Abstract
Both spontaneous and chemically induced rodent models of autoimmune nephritis and autoantibody production have been explored to understand mechanisms involved in human systemic lupus erythematosus (SLE). While it has been known for decades that women are more susceptible than men to SLE, mechanisms underlying this female preponderance remain unclear. One chemically induced model involves injection of hydrocarbon oils such as pristane into otherwise normal mouse strains, which results in the development of autoantibodies and inflammation in organs such as kidney and liver. It is unknown whether lupus-like disease induced by chemicals would exhibit a sex bias in disease susceptibility. Here, we show that SJL/J female mice injected with pristane display greater mortality, kidney disease, serum anti-nuclear and anti-dsDNA antibodies than their male siblings. This is the first evidence that a female sex bias exists in a chemically induced lupus model.
Keywords: Systemic lupus erythematosus; Pristane; Autoantibodies; Nephritis, sex bias; Anti-nuclear antibody; Anti-dsDNA antibody
Introduction
Human systemic lupus erythematosus (SLE) is characterized by inflammation and fibrosis in multiple organs, including kidneys, and the presence of autoantibodies against a variety of self-antigens [1–3]. The majority of autoimmune diseases, including SLE, affect females more frequently than males [4]. In SLE, there is a female to male ratio of 9:1. Many spontaneous models of SLE in mice also demonstrate a female preponderance and have been used as models to understand mechanisms underlying the development of lupus nephritis. In (NZBxNZW)F1 mice, females spontaneously develop IgG antibodies to DNA and severe immune complex glomerulonephritis (GN) earlier than males. Females also have decreased survival as compared to males [5]. The NZM.2328 strain also develops anti-DNA antibodies as well as both chronic and acute GN, and this too is characterized by a female preponderance [6]. Finally, (NZBxSJL)F1 mice spontaneously develop a less acute form of lupus, which displays a sex bias in that females develop more anti-DNA antibodies, more severe GN and increased mortality as compared to males [7].
In addition to spontaneous lupus, humans may develop drug-induced lupus erythematosus (DILE) after taking certain medications such as hydralazine, procainamide, isoniazid, methyldopa, chlorpromazine, quinidine or minocycline [8]. Whether or not there is a sex bias in susceptibility to DILE is unknown. Studies examining the numbers of men and women affected by DILE are confounded by the fact that many of these drugs are used more frequently in one sex than another [9]. For example, procainamide and hydralazine, the two drugs most strongly associated with the development of DILE, are prescribed for high blood pressure and cardiac arrhythmias, conditions that affect men more often than women [9,10].
Animal models have been described for DILE in humans. Strains of lupus prone mice have been treated with drugs known to cause DILE in humans, mainly procainamide and hydralazine [10–14]. In addition, strains of lupus prone and non-lupus prone mice treated with environmental chemicals have been used as DILE models [15–17]. Treatment of the murine SJL/J strain with the hydrocarbon oil, pristane, induces a lupus-like syndrome characterized by nephritis, arthritis, serositis and the presence of autoantibodies (e.g. anti-nuclear, anti-Sm, anti-dsDNA and anti-ribosomal P) [16]. Since it is currently unknown whether a sex bias exists in any chemically induced lupus model, the purpose of this study was to determine whether a sex bias exists in pristane-induced lupus in SJL/J mice.
Materials and methods
Mice
Male and female SJL/J mice, 5–8 weeks old, were purchased from the Jackson Laboratory (Bar Harbor, ME). All studies were performed in accordance with the National Institutes of Health Animal Protection Guidelines and were approved by the UCLA Chancellor’s Committee on Animal Research.
Induction of pristane-induced lupus
Mice were injected i.p. once with 0.5 ml of pristane (2,6,10,14-tetramethyl-pentadecane; Sigma-Aldrich, St. Louis, MO), as described [16,18]. Others were injected with saline as a control. Age at injection was 6 weeks in two experiments and 11 weeks in one experiment. Mice were monitored daily for signs of disease (lethargy, ruffled fur, distended abdomen) and survival rates were attained. In some cases, mice required euthanasia before natural death due to their moribund state. For survival graphs, death was assumed to occur 2 weeks after euthanasia. Mice underwent serial eye bleeds via retro-orbital puncture at 2, 4 and 6 months and sera were frozen for analysis of autoantibodies.
Assessment of nephritis
Kidney disease was assessed as follows: Proteinuria was measured weekly on a 0–4+ scale using a colorimetric assay strip for albumin (Albustix: Bayer, Elkhart, IN), where 0 is absent, 1+ is <30 mg/dl (mild), 2+ is 100 mg/dl (moderate), 3+ is 300 mg/dl and 4+ is <2000 mg/dl (severe). Furthermore, in later stages of disease when proteinuria levels were equal to or greater than 100 mg/dl, blood urea nitrogen (BUN) levels were measured by impregnating Azostix (Bayer) with a drop of fresh blood and using the following scale: 1+ (normal), 2+ (mild) is 15–30 mg/dl and 3+ (severe) is >30 mg/dl. When mice displayed 3+ proteinuria on two occasions and elevated BUN, they were perfused with saline and sacrificed. In another separate experiment, female and male mice were all sacrificed at the same time point, 21 weeks after disease induction.
Upon sacrifice, kidneys were collected for pathologic studies. Kidney sections were stained with standard H&E and periodic acid-Schiff (PAS). Sections were scored by two observers for active and chronic kidney lesions (glomerular cellularity and necrosis, glomerulosclerosis, interstitial infiltration, tubular atrophy, interstitial fibrosis and vasculitis) and the mean individual scores were summed to obtain a composite kidney biopsy index, as described [18,19].
Detection of antibodies
Approximately 21 weeks post-pristane or saline injection, serum samples were assessed for ANA IgG by QUANTA Lite ANA ELISA (INOVA Diagnostics, Inc., San Diego, CA), adapted to test mouse serum. The serum samples were also assessed for anti-dsDNA antibody (IgG, IgG1, IgG2a), as well as total IgG, IgG1, IgG2a and IgE levels by ELISA, as described [20]. ANA and anti-dsDNA values were expressed as attributed units per milliliter using a reference-positive standard of pooled serum. IgE and total immunoglobulin ELISA values were expressed as OD405nM units. IgE and IgG1 antibodies were purchased from BD PharMingen (San Diego, CA) and IgG and IgG2a antibodies were purchased from Southern Biotechnology Associates, Inc. (Birmingham, AL).
Statistical analysis
Survival curves between the sexes within any one experiment were computed using the Kaplan–Meier method and compared using the log rank test. To control for the effect of experiment, overall survival between males and females was compared using the Cox proportional hazards model with experiment as the covariate. Disease scores and antibody levels were compared using GraphPad Prism software (San Diego, CA). Student’s t tests were used if data followed a normal distribution; otherwise, the Mann–Whitney test was used.
Results
Pristane-induced lupus in SJL/J females was characterized by reduced survival as compared to males
We addressed whether a chemically induced lupus model might demonstrate a sex bias. Female and male pristane-injected mice were monitored daily for signs of disease. Survival data were collected from three separate experiments. Females exhibited earlier mortality, as they began dying at 16 weeks of age while males began dying at 24 weeks of age. By 35 weeks, only 37.5% of females had survived, whereas greater than 80% of males had survived, p<0.0017 (Fig. 1). Overall, females were 3.4 times more likely to die at any given point in time than males.
Figure 1.
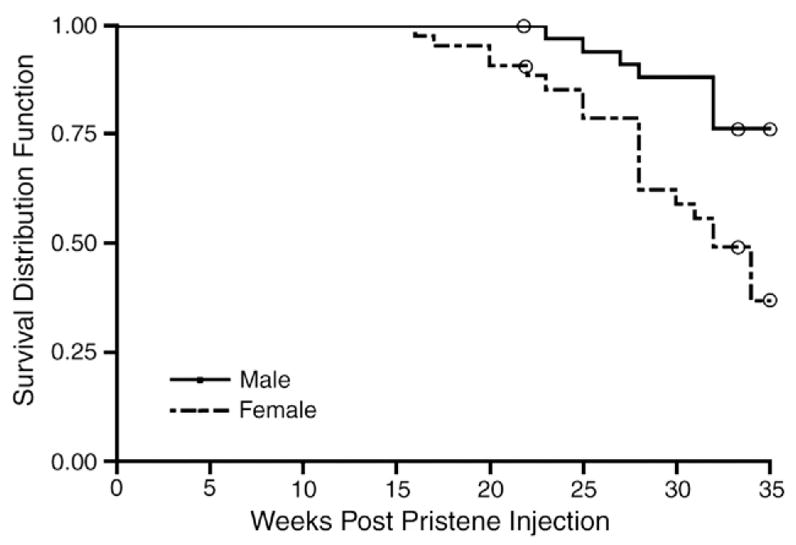
Reduced survival in female pristane-induced lupus mice. Female (n=44) and male (n=49) mice were injected with pristane and animals monitored for survival (p<0.0017; Kaplan–Meier log rank test). 19 females and 8 males, which were sacrificed during study for tissue harvesting, were censored from analysis (open circles). There was no difference in survival outcome among the three experiments (Cox proportional hazards model).
Nephritis was more severe in SJL/J females with pristane-induced lupus as compared to SJL/J males
We next addressed whether renal pathology differed between the sexes. Two separate experiments, one sacrificed at 32 weeks post-pristane injection and another at 21 weeks post-injection were examined. H&E- and PAS-stained sections of kidneys from both experiments were scored for renal histological changes, as described [21]. Sections revealed typical signs of lupus nephritis including epithelial and endothelial deposits, focal proliferative nephritis, diffuse glomerular hypercellularity, cellular and fibrous crescents, tubular dilatation and atrophy, tubular casts as well as epithelial cells and macrophages in tubular lumens (Fig. 2). These changes were more severe in female mice. Semi-quantitative kidney pathology scores are shown in Fig. 3. At the late stage in the disease (32 weeks post-pristane injection), the female SJL/J mice experienced significantly more severe pristane-induced lupus pathology overall than their male siblings, p<0.03 (Fig. 3A). At an earlier stage (21 weeks post-pristane injection), females experienced significantly more glomerular hypercellularity and necrosis, p<0.0128, and also displayed a trend for greater glomerulosclerosis, greater cellular crescent formation and greater interstitial infiltration, while there was no sex difference in tubular disease (Fig. 3B).
Figure 2.
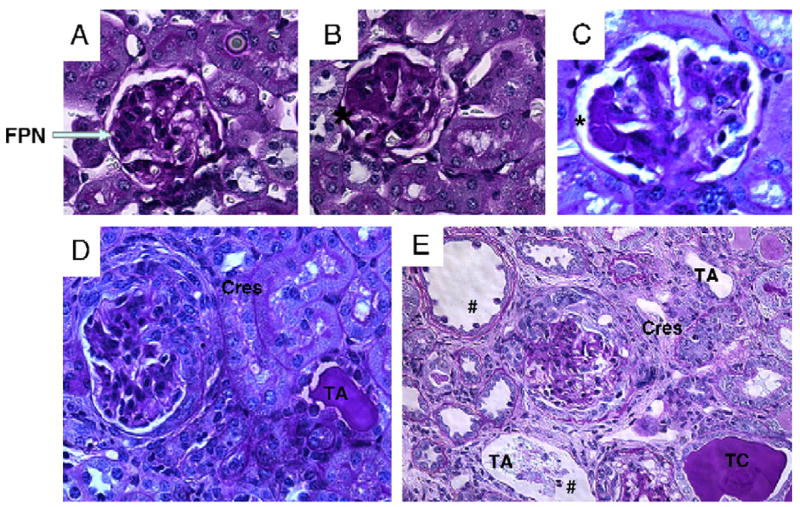
Representative renal histology from pristane-induced lupus SJL/J mice. Mice were injected with pristane at 6 weeks of age and sacrificed at 32 weeks. Kidneys were harvested, fixed in 4% formalin and paraffin sections were stained with H&E and PAS. (A, D) Representative sections from male and (B, C, E) female mice are shown. These figures show typical epithelial and endothelial deposits (denoted by *), focal proliferative nephritis (FPN), diffuse glomerular hypercellularity (in D and E), cellular and fibrous crescents (Cres), tubular dilatation and atrophy (TA) and casts (TC) and tubular epithelial cell and macrophages (#) in tubular lumen. Summary of disease grades with differences in female versus male is shown in Fig. 3.
Figure 3.
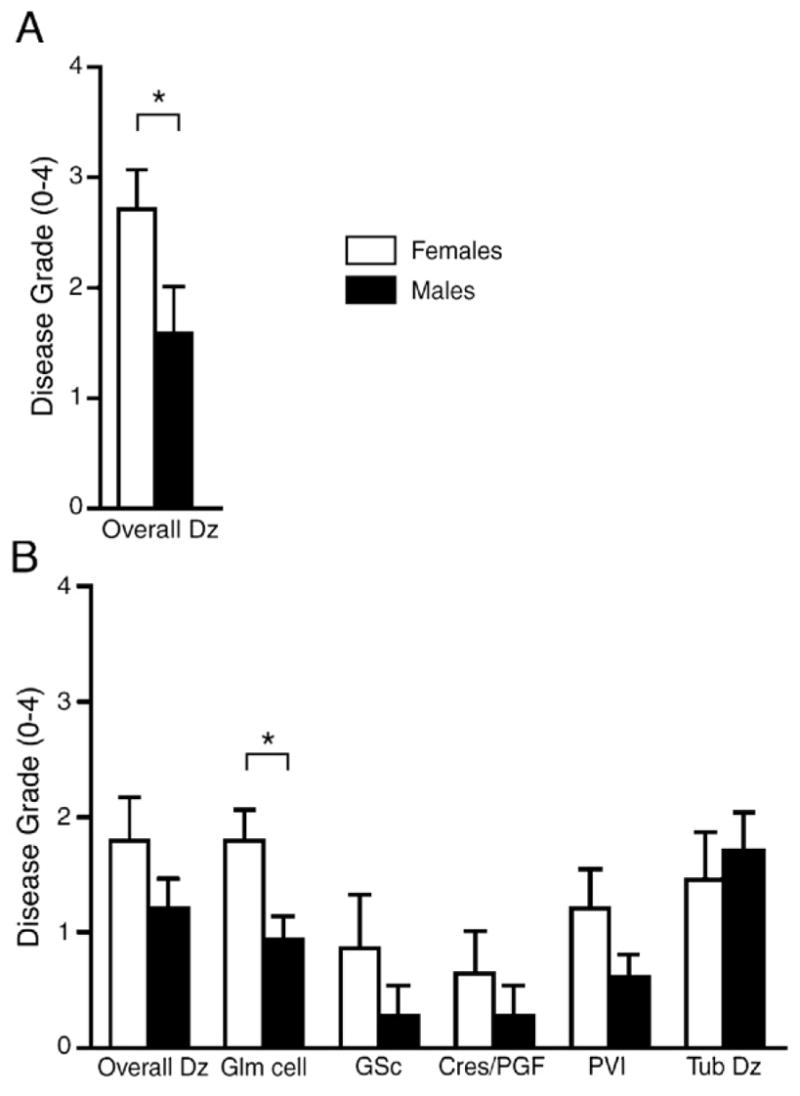
Female pristane-induced lupus mice have significantly greater kidney disease pathology as compared to males. (A) In one experiment, 8 female and 12 male pristane-injected mice were sacrificed at 32 weeks post-injection. (B) In a second experiment, twelve female and fifteen male pristane-injected mice were sacrificed at 21 weeks post-injection. H&E- and PAS-stained kidney sections from both experiments were scored for lesions. Kidney pathology scores were quantified and statistically analyzed by the Wilcoxon test. (A) Note that at a late stage in the disease (32 weeks post-pristane injection), female mice experienced significantly more severe pristane-induced lupus disease pathology overall than their male siblings, p<0.03. (B) At an earlier stage (21 weeks post-pristane injection), females experienced significantly more glomerular hypercellularity and necrosis (Glm cell), p<0.0128, and also displayed a trend for greater disease pathology overall, greater glomerulosclerosis (GSc), greater cellular crescent formation (Cres/PGF) and greater interstitial infiltration (PVI), while there was no sex difference in tubular disease (Tub dz). Histograms show means and SEM for mice in each group. *p<0.05.
Autoantibody levels were higher in pristane-induced lupus in SJL/J females as compared to SJL/J males
ANA and anti-dsDNA autoantibodies, as well as autoantibodies to other nuclear proteins, are hallmark features of SLE. Levels of ANA, anti-dsDNA antibody, as well as IgG1 and IgG2a isotypes of anti-dsDNA antibody were determined in the sera of female and male mice injected with pristane. At 21 weeks, females produced higher levels of ANA IgG, p=0.0002 (Fig. 4A); anti-dsDNA IgG antibody, p=0.03 (Fig. 4B); anti-dsDNA IgG1 antibody, p=0.024 (Fig. 4C); and IgG2a antibody, p=0.039 (Fig. 4D) than males. In addition, we measured OD405nM levels of total IgG antibody and its isotypes and found that all levels were significantly higher in female sera as compared to males [total IgG (females=1.34±0.05, males=1.09±0.07, p=0.019), total IgG1 (females=1.08±0.20, males=0.46±0.13, p=0.016) and total IgG2a (females=1.06±0.12, males=0.78±0.08, p=0.023)]. A similar trend was found for serum IgE levels, but this difference did not reach significance (females= 0.21±0.071, males=0.08±0.004, p=0.076). To insure that the sex differences in autoantibody production were due to pristane injection and not inherent to the SJL/J strain, autoantibodies were also determined in serum from age-matched SJL/J mice injected with saline. At 21 weeks, ANA and anti-dsDNA antibody levels from sera of female and male SJL/J mice injected with saline were significantly lower than female and male SJL/J mice injected with pristane, very similar to levels from Balb/c negative controls, and there was no significant difference between the sexes in these low levels (ANA: PBS-treated females=2.882±0.384, males=2.539±1.416; anti-dsDNA: PBS-treated females=5.566±1.006, males=2.523±1.106).
Figure 4.
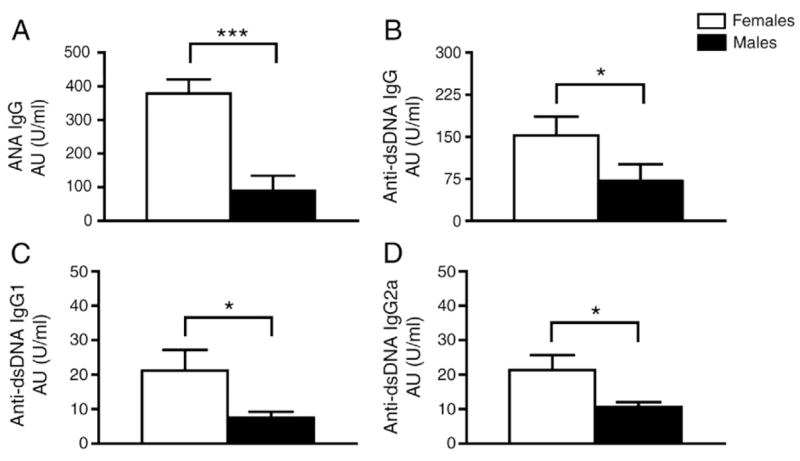
Autoantibody levels were significantly higher in sera from female, as compared to male SJL/J mice with pristane-induced lupus. (A) Sixteen weeks post-pristane injection, female mice (n=9) produced more serum IgG ANA than male mice (n=13), p=0.0002. (B) Female mice (n=29) produced more serum anti-dsDNA IgG Ab than male mice (n=31), p=0.03. (C and D) Female mice (n=10) produced more serum anti-dsDNA IgG1 and IgG2a Ab than male mice (n=13), p=0.024 and p=0.039, respectively. Autoantibody levels were determined using a reference-positive standard of pooled serum and expressed as attributed units per milliliter. Histograms show means and SEM for mice in each group. *p<0.05; ***p<0.0005. ANA and anti-dsDNA data are representative of two independent experiments on different sets of mice.
Discussion
Human SLE affects females more frequently than males with a ratio of 9:1. This sex difference may be due to sex hormones, sex chromosomes or both. To begin to understand this sex difference, animal models of SLE can be informative. Human SLE is heterogeneous, with etiologies involving both genetic background and environmental exposure. The use of more than one murine disease model is therefore needed, each contributing insights which bear some relevance to various subtypes of human SLE. Analysis of kidney disease in large cohorts of patients with SLE reveals that the histological pattern of kidney involvement varies from mild mesangial and focal lesions to diffuse proliferative and interstitial nephritis [1,22]. Different animal models of SLE probably represent these various subsets of the human disease. For example, mesangial and focal lesions, typically seen in about 35–60% of pristane-injected BALB/c [18,23] mice, are found in approximately 50% of SLE patients who undergo kidney biopsy. On the other hand, diffuse proliferative nephritis, typically seen in almost 100% of female (NZBxNZW)F1, NZM.2328 and MRL-lpr mice, is found in 34% of SLE patients [22]. Taken together, these observations suggest that the pristane-induced lupus model is probably as relevant as the other genetically lupus-prone strains for investigations into the mechanisms of lupus nephritis. In this paper we describe for the first time a sex difference in susceptibility to pristane-induced lupus. Use of this model has some advantages over spontaneous models in that sex differences in early and late pathogenic events can now be determined since the time point of disease induction is known.
A role of sex hormones in lupus has been clearly demonstrated. Endogenous testosterone is clearly protective in murine lupus since castration or use of a testosterone blocker accelerates disease in the (NZBxNZW)F1 and (NZBxSJL)F1 models [7,24–26]. In addition, exogenous testosterone treatment is protective in these spontaneous models, as well as in BALB/c mice immunized with human anti-DNA antibodies [7,24,25,27–30]. The effect of endogenous estrogens is unclear since ovariectomy of the ROP Os/+ strain [31] or use of an estrogen blocker in (NZBxNZW)F1 [32] and BALB/c [33] mice accelerates lupus-like symptoms, where as ovariectomy has no effect in the (NZBxNZW)F1 and (NZBxSJL)F1 models [7,24,25]. The effect of exogenous estrogen treatment is also unclear since exogenous estrogen treatment exacerbates disease in the (NZBxNZW)F1 model [17,24], as well as in BALB/c mice immunized with human anti-DNA antibodies [28], but ameliorates disease in the ROP Os/+ model [31]. The hormonal contribution to the sex bias in susceptibility to murine lupus would reflect the effect of endogenous circulating levels of sex hormones, not effects of exogenous hormone treatment. Overall, it appears that the hormonal contribution to the increased susceptibility of females to murine lupus primarily entails a protective effect of endogenous androgens, while the role of endogenous estrogens is less clear.
An effect of sex hormones in murine lupus does not rule out additional effects of sex chromosomes in the sex bias in susceptibility. In one model of SLE, male BXSB mice with the Yaa (Y chromosome-linked autoimmune acceleration) gene spontaneously develop a severe case of the autoimmune syndrome in which more than half of them die before 6 months of age, whereas females of the same strain do not develop autoimmune disease until one year later [34]. This well-documented Y chromosome effect in lupus pathogenesis was recently shown to be associated with increased expression of Toll Like Receptor 7 (TLR7) [35]. However, the Yaa gene effect is a strain-specific Y chromosome effect, unique to the BXSB strain and other strains consomic for the BXSB Y chromosome [36–39]. In the outbred human population, sexual dimorphisms occur in numerous models of autoimmunity involving numerous strains. Thus, to model a sex chromosome complement effect in the sex difference in human autoimmune disease, one would require that it not be strain specific but be present across numerous genetic backgrounds. It would also need to be consistent with there being disease acceleration in females, not males. Therefore, a role of sex chromosome complement in the female preponderance for autoimmune disease remains unclear at present. Further complicating this issue is the recent finding that compensatory relationships may exist between sex hormones and sex chromosomes [40]. Informative mice were used whereby the testis-determining Sry gene was “moved” from the Y chromosome to an autosome by successive deletion from the Y chromosome with introduction of an Sry transgene onto an autosome. Thus, the inheritance of the gene causing testicular differentiation was separated from the Y chromosome, permitting the study of effects of the sex chromosome complement in the absence of the confounding effect of differences in sex hormones. These informative mice were backcrossed onto the SJL background. The pristane-induced lupus model in SJL mice characterized by greater susceptibility in females described herein can now be used to unravel sex chromosome versus sex hormone-based mechanisms underlying the female preponderance to pristane-induced lupus.
Finally, it is remarkable that in a common genetic background, the SJL/J, there is a female preponderance to two very immunologically distinct diseases, experimental autoimmune encephalomyelitis (EAE) and SLE. EAE is a prototypic Th1-mediated autoimmune disease induced by myelin protein specific T lymphocytes, which serves as a model for multiple sclerosis. It affects female mice more frequently and more severely than male SJL/J mice [41–44], and differences in Th1 cytokine production are thought to underlie this female preponderance, at least in part [42–44]. As is the case with murine lupus, castration exacerbates disease [45,46], while the effect of ovariectomy is unclear [46,47]. This manuscript now demonstrates that pristane-induced lupus also affects female SJL/J mice more frequently and more severely than male SJL/J mice. Differences in autoantibody production may underlie this female preponderance. The presence of a female preponderance to two such immunopathologically distinct diseases reveals the pervasiveness of the sex influence on autoimmunity in this common genetic background. The pervasiveness of the sex influence in this strain is consistent with the observation that numerous human autoimmune diseases, entailing a variety of immunopathogenic mechanisms, demonstrate a female preponderance. Unraveling the basis for the sexual dimorphism in these two distinct autoimmune disease models in this strain will yield insights into the female bias in SLE, multiple sclerosis and other autoimmune diseases.
Acknowledgments
This work is partly supported by the JCCC, the UCLA AIDS Institute, the David Geffen School of Medicine at UCLA and the National Institutes of Health awards (AI50839 to R.R.V. and AR47322 and AR50797 and DK69282 to R.R.S.).
References
- 1.Singh RR. SLE: translating lessons from model systems to human disease. Trends Immunol. 2005;26:572–579. doi: 10.1016/j.it.2005.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mills JA. Systemic lupus erythematosus. N Engl J Med. 1994;330:1871–1879. doi: 10.1056/NEJM199406303302608. [DOI] [PubMed] [Google Scholar]
- 3.Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303–306. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 4.Yu CY, Whitacre CC. Sex, MHC and complement C4 in autoimmune diseases. Trends Immunol. 2004;25:694–699. doi: 10.1016/j.it.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Howie JB, Helyer BJ. The immunology and pathology of NZB mice. Adv Immunol. 1968;9:215–266. doi: 10.1016/s0065-2776(08)60444-7. [DOI] [PubMed] [Google Scholar]
- 6.Waters ST, Fu SM, Gaskin F, Deshmukh US, Sung SS, Kannapell CC, Tung KS, McEwen SB, McDuffie M. NZM2328: a new mouse model of systemic lupus erythematosus with unique genetic susceptibility loci. Clin Immunol. 2001;100:372–383. doi: 10.1006/clim.2001.5079. [DOI] [PubMed] [Google Scholar]
- 7.Dumont F, Monier JC. Sex-dependent systemic lupus erythematosus-like syndrome in (NZB X SJL)F1 mice. Clin Immunol Immunopathol. 1983;29:306–317. doi: 10.1016/0090-1229(83)90032-6. [DOI] [PubMed] [Google Scholar]
- 8.Antonov D, Kazandjieva J, Etugov D, Gospodinov D, Tsankov N. Drug-induced lupus erythematosus. Clin Dermatol. 2004;22:157–166. doi: 10.1016/j.clindermatol.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 9.Yung RL, Richardson BC. Drug-induced lupus. Rheum Dis Clin North Am. 1994;20:61–86. [PubMed] [Google Scholar]
- 10.Yung RL, Quddus J, Chrisp CE, Johnson KJ, Richardson BC. Mechanism of drug-induced lupus. I. Cloned Th2 cells modified with DNA methylation inhibitors in vitro cause autoimmunity in vivo. J Immunol. 1995;154:3025–3035. [PubMed] [Google Scholar]
- 11.Cannat A, Seligmann M. Induction by isoniazid and hydrallazine of antinuclear factors in mice. Clin Exp Immunol. 1968;3:99–105. [PMC free article] [PubMed] [Google Scholar]
- 12.Kretz-Rommel A, Duncan SR, Rubin RL. Autoimmunity caused by disruption of central T cell tolerance. A murine model of drug-induced lupus. J Clin Invest. 1997;99:1888–1896. doi: 10.1172/JCI119356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yung R, Chang S, Hemati N, Johnson K, Richardson B. Mechanisms of drug-induced lupus. IV. Comparison of procainamide and hydralazine with analogs in vitro and in vivo. Arthritis Rheum. 1997;40:1436–1443. doi: 10.1002/art.1780400811. [DOI] [PubMed] [Google Scholar]
- 14.Uetrecht J. Current trends in drug-induced autoimmunity. Autoimmun Rev. 2005;4:309–314. doi: 10.1016/j.autrev.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Singh AK, Yang JQ, Parekh VV, Wei J, Wang CR, Joyce S, Singh RR, Van Kaer L. The natural killer T cell ligand alpha-galactosylceramide prevents or promotes pristane-induced lupus in mice. Eur J Immunol. 2005;35:1143–1154. doi: 10.1002/eji.200425861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satoh M, Richards HB, Shaheen VM, Yoshida H, Shaw M, Naim JO, Wooley PH, Reeves WH. Widespread susceptibility among inbred mouse strains to the induction of lupus autoantibodies by pristane. Clin Exp Immunol. 2000;121:399–405. doi: 10.1046/j.1365-2249.2000.01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sobel ES, Gianini J, Butfiloski EJ, Croker BP, Schiffenbauer J, Roberts SM. Acceleration of autoimmunity by organ-ochlorine pesticides in (NZB x NZW)F1 mice. Environ Health Perspect. 2005;113:323–328. doi: 10.1289/ehp.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang JQ, Singh AK, Wilson MT, Satoh M, Stanic AK, Park JJ, Hong S, Gadola SD, Mizutani A, Kakumanu SR, Reeves WH, Cerundolo V, Joyce S, Van Kaer L, Singh RR. Immunoregulatory role of CD1d in the hydrocarbon oil-induced model of lupus nephritis. J Immunol. 2003;171:2142–2153. doi: 10.4049/jimmunol.171.4.2142. [DOI] [PubMed] [Google Scholar]
- 19.Yang JQ, Saxena V, Xu H, Van Kaer L, Wang CR, Singh RR. Repeated alpha-galactosylceramide administration results in expansion of NK T cells and alleviates inflammatory dermatitis in MRL-lpr/lpr mice. J Immunol. 2003;171:4439–4446. doi: 10.4049/jimmunol.171.8.4439. [DOI] [PubMed] [Google Scholar]
- 20.Fan GC, Singh RR. Vaccination with minigenes encoding V (H)-derived major histocompatibility complex class I-binding epitopes activates cytotoxic T cells that ablate autoantibody-producing B cells and inhibit lupus. J Exp Med. 2002;196:731–741. doi: 10.1084/jem.20020223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh RR, Saxena V, Zang S, Li L, Finkelman FD, Witte DP, Jacob CO. Differential contribution of IL-4 and STAT6 vs STAT4 to the development of lupus nephritis. J Immunol. 2003;170:4818–4825. doi: 10.4049/jimmunol.170.9.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daniel L, Sichez H, Giorgi R, Dussol B, Figarella-Branger D, Pellissier JF, Berland Y. Tubular lesions and tubular cell adhesion molecules for the prognosis of lupus nephritis. Kidney Int. 2001;60:2215–2221. doi: 10.1046/j.1523-1755.2001.00055.x. [DOI] [PubMed] [Google Scholar]
- 23.Richards HB, Satoh M, Jennette JC, Croker BP, Yoshida H, Reeves WH. Interferon-gamma is required for lupus nephritis in mice treated with the hydrocarbon oil pristane. Kidney Int. 2001;60:2173–2180. doi: 10.1046/j.1523-1755.2001.00045.x. [DOI] [PubMed] [Google Scholar]
- 24.Roubinian JR, Talal N, Greenspan JS, Goodman JR, Siiteri PK. Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies, and glomerulonephritis in NZB/NZW F1 mice. J Exp Med. 1978;147:1568–1583. doi: 10.1084/jem.147.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dumont F. Effects of ovariectomy and androgen treatment on the thymic pathology of NZB X SJL mice. Thymus. 1985;7:37–48. [PubMed] [Google Scholar]
- 26.Walker SE, Besch-Williford CL, Keisler DH. Accelerated deaths from systemic lupus erythematosus in NZB x NZW F1 mice treated with the testosterone-blocking drug flutamide. J Lab Clin Med. 1994;124:401–407. [PubMed] [Google Scholar]
- 27.Lucas JA, Ahmed SA, Casey ML, MacDonald PC. Prevention of autoantibody formation and prolonged survival in New Zealand black/New Zealand white F1 mice fed dehydroisoan-drosterone. J Clin Invest. 1985;75:2091–2093. doi: 10.1172/JCI111929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blank M, Mendlovic S, Fricke H, Mozes E, Talal N, Shoenfeld Y. Sex hormone involvement in the induction of experimental systemic lupus erythematosus by a pathogenic anti-DNA idiotype in naive mice. J Rheumatol. 1990;17:311–317. [PubMed] [Google Scholar]
- 29.Walker SE, Keisler LW, Caldwell CW, Kier AB, vom Saal FS. Effects of altered prenatal hormonal environment on expression of autoimmune disease in NZB/NZW mice. Environ Health Perspect. 1996;104(Suppl 4):815–821. doi: 10.1289/ehp.96104s4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melez KA, Boegel WA, Steinberg AD. Therapeutic studies in New Zealand mice. VII. Successful androgen treatment of NZB/NZW F1 females of different ages. Arthritis Rheum. 1980;23:41–47. doi: 10.1002/art.1780230108. [DOI] [PubMed] [Google Scholar]
- 31.Elliot SJ, Karl M, Berho M, Potier M, Zheng F, Leclercq B, Striker GE, Striker LJ. Estrogen deficiency accelerates progression of glomerulosclerosis in susceptible mice. Am J Pathol. 2003;162:1441–1448. doi: 10.1016/S0002-9440(10)64277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sthoeger ZM, Zinger H, Mozes E. Beneficial effects of the anti-oestrogen tamoxifen on systemic lupus erythematosus of (NZBxNZW)F1 female mice are associated with specific reduction of IgG3 autoantibodies. Ann Rheum Dis. 2003;62:341–346. doi: 10.1136/ard.62.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sthoeger ZM, Bentwich Z, Zinger H, Mozes E. The beneficial effect of the estrogen antagonist, tamoxifen, on experimental systemic lupus erythematosus. J Rheumatol. 1994;21:2231–2238. [PubMed] [Google Scholar]
- 34.Murphy ED, Roths JB. A Y chromosome associated factor in strain BXSB producing accelerated autoimmunity and lympho-proliferation. Arthritis Rheum. 1979;22:1188–1194. doi: 10.1002/art.1780221105. [DOI] [PubMed] [Google Scholar]
- 35.Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 36.Izui S, Merino R, Fossati L, Iwamoto M. The role of the Yaa gene in lupus syndrome. Int Rev Immunol. 1994;11:211–230. doi: 10.3109/08830189409061728. [DOI] [PubMed] [Google Scholar]
- 37.Kuroki A, Moll T, Lopez-Hoyos M, Fossati-Jimack L, Ibnou-Zekri N, Kikuchi S, Merino J, Merino R, Izui S. Enforced Bcl-2 expression in B lymphocytes induces rheumatoid factor and anti-DNA production, but the Yaa mutation promotes only anti-DNA production. Eur J Immunol. 2004;34:1077–1084. doi: 10.1002/eji.200424859. [DOI] [PubMed] [Google Scholar]
- 38.Izui S, Iwamoto M, Fossati L, Merino R, Takahashi S, Ibnou-Zekri N. The Yaa gene model of systemic lupus erythematosus. Immunol Rev. 1995;144:137–156. doi: 10.1111/j.1600-065x.1995.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 39.Fossati L, Iwamoto M, Merino R, Izui S. Selective enhancing effect of the Yaa gene on immune responses against self and foreign antigens. Eur J Immunol. 1995;25:166–173. doi: 10.1002/eji.1830250128. [DOI] [PubMed] [Google Scholar]
- 40.Palaszynski KM, Smith DL, Kamrava S, Burgoyne PS, Arnold AP, Voskuhl RR. A yin-yang effect between sex chromosome complement and sex hormones on the immune response. Endocrinology. 2005;146:3280–3285. doi: 10.1210/en.2005-0284. [DOI] [PubMed] [Google Scholar]
- 41.Voskuhl RR, Pitchekian-Halabi H, MacKenzie-Graham A, McFarland HF, Raine CS. Gender differences in autoimmune demyelination in the mouse: implications for multiple sclerosis. Ann Neurol. 1996;39:724–733. doi: 10.1002/ana.410390608. [DOI] [PubMed] [Google Scholar]
- 42.Cua DJ, Hinton DR, Stohlman SA. Self-antigen-induced Th2 responses in experimental allergic encephalomyelitis (EAE)-resistant mice. Th2-mediated suppression of autoimmune disease. J Immunol. 1995;155:4052–4059. [PubMed] [Google Scholar]
- 43.Bebo BF, Jr, Vandenbark AA, Offner H. Male SJL mice do not relapse after induction of EAE with PLP 139-151. J Neurosci Res. 1996;45:680–689. doi: 10.1002/(SICI)1097-4547(19960915)45:6<680::AID-JNR4>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 44.Dalal M, Kim S, Voskuhl RR. Testosterone therapy ameliorates experimental autoimmune encephalomyelitis and induces a T helper 2 bias in the autoantigen-specific T lymphocyte response. J Immunol. 1997;159:3–6. [PubMed] [Google Scholar]
- 45.Bebo BF, Jr, Zelinka-Vincent E, Adamus G, Amundson D, Vandenbark AA, Offner H. Gonadal hormones influence the immune response to PLP 139-151 and the clinical course of relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol. 1998;84:122–130. doi: 10.1016/s0165-5728(97)00214-2. [DOI] [PubMed] [Google Scholar]
- 46.Palaszynski KM, Loo KK, Ashouri JF, Liu HB, Voskuhl RR. Androgens are protective in experimental autoimmune encephalomyelitis: implications for multiple sclerosis. J Neuroimmunol. 2004;146:144–152. doi: 10.1016/j.jneuroim.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 47.Offner H, Adlard K, Zamora A, Vandenbark AA. Estrogen potentiates treatment with T-cell receptor protein of female mice with experimental encephalomyelitis. J Clin Invest. 2000;105:1465–1472. doi: 10.1172/JCI9213. [DOI] [PMC free article] [PubMed] [Google Scholar]


