRF is involved in both light-enhanced startle and anxiety. It has been demonstrated (Walker and Davis, 2006) that light-enhanced startle is mediated by the bed nucleus of the stria terminalis, which also mediates anxiety and CRF-enhanced startle (Davis et al., 1997; Walker and Davis, 1997b). Finally, the time courses of both light-enhancement and CRF-enhancement of the acoustic startle response (i.e., slow onset and prolonged action) are consistent with those of anxiety-associated changes but distinct from the rapid onset and termination of fear-potentiated startle responses, which are mediated by the central nucleus of the amygdala (Swerdlow et al., 1986; Davis et al., 1997; Walker and Davis, 1997a; Davis and Shi, 1999; de Jongh et al., 2003). These pharmacologic, anatomic, mechanistic and temporal characteristics strongly support the suggestion that light-enhanced startle is a useful index of rat situational anxiety. In the current study, bright light increased overall startle response, even in the LOW and MID groups. There were no significant effects of Control vs Shock treatments in the LOW and MID groups; in the HIGH group Shock treatment increased startle responses, but there was not a significant interaction between Shock response and dark vs light conditions. With the relative consistency of bright light stimulation of startle responses in all three pre-treatment startle groups, it would be reasonable to hypothesize that extrahypothalamic CRF-dependent mechanisms discussed above as mediating light enhancement may not have primary roles in the differential [TS+R]-induced increases in basal startle in the HIGH pre-treatment startle rats, although further studies would be required to specifically address this issue.
Regulation of adrenal glucocorticoid secretion has been the focus of many PTSD studies in humans, but experimental results have been notably variable, consistent with the complexity of regulation of the hypothalamo-pituitary-adrenal (HPA) axis and the varied causes and courses of PTSD (Yehuda, 2002). This complexity is further confounded by conditions which are commonly co-morbid with PTSD and which also alter HPA regulation, such as depression, substance abuse and other psychiatric disorders (Jacobsen et al., 2001). In the current study, rats were decapitated and trunk blood collected in the morning, a time consistent with the circadian nadir of rat circulating corticosterone levels. Plasma corticosterone was elevated only in the pretreatment HIGH responder group which had then received [TS+R] treatment, i.e., the same sub-group which exhibited [TS+R]-induced increased acoustic startle. It cannot be determined from the present results whether the increase reflected tonically increased basal corticosterone levels vs stress-induced HPA responses associated with transfer of the rats to the room where decapitation would be performed - to which the rats had been acclimated on each of three previous days but which nonetheless could potentially have been perceived to be similar to previous transfers to another room where the shock vs no shock and weekly reminders were performed. It is significant that, in a study in which a single 10-minute exposure to a predator induced increased levels of ‘anxiety/PTSD’ -like behavior seven days later in only 25% of exposed rats, only this same subgroup of rats exhibited significantly higher plasma corticosterone concentrations (Cohen et al., 2003), consistent with our results. However, another study by Cohen et al. (Cohen et al., 2006) demonstrated that strains of rats exhibiting markedly different acoustic startle and anxiety-like behavior nonetheless exhibited similar “basal” plasma corticosterone levels, although predisposition to acoustic startle and behavioral effects of trauma appeared to be linked to blunted HPA responsiveness (Cohen et al., 2006). Further studies will be required to resolve the mechanisms and significance of the HPA changes revealed in the current study. Nonetheless, our results clearly demonstrate that the only subset of rats which exhibited increased [TS+R]-induced acoustic startle response was also the only subset which exhibited evidence of altered HPA regulation, consistent with PTSD.
Milde et al. (Milde et al., 2003) demonstrated that only rats with low initial plasma corticosterone levels exhibited increased acoustic startle responses 19 days after footshock stress, and suggested that “low corticosterone secretion may represent a marker for vulnerability to long term effects of shocks as indicated by increased startle responses...”. In the current study, plasma corticosterone levels in the HIGH responder Control (i.e., non-shocked) rats (analogous to initial determinations in rats in the Milde et al. study which subsequently developed shock-induced increased acoustic startle) were not different from LOW- or MID-responder Control rats. The reason(s) for this potential discrepancy between results of our study and the study of Milde et al. are not clear. Blood collection for both studies was performed at approximately the same time in the morning, but blood collection in the Milde et al. study was performed after inhalation anesthesia and subsequent surgical exposure of the jugular vein, whereas in the present study trunk blood was collected at decapitation, so different levels of acute stress may have played an important role. Consistent with this hypothesis, mean basal corticosterone levels in the Milde et al. study were >100 ng/ml in their low corticosterone group and >200 ng/ml in their high corticosterone group, whereas mean basal corticosterone levels in the present study were <50 ng/ml in all Control groups. This suggests that the relatively non-stressed lower levels in the present study may have introduced a “floor” effect, limiting the ability to demonstrate decreased basal HPA activation. It is also reasonable to hypothesize that the potential repeated stress associated with the weekly reminder sessions, which we hypothesized may have been responsible for the % increase in basal startle response between initial characterization and post-[TS+R] testing of even Control rats, may have also contributed basal HPA activation variability sufficient to compromise evaluations of the low basal corticosterone levels in the current study. Consequently, the potential role of pre-existing basal corticosterone levels in determining vulnerability to this [TS+R] paradigm remains to be resolved.
Overall, our results suggest that appropriate testing of pre-existing acoustic startle response can prospectively identify a subgroup (in this case, approximately 1/3) of rats which are, on average, predisposed (and, conversely, the 2/3 which are resistant) to developing a persistent PTSD-like syndrome following a traumatic experience and subsequent contextual reminders. The relatively small vulnerable subset of animals developing this response is consistent with the small proportion of severely traumatized humans who develop PTSD, suggesting that corresponding testing of humans before exposure to anticipated traumatic stress could potentially help to identify individuals most likely to subsequently develop PTSD, perhaps allowing early initiation of preventive treatment. Furthermore, this animal model may allow resolution of mechanisms specifically mediating development of PTSD in the vulnerable subgroup. An additional goal is to use this model to begin investigating interventions to effectively prevent or reverse the PTSD-like syndrome in this subgroup, as well as investigate the utility of these interventions in preventing or treating other pathologies highly correlated with PTSD, such as alcohol abuse.
Acknowledgments
This work was supported by the Department of Veterans Affairs and by National Institute on Alcohol Abuse and Alcoholism / National Institutes of Health grants AA10567 and AA013881.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamec R, Walling S, Burton P. Long-lasting, selective anxiogenic effects of feline predator stress in mice. Physiol. Behav. 2004;83:401–410. doi: 10.1016/j.physbeh.2004.08.029. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual IV. American Psychiatric Press, Inc.; Washington DC: 1994. [Google Scholar]
- Butler RW, Braff DL, Rausch JL, Jenkins MA, Sprock J, Geyer MA. Physiological evidence of exaggerated startle response in a subgroup of Vietnam Veterans with combat-related PTSD. Am. J. Psychiatry. 1990;147:1308–1312. doi: 10.1176/ajp.147.10.1308. [DOI] [PubMed] [Google Scholar]
- Cohen H, Zohar J. An animal model of posttraumatic stress disorder. Ann. N. Y. Acad. Sci. 2004;1032:167–178. doi: 10.1196/annals.1314.014. [DOI] [PubMed] [Google Scholar]
- Cohen H, Zohar J, Gidron Y, Matar MA, Belkind D, Loewenthal U, Kozlovsky N, Kaplan Z. Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biol. Psychiatry. 2006;59:1208–1218. doi: 10.1016/j.biopsych.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Cohen H, Zohar J, Matar M. The relevance of differential response to trauma in an animal model of posttraumatic stress disorder. Biol. Psychiatry. 2003;53:463–473. doi: 10.1016/s0006-3223(02)01909-1. [DOI] [PubMed] [Google Scholar]
- Connor KM, Davidson JR. Familial risk factors in posttraumatic stress disorder. Ann. N. Y. Acad. Sci. 1997;821:35–51. doi: 10.1111/j.1749-6632.1997.tb48267.x. [DOI] [PubMed] [Google Scholar]
- Davis M, Shi C-J. The extended amygdala: Are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Ann. N. Y. Acad. Sci. 1999;877:281–291. doi: 10.1111/j.1749-6632.1999.tb09273.x. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Roles of the amygdala and bed nucleus of the stria terminalis in fear and anxiety measured with the acoustic startle reflex. Possible relevance to PTSD. Ann. N. Y. Acad. Sci. 1997;821:305–331. doi: 10.1111/j.1749-6632.1997.tb48289.x. [DOI] [PubMed] [Google Scholar]
- de Jongh R, Groenink L, van der Gugten J, Olivier B. The light-enhanced startle paradigm as a putative animal model for anxiety: Effects of chlordiazepoxide, flesinoxan and fluvoxamine. Psychopharmacology. 2002;159:176–180. doi: 10.1007/s002130100914. [DOI] [PubMed] [Google Scholar]
- de Jongh R, Groenink L, van der Gugten T, Olivier B. Light-enhanced and fear-potentiated startle: temporal characteristics and effects of α-helical corticotropin-releasing hormone. Biol. Psychiatry. 2003;54:1041–1048. doi: 10.1016/s0006-3223(03)00468-2. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, McNish KA, Davis M. Lesions of the bed nucleus of the stria terminalis block sensitization of acoustic startle reflex produced by repeated stress, but not fear-potentiated startle. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1998;22:625–648. doi: 10.1016/s0278-5846(98)00028-1. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Swerdlow NR. Measurement of startle response, prepulse inhibition, and habituation. Current Protocols Neurosci. 1998;(Supplement 3):8.7.1–8.7.15. doi: 10.1002/0471142301.ns0807s03. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin. Neurophysiol. 2003;114:1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Dierker L, Merikangas KR. Fear-potentiated startle in adolescent offspring of parents with anxiety disorders. Biol. Psychiatry. 1998;44:990–997. doi: 10.1016/s0006-3223(98)00188-7. [DOI] [PubMed] [Google Scholar]
- Grillon C, Warner V, Hille J, Merikangas KR, Bruder GE, Tenke CE, Nomura Y, Leite P, Weissman MM. Families at high and low risk for depression: a three-generation startle study. Biol. Psychiatry. 2005;57:953–960. doi: 10.1016/j.biopsych.2005.01.045. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Southwick SM, Kosten TR. Substance use disorders in patients with posttraumatic stress disorder: a review of the literature. Am. J. Psychiatry. 2001;158:1184–1190. doi: 10.1176/appi.ajp.158.8.1184. [DOI] [PubMed] [Google Scholar]
- Koch M, Schnitzler HU. The acoustic startle response in rats - circuits mediating evocation, inhibition and potentiation. Behav. Brain Res. 1997;89:35–49. doi: 10.1016/s0166-4328(97)02296-1. [DOI] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848:141–152. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- Korte SM, Korte-Bouws GA, Bohus B, Koob GF. Effect of corticotropin-releasing factor antagonist on behavioral and neuroendocrine responses during exposure to defensive burying paradigm in rats. Physiol. Behav. 1994;56:115–120. doi: 10.1016/0031-9384(94)90268-2. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Webb E, Grillon C, Cooney N, Casal L, Morgan CAI, Southwick SM, Davis M, Charney DS. Evidence of acoustic startle hyperreflexia in recently detoxified early onset male alcoholics: modulation by yohimbine and m-chlorphenylpiperazine (mCPP) Psychopharmacology. 1997;131:207–215. doi: 10.1007/s002130050285. [DOI] [PubMed] [Google Scholar]
- Kulka RA, Schlenger WE, Fairbank JA, Hough RL, Jordan BK, Marmar CR, Weiss DS. Trauma and the Vietnam War Generation: Report of Findings from the National Vietnam Veterans Readjustment Study. Bruner and Mazel; New York, NY: 1990. [Google Scholar]
- Lee Y, López DE, Meloni EG, Davis M. A primary acoustic startle pathway: Obligatory role of the cochlear root neurons and the nucleus reticularis pontis caudalis. J. Neurosci. 1996;16:3775–3789. doi: 10.1523/JNEUROSCI.16-11-03775.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvart H, Maccari S, Ducrocq F, Thomas P, Darnaudéry M. Long-term behavioural alterations in female rats after a single intense footshock followed by situational reminders. Psychoneuroendocrinology. 2005;30:316–324. doi: 10.1016/j.psyneuen.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Louvart H, Maccari S, Lesage J, Léonhart M, Dickes-Coopman A, Darnaudéry M. Effects of a single footshock followed by situational reminders on HPA axis and behaviour in the aversive context in male and female rats. Psychoneuroendocrinology. 2006;31:92–99. doi: 10.1016/j.psyneuen.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Maier SF. Exposure to the stressor environment prevents the temporal dissipation of behavioral depression/learned helplessness. Biol. Psychiat. 2001;49:763–773. doi: 10.1016/s0006-3223(00)01095-7. [DOI] [PubMed] [Google Scholar]
- Milde AM, Sundberg H, Roseth AG, Murison R. Proactive sensitizing effects of acute stress on acoustic startle responses and experimentally induced colitis in rats: relationship to corticosterone. Stress. 2003;6:49–57. doi: 10.1080/1025389031000075808. [DOI] [PubMed] [Google Scholar]
- Morgan CAI, Southwick SM, Grillon C, Davis M, Krystal JH, Charney DS. Yohimbine-facilitated acoustic startle reflex in humans. Psychopharmacology. 1993;110:342–346. doi: 10.1007/BF02251291. [DOI] [PubMed] [Google Scholar]
- Pitman RK. Post-traumatic stress disorder, conditioning, and network theory. Psychiatr. Ann. 1988;18:182–189. [Google Scholar]
- Pynoos RS, Ritzmann RF, Steinberg AM, Goenjian A, Prisecaru I. A behavioral animal model of posttraumatic stress disorder featuring repeated exposure to situational reminders. Biol. Psychiatry. 1996;39:129–134. doi: 10.1016/0006-3223(95)00088-7. [DOI] [PubMed] [Google Scholar]
- Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J. Consult. Clin. Psychol. 1993;61:984–991. doi: 10.1037//0022-006x.61.6.984. [DOI] [PubMed] [Google Scholar]
- Sandbak T, Rimol LM, Jellestad FK, Murison R. Relating acoustic startle reactivity and plasticity to alcohol consumption in male Wistar rats. Physiol. Behav. 2000;68:723–733. doi: 10.1016/s0031-9384(99)00239-5. [DOI] [PubMed] [Google Scholar]
- Servatius RJ, Ottenweller JE, Bergen MT, Soldan S, Natelson BJ. Persistent stress-induced sensitization of adrenocortical and startle responses. Phsysiol. Behav. 1994;56:945–954. doi: 10.1016/0031-9384(94)90328-x. [DOI] [PubMed] [Google Scholar]
- Shalev AY, Peri T, Brandes D, Freedman S, Orr SP, Pitman RK. Auditory startle response in trauma survivors with posttraumatic stress disorder: a prospective study. Am. J. Psychiatry. 2000;157:255–261. doi: 10.1176/appi.ajp.157.2.255. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Vale WW, Koob GF. Corticotropin-releasing factor potentiates acoustic startle in rats: Blockade by chlordiazepoxide. Psychopharmacology. 1986;88:147–152. doi: 10.1007/BF00652231. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Anxiogenic effects of high illumination levels assessed with the acoustic startle response. Biol. Psychiatry. 1997a;42:461–471. doi: 10.1016/S0006-3223(96)00441-6. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J. Neurosci. 1997b;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M. Light-enhanced startle: further pharmacological and behavioral characterization. Psychopharmacology. 2006;159:304–310. doi: 10.1007/s002130100913. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Current status of cortisol findings in post-traumatic stress disorder. Psychiatr. Clin. North Am. 2002;25:341–368. doi: 10.1016/s0193-953x(02)00002-3. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Frankland PW. The acoustic startle reflex: Neurons and connections. Brain Res. Rev. 1996;21:301–314. doi: 10.1016/0165-0173(96)00004-5. [DOI] [PubMed] [Google Scholar]