Abstract
Asthma disproportionately affects inner-city, minority children in the U.S. Outdoor pollutant concentrations, including particulate matter (PM), are higher in inner-cities and contribute to childhood asthma morbidity. Although children spend the majority of time indoors, indoor PM exposures have been less extensively characterized. There is a public health imperative to characterize indoor sources of PM within this vulnerable population to enable effective intervention strategies. In the present study, we sought to identify determinants of indoor PM in homes of Baltimore inner-city pre-school children.
Children ages 2-6 (n=300) who were predominantly African-American (90%) and from lower socioeconomic backgrounds were enrolled. Integrated PM2.5 and PM10 air sampling was conducted over a 3-day period in the children’s bedrooms and at a central monitoring site while caregivers completed daily activity diaries. Homes of pre-school children in inner-city Baltimore had indoor PM concentrations that were twice as high as simultaneous outdoor concentrations. The mean indoor PM2.5 and PM10 concentrations were 39.5±34.5 μg/m3 and 56.2±44.8 μg/m3, compared to the simultaneously measured ambient PM2.5 and PM10 (15.6±6.9 and 21.8±9.53 μg/m3, respectively). Common modifiable household activities, especially smoking and sweeping, contributed significantly to higher indoor PM, as did ambient PM concentrations. Open windows were associated with significantly lower indoor PM. Further investigation of the health effects of indoor PM exposure is warranted, as are studies to evaluate the efficacy of PM reduction strategies on asthma health of inner-city children.
Keywords: Particulate matter, Air pollution, Asthma, Pediatric, Urban
Introduction
Asthma is the most common chronic disease of childhood in the United States (ALA, 2006). For reasons that are still not entirely clear, inner-city minority children are disproportionately affected by asthma. (CDC, 2006). Asthma is not only more prevalent among this population but mortality is higher and morbidity is more severe, including higher rates of emergency department visits and hospitalizations (ALA Epidemiology and Statistics Unit, 2005).
Multiple factors have been suggested to explain the urban and racial disparities in asthma health, including higher exposure to ambient air pollutants. Ambient pollutants, such as airborne particulate matter (PM) exposure, have been linked to more severe respiratory symptoms and decreased lung function among asthmatics, as well as increased mortality in the general population (Delfino et al., 2004; Mar et al., 2004; McConnell et al., 2003; Samet et al., 2000). It is notable that racial and ethnic minorities are more likely to live in the inner-city, where ambient PM concentrations are higher than in other more suburban settings (ALA Lung Disease Data in Culturally Diverse Communities, 2005). In addition, it has been shown that indoor PM concentrations in inner city homes are more than three times greater than suburban home environments. (Simons et al., 2006) Differences in exposure to indoor as well as outdoor PM may be partially responsible for increased inner-city asthma burden
While the evidence for the effect of ambient PM is substantial, there is now also growing evidence for the effect of indoor PM on asthma health. For example, small panel studies in children have found that exposure to elevated indoor PM concentrations is associated with lower lung function (Koenig et al., 2005; Trenga et al., 2006). While these latter studies of the effect of indoor PM on asthma have not focused on racial and ethnic minorities, this evidence suggests that indoor PM may have harmful effects on the respiratory health of young children generally. Further evidence underscoring the importance of indoor air as a highly relevant exposure for young children is that most Americans, including young children, spend over 85% of their time indoors (Klepeis et al., 2001). Thus, indoor PM exposure likely contributes more to the personal exposure of pre-school age children than outdoor PM exposure.
There have been several large studies investigating particulate matter in U.S. homes (Breysse et al., 2005; Wallace, 1996; Wallace et al., 2003). However, substantial gaps still exist in our understanding of the determinants of PM in the homes of very young, minority children. Most of the study populations were comprised of adults and older children living in cities throughout the U.S. (Wallace, 1996), and few of these studies focused on minorities. One of the more recent studies that included inner-city residents enrolled graduates of an environmental intervention study which means that the results may not represent the natural state of inner-city homes (Wallace et al., 2003). These studies all found smoking to be a major predictor of indoor particulate in the homes of smokers. Cooking activities were also found to be significant contributors to PM concentration in a subset of these studies (Ozkaynak et al., 1996, Wallace et al., 2003). Whether the results pertain to the especially vulnerable subset of pre-school inner-city minority children is unclear.
Given the impressive excess burden of asthma on young, inner-city children, there is an urgent need for strategies to limit potentially harmful exposures. Because the indoor environment is unique compared to the outdoor environment (Wallace et al., 2003) and because young children spend most of their time indoors, exposures from indoor environments in inner-cities warrant further investigation. In order to design effective strategies to limit indoor PM, it is essential to understand sources and determinants. To fill this gap in our knowledge, we conducted a study to identify predictors of in-home PM exposure in young inner-city minority children, using information from daily accounts of activities that occurred in the homes and simultaneous environmental PM monitoring.
Materials and Methods
Study participants
The study population consisted of children who participated in the Baltimore Indoor Environmental Study of Asthma in Kids (BIESAK). The BIESAK is an important research component of the Johns Hopkins Center for Childhood Asthma in the Urban Environment (CCAUE). The BIESAK recruited children with and without asthma in order to understand the effect of environmental exposures on the development of asthma. The children were between two and six years of age upon entering the study and resided in East Baltimore in one of nine contiguous zip codes in an area that was approximately 4 square miles. The study catchment area was developed in conjunction with a community advisory board to represent this predominantly African American region. The children were identified from a sample of those with health care encounters within the 12 previous months at Johns Hopkins Community Physicians or Bayview Pediatrics, the medical centers that provide the majority of care to children living in these zip codes. Eligibility criteria for asthmatic participants included 1) care-giver report of physician-diagnosed asthma, 2) symptoms or use of asthma medication within 12 months, and 3) at least one health care encounter for asthma within 12 months. Non-asthmatic participants had 1) at least one health care encounter in the previous 12 months and 2) a caregiver report of never having physician-diagnosed asthma. Participants were recruited from September 2001 through December 2003. Written informed consent was obtained from parents or legal guardians. The Johns Hopkins Medical Institutional Review Board approved the study protocol.
Environmental monitoring
Integrated air sampling was performed in the child’s bedroom over a 3-day period. The children’s bedrooms were chosen as the indoor monitoring site because the bedroom represents an environment where the child was expected to spend a substantial portion of time while indoors. Air sampling was conducted continuously over 72 hours using PM10 and PM2.5 4 L/min MSP™ impactors (St. Paul, MN) loaded with 37mm, 2.0 μm pore size, PALL Teflo™ PTFE membrane filters with polypropylene support rings (Pall Corporation, Ann Arbor, MI). Inlet flow rates were checked at the beginning and end of each sampling period using primary standards (BIOS DryCal™, Bios International Corporation, Butler, NJ). PM gravimetric analysis was conducted on a Metler T5 microbalance, after filters were pre-equilibrated for 24 hours at a constant temperature and humidity. Temperature and humidity were measured concurrently using a HOBO temperature and humidity data logger (Onset Corporation, Pocasset, MA). To understand the contribution of outdoor PM to the indoor concentrations, concurrent ambient PM data were obtained from an ambient monitoring site operated and maintained by the CCAUE and located within the study area. When matched 3-day ambient sampling data were not available from the CCAUE site, ambient PM data were obtained from a Maryland Department of the Environment ambient morning site also located within the study area.
Participant Interviews and Identification of Particulate Sources
Data were collected using an interviewer-administered survey with closed-ended questions. Caregivers were asked questions related to demographics, housing characteristics, and environmental control practices. In addition, caregivers were asked to complete a standardized household daily activity diary that described activities that occurred in the home during the period of monitoring. This diary was completed during each day of environmental monitoring and included detailed information about activities that occurred during morning, afternoon, and evening/overnight time periods. For example, caregivers were asked to report how many cigarettes were smoked in the home in the morning (6am to noon), in the afternoon (noon to 6 pm), and in the evening (6 pm to 6 am). Caregivers were asked to report the number of windows that were open for more than 10 minutes during each of these time periods. They were also asked to report whether or not other common household activities (stove use, oven use, burned food, sweeping, vacuuming, air conditioning, air purifier use, candles/incense) occurred during each of these time periods and the frequency with which these activities occurred when appropriate. Caregivers also reported the number of hours that the child was in the room where monitoring occurred and in the home during the monitoring period.
Statistical Analysis
We used descriptive statistics to characterize the participants, samples, and reported activities using proportions, means, and medians, as appropriate. Non-parametric tests were used to compare categories of activities (Kruskal-Wallis) and to evaluate trends (Cuzick’s test for trend). To identify the relationship between the indoor PM concentrations and the reported activities, linear regression models were constructed. Statistical significance was interpreted as p <0.05. Covariates were analyzed as dichotomous (ever versus never reporting an activity during the monitoring period), continuous, and/or categorical variables. Colinearity was investigated using variance inflation factors. Multivariate regression models were constructed and included variables (dichotomous or categorical) that were significant in the bivariate analyses. In addition, stepwise regression was performed to investigate other potentially significant relationships in the multivariate model. Stratified analyses were examined to evaluate the effect of asthma status (asthmatic versus non-asthmatic) and season on the relationship between PM and household activities. As associations between activities and PM did not differ between children with and without asthma, data were presented in aggregate. Likewise, analyses stratified by season did not reveal significantly different relationships between household activities and indoor PM levels and these data were also presented in aggregate. Analyses were performed using StataSE, 8.0. (StataCorp., 2003)
Results
The children were ages 2 to 6 years old and predominantly African-American (90%) (Table1). Most children had public health insurance and came from households with low annual incomes (<$25,000). No significant demographic differences in baseline characteristics were found between the 150 children with and the 150 children without asthma, except that there were more males in the asthmatic group (58% versus 43%). The children spent an average of 14 out of 24 hours indoors in their own home and the majority of the time indoors (57%) was spent in the room where the monitoring occurred. The study was conducted across all seasons with 20% of homes studied in the winter, 33% in the spring, 18% in the summer, and 29% in the fall.
Table 1.
Participant Characteristics
| Characteristic | (N=300) |
|---|---|
| Age (years)(mean)(range) | 4.4(2-6) |
| Race (%) | |
| African American | 90 |
| Caucasian | 6 |
| Other | 4 |
| Gender (% male) | 50 |
| Annual Household Income (%)* | |
| <$25,000 | 74 |
| $25,000-50,000 | 20 |
| >$50,000 | 6 |
| Health Insurance (%) | |
| Public | 88 |
| Private | 10 |
| Self-pay | 2 |
| Time In-home (hours/day) (median) | 14 |
| Time in room where monitoring occurred (hours/day)(median) | 8 |
a substantial number (123) of participants did not provide this information
Based on 2000 U.S. Census data, the zip codes of homes included in the study represented a geographic region that is more than 99% urban (U.S. Census Bureau, 2000). Most of the homes (79%) were rowhomes (homes that share adjacent walls) that were close to the street (within 25 feet) and all were within 2 miles of the central monitoring site. The average PM2.5 concentration in the homes was (mean± s.d.) 39.5 ± 34.5 μg/m3 (Figure 1). The average PM10 concentration was 56.2 ± 44.8 μg/m3. The indoor concentrations were significantly higher that the mean ambient PM2.5 and PM10 concentrations measured at central monitoring sites, which were 15.6 ± 6.9 and 21.8 ± 9.5 μg/m3, respectively (p<0.01 for both comparisons). Over 75% of indoor PM2.5 samples exceeded the EPA annual limit for ambient PM2.5 (15μg/m3) and 47% of indoor PM10 samples were above the EPA annual limit for ambient PM10 (50 μg/m3) (EPA Revised PM Standards 1997). Even using the EPA 24-hour standard, 15% and 10% would have exceeded the respective PM2.5 and PM10 thresholds that were in effect at the time of the study (EPA,1997).
Figure 1. Indoor PM Concentrations in Inner-City Baltimore Homes.
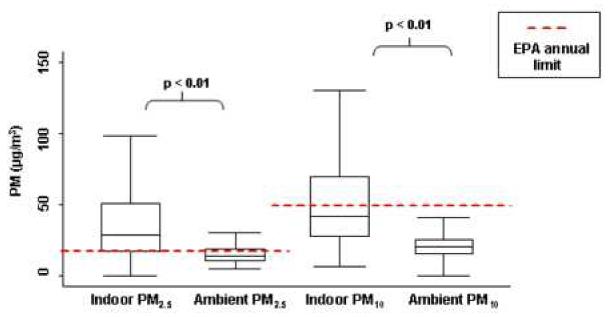
These box and whisker plots demonstrate the PM values measured in the homes and simultaneous ambient levels. The boxes show the interquartile ranges (IQR) and the heavy dark lines are the medians. Whiskers represent closest value within1.5X the IQR of PM values. In-home PM concentrations were significantly higher than ambient PM concentrations and in most cases. Over 75% of homes exceeded the EPA annual limit for ambient PM2.5 and 47% of homes exceeded the annual limit for PM10.
Activities with the potential to generate PM were common during the 3-day monitoring period (Table 2). Over half of the respondents (56%) reported smoking in the home. Cleaning activities were frequently reported: 85% of households reported at least one episode of sweeping and 37% reported at least one episode of vacuuming. Cooking activities were also common: 92% reported stove use and 49% reported oven use. Climate control measures that were reported by caregivers included open windows, space heaters, air conditioners, and rarely, air purifiers. None of the reported activities differed significantly in homes of children with and without asthma (data not shown).
Table 2.
Bivariate Analysis of Predictors and Modifiers of Indoor PM2.5 Concentrations
| Activity (reported versus not reported during the monitoring period) | PM2.5 (μg/m3) | PM10 (μg/m3) | |||||
|---|---|---|---|---|---|---|---|
| % Reporting activity | Coefficient | 95% Confidence Interval | P-value | Coefficient | 95% Confidence Interval | P-value | |
| Smoking | 56 | 26.0 | 18.3, 33.8 | <0.01 | 28.4 | 18.0, 38.7 | <0.01 |
| Sweeping | 85 | 13.0 | 2.9, 23.0 | 0.01 | 16.4 | 3.6, 29.2 | 0.01 |
| Stove use | 92 | 5.8 | -9.6, 21.1 | 0.46 | 6.6 | -13.1, 26.3 | 0.51 |
| Burned food | 6 | 4.2 | -13.7, 22.2 | 0.65 | 3.7 | -20.6, 28.0 | 0.77 |
| Oven | 49 | 3.0 | -5.3, 11.3 | 0.48 | 5.9 | -4.9, 16.7 | 0.28 |
| Candles/incense | 33 | 0.7 | -8.2, 9.5 | 0.89 | -0.03 | -11.5, 11.4 | 0.99 |
| Open Windows | 85 | -2.0 | -14.2, 10.2 | 0.75 | 9.9 | -5.7, 25.5 | 0.22 |
| Vacuuming | 37 | -1.7 | -10.3, 6.9 | 0.70 | -4.3 | -15.4, 6.9 | 0.46 |
| Air conditioning | 30 | -1.9 | -15.7, 11.8 | 0.69 | -6.3 | -18.1, 5.6 | 0.30 |
| Space heater | 7 | -3.2 | -19.7, 13.3 | 0.70 | 1.9 | -18.7, 22.4 | 0.86 |
| Air purifier | 1 | -20.5 | -88.1, 47.1 | 0.55 | -5.4 | -56.8, 46.0 | 0.84 |
Bivariate Analyses
There were no statistically significant differences in PM2.5 or PM10 based on temperature, humidity, or season (data not shown). In the bivariate analyses, smoking and sweeping were strongly associated with higher indoor PM concentrations (Table 3). The homes with a smoker had very high PM concentrations compared to those without a smoker. For example, the mean PM2.5 concentration was 26 μg/m3 higher in homes with a smoker than in homes without a smoker (smoking vs. non-smoking home: 51 vs. 25 μg/m3, (p<0.01)). There was a dose-response relationship found between the number of cigarettes smoked during the monitoring period and PM concentrations (p<0.05) (Figures 2a and 2b). Likewise, homes in which any sweeping occurred during the monitoring period had higher PM concentrations compared to those in which no sweeping was reported. Homes in which sweeping occurred had PM2.5 concentrations that were 13 μg/m3 higher (p<0.01) and PM10 concentrations that were 16 μg/m3 higher (p<0.01) than homes where sweeping did not occur. There was also a dose-response relationship between the number of sweeping events and the PM concentrations (p<0.05) (Figures 2a and 2b). PM concentrations were higher, on average, for greater number of times the stove was used during the monitoring period (p<0.05) (Figures 2a and 2b). Other activities, such as burning food, oven use, vacuuming, and lighting candles or incense had small and non-statistically significant effects on indoor PM concentrations (Table 2). The use of air conditioning did not significantly impact indoor PM concentrations, even in homes that were evaluated during the summer months. The presence of an air cleaner in the home was associated with a 20 μg/m3 decrease in PM2.5 concentration and a 5.4 μg/m3 decrease in PM10 concentration; however, air cleaners were rarely used and these relationships were not statistically significant.
Table 3. Multivariate Analysis of Predictors of Indoor PM Concentrations.
Linear regression was used to estimate the relationship between household activities reported and PM levels measured during the monitoring period
| Activity | PM2.5 (μg/m3) | PM10 (μg/m3) | ||||
|---|---|---|---|---|---|---|
| Coefficient | 95% Confidence Interval | P-value | Coefficient | 95% Confidence Interval | P-value | |
| Smoking (per cigarette) | 0.42 | 0.27, 0.57 | < 0.001 | 0.56 | 0.33, 0.80 | < 0.001 |
| Sweeping (per sweeping event) | 2.28 | 0.96, 3.59 | 0.001 | 3.65 | 1.61, 5.68 | < 0.001 |
| Open windows (per open window) | -0.88 | -1.59, 0.17 | 0.02 | -0.61 | -1.72, 0.50 | 0.28 |
| Ambient PM (per μg/m3) | 0.58 | 0.08, 1.09 | 0.02 | 0.29 | -0.26, 0.84 | 0.30 |
| Stove (per stove use) | 0.28 | -1.03, 1.60 | 0.67 | -0.17 | -2.18, 1.84 | 0.87 |
Figure 2a and 2b. Predictors of Elevated Indoor PM2.5 and PM10 Concentrations by Events over the 3-day Period.
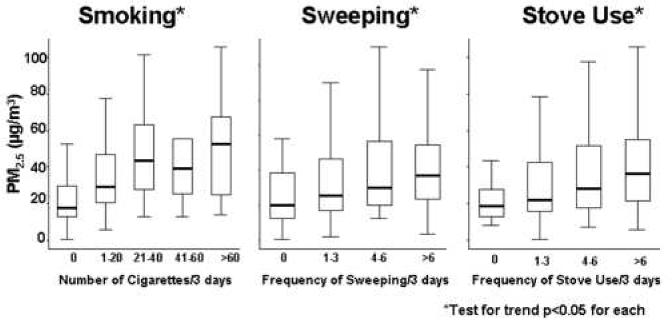
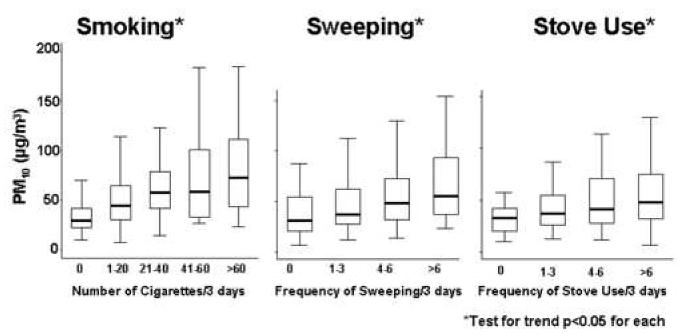
Box and whisker plots demonstrate the dose response effect seen with increased frequency of smoking, sweeping, and stove use over the 3-day monitoring period that lead to increased indoor PM levels. The median values are displayed within boxes that contain the middle 50% of PM values. The whiskers extend to the closest value within 1.5 times the interquartile range.
Multivariate Analyses
In multivariate analyses (Table 3), cigarette smoking remained strongly associated with elevated indoor PM concentrations for both PM2.5 and PM10, even after adjusting for other activities in the home. For example, for each cigarette smoked during the monitoring period, PM2.5 was 0.42 μg/m3 higher (p<0.001). Sweeping also remained significantly associated with elevated indoor PM2.5 and PM10. PM10 was 3.6 μg/m3 higher with each sweeping event that was reported during the monitoring period. When included in the multivariable model, stove use was no longer significantly associated with PM2.5 or PM10 concentrations. Open windows were associated with lower PM2.5 and PM10 concentrations, but this relationship was only significant for PM2.5. For each window that was open for more than 10 minutes per day PM2.5 was, on average, 0.88 μg/m3 lower. As expected, ambient PM also contributed to the measured indoor PM concentrations. The relationship was between ambient PM2.5 and indoor PM2.5 was statistically significant but the relationship for PM10 was not. For every 1 μg/m3 increase in the ambient PM2.5, there was a 0.58 μg/m3 increase in the indoor PM2.5 (p=0.02) and for every every 1 μg/m3 increase in the ambient PM10, there was a 0.29 μg/m3 increase in the indoor PM10 concentration (p=0.30).
Discussion
In this study of homes of African American pre-school inner-city children, we found that indoor PM concentrations in the children’s bedrooms were twice as high as outdoor concentrations and in many cases, exceeded the EPA outdoor annual limit. Indoor activities, such as smoking and sweeping, were substantial contributors to indoor PM. It is notable for the goal of reducing indoor PM that these activities are modifiable. As expected, ambient PM concentrations were also positively associated with indoor concentrations. Consistent with our finding that the indoor PM concentrations were higher than simultaneously measured ambient levels, keeping windows open appeared to lower the PM concentration in home indoor air. Given that the in-home PM concentrations were elevated and that higher indoor PM concentrations have been linked to symptoms and lower lung function in children with asthma (Koenig et al., 2005), these results can point to feasible PM reduction strategies to improve home indoor air quality in susceptible young children, including those with asthma.
Findings from the present study are consistent with certain findings reported previously by other investigators (Wallace, 1996; Wallace et al., 2003). Our study extends previous findings by focusing on an especially susceptible population, African-American pre-school children with asthma living in an inner-city environment. Smoking has been described as a major source of indoor particulate over the last several decades and our results suggest that smoking continues to be a significant contributor to PM exposure. The difference in PM2.5 of 26 μg/m3 is similar to the range of 25 to 45 μg/m3 that has been previously reported (Breysse et al., 2005; Wallace, 1996; Wallace et al., 2003). It is disappointing that smoking has the same impact on indoor PM as it did in studies from several years ago, which demonstrates that in-home smokers are not taking effective precautions to limit the impact of smoking on the home environment. This observation implies that despite the known health risks of second hand smoke to very young children in the home (Cook et al., 1998; Cook et al., 1999; Corbo et al., 1996; Mannino et al., 2001; Moshammer et al., 2006), parents are not taking precautions to protect their children and that public health messages have not effectively led to changes in smoking behaviors in the home.
Cooking activities have also been previously reported as a source of indoor PM (Ozkaynak et al., 1996; Wallace et al., 2003). However, we were not able to confirm such an effect in our study population by looking at stove use, oven use and reports of burned food. While we found a dose-response relationship between stove use and indoor PM levels, this was no longer significant after adjusting for other household activities in the multivariate model. The difference between the findings of our study and those of previous studies may be due, in part, to the method of environmental assessment. While previous studies have typically monitored a common living space, our study differs in that environmental monitoring was conducted in the children’s bedrooms which may have been farther away from the cooking area. Different methods of ascertaining household activities could also have contributed to the differences with respect to cooking activities. For example, in Wallace’s study, respondents were asked at the end of 2 weeks to report events that had occurred during monitoring, while we asked respondents to record events in a daily diary. It is possible that events recalled weeks after they occurred (e.g., an occasion of burned food) would be more intense, which could be more strongly tied to PM concentrations. Finally, regional or cultural differences in cooking methods could account for some of the differences between study results. As our study suggests that cooking activities are not predictive of elevated PM levels in the bedroom and previous findings suggest that indoor PM levels in common household areas are elevated in association with cooking, consideration should be given to moving children to more distant areas of the home during times that cooking occurs to avoid PM exposure.
A finding unique to our study, was the strong, independent effect that sweeping had on elevation of PM. Each sweeping event that was reported during the 3-day monitoring was associated with an increase in PM10 of 3-4 μg/m3. While household cleaning is necessary, our study suggests that the method of cleaning may be predictive of PM concentrations. Sweeping showed a significantly positive relationship with indoor PM concentrations while vacuuming did not. This distinction has not been previously reported to our knowledge and may have implications for future recommendations about strategies to limit PM concentrations. While use of vacuum cleaners, including those with HEPA filters, have not been shown to improve children’s asthma (Costovic and Wijk, 2005), our study findings would suggest that in the interest of keeping airborne PM concentrations lower, vacuum cleaners may be preferred to sweeping. Indeed, parents of children with asthma should be reminded that cleaning activities should ideally be performed when the child is out of the home.
Certain household conditions, including open windows, were associated with lower indoor PM concentrations. While a previous study reported that open windows reduced indoor PM in homes with smokers (Wallace et al., 2003), we have extended this finding by showing this effect regardless of smoking status. It is notable that this finding applied to the exclusively urban community that comprised the study population, an environment where this finding might not be expected. While the use of air cleaners was rare in our study, they appeared to have a marked negative effect on indoor PM concentrations.
Our study has several strengths and weaknesses. Our study was designed to minimize recall bias by asking caregivers to complete an intensive survey (survey completed at least 3 times daily) about household activities over a relatively short duration of time (3-day monitoring period). Even this method can be affected by recall and by issues such as underreporting based on social desirability (e.g., may not want to reveal how much smoking actually occurs) which may have limited our ability to demonstrate certain associations. We recorded PM concentrations in the child’s bedroom, as this location was expected to represent the majority of time of in-home exposure. However, certain activities that generated PM occurred in other locations in the home so we may have underestimated the effect of certain sources of PM on other living areas (e.g., cooking activity and kitchen PM). Since our study was conducted entirely in an urban setting, mostly in row homes, we cannot be sure if similar associations would be found in other residential settings. Nonetheless, our findings are highly relevant to young, urban dwelling children, who are markedly affected by asthma in United States (American Lung Association. Lung Disease Data in Culturally Diverse Communities, 2005).
The findings from the present study highlight that environmental recommendations given to those with respiratory disease should be quite specific. For example, current international guidelines recommend staying indoors as a means to avoid unfavorable outdoor environmental conditions (Global Strategy for Asthma Management and Prevention, 2006). This advice is warranted as staying indoors on high ozone days will effectively limit ozone exposure (Gold DR et al., 996; Lee K et al., 2004). However, the results of our study suggest that retreating indoors may not be an effective means of avoiding high PM exposure, even in homes without smokers. Thus, the recommendation should be carefully crafted to specify the characteristics of a favorable indoor environment.
In conclusion, our study demonstrated that in a population of inner-city, predominantly lower income, African-American pre-school children, common household activities and ambient PM both contribute to indoor PM concentrations. The children in our study spent a remarkable proportion of their time in their own homes, where they were exposed to PM concentrations that were markedly higher than those found outdoors. For children who are vulnerable to the effects of airborne PM, strategies to reduce PM exposure should include keeping the child away from the home when sweeping occurs, improved ventilation of the house including the use of open windows, and especially avoidance of indoor tobacco smoke. While it is clearly difficult for some families to implement complex or expensive modifications, this study points to a few simple, targeted changes that could have substantial impact on indoor air quality. Further studies are still needed to determine the most efficacious, feasible and affordable methods for improving indoor air quality for the sake of the respiratory health of young children.
Acknowledgments
Funding Sources:
This research was supported by the NIEHS (PO1 ES 09606) and EPA (PO1 R-826724) and the Johns Hopkins NIEHS Center in Urban Environmental Health (P30 ES 03819)
This research was approved by Johns Hopkins Institutional Review Board number 2, headed by David Cornblath (IRB # 01012602, “Cohort study of environmental exposures, environmental control measures, and outcomes of children with asthma”).
Abbreviations
- BIESAK
Baltimore Indoor Environmental Study of Asthma in Kids
- CCAUE
Center for Childhood Asthma in the Urban Environment
- EPA
Environmental Protection Agency
- IQR
Interquartile range
- L/min
Liters per minute
- mm
millimeter
- PM
Particulate Matter
- μg/m3
micrograms per cubic meter
- μm
micrometer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ALA (American Lung Association) [accessed 13 February 2007];Lung Disease Data in Culturally Diverse Communities. 2005 Available: www.lungusa.org.
- ALA (American Lung Association) Epidemiology and Statistics Unit Research and Scientific Affairs. [accessed 13 February 2007];Trends in Asthma Morbidity and Mortality. 2005 Available: www.lungusa.org.
- ALA (American Lung Association) [accessed 12 February 2007];Lung Disease Data. 2006 Available: www.lungusa.org.
- Breysse PN, Buckley TJ, Williams D, Beck CM, Jo S, Merriman B, et al. Indoor exposures to air pollutants and allergens in the homes of asthmatic children in inner-city Baltimore. Environmental Research. 2005;98:167–76. doi: 10.1016/j.envres.2004.07.018. [DOI] [PubMed] [Google Scholar]
- CDC. (Center for Disease Control) [accessed 19 January 19];National Center for Health Statistics - FASTATS. 2006 Available: www.cdc.gov.
- Cook DG, Strachan DP. Health effects of passive smoking-10: Summary of effects of parental smoking on the respiratory health of children and implications for research. Thorax. 1999;54(4):357–66. doi: 10.1136/thx.54.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DG, Strachan DP, Carey IM. Health effects of passive smoking. 9. Parental smoking and spirometric indices in children. Thorax. 1998;53(10):884–93. doi: 10.1136/thx.53.10.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbo GM, Agabiti N, Forastiere F, Dell’Orco V, Pistelli R, Kriebel D, et al. Lung function in children and adolescents with occasional exposure to environmental tobacco smoke. Am J Respir Crit Care Med. 1996;154(3 Pt 1):695–700. doi: 10.1164/ajrccm.154.3.8810607. [DOI] [PubMed] [Google Scholar]
- Custovic A, Wijk RG. The effectiveness of measures to change the indoor environment in the treatment of allergic rhinitis and asthma: ARIA update (in collaboration with GA(2)LEN) Allergy. 2005;60(9):1112–5. doi: 10.1111/j.1398-9995.2005.00934.x. [DOI] [PubMed] [Google Scholar]
- Delfino RJ, Quintana PJ, Floro J, Gastanaga VM, Samimi BS, Kleinman MT, et al. Association of FEV1 in asthmatic children with personal and microenvironmental exposure to airborne particulate matter. Environ Health Perspect. 2004;112(8):932–41. doi: 10.1289/ehp.6815. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA’s (Environmental Protection Agency’s) Revised Particulate Matter Standards. [accessed 23 February 2007];Fact Sheet. 1997 Available: http://www.epa.gov/ttn/oarpg/t1/fact_sheets/pmfact.pdf.
- [accessed 15 February 2007];Global Strategy for Asthma Management and Prevention. 2006 Available: www.ginasthma.org.
- Gold DR, Allen G, Damokosh A, Serrano P, Hayes C, Castillejos M. Comparison of outdoor and classroom ozone exposures for school children in Mexico City. J Air Waste Manag Assoc. 1996;46(4):335–42. [PubMed] [Google Scholar]
- Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, et al. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Expo Anal Environ Epidemiol. 2001;11(3):231–52. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- Koenig JQ, Larson TV, Hanley QS, Rebolledo V, Dumler K, Checkoway H, et al. Pulmonary function changes in children associated with fine particulate matter. Environ Res. 1993;63(1):26–38. doi: 10.1006/enrs.1993.1123. [DOI] [PubMed] [Google Scholar]
- Koenig JQ, Mar TF, Allen RW, Jansen K, Lumley T, Sullivan JH, et al. Pulmonary effects of indoor- and outdoor-generated particles in children with asthma. Environ Health Perspect. 2005;113(4):499–503. doi: 10.1289/ehp.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Parkhurst WJ, Xue J, Ozkaynak AH, Neuberg D, Spengler JD. Outdoor/Indoor/Personal ozone exposures of children in Nashville, Tennessee. J Air Waste Manag Assoc. 2004;54(3):352–9. doi: 10.1080/10473289.2004.10470904. [DOI] [PubMed] [Google Scholar]
- Mannino DM, Moorman JE, Kingsley B, et al. Health effects related to environmental tobacco smoke exposure in children in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med. 2001;155(1):36–41. doi: 10.1001/archpedi.155.1.36. [DOI] [PubMed] [Google Scholar]
- Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma - United States, 1980-1999. Morbidity and Mortality Weekly Report. 2002;51(SS01):1–13. [PubMed] [Google Scholar]
- Mar TF, Larson TV, Stier RA, Claiborn C, Koenig JQ. An analysis of the association between respiratory symptoms in subjects with asthma and daily air pollution in Spokane, Washington. Inhal Toxicol. 2004;16:809–15. doi: 10.1080/08958370490506646. [DOI] [PubMed] [Google Scholar]
- McConnell R, Berhane K, Gilliland F, et al. Prospective Study of Air Pollution and Bronchitic Symptoms in Children with Asthma. Am J Respir Crit Care Med. 2003;168(7):790–797. doi: 10.1164/rccm.200304-466OC. [DOI] [PubMed] [Google Scholar]
- Moshammer H, Hoek G, Luttmann-Gibson H, et al. Parental smoking and lung function in children: an international study. Am J Respir Crit Care Med. 2006;173(11):1255–63. doi: 10.1164/rccm.200510-1552OC. [DOI] [PubMed] [Google Scholar]
- Ozkaynak H, Xue J, Spengler JD, Wallace LA, Pellizzari ED, Jenkins P, et al. Personal exposure to airborne particles and metals: results from the particle TEAM study in Riverside, CA. J Expo Anal Environ Epidemiol. 1996;6:57–78. [PubMed] [Google Scholar]
- Samet JM, Dominici F, Curriero FC, et al. Fine particulate air pollution and mortality in 20 U.S. cities, 1987-1994. N Engl J Med. 2000;343(24):1742–9. doi: 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- Simons E, Curtin-Brosnan J, Buckley T, Breysse P, Eggleston PA. Indoor Environmental Differences between Inner City and Suburban Homes of Children with Asthma. Journal of Urban Health. 2006 doi: 10.1007/s11524-007-9205-3. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statacorp . Stata Statistical Software: Release 8.0. Stata Corporation; College Station, TX: 2003. [Google Scholar]
- Trenga CA, Sullivan JH, Schildcrout JS, et al. Effect of particulate air pollution on lung function in adult and pediatric subjects in a Seattle panel study. Chest. 2006;129(6):1614–22. doi: 10.1378/chest.129.6.1614. [DOI] [PubMed] [Google Scholar]
- U.S.Census Bureau Decennial census. [accessed January 8, 2007];Census 2000 summary file 1. Available: http://factfinder.census.gov/
- Wallace LA. Indoor Particles: A Review. J Air and Waste Manage Assoc. 1996;46:98–126. doi: 10.1080/10473289.1996.10467451. [DOI] [PubMed] [Google Scholar]
- Wallace LA, Mitchell H, O’Connor GT, et al. Particle concentrations in inner-city homes of children with asthma: the effect of smoking, cooking, and outdoor pollution. Environ Health Perspect. 2003;111(9):1265–72. doi: 10.1289/ehp.6135. [DOI] [PMC free article] [PubMed] [Google Scholar]


