Abstract
The nuclear envelope of eukaryotic cells provides a barrier separating nucleus from cytoplasm, thereby regulating the exchange of macromolecules between both compartments. However, in cells exposed to severe forms of stress this barrier may break down, resulting in the mixing of nuclear and cytoplasmic contents. We show here that the fusion protein GFP-β-galactosidase can be used to evaluate the intactness of nuclear envelopes in HeLa cells that have been exposed to heat and oxidative stress. GFP-β-galactosidase is restricted to the cytoplasm of interphase cells, but enters the nucleus when nuclear membranes are disrupted. For comparison, we have analyzed the barrier function of nuclear membranes with antibodies against lamin B. Treatment of fixed cells with digitonin permeabilizes the plasma membrane, but leaves nuclear envelopes intact. Consequently, after digitonin incubation antibodies to lamin B can bind their antigen only if nuclear membranes are damaged. For various heat and oxidative stress conditions, we have compared the distribution of GFP-β-galactosidase with the accessibility of lamin B to antibodies. Our results demonstrate that nuclear envelopes are permeable to antibodies whenever GFP-β-galactosidase enters the nucleus. GFP-β-galactosidase is therefore a useful tool for evaluating the disintegration of the nuclear envelope and identifying cells in which a mixing of nuclear and cytoplasmic material takes place.
Keywords: Stress, nucleus, nuclear envelope, GFP
Changes in the environment of an organism may lead to cell injury and damage of organelles, such as the nucleus. Ultimately this may promote death, either by apoptosis or necrosis, if cells fail to adapt and repair stress-induced damage.1–4 Hallmarks of stress-dependent injuries to nuclei are the degradation of nucleoporins and the disintegration of the nuclear membranes, resulting in the mixing of nuclear and cytoplasmic contents (reviewed in ref. 5).6,7 In interphase cells, nuclear pore complexes prevent the free movement between nucleus and cytoplasm of molecules that exceed the size of the nuclear pore complex diffusion channel. Therefore, cytoplasmic proteins with a molecular mass larger than 40 kD are unable to enter the nucleus unless they provide a signal for nuclear import (reviewed in ref. 8). Since the barrier function of the nuclear envelope can break down in cells upon severe stress, our goal was to develop an essay to follow this event in culture cells exposed to different growth conditions. We show here that GFP-β-galactosidase (GFP-β-gal), a large tetrameric protein with a molecular mass of more than 500 kD, is an ideal reporter protein to evaluate the impact of stress on the integrity of the nuclear envelope in interphase cells of higher eukaryotes.
MATERIALS AND METHODS
Cell Culture, Transfection, and Stress Exposure
The GFP-β-gal gene was expressed in transiently transfected HeLa cells by introducing plasmid pHM830 using standard procedures.9,10 At 24 h post-transfection, HeLa cells were exposed to various stresses as shown in Table 1. For oxidant exposure, diethyl maleate (DEM, Aldrich, Milwaukee, WI) was diluted in ethanol and added together with growth medium. Final concentrations of ethanol were 0.08% for control and oxidant-treated cells.
TABLE 1.
Disruption of the Nuclear Envelope in Response to Changing Growth Conditions
| Staining with Antibodies to Lamin B | |||
| Treatment | Distribution of GFP-β-gal | Digitonin | Triton X-100 |
| None | C > > N | No NE staining | NE staining |
| 45.5°C, 30 min | C > > N | No NE staining | NE staining |
| 48°C, 30 min | C > > N | No NE staining | NE staining |
| 55°C, 30 min | N + C | NE staining | NE staining |
| 0.08% Ethanol, 4 h | C > >N | No NE staining | NE staining |
| 1 mM DEM, 4 h | C > >N | No NE staining | NE staining |
| 2 mM DEM, 4 h | C > >N | No NE staining | NE staining |
| 5 mM DEM, 4 h | N + C, C> > N | NE staining, No NE staining | NE staining |
| 20 mM H2O2, 1 h | N + C | NE staining | NE staining |
HeLa cells were incubated under control or stress conditions as indicated. At the end of the treatment, cells were fixed, DNA stained with 4,′6-diamidino-2-phenylindole, and GFP-β-gal was localized by fluorescence microscopy. For lamin B antibodies, fixed cells were treated with either digitonin or Triton X-100 before incubation with lamin B-specific antibodies. C > > N, GFP-β-gal is restricted to the cytoplasm; N + C, GFP-β7-gal can be detected in nucleus and cytoplasm; NE, nuclear envelope. GFP-β-gal, GFP-β-galactosidase; DEM, diethyl maleate.
Localization of the Reporter Protein GFP-β-Gal
Stressed cells were fixed at room temperature for 25 min with 3.7% formaldehyde in phosphate buffered saline (PBS). Nuclei were stained with 4,′6-diamidino-2-phenylindole (DAPI) and the distribution of GFP-β-gal was followed by fluorescence microscopy using a Nikon Optiphot at 400× magnification and photographed with Kodak T-MAX 400 films. Negatives were scanned and processed with Photoshop 8.0.
Indirect Immunofluorescence
All steps were carried out at room temperature, essentially as described.11 Control and stressed cells were fixed in 3.7% formaldehyde/PBS and incubated on ice for 3 min with 40 μg/mL digitonin in PBS/2 mg/mL bovine serum albumin (PBS/BSA) to permeabilize the plasma membrane. This treatment leaves the nuclear envelope intact, and antibodies against lamin B cannot bind their antigen, unless the nuclear membranes have been perforated. In control experiments, all membranes of fixed cells were permeabilized for 5 min with 0.1% Triton X-100 in PBS/BSA. Upon permeabilization with digitonin or Triton X-100, nonspecific binding sites were blocked with PBS/BSA for 1h and samples were incubated overnight with antibodies against lamin B (sc-6217; Santa Cruz, CA), diluted 1:2,000 in PBS/BSA. Upon washing, anti-lamin B antibodies were detected by incubating 2h with Cy3-labeled secondary antibodies, diluted 1:2,000 (Jackson ImmunoResearch,West Grove, PA). Samples were washed and DNA stained with DAPI. Mounting of slides, fluorescence microscopy, and processing of the images were carried out as described for GFP-β-gal (see above).
RESULTS AND DISCUSSION
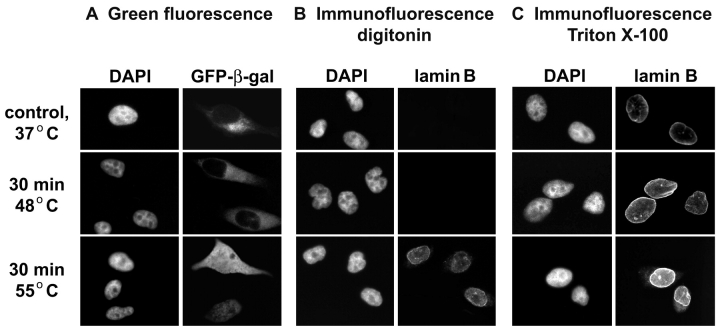
In the experiments described here, we have tested several conditions that are relevant to the pathophysiology and diseases in humans, heat shock, and oxidative stress.1–4 Under normal growth conditions, GFP-β-gal is restricted to the cytoplasm (Fig. 1A). Likewise, exposure to a 30-min heat shock at 45.5°C or 48°C did not relocate the reporter protein, suggesting that the nuclear envelope still provides an efficient barrier for large molecules. When cells were exposed to severe heat stress at 55°C, however, GFP-β-gal was detected in nuclei (Fig. 1A, Table 1).
FIGURE 1.
Localization of the reporter protein GFP-β-gal in control and stressed HeLa cells. A: HeLa cells transiently synthesizing GFP-β-gal were kept at 37°C or exposed to a 30-min period of heat shock at 48°C or 55°C. Cells were fixed, GFP-β-gal located by fluorescence microscopy and nuclei visualized with DAPI. B, C: The integrity of nuclear envelopes was monitored further with antibodies to lamin B in cells treated with digitonin (B) or Triton X-100 (C). In cells incubated with digitonin, antibodies do not bind to lamin B if the nuclear envelope is intact.
We compared the results for GFP-β-gal with those for an independent assay, to see whether antibodies against lamin B can pass through nuclear membranes in heat-shocked cells. No staining of lamin B was detected in digitonin-treated control samples or cells incubated at 48°C. By contrast, heat shock at 55°C punctured the nuclear envelope and provided access of lamin B-antibodies to the nuclear lamina of digitonin-treated cells (Fig. 1B). In control experiments, permeabilization of cellular membranes with Triton X-100 promoted binding of antibodies to the nuclear lamina, demonstrating that epitopes recognized by the antibodies are present for all the conditions tested (Fig. 1C).
We further determined whether GFP-β-gal localization could be used as a more general tool to evaluate the intactness of the nuclear envelope in cells exposed to various types of stress; Table 1 summarizes the results obtained for the localization of GFP-β-gal and binding of lamin B antibodies after different treatments. To test the effect of oxidants, HeLa cells were incubated with increasing concentrations of diethyl maleate, a compound that reduces glutathione levels and thereby generates oxidative stress (see refs. 11 and 12 and refs. cited in Molteni et al.12). Upon a 4-h incubation at 37°C with 0.08% of the solvent ethanol, 1 or 2 mM DEM, GFP-β-gal was restricted to the cytoplasm, as evident by the lack of green fluorescence in nuclei. However, after treatment with 5 mM DEM the reporter protein could be detected in the nucleus, and lamin B antibodies also bound the nuclear lamina of digitonin-treated cells (Table 1). Likewise, exposure to severe oxidative stress by incubation with 20 mM hydrogen peroxide for 1 h redistributed GFP-β-gal throughout nucleus and cytoplasm and gave antibodies against lamin B access to the nuclear lamina (Table 1).9
CONCLUSION
Our studies demonstrate that for all of the conditions that promoted entry of GFP-β-gal into the interphase nucleus, lamin B was accessible to antibodies in digitonin-permeabilized cells. Therefore, both methods can be used to evaluate the intactness of nuclear envelopes. The experiments shown here were carried out with formaldehyde-treated cells, but the same approach should be applicable to unfixed cells. When compared with indirect immunofluorescence with antibodies to lamin B, the localization of GFP-β-gal provides a simple and fast assay to monitor the disruption of the nuclear membranes in mammalian interphase cells. GFP-β-gal will be useful to follow changes in nuclear envelope permeability in real time in a variety of applications. This not only applies to conditions of severe environmental stresses, but also to a variety of viral infections that affect the integrity of nuclear pore complexes and nuclear envelopes (reviewed in ref. 13).14 Furthermore, GFP-β-gal can be employed as a reporter protein in eukaryotic cells that do not synthesize lamins, such as plants or the simple eukaryote Saccharomyces cerevisiae.15
Acknowledgments
US is a chercheur national of Fonds de la Recherche en Santé (FRSQ) and supported by grants from the Canadian Institutes of Health Research and the Heart and Stroke Foundation of Quebec. MK is supported by a fellowship from FRSQ.
REFERENCES
- 1.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 2000;408:239–247. [DOI] [PubMed] [Google Scholar]
- 2.Kumar D, Lou H, Singal PK. Oxidative stress and apoptosis in heart dysfunction. Herz 2002;27:662–668. [DOI] [PubMed] [Google Scholar]
- 3.Martindale JL, Holbrook NJ. Cellular response to oxidative stress: Signaling for suicide or survival. J Cell Physiol 2002;192:1–15. [DOI] [PubMed] [Google Scholar]
- 4.Sitia, R, Molteni SN. Stress, protein (mis)folding, and signaling: the redox connection. Sci STKE 2004;pe27(2004). [DOI] [PubMed]
- 5.Buendia B, Courvalin J-C, Collas P. Dynamics of the nuclear envelope at mitosis and during apoptosis. Cell Mol Life Sci 2001;58:1781–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrando-May E, Cordes V, Biller-Ckovric I, Mirkovic J, Görlich D, Nicotera P. Caspases mediate nucleoporin cleavage but not early distribution of nuclear transport factors and modulation of nuclear permeability in apoptosis. Cell Death Differ 2001;8:495–505. [DOI] [PubMed] [Google Scholar]
- 7.Faleiro L, Lazenik Y. Caspases disrupt the nuclearcytoplasmic barrier. J. Cell Biol 2000;151:951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weis K. Regulating access to the genome: Nucleocytoplasmic transport throughout the cell cycle. Cell 2003;112:441–451. [DOI] [PubMed] [Google Scholar]
- 9.Sorg G, Stamminger T. Mapping of nuclear localization signals by simultaneous fusion to green fluorescent protein and to β-galactosidase. BioTechniques 1999;26: 858–862. [DOI] [PubMed] [Google Scholar]
- 10.Kodiha M, Chu A, Matusiewicz N, Stochaj U. Multiple mechanisms promote the inhibition of classical nuclear import upon exposure to severe oxidative stress. Cell Death Differ 2004;11:862–874. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz E, Siow RC, Bartlett SR, et al. Vitamin C inhibits diethylmaleate-induced l-cystine transport in human vascular smooth muscle cells. Free Radical Biol Med 2003;34:103–110. [DOI] [PubMed] [Google Scholar]
- 12.Molteni SN, Fassio A, Ciriolo MR, et al. Glutathione limits Ero1-dependent oxidation in the endoplasmatic reticulum. J Biol Chem 2004;279:32667–32673. [DOI] [PubMed] [Google Scholar]
- 13.Whittaker GR. Virus nuclear import. Adv Drug Del Rev 2003;55:733–747. [DOI] [PubMed] [Google Scholar]
- 14.Belov GA, Lidsky PV, Mikitas OV, et al. Bidirectional increase in permeability of nuclear envelope upon poliovirus infection and accompanying alterations of nuclear pores. J Virol 2004;78:10166–10177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chughtai ZS, Rassadi R, Matusiewicz N, Stochaj U. Starvation promotes nuclear accumulation of the hsp70 Ssa4p in yeast cells. J Biol Chem 2001;276:20261–20266. [DOI] [PubMed] [Google Scholar]