Abstract
Glycosylation is the most common form of posttranslational modification of proteins (50–80%). The isolation, discovery, and subsequent identification of glycosylated peptides and proteins is becoming more and more important in glycoproteomics and diagnosis. MALDI-TOF mass spectrometry is an ideal technique for identifying peptides and proteins and their corresponding modifications. The enrichment of glycosylated peptides and proteins from different sources can be attained by affinity chromatography supported by functionalized magnetic particles. Covalent coating of magnetic beads with Concanavalin A (ConA) and diboronic acid was performed by carbodiimide and poly-glutaraldehyde methods, respectively. The functionalized beads were employed to establish and optimize protocols for the binding and detection of glycosylated peptides and proteins with respect to an automated workflow and the subsequent detection and identification by MALDI-TOF mass spectrometry. For several model proteins, the capture and identification could be demonstrated by SDS-PAGE and MALDI-TOF mass spectrometry. According to the type of glycosylation (high man-nose, hybrid, or complex type) the different proteins were enriched by ConA or boronic acid–functionalized beads.
Keywords: Glycosylation, Concanavalin A, boronic acid, magnetic particles, MALDI-TOF MS
Protein glycosylation and glycoproteomics are growing fields of interest due to the relationship between glycosylation degree and type and the health status of cells. The discovery and identification of glycosylated peptides and proteins and the analyses of their glycostructures are increasingly important in diagnosis and proteomics. In particular, missing, aberrant, or additional glycosylations are known to be linked to certain diseases and may be utilized as markers for diagnosis and/or therapeutic monitoring.1 Direct mass-spectrometric detection of glycosylated peptides and proteins from complex sources such as blood serum/plasma, urine, and spinal fluid is quite difficult due to saline contaminants disturbing the MS ionization process. Furthermore, highly abundant proteins (human albumin in blood serum) interfere with the mass spectrometric analysis. Therefore, enrichment and purification are essential prior to MALDI-TOF MS analysis and the subsequent identification and structure elucidation. The diagnostic applications require an automated workflow to assure ease of handling, good reproducibility, rapid analysis time, and high-throughput capability. The use of MALDI-TOF mass spectrometry and magnetic particles for identifying peptides and proteins and their corresponding modifications ideally fulfills those requirements.
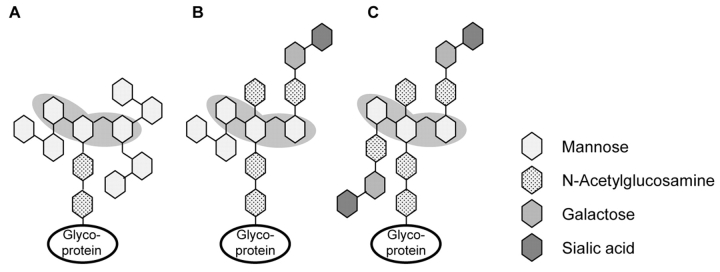
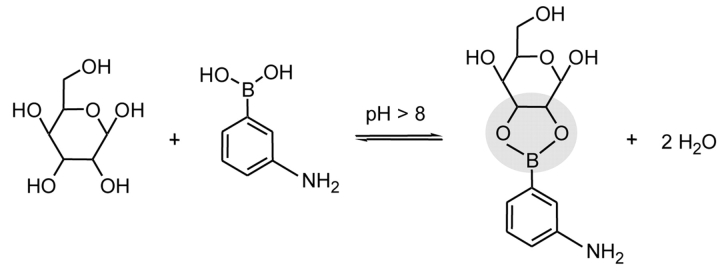
Different approaches for the specific isolation of glycosylated peptides and proteins have been described, e.g., lectin affinity chromatography or boronic acid chromatography. Concanavalin A (ConA) is a lectin that specifically binds mannosyl and glucosyl residues containing unmodified hydroxyl groups at positions C3, C4, and C6.2,3 It can be utilized for the targeted binding of certain oligosaccharide structures of N-glycosylated proteins.4 The pentacore of high mannose, hybrid, or bi-antennary complex–type oligosaccharide structures is shown in Figure 1. In contrast, phenyl boronic acid covalently binds molecules containing cis-diol groups.5 Glycopeptides and glycoproteins equipped with saccharides like mannose, galactose, or glucose can form heterocyclic diesters that are stable under alkaline conditions (Figure 2). Additionally, boronic acid facilitates the enrichment of the more heterogeneous O-linked oligosaccharides.
FIGURE 1.
Scheme of different N-glycan structures. A: high-mannose type; B: hybrid type; and C: bi-antennary complex type. The binding motif of ConA is underlaid in gray.
FIGURE 2.
Scheme describing the formation of the heterocyclic boronic acid diester derived from mannose and 3-aminophenyl boronic acid.
Carboxyl-functionalized magnetic beads were covalently coated with ConA and di-boronic acid utilizing carbodiimide and poly-glutaraldehyde cross-linking, respectively. The functionalized beads were employed to establish and optimize protocols for the binding and detection of glycosylated peptides and proteins with respect to an automated workflow and the subsequent detection and identification by MALDI-TOF mass spectrometry.
MATERIALS AND METHODS
Model proteins and chemicals were purchased from Sigma-Aldrich, Deisenhofen, Germany. Carboxyl- and boronic acid–functionalized magnetic particles were obtained from Chemicell, Berlin, Germany.
Carboxyl-functionalized magnetic beads were covalently modified with ConA employing the carbodiimide method according to the protocol from Dynal Biotech, Oslo, Norway, with a few modifications. The resulting beads are available as MB-LAC-ConA Kit (magnetic bead-lectin affinity chromatography with Concanavalin A) including all buffers from Bruker Daltonik, Leipzig, Germany.
Binding and Elution of Proteins
Binding of samples on MB-LAC ConA beads was performed according to the manufacturer’s recommendations. Briefly, beads and sample were incubated for 1 h at RT under gentle agitation in binding buffer. The beads were separated from the supernatant using a magnetic separation device (Bruker Daltonik, Leipzig, Germany). After washing, the bound glycoproteins/peptides were eluted under acidic conditions.
Boronic acid–functionalized beads were loaded with glycoprotein containing sample under slightly alkaline (pH 8.5) conditions. After incubation for 1 h at RT under gentle shaking, the bound protein species were eluted under acidic conditions.
Mass Spectrometry
For MS analysis the eluted samples were applied onto Anchor-Chip 600 targets (Bruker Daltonik, Germany) using 2,5-dihydroxyacetophenone (2,5-DHAP) (Bruker Daltonik, Germany) as matrix according to the manufacturer’s recommendations. MS measurements were performed on an autoflex II TOF/TOF. Spectra were acquired in the linear mode.
SDS-PAGE
For SDS-PAGE, samples were mixed with 5X loading buffer, boiled for 5 min at 95°C, and applied onto a discontinuous 12.5% or 15% poly-acrylamid gel according to the protocol of Laemmli.6 After running, the gels were stained with Coomassie blue.
RESULTS AND DISCUSSION
Enrichment of Glycoproteins by ConA Beads
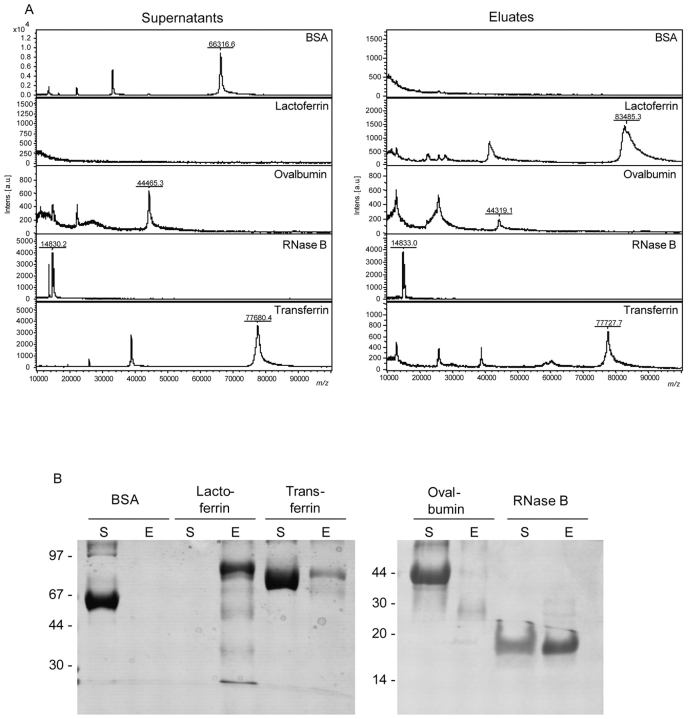
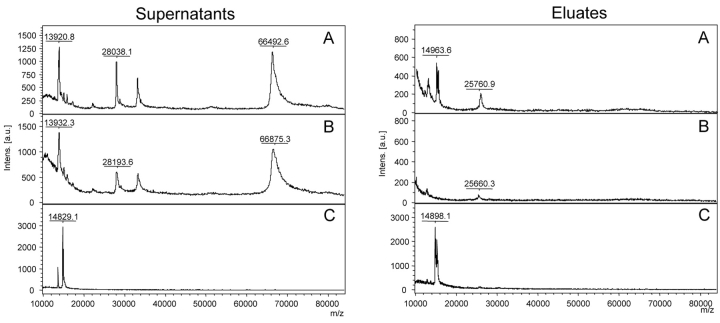
Carboxyl-functionalized magnetic particles were employed to couple ConA onto the beads using the carbodiimide method. The quality and the specificity of the MB-LAC-ConA beads were demonstrated by the capturing of different model proteins, i.e., bovine serum albumin (BSA), bovine lactoferrin, ovalbumin (OVA), bovine RNase B, and bovine transferrin (1–5 μg, corresponding to 10–300 pmol of protein). BSA served as non-glycosylated negative control. All other proteins contain N-linked oligosaccharide structures of different N-glycan types. Binding of proteins on MB-LAC-ConA beads (20 μL) was performed under neutral conditions for 1 h at RT. After binding, washing, and elution, the supernatants and the eluates were analyzed by MALDI-TOF mass spectrometry (Figure 3A) and SDS-PAGE (Figure 3B). Preparations for MALDI-TOF MS were performed with 2,5-DHAP on Anchor-ChipTM 600 targets, and spectra were acquired on an autoflex II TOF/TOF.
FIGURE 3.
Analysis of supernatants and eluates of model proteins after binding on ConA magnetic beads by MALDI-TOF MS (A) and SDS-PAGE (B). Samples were prepared with 2,5-DHAP on Anchor-Chip targets for MALDI-TOF MS analyses and measured on an autoflex II TOF/TOF.
As shown in Figure 3, nonspecific binding of BSA was not observed either by SDS-PAGE or by the more sensitive MALDI-TOF MS. In contrast, the other model proteins were bound, demonstrating the efficiency of the MB-LAC-ConA beads. Lactoferrin was completely captured and therefore found only in the eluate. This result is in agreement with the known five and four N-glycosylation sites of lactoferrin isoform-a and -b, respectively, all presenting the binding motif of ConA. The asparagines at positions 233 and 545 contain highmannose structures. At positions 368 and 476, N-glycans of the hybrid-type oligosaccharide have been described. Position 281, which is glycosylated only in isoform-a, contains a heterogeneous bi-antennary complex structure.7
In contrast to the binding of lactoferrin, the binding of OVA was less efficient. The experimental findings can be explained by the reduced number of glycosylation sites. The only glycosylation site of OVA is situated at position 293, which carries a heterogeneous N-glycan structure belonging predominantly to the hybrid type with up to 45 different isoforms.8 More-over, all commercially available OVA preparations contain co-purified glycoproteins such as riboflavinbinding protein and ovomucoid.9 Since these proteins are also glycosylated, they compete against OVA for the ConA binding sites and are preferentially bound (Figure 3). These proteins were detected by SDS-PAGE as a smear and by MS analysis as a broad peak from 20 to 30 kDa, respectively. The amount of bound OVA was in the range of the detection limit for Coomassie-stained SDS gels, so that only a weak band was visible on the gel. The MS analysis revealed a small peak at the expected mass of 44 kDa for OVA.
RNase B, a glycoprotein containing one high-mannose glycan structure at position N60,10 was captured by magnetic ConA beads with an efficiency of about 50%, corresponding to 150 pmol of protein. This protein exactly fitted the binding motif of ConA and was easily detected by MS.
Binding of transferrin was incomplete because of its N-glycan structure. Transferrin contains two N-glycosylation sites at positions N413 and N611, bearing bi- and tri-antennary N-glycan structures.11 The oligosaccharide at position N611 additionally bears a fucosylated core structure.12 The binding motif of ConA does not include tri-antennary structures, due to the lack of the free hydroxyl groups of the oligosaccharide pentacore structure. Further-more, the presence of fucose on the first GlcNAc residue of a bi-antennary oligosaccharide structure dramatically decreases the binding affinity of ConA for such glycans.1 Therefore, only the non-fucosylated bi-antennary portion of transferrin could be bound by the magnetic ConA beads and detected after elution.
Enrichment of Glycoproteins by Boronic Acid Beads
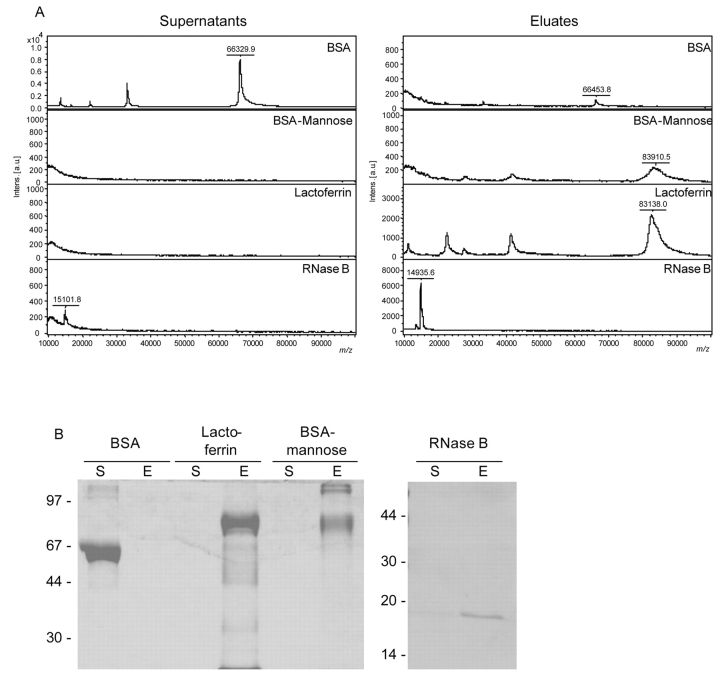
Boronic acid beads were purchased commercially. 3-Aminophenyl-di-boronic acid was coupled via poly-glutaraldehyde on carboxyl-functionalized magnetic beads. After binding of model proteins, washing and elution supernatants and eluates were subjected to SDS-PAGE and MALDI-TOF MS analysis.
As can be seen from Figure 4, the more sensitive MS measurement revealed a slightly unspecific background binding of the nonglycosylated BSA on boronic acid beads. The amount was below the detection limit of the SDS-PAGE.
FIGURE 4.
Analysis of supernatants and eluates of model proteins after binding on boronic acid–functionalized magnetic beads by MALDI-TOF MS (A) and SDS-PAGE (B) Samples were prepared with 2,5-DHAP on Anchor-Chip 600 targets for MALDI-TOF MS analysis and measured on an autoflex II TOF/TOF.
BSA-mannose is a chemically modified BSA containing 20 to 30 residues of α-mannose per mole of protein. Thereby, this protein provides a high number of reaction sites for the formation of the heterocyclic boronic acid diester, allowing an efficient capturing by the boronic acid–functionalized magnetic particles. The elution of BSA-mannose was very inefficient, as could be demonstrated by SDS-PAGE and MS analysis. This might be due to the relatively high numbers of reaction sites that can repeatedly react with other boronic acid molecules after elution according to the equilibrium constant of the diester formation. Lactoferrin was completely bound, corresponding to the four to five N-glycosylation sites containing mannose residues.7 Furthermore, RNase B was successfully bound by about 90%, according to the high-mannose type glycosylation of this protein.10 This amount corresponded to 270 pmol of RNase B.
Demonstration of Specificity
The specificity of the binding of glycoproteins on ConA beads was demonstrated by competition experiments. Binding of model proteins on magnetic ConA beads was performed in the presence of 500 mM α-methyl-mannoside, which competed against the N-glycan structures for the saccharide binding sites of the lectin. The supernatants and the eluate were analyzed by MALDI-TOF MS. The spectra revealed no association of glycoproteins onto the beads (data not shown). The same experiments for the boronic acid beads are under current investigation.
Capturing of RNase B from Defined Protein Mixtures
The capability of the magnetic ConA beads to capture a glycoprotein from a defined protein mixture was investigated (Figure 5). RNase B (60 pmol) was mixed with Protein Standard II (Bruker Daltonics, Germany) containing trypsinogen (18.6 pmol), protein A (38.6 pmol) and BSA (96 pmol). RNase B could be successfully enriched and separated from the nonglycosylated species. The main portion of RNase B was found in the eluate. No nonspecific binding of the other proteins was observed.
FIGURE 5.
Specific capturing of RNase B from protein standard II mix (Bruker Daltonik, Germany) using magnetic ConA beads. The mixture contained BSA, protein A, trypsinogen, and RNase B. The spectra present the original sample (A), the supernatant after ConA binding (B), and the eluate (C). Samples were prepared with 2,5-DHAP on Anchor-ChipTM 600 targets and measured on an autoflex II TOF/TOF.
Capturing of RNase B from Complex Protein Sources
The magnetic ConA beads were further evaluated with respect to their efficiency towards heterogeneous complex samples. For this purpose, 5 μL of human blood serum were diluted with binding solution and spiked with 30 pmol of RNase B. The magnetic ConA beads were employed to capture the added glycoprotein from the complex protein mixture.
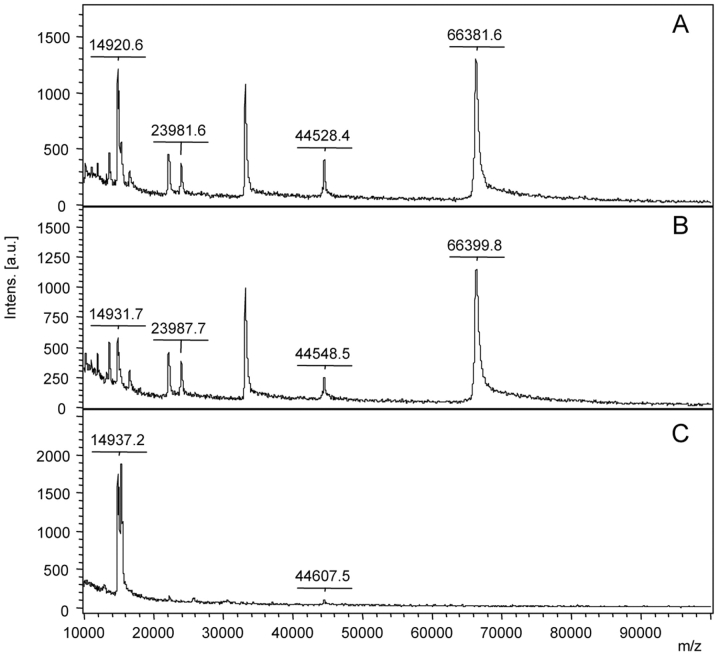
Figure 6 shows the successful combination of the enrichment of RNase B (30 pmol) from spiked serum samples by ConA capturing (20 μL beads) and the subsequent MALDI-TOF MS analysis. Prominent nonglycosylated serum proteins like HSA (m/z 66,000) and apolipoprotein A (m/z 28,000) were not bound and could be detected in the supernatant. Residual RNase B was undetectable in the supernatant. Surprisingly, only ConA (m/z 25,750) was visible as a clear peak, but no further serum proteins were detectable. This might be due to the high number of different glycosylated species, each in a very low absolute amount that was not sufficient for detection.
FIGURE 6.
MALDI-TOF MS analyses of supernatants and eluates from RNase B–spiked serum (A), unspiked serum as control (B), and RNase B (C) as reference after incubation with ConA beads. The peak at m/z 15.300 represents RNase B. samples were prepared with 2,5-DHAP on Anchor-Chip 600 targets and measured on an autoflex II TOF/TOF.
CONCLUSIONS
Carboxyl-functionalized magnetic particles were successfully coated with ConA by the carbodiimide method. The resulting beads were employed to specifically bind model proteins containing N-glycans of different oligosaccharide types. No binding of proteins was observed under competing conditions in the presence of an excess of free mannose, demonstrating specificity of binding. Similarly, di-boronic acid-functionalized beads were validated by the capturing of different model proteins. RNase B was efficiently isolated from different complex sources—e.g., spiked human blood serum—by ConA beads. An amount of 30 pmol of spiked protein could be detected after recovery by mass spectrometry.
Employing magnetic particles as a solid support for functionalization allows for easy automation and high-throughput analyses. All protocols for binding, washing, and elution of enriched proteins and peptides were optimized for subsequent MALDI-TOF-MS analyses.
Acknowledgments
This work was supported by Sächsische Aufbaubank grant 7495/1185.
REFERENCES
- 1.Durand G, Seta N. Protein glycosylation and diseases: Blood and urinary oligosaccharides as markers for diagnosis and therapeutic monitoring. Clin Chem 2000;46:795–805. [PubMed] [Google Scholar]
- 2.Kamra A, Gupta MN. Cross-linked concanavalin A-O- (diethylaminoethyl) -cellulose—an affinity medium for concanavalin A-interacting glycoproteins. Anal Biochem 1987;164:405–410. [DOI] [PubMed] [Google Scholar]
- 3.Yahara I, Edelman GM. Restriction of the mobility of lymphocyte immunoglobulin receptors by Concanavalin A. Proc Natl Acad Sci USA 1972;69:608–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein IJ, Hollerman CE, Smith EE. Protein-carbohydrate interaction. II. Inhibition studies on the interaction of Concanavalin A with polysaccharides. Biochemistry 1965;41:876–883. [DOI] [PubMed] [Google Scholar]
- 5.Rawn JD, Lienhard GE. The binding of boronic acids to chymotrypsin. Biochemistry 1974;13:3124–3130. [DOI] [PubMed] [Google Scholar]
- 6.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–685. [DOI] [PubMed] [Google Scholar]
- 7.Wei Z, Nishimura T, Yoshida S. Characterisation of glycans in lactoferrin isoform, lactoferrin-a. J Dairy Sci 2001;84:2584–2590. [DOI] [PubMed] [Google Scholar]
- 8.Sheares BT, Robbins PW. Glycosylation of ovalbumin in a heterologous cell: Analysis of oligosaccharide chains of the cloned glycoprotein in mouse L cells. Proc Natl Acad Sci USA 1986;83:1993–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harvez DJ, Wing DR, Kuester B, Wilson IBH. Composition of N-linked carbohydrates from ovalbumin and co-purified glycoproteins. J Am Soc Mass Spectrom 2000;11:564–571. [DOI] [PubMed] [Google Scholar]
- 10.Liu T, Li JD, Zeng R, Shao XX, Wang KY, Xia QC. Capillary electrophoresis-electrospray mass spectrometry for the characterization of high-mannose-type N-glycosylation and differential oxidation in glycoproteins by charge reversal and protease/glycosidase digestion. Anal Chem 2001;73:5875–5885. [DOI] [PubMed] [Google Scholar]
- 11.Fu D, van Halbeek H. N-glycosylation site mapping of human serotransferrin by serial lectin affinity chromatography, fast atom bombardment-mass spectrometry, and 1H nuclear magnetic resonance spectroscopy. Anal Biochem 1992;206:53–63. [DOI] [PubMed] [Google Scholar]
- 12.Satomi Y, Shimonishi Y, Hase T, Takao T. Site-specific carbohydrate profiling of human transferrin by nanoflow liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom 2004;18:2983–2988. [DOI] [PubMed] [Google Scholar]