Abstract
Polychlorinated biphenyls (PCBs) are ubiquitous environmental contaminants that have been demonstrated to be toxic to the dopamine (DA) systems of the central nervous system. One proposed mechanism for PCB-induced DA neurotoxicity is inhibition of the vesicular monoamine transporter (VMAT); such inhibition results in increased levels of unsequestered DA and DA metabolism leading to oxidative stress. We have used an organotypic co-culture system of developing rat striatum and ventral mesencephalon (VM) to determine whether alterations in the vesicular storage of DA, resulting from PCB exposure and consequent induction of oxidative stress, leads to GABA and DA neuronal dysfunction. 24 hr exposure to an environmentally relevant mixture of PCBs reduced tissue DA and GABA concentrations, increased medium levels of DA and measures of oxidative stress in both the striatum and VM. Alterations in neurochemistry and increases in measures of oxidative stress were blocked in the presence of n-acetylcysteine (NAC). Although NAC treatment did not alter PCB-induced changes in DA neurochemistry, it did protect against reductions in GABA concentration. To determine whether alterations in the vesicular storage of DA were responsible for PCB-induced oxidative stress and consequent reductions in GABA levels, we depleted DA from the co-cultures using α-methyl-p-tyrosine (AMPT). AMPT reduced striatal and VM DA levels by 90% and 70%, respectively. PCB exposure, following DA depletion, neither increased levels of oxidative stress nor resulted in GABA depletion. These results suggest that PCB-induced alterations in the vesicular storage of DA, resulting in increased levels of unsequestered DA, leads to increased oxidative stress, depletion of tissue glutathione, and consequent reductions in tissue GABA concentrations.
Keywords: PCBs, dopamine, oxidative stress, α-methyl-p-tyrosine, GABA
INTRODUCTION
Although production of polychlorinated biphenyls (PCBs) in the United States ceased in 1977 due to their possible carcinogenic effects, the persistent nature of PCBs in the environment is still a cause for concern (ATSDR, 2000). PCB exposure in humans is also associated with developmental behavioral deficits in children (Jacobson and Jacobson, 1996; Stewart et al., 2003), as well as increased risk for Parkinson’s disease (PD) mortality in those occupationally exposed as adults (Steenland et al., 2006).
PCBs have been shown to be neurotoxic to dopamine (DA) neurons in a number of in vitro and in vivo models. Results from in vivo rat studies have shown that adult PCB exposure alters extra-neuronal DA concentrations (Seegal et al., 2002), and eventually reduces the levels of frontal cortical and striatal DA (Seegal et al., 1997). Other studies, in both adult non-human primates and rats exposed to PCBs for several weeks, revealed a reduction in the number of tyrosine hydroxylase positive (TH+) DA neurons in the substantia nigra (SN) (Seegal, Chishti, and Brosch, unpublished observations). Similar losses in the numbers of TH+ DA neurons and in striatal DA are observed in the brains of individuals who have been diagnosed with PD (Gelb et al., 1999). Interestingly, even a single in vivo PCB exposure of mice reduced the expression of brain dopamine transporter (DAT) and vesicular monoamine transporter (VMAT) proteins (Richardson and Miller, 2004).
In vitro, PCBs are potent competitive inhibitors of the monoamine transporters, DAT and VMAT, while having less effect on the transport of serotonin, glutamate, and GABA transporters (Mariussen et al., 1999; Mariussen et al., 2001; Bemis & Seegal 2004). PCB-induced inhibition of VMAT can result in the accumulation of non-sequestered DA which, in turn, leads to increased production of the DA metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) (Bemis & Seegal 2004, Teng et al., 1997). In addition, unsequestered DA, can result in free-radical formation, either through auto-oxidation or through the metabolism of DA to DOPAC (Fahn & Cohen, 1992; Hermida-Ameijeiras et al., 2004). Increased oxidative stress can, in turn, lead to the dysfunction and eventual death of DA neurons. In fact, when the MN9D DA cell line was exposed to PCBs, neuronal death ensued, arising from increased oxidative stress (Lee and Opanashuk, 2004; Lee et al., 2006). Recently, rats exposed to PCBs, were found to have decreased levels of glutathione, a major cellular antioxidant, as well as increased levels of lipid peroxidation products and hydrogen peroxide (H2O2) in several brain regions (Muthuvel et al., 2006; Venkataraman et al., 2007).
Our studies of DA and GABA development and the neurotoxicological consequences of PCB exposure in organotypic co-cultures of developing rat striatum and ventral mesencephalon (VM) (Lyng et al., 2007a, 2007b) have provided a framework to further study the mechanisms of PCB-induced neurotoxicity. There have been reports that PCB-induced inhibition of VMAT subsequently affects DA neurochemistry (Mariussen et al., 1999; Mariussen et al., 2001; Bemis & Seegal 2004; Seegal et al., 1997; Lyng et al., 2007b). Additionally, DA metabolism and autooxidation lead to increased oxidative stress (Fahn & Cohen, 1992; Hermida-Ameijeiras et al., 2004), and PCB exposure leads to oxidative stress and death of DA and GABA neurons (Lee and Opanashuk, 2004; Lee et al., 2006; Lyng et al., 2007b).
The organotypic co-culture system, originally developed by Stoppini et al. (1991) and modified by Snyder-Keller et al. (2001), provides an in vitro model of the nigrostriatal DA system that closely mimics that of the intact animal (Lyng et al., 2007a). Exposure of co-cultures to PCBs, for periods ranging from 1 to 14 days, resulted in depletion of tissue DA, DA associated proteins (TH and DAT), GABA, the proteins glutamic acid decarboxylase (GAD 65/67), as well as increased DA neuronal death (Lyng et al., 2007b). The loss of GABA-ergic neurons prior to the loss of DA neurons and hypothesized to be due to increased oxidative stress was especially interesting (Lyng et al., 2007b). Indeed, Gramsbergen et al., (2002) reported similar findings – that increased oxidative stress due to GSH depletion in organotypic cultures reduced GABA concentrations prior to reductions in DA content. Based on the above findings, we hypothesize that PCB-induced inhibition of VMAT leads to increased intra-neuronal unsequestered DA that, in turn, forms reactive intermediates, results in oxidative damage and subsequent reductions in both DA and GABA neuronal function.
In order to test that hypothesis, we used organotypic co-cultures of developing rat striatum and VM exposed to a single-dose exposure to PCBs for 24 hr - a duration/dose that has been shown to increase FJB fluorescence - alters tissue and media DA levels and decreases expression of GAD 65/67 protein (Lyng et al., 2007b). We analyzed levels of DA and GABA neurochemistry, as well as indicators of oxidative stress, including 123-dihydrorhodamine and total GSH levels. These assessments were made with and without antioxidant pretreatment, as well as following depletion of co-culture DA using α-methyl-p-tyrosine (AMPT).
MATERIALS AND METHODS
Preparation of VM and Striatum Organotypic Co-Cultures
Organotypic co-cultures of VM and striatum were prepared based on the methods described by Lyng et al. (2007a) and Snyder-Keller et al. (2001). Under aseptic conditions, E14 and E21 fetuses (the presence of sperm after mating was considered embryonic day E0) were removed from timed-pregnant Sprague-Dawley rats (Taconic Farms, Germantown, NY) under isoflurane anesthesia of the dam. The fetuses were immediately decapitated and the brains removed in ice-cold Ham’s F-12 medium (Gibco, Carlsbad, CA).
The VM was removed from E14 brains and co-cultured with E21 striatum that had been dissected from a 300-μm thick vibratome-sectioned forebrain. The tissues were placed approximately 1 mm apart on a Transwell Permeable Support (0.4-μm pore size) in Costar clear six-well culture trays (Corning, Acton, MA), with two co-cultures per well. For the first 3 days the medium consisted of Neurobasal medium (Gibco) supplemented with 20% horse serum, 2% antioxidant-free B-27 supplement (Gibco), penicillin/streptomycin/neomycin (100 μg/ml), bicarbonate (1.2 mg/ml), HEPES (4.5 mg/ml), and glutamine (2 mM). For the remaining time in vitro the medium was composed as described above, except that it did not contain serum. Unlike procedures described in Lyng et al. (2007b), co-cultures for the experiments reported here were incubated at 37°C in a 5% CO2/95% room air humidified incubator for a total of 7 days in vitro (DIV) prior to experimental manipulation, in order to provide a more static in vitro model of the nigrostriatal DA system. Because the number of day in-vitro (DIV) influence the response of the co-culture to PCBs, it is not possible to directly compare the effects of 24h exposure to PCBs reported here and in Lyng et al. (2007b). All animal procedures were approved by the Institutional Animal Care and Use Committee of the Wadsworth Center.
Co-Culture Treatments
We used the same environmentally relevant Fox River (FR) PCB mixture that was used in our previous report (Lyng et al., 2007b), which had been previously characterized by Kostyniak et al. (2005). The FR PCB mixture consists of 35% Aroclor (A) 1242, 35% A1248, 15% A1254, and 15% A1260. PCB stocks solutions were made in dimethylformamide (DMF) and diluted 1000X into culture medium to obtain the desired concentration of PCBs (8 μM), a dose that we have previously shown to induce reductions in co-culture DA and GABA concentrations after 24 hr (Lyng et al., 2007b). DMF concentrations were 0.1% in both PCB-and vehicle-treated samples. Hydrogen peroxide (Sigma, St. Louis, MO) served as a positive control and was used at a concentration of 10 μM.
For antioxidant treatment experiments n-acetylcysteine (NAC) (Sigma) was dissolved into co-culture medium to achieve a final concentration of 10 mM. NAC pretreatment began on DIV 7, 30 min prior to the addition of 8 μM PCB or 10 μM H2O2-supplemented medium. PCB and/or H2O2 exposure continued for 24 hr. Co-cultures were removed for analyses following the 24-hr exposure to PCBs, H2O2, or DMF vehicle with or without NAC supplementation.
Depletion of DA in the VM and striatum co-cultures was accomplished using AMPT (Sigma, St. Louis, MO). DIV 7 co-cultures were treated with 250 μM AMPT for 24 hr prior to 24-hr exposure to 8 μM PCBs. The concentration of AMPT used here was based on pilot studies that demonstrated that this concentration resulted in significant decreases in striatal and VM DA concentration.
High Performance Liquid Chromatography with Electrochemical Detection
High performance liquid chromatography with electrochemical detection (HPLC-ECD) was used to determine the levels of DA, DOPAC and HVA within the striatum, VM and medium, as well as levels of GABA in the striatum and VM (Bemis and Seegal 2004; Peinado et al., 2004). Medium concentrations of GABA were below the detection limit and thus not quantified. All neurochemical measurements were corrected for the protein content of the co-cultures using the bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL). All samples were prepared for neurochemical analyses by separating the VM and striatum and sonicating each in 150 μl of 0.2M HClO4. Co-culture medium was collected and diluted 1:1 in 0.4M HClO4. Following centrifugation, one half of the volume of each supernatant was used for DA, DOPAC, and HVA determination using HPLC-ECD while the other half was derivatized for detection of GABA using HPLC-ECD, based on the methods described by Peinado et al. (2004). The remaining tissue pellet was dissolved in 0.1M NaOH for BCA protein analysis (Pierce). Neurochemical data are presented as nanograms (ng) of neurotransmitter or metabolite per milligram (mg) of protein in the corresponding co-culture.
Glutathione Analysis
Total glutathione concentrations, including reduced (GSH) and oxidized (GSSG) forms, were determined by the enzymatic recycling method described by Tietze (1969) with reagents obtained from Sigma (St Louis, MO). Briefly, the VM and striatum were separated, and each homogenized in 200 μl of 5% 5-sulfosalicylic acid (SSA) and kept on ice for 10 min to deproteinize the samples. The samples were centrifuged, and the supernatants were used to determine the levels of total glutathione. 10μl of each sample/standard was added in duplicate to a 96-well micro-titer plate; the kinetic assay was initiated with the addition of 5,5′-dithiobis-(2-nitrobenzoic acid), glutathione reductase, and NADPH. The increase in absorbance was measured at 412 nm over a 3-min period. The protein content of each sample was determined using the BCA protein assay (Pierce).
RH-123 Imaging and Quantitative Analysis
We assessed oxidative stress in co-cultures by determining the extent of conversion/oxidation of the non-fluorescent 123-dihydrorhodamine (DHR) to the fluorescent rhodamine (RH) molecule, RH-123 Co-cultures were placed in tissue culture plates (previously described) in medium containing 5μM DHR (Molecular Probes, Eugene, OR) for 30 min, in order to load the tissue prior to any experimental manipulations. After the 30-min loading, the co-cultures were rinsed twice with fresh medium to remove excess DHR and then underwent their specific experimental manipulations (e.g., PCB, H2O2, NAC, or AMPT treatment).
RH-123 fluorescence that resulted from the conversion of DHR was assessed by imaging the entire co-culture under a fluorescent stereomicroscope using fixed image capturing settings. The striatum and VM of the images were outlined in Image J (NIH Image), and the RH-123 fluorescent intensity determined. H2O2 and vehicle-treated co-cultures were also included as positive and negative controls, respectively. All image collection and analyses were carried out in a blinded manner.
Statistical Procedures
All data were analyzed using one-way analysis of variance (ANOVA) and included Bonferroni-corrected post hoc t tests (SPSS v.15 statistical analysis software) where a p-value ≤ 0.05 was considered significant. All results are based on sample sizes (n) of at least 5 co-cultures for each treatment group. All data are expressed as the mean ± SEM.
RESULTS
PCB-Induced Neurochemical Changes: Effects of NAC Treatment
Exposure to 8 μM FR PCBs for 24 hr significantly reduced levels of striatal and VM DA (Fig. 1A) and GABA (Fig. 1B) compared to vehicle control. Levels of striatal DA were decreased by nearly 40% (p ≤ .001) while VM DA levels were decreased by almost 30% (p ≤ .01). Levels of medium DA increased to 230% of the level observed in controls (p ≤ .05) (Fig. 1A). Although there was a trend for PCB-induced increases in medium DOPAC and HVA, neither value reached statistical significance (data not shown). This disparity in results, compared to results reported in Lyng et al. (2007b), most likely reflects the fact that the co-cultures used in these experiments were exposed to PCBs from DIV 7, rather than DIV 3 reflecting differential sensitivity based on the number of days in-vitro prior to PCB-exposure. PCB-induced reductions in GABA concentrations approached 45% (p ≤ .10) in the VM and 40% in the striatum (p ≤ .05). H2O2-treated positive control co-cultures were significantly depleted of all neurotransmitters assessed (DA and GABA).
Fig. 1.
Changes in DA (A) and GABA (B) concentrations in the striatum, VM, and medium of organotypic co-cultures following a 24-hr exposure to vehicle control, 8 μM FR PCBs, or10 μM H2O2, each in the presence or absence of 1mM n-acetylcysteine (NAC). Samples underwent HPLC-ECD for determination of DA and GABA content, and each value represents the mean ± SEM of 7–12 samples. Within a region, those bars labeled with different letters denote values significantly different from one another (p≤.05).
Treatment with the anti-oxidant NAC alone did not alter DA levels. However, NAC pretreatment provided significant protection against PCB-induced striatal GABA depletion (p ≤ .05) and demonstrated a trend for protection within the VM (not significant (ns), p ≤ .10). NAC treatment prevented H2O2-induced depletion of all neurotransmitters.
PCBs Induce Oxidative Stress in Organotypic Co-Cultures: Effects of NAC Treatment RH-123 Fluorescence
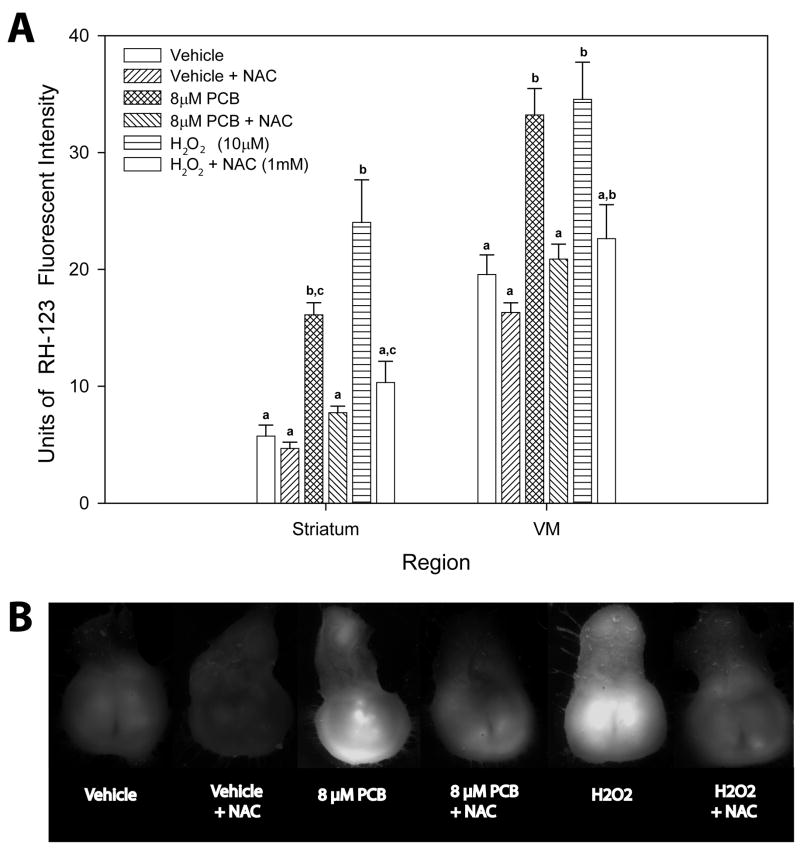
24-hr PCB exposure increased RH-123 fluorescence in both the striatum and VM (Fig. 2A), an effect that was attenuated with NAC treatment. Specifically, 24-hr exposure to 8 μM FR PCB increased striatal fluorescence to 280% of control (p ≤ .05), and VM DHR fluorescence to 170% of control (p ≤ .01) while exposure to H2O2 for 24-hr exposure resulted in an even larger increase in DHR fluorescence: striatal levels increased by 380% (p ≤ .001), while VM fluorescence increased to 175% (p ≤ .01) of control levels. Treatment with NAC eliminated both the PCB and H2O2-induced increases in RH-123 fluorescence.
Fig. 2.
Changes in the fluorescent intensity of RH-123 from the VM and striatum of organotypic co-cultures following a 24-hr exposure to vehicle control, 8 μM FR PCBs, or 10 μM H2O2, each in the presence or absence of 1mM NAC (A). Representative images of RH-123 fluorescence from selected treatment groups (B). Each value represents the mean ± SEM of 5–6 samples. Within a region, those bars labeled with different letters denote values significantly different from one another (p≤.05).
Total Glutathione Levels
Total GSH (GSH + GSSG) content was reduced in the striatum and VM of PCB andH2O2-exposed co-cultures, but not following NAC treatment (Fig. 3). Exposure to 8 μM FR PCBs for 24 hr resulted in a 44% reduction in striatal total GSH levels (p ≤ .05) and a 35% reduction in VM levels (p ≤ .05). Co-cultures treated with H2O2 as a positive control were even more strongly depleted of total GSH: levels decreased by over 76% in the striatum (p ≤ .001), and by 58% in the VM (p ≤ .001). Similar to the increases in RH-123 fluoresence reported earlier, pretreatment with NAC eliminated both the PCB- and H2O2-induced reductions in total GSH.
Fig. 3.
Changes in the total GSH content of the VM and striatum of organotypic co-cultures following a 24-hr exposure to vehicle control, 8 μM FR PCBs, or 10 μM H2O2, each in the presence or absence of 1mM NAC. Each value represents the mean ± SEM of 8–10 samples. Within a region, those bars labeled with different letters denote values significantly different from one another (p≤.05).
PCB-Induced Neurochemical Changes: Effects on DA Depletion with AMPT
Organotypic co-cultures were exposed to 250 μM AMPT for 24 hr in order to significantly deplete levels of both striatal and VM DA (Fig. 4A). AMPT alone did not alter GABA levels in either region (Fig. 4B). Co-cultures exposed to PCBs following AMPT no longer exhibited PCB-induced effects on DA, most likely due to the remaining low levels of DA. Most importantly, PCB-induced reductions in GABA were no longer observed following DA depletion with AMPT (Fig. 4B). Specifically, 24-hr exposure to 8 μM FR PCBs depleted DA levels by 45% in the striatum (p ≤ .001) and by 39% in the VM (p ≤ .001), while AMPT treatment resulted in even greater depletions of DA, with striatal DA reduced by nearly 90% (p ≤ .001) and VM DA by almost 70% (p ≤ .001) (Fig. 4A).
Fig. 4.
Changes in DA (A) and GABA (B) concentrations in the striatum, VM, and medium of organotypic co-cultures following a 24-hr exposure to vehicle control or 8 μM FR PCBs with or without prior DA depletion using 250 μM AMPT. Samples underwent HPLC-ECD for determination of DA and GABA content, and each value represents the mean ± SEM of 7–12 samples. Within a region, those bars labeled with different letters denote values significantly different from one another (p≤.05).
PCBs Induce Oxidative Stress in Organotypic Co-Cultures: Effects of DA Depletion On RH-123 Fluorescence
AMPT alone did not significantly alter RH-123 fluorescence in either the striatum or VM (Fig. 5). However, RH-123 fluorescence increased in the striatum and in the VM to 240% and 148% of control levels, respectively, following a 24-hr exposure to 8 μM FR PCB - an effect blocked following pre-exposure to 250 μM AMPT.
Fig. 5.
Changes in the fluorescent intensity of RH-123 from the VM and striatum of organotypic co-cultures following a 24-hr exposure to vehicle control or 8 μM FR PCBs with or without prior DA depletion using 250 μM AMPT (A). Representative images of RH-123 fluorescence from selected treatment groups (B). Each value represents the mean ± SEM of 5–6 samples. Within a region, those bars labeled with different letters, denote values significantly different from one another (p≤.05).
Total Glutathione Levels
AMPT alone did not significantly alter GSH content in the striatum and VM of co-cultures compared to vehicle control (Fig. 6). Similar to the PCB-induced depletion of GSH reported in Figure 3, 24-hr exposure to 8 μM FR PCB depleted total GSH in the striatum by more than 52% (p ≤ .001), and in the VM by more than 40% (p ≤ .01). On the other hand, AMPT prevented the aforementioned PCB-induced reductions in striatal GSH levels. However, AMPT treatment did not alter the PCB-induced reductions in total GSH within the VM to the same extent. This regional difference in protection following AMPT induced depletion of DA may reflect pharmacokinetic differences in the ability of AMPT to fully penetrate the thicker VM section.
Fig. 6.
Changes in the total GSH content of the VM and striatum of organotypic co-cultures following a 24-hr exposure to vehicle control or 8 μM FR PCBs with or without prior DA depletion using 250 μM AMPT. Each value represents the mean ± SEM of 8–10 samples. Within a region, those bars labeled with different letters denote values significantly different from one another (p≤.05).
DISCUSSION
We have shown that 24-hr exposure of organotypic co-cultures of VM and striatum to PCBs reduces striatal and VM DA and GABA concentrations while increasing levels of medium DA. We also show, for the first time, that PCBs reduce tissue GABA concentrations in co-cultures most likely due to induction of oxidative stress. PCB-induced inhibition of the VMAT (Mariussen et al., 2001; Bemis and Seegal, 2004) may account for both the direct effects on tissue levels of DA and the trend for increased DOPAC, given that elevations of intraneuronal or unsequestered DA result in greater metabolism of DA to DOPAC and eventual downregulation of DA synthesis (Cerrito and Raiteri, 1980; Wolf and Roth, 1990). These changes are, however, not sufficient to explain the reductions in GABA concentrations. Although NAC does not alter PCB-induced depletion of tissue DA, it does protect against depletion of GABA suggesting that PCB-induced increases in oxidative stress lead to reductions in GABA concentrations. Indeed, using either a similar organotypic culture technique (Gramsbergen et al., 2002) or a dissociated cell culture model (Nakamura et al., 2000), depletion of GSH content with L-buthionine sulfoximine (BSO), depleted tissue GABA content and increased GABA neuronal death prior to any observed reductions in DA or the loss of DA neurons.
These PCB-induced increases in oxidative stress likely result from inhibition of VMAT, which leads to sustained elevations in unsequestered DA that, in turn, can lead to increased oxidative stress (Graham, 1978; Hastings, 1995; Fahn and Cohen, 1992; Lee and Opanashuk, 2004) due either to non-enzymatic oxidation by molecular oxygen to form H2O2 and quinones, or metabolism via monoamine oxidase (MAO) to DOPAC and H2O2 (Hermida-Ameijeiras et al., 2004). H2O2, in turn, can be reduced via the Fenton reaction in the presence of ferrous iron (Fe2+), to produce the highly reactive and toxic hydroxyl radical (•OH) (Hermida-Ameijeiras et al., 2004). Indeed, VMAT inhibition induces formation of reactive oxygen species and results in degeneration of mature DA neurons in culture, whereas inhibition of DAT does not produce these same toxic effects (Murase and McKay, 2006). In fact, a recent study by Caudle et al. (2007) that used mice that express extremely low levels of VMAT demonstrated that these mice are under oxidative stress that eventually leads to degeneration of the nigrostriatal DA system.
PCBs have also been shown to induce oxidative stress and subsequent neurotoxicity. Lee and Opanashuk (2004) have shown that PCB exposure in MN9D DA cell lines results in the formation of oxidative products and depletion of intracellular GSH while Lee et al. (2006) suggested that PCB-induced increases in heme-oygenase-1 and corresponding increases in Fe2+ at least partly explain the PCB-induced oxidative stress and eventual toxicity reported earlier. In vivo, rats exposed to A1254 have increased levels of oxidative damage (e.g., increased lipid peroxidation and H2O2, as well as decreased GSH) in a number of brain regions; interestingly, vitamin C treatment protected against these effects, although the exact mechanism of the changes was not explored (Muthuvel et al., 2006; Venkataraman et al., 2007).
In order to determine whether PCB-induced changes in the vesicular storage of DA are responsible for the reductions in concentrations of GABA and eventual DA neuronal loss we recently reported (Lyng et al., 2007b); we depleted DA from the co-culture system using AMPT. AMPT is a tyrosine analog that competitively inhibits DA synthesis, eventually depletes vesicular stores of DA (Brogden et al., 1981) and thereby reduces DA content in organotypic and other neuronal preparations. PCBs may also reduce DA concentration through the inhibition of TH (Seegal, 1996); however this results from inhibition of VMAT which results in increased cytosolic DA and subsequent feedback inhibition of TH - not through competitive inhibition, as is the case with AMPT. Thus, both AMPT and PCBs may have similar effects on the function of TH acting, however, through very different mechanisms. AMPT-induced depletion of DA has been shown to be protective of DA neurons in many models of neurotoxicity. In vivo, AMPT reduces the behavioral motor deficits associated with MPTP exposure (Leng et al., 2004) while in vitro, AMPT treatment protects against MPP+ (Chen et al., 2005) and methamphetamine-induced (Pubill et al., 2005) oxidative stress and subsequent neurotoxicity. Thus, by depleting the co-cultures of DA we demonstrated that PCB-induced increases in oxidative stress were dependent on the presence of DA. In fact, PCB-induced reductions in tissue GSH and increased RH-123 fluorescence were attenuated in the VM and to an even greater degree in the striatum following AMPT treatment. This difference, we hypothesize, maybe due to the greater thickness of the VM tissue, relative to that of the striatum. Coincident with the reductions in oxidative stress, AMPT pre-treatment significantly inhibited PCB-induced reductions GABA, suggesting that GABA loss maybe due to the effects of DA-derived oxidative stress including the depletion of tissue GSH. The PCB-induced reductions in GABA content is not considered a normal in vivo response to DAergic neurotoxicants in the basal ganglia and in disease states such as PD. In vivo, reports suggest that when DA neurons are disrupted via neurotoxicant administration, an increase in GABA neuronal activity results (Stephenson et al., 2005; Diaz et al., 2003). We suggest that contaminant (e.g., PCB) induced DA dysfunction in-vivo may either not result in such marked elevations in basal ganglia oxidative stress or that higher levels of GSH may buffer changes in oxidative stress.
In conclusion, we suggest that PCB-induced neuronal toxicity originates in DA neuronal terminals through inhibition of VMAT and consequent increases in DA metabolism and oxidative stress. In turn, we have shown that increased oxidative stress results in significant depletion of GSH resulting in significant loss of organotypic GABA concentrations. This reduction in anti-oxidant defense mechanisms is likely to lead to additional neuronal damage and eventual loss of both VM and striatal GABA neurons, as observed in our previous studies using longer-term exposure to PCBs (Lyng et al., 2007b). These acute changes in oxidative stress and neurochemistry observed following PCB exposure allow us to define, at least in part, the mechanisms responsible for PCB-induced neurotoxicity. Our results, along with findings from numerous other published reports on PCB-induced neurotoxicity, add to the growing understanding of the roles of this ubiquitous environmental contaminant in neurodegenerative disease.
Acknowledgments
We would like to thank Dr. A. Snyder-Keller for her assistance with the organotypic co-culture technique and Dr. D.A. Lawrence for his expert advice regarding measures of oxidative stress. This work was supported by the NIEHS/USEPA Centers for Children’s Environmental Health and Disease Prevention Research Grants ES11263 and 829390 to RFS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for polychlorinated biphenyls (PCBs) Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service; 2000. [PubMed] [Google Scholar]
- Bemis JC, Seegal RF. PCB-induced inhibition of the vesicular monoamine transporter predicts reductions in synaptosomal dopamine content. Toxicol Sci. 2004;80(2):288–295. doi: 10.1093/toxsci/kfh153. [DOI] [PubMed] [Google Scholar]
- Brogden RN, Heel RC, Speight TM, Avery GS. Alpha-methyl-p-tyrosine: a review of its pharmacology and clinical use. Drugs. 1981;21:81–89. doi: 10.2165/00003495-198121020-00001. [DOI] [PubMed] [Google Scholar]
- Caudle WM, Richardson JR, Wang MZ, Taylor TN, Guillot TS, McCormack AL, Colebrooke RE, DiMonte DA, Emson PC, Miller GW. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci. 2007;27(30):8138–8148. doi: 10.1523/JNEUROSCI.0319-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerrito F, Raiteri M. Dopamine biosynthesis is regulated by the amine newly recaptured by dopaminergic nerve endings. Eur J Pharmacol. 1980;68(4):465–470. doi: 10.1016/0014-2999(80)90421-5. [DOI] [PubMed] [Google Scholar]
- Chen CXQ, Huang SY, Zhang L, Liu YJ. Synaptophysin enhances the neuroprotection of VMAT2 in MPP +-induced toxicity in MN9D cells. Neurobiol of Disease. 2005;19(3):419–426. doi: 10.1016/j.nbd.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Diaz MR, Barroso-Chinea P, Acevedo A, Gonzalez-Hernandez T. Effects of dopaminergic cell degeneration on electrophysiological characteristics and GAD65/GAD67 expression in the substantia nigra: different action on GABA cell populations. Movement Disorders. 2003;18(3):254–266. doi: 10.1002/mds.10345. [DOI] [PubMed] [Google Scholar]
- Fahn S, Cohen G. The oxidant stress hypothesis in Parkinson’s: evidence supporting it. Ann Neurol. 1992;32:804–812. doi: 10.1002/ana.410320616. [DOI] [PubMed] [Google Scholar]
- Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- Graham DG. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol Pharmacol. 1978;14(4):633–643. [PubMed] [Google Scholar]
- Gramsbergen JB, Sandberg M, Dall AM, Kornblit B, Zimmer J. Glutathione depletion in nigrostriatal slice cultures: GABA loss, dopamine resistance and protection by the tetrahydrobiopterin precursor sepiapterin. Brain Res. 2002;935:47–58. doi: 10.1016/s0006-8993(02)02451-4. [DOI] [PubMed] [Google Scholar]
- Hastings TG. Enzymatic oxidation of dopamine: the role of prostaglandin H synthase. J Neurochem. 1995;64(2):919–924. doi: 10.1046/j.1471-4159.1995.64020919.x. [DOI] [PubMed] [Google Scholar]
- Hermida-Ameijeiras A, Méndez-Álvarez E, Sánchez-Iglesias S, Sanmartín-Suárez C, Soto-Otero R. Autoxidation and MAO-mediated metabolism of dopamine as a potential cause of oxidative stress: role of ferrous and ferric ions. Neurochem Internat. 2004;45:103–116. doi: 10.1016/j.neuint.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N Eng J Med. 1996;335:783–789. doi: 10.1056/NEJM199609123351104. [DOI] [PubMed] [Google Scholar]
- Kostyniak PJ, Hansen LG, Widholm JJ, Fitzpatrick RD, Olson JR, Helferich JL, Kim KH, Sable HJK, Seegal RF, Pessah IN, Schantz SL. Formulation and characterization of an experimental PCB mixture designed to mimic human exposure from contaminated fish. Toxicol Sci. 2005;88:400–411. doi: 10.1093/toxsci/kfi338. [DOI] [PubMed] [Google Scholar]
- Lee DW, Gelein RM, Opanashuk LA. Heme-oxygenase-1 promotes polychlorinated biphenyl mixture Aroclor 1254-induced oxidative stress and dopaminergic cell injury. Toxicol Sci. 2006;90(1):159–167. doi: 10.1093/toxsci/kfj052. [DOI] [PubMed] [Google Scholar]
- Lee DW, Opanashuk LA. Polychlorinated biphenyl mixture Aroclor 1254-induced oxidative stress plays a role in dopaminergic cell injury. Neurotoxicol. 2004;25:925–939. doi: 10.1016/j.neuro.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Leng A, Mura A, Hengerer B, Feldon J, Ferger B. Effects of blocking the dopamine biosynthesis and of neurotoxic dopamine depletion with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on voluntary wheel running in mice. Beh Brain Res. 2004;154:375–383. doi: 10.1016/j.bbr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Lyng GD, Snyder-Keller A, Seegal RF. Dopaminergic development of prenatal ventral mesencephalon and striatum in organotypic co-cultures. Brain Res. 2007a;1133:1–9. doi: 10.1016/j.brainres.2006.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyng GD, Snyder-Keller A, Seegal RF. Polychlorinated Biphenyl-Induced Neurotoxicity in Organotypic Co-Cultures of Developing Rat Ventral Mesencephalon and Striatum. Toxicol Sci. 2007b;97(1):128–140. doi: 10.1093/toxsci/kfm027. [DOI] [PubMed] [Google Scholar]
- Mariussen E, Andersson PL, Tysklind M, Fonnum F. Effect of polychlorinated biphenyls on the uptake of dopamine into rat brain synaptic vesicles: a structure-activity study. Toxicol Appl Pharmacol. 2001;175:176–183. doi: 10.1006/taap.2001.9231. [DOI] [PubMed] [Google Scholar]
- Murase S, McKay RD. A specific survival response in dopamine neurons at most risk in Parkinson’s disease. J Neurosci. 2006;26(38):9750–9760. doi: 10.1523/JNEUROSCI.2745-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuvel R, Venkataraman P, Krishnamoorthy G, Gunadharini DN, Kanagaraj P, Jone Stanley A, Srinivasan N, Balasubramanian K, Aruldhas MM, Arunakaran J. Antioxidant effect of ascorbic acid on PCB (Aroclor 1254) induced oxidative stress in hypothalamus of albino rats. Clin Chim Acta. 2006;365(1–2):297–303. doi: 10.1016/j.cca.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Won L, Heller A, Kang UJ. Preferential resistance of dopaminergic neurons to glutathione depletion in a reconstituted nigrostriatal system. Brain Res. 2000;873:203–211. doi: 10.1016/s0006-8993(00)02425-2. [DOI] [PubMed] [Google Scholar]
- Peinado V, González JC, Leret ML. Effect of 17-beta-estradiol on dopamine, serotonine and GABA striatal levels in 6-hydroxydopamine-treated rats. Toxicol. 2004;204:155–160. doi: 10.1016/j.tox.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Pubill D, Chipana C, Camins A, Pallàs M, Camarasa J, Escubedo E. Free radical production induced by methamphetamine in rat striatal synaptosomes. Toxicol Appl Pharmacol. 2005;204(1):57–68. doi: 10.1016/j.taap.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Miller GW. Acute exposure to Aroclor 1016 or 1254 differentially affects dopamine transporter and vesicular monoamine transporter 2 levels. Toxicol Lett. 2004;148:29–40. doi: 10.1016/j.toxlet.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Seegal RF, Okoniewski RJ, Brosch KO, Bemis JC. Polychlorinated biphenyls alter extraneuronal but not tissue dopamine concentrations in adult rat striatum: An in vivo microdialysis study. Environ Health Perpsect. 2002;110(11):1113–1117. doi: 10.1289/ehp.021101113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegal RF, Brosch KO, Okoniewski RJ. Effects of in utero and lactational exposure of the laboratory rat to 2,4,2′,4′- and 3,4,3′,4′-tetrachlorobiphenyl on dopamine function. Toxicol Appl Pharmacol. 1997;146:95–103. doi: 10.1006/taap.1997.8226. [DOI] [PubMed] [Google Scholar]
- Seegal RF. Epidemiological and Laboratory Evidence of PCB-Induced Neurotoxicity. Crit Rev Toxicol. 1996;26(6):709–737. doi: 10.3109/10408449609037481. [DOI] [PubMed] [Google Scholar]
- Snyder-Keller A, Costantini LC, Graber DJ. Development of striatal patch/matrix organization in organotypic co-cultures of perinatal striatum, cortex, and substantia nigra. Neurosci. 2001;103(1):97–109. doi: 10.1016/s0306-4522(00)00535-2. [DOI] [PubMed] [Google Scholar]
- Steenland K, Hein MJ, Cassinelli RT, Prince MM, Nilsen NB, Whelan EA, Waters MA, Ruder AM, Schnorr TM. Polychlorinated biphenyls and neurodegenerative disease mortality in an occupational cohort. Epidemiology. 2006;17(1):8–13. doi: 10.1097/01.ede.0000190707.51536.2b. [DOI] [PubMed] [Google Scholar]
- Stephenson DT, Li Q, Simmons C, Connell MA, Meglasson MD, Merchant K, Emborg ME. Expression of GAD65 and GAD67 immunoreactivity in MPTP-treated monkeys with or without L-DOPA administration. Neurobiology of Disease. 2005;20:347–359. doi: 10.1016/j.nbd.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Stewart PW, Reihman J, Lonky EI, Darvill TJ, Pagano J. Cognitive development in preschool children prenatally exposed to PCBs and MeHg. Neurotoxicol Teratol. 2003;25:11–22. doi: 10.1016/s0892-0362(02)00320-3. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs P-A, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Meth. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Teng L, Crooks PA, Sonsalla PK, Dwoskin LP. Lobeline and nicotine evoke [3H] overflow from rat striatal slices preloaded with [3H] dopamine: Differential inhibition of synaptosomal and vesicular [3H] dopamine uptake. J Pharmacol Exp Ther. 1997;280:1432–1444. [PubMed] [Google Scholar]
- Tietze F. Enzymatic methods for quantitative determination of nanogram amounts of total and oxidized glutathione. Application to mammalian blood and other tissues. Anal Chem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Venkataraman P, Muthuvel R, Krishnamoorthy G, Arunkumar A, Sridhar M, Srinivasan N, Balasubramanian K, Aruldhas MM, Arunakaran J. PCB (Aroclor 1254) enhances oxidative damage in rat brain regions: Protective role of ascorbic acid. Neurotoxicol. 2007;28(3):490–498. doi: 10.1016/j.neuro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Roth RH. Autoreceptor regulation of dopamine synthesis. Ann NY Acad Sci. 1990;604:323–343. doi: 10.1111/j.1749-6632.1990.tb32003.x. [DOI] [PubMed] [Google Scholar]