Abstract
Surface labeling of Escherichia coli ribosomes with the use of the tritium bombardment technique has revealed a minor unidentified ribosome-bound protein (spot Y) that is hidden in the 70S ribosome and becomes highly labeled on dissociation of the 70S ribosome into subunits. In the present work, the N-terminal sequence of the protein Y was determined and its gene was identified as yfia, an ORF located upstream the phe operon of E. coli. This 12.7-kDa protein was isolated and characterized. An affinity of the purified protein Y for the 30S subunit, but not for the 50S ribosomal subunit, was shown. The protein proved to be exposed on the surface of the 30S subunit. The attachment of the 50S subunit resulted in hiding the protein Y, thus suggesting the protein location at the subunit interface in the 70S ribosome. The protein was shown to stabilize ribosomes against dissociation. The possible role of the protein Y as ribosome association factor in translation is discussed.
Keywords: ribosomal proteins, ribosome dissociation, ribosomal surface, tritium labeling
The hot tritium bombardment technique is based on replacement of hydrogen by tritium in covalent bonds of thin surface layer in macromolecules (1). The technique appears to be the most direct approach for studies of protein topography on surfaces of biological structures. Protein exposure on the surfaces of viruses (1–3), membranes (4), and ribosomes (5–9) has been determined by the use of this technique.
Studying proteins of the ribosome surface with this method has lead to the finding that unidentified minor component of the ribosome corresponding to spot Y on the ribosomal protein map becomes highly exposed on dissociation of the 70S ribosome into subunits (5, 7, 9). The reassociation of ribosomal subunits by increasing Mg2+ concentration resulted in reshielding of the protein Y, suggesting its location at the ribosomal subunit interface (9). In this study, the protein was identified and isolated. Its interface location was confirmed by experiments on hot tritium bombardment. The protein was shown to support the 70S ribosome in associated state at low concentration of Mg2+.
Materials and Methods
Materials.
Buffer reagents were obtained from Sigma, acrylamide and methylenebisacrilamide were from Fluka, urea was from Bio-Rad, DNase I was from Serva, Butyl-Toyopearl 650S from Toyo Soda (Tokyo), and DEAE-Sepharose Fast Flow from Pharmacia. Sucrose, acetone, hydrogen peroxide, and acetic acid were from ReaKhim (Moscow, Russia).
Preparation of 70S Ribosomes and Total Ribosomal Protein.
Ribosomes were prepared from Escherichia coli MRE-600 cells according to Staehelin et al. (10) with modifications described in ref. 9. Salt-washed ribosomes used in binding assay, as well as ribosomal subunits, were prepared as described by Gavrilova et al. (11) with substitution of pelleting by ultracentrifugation for precipitation with (NH4)2SO4 at the final stage of the procedure.
The standard procedure of acetic acid extraction and precipitation with acetone (12) was used to prepare the total ribosomal protein. Precipitation with 7.5 volumes of acetone (9) was applied to ensure the quantitative yield of each individual protein.
Two-Dimensional Polyacrylamide/Urea/SDS Gel Electrophoresis.
To exclude the loss of cysteine-containing proteins during electrophoretic separation, the total ribosomal protein was first modified with iodoacetamide. The protein precipitate (see above) was dissolved in 10 M urea containing 10 mM 2-mercaptoethanol and incubated for 1 hr at 37°C. Then Tris-HCl (pH20 9.0) and iodoacetamide were added to the final concentrations 100 mM and 50 mM, respectively. After a 3-min incubation 2-mercaptoethanol was added to the concentration 300 mM to quench unreacted iodoacetamide, and the sample was immediately subjected to fast gel-filtration on Sephadex G-25 spin column in 8 M urea containing 1% (vol/vol) 2-mercaptoethanol and 10 mM Bis-Tris-OAc (pH20 4.2). Basic fuchsin was added to the eluted material before electrophoretic separation.
The first-dimension electrophoresis was performed according to Madjar et al. (13) with modifications. Glass tubes of 140 mm length and 2 mm inside diameter were used. Before sample loading, a preelectrophoreses was run for 4 hr and 1.5 mA/gel with electrode buffer containing 10 mM methyl ester of l-cysteine in 40 mM Bis-Tris-OAc (pH20 5.5). The first-dimension gels were combined with the second-dimension gels without any dialysis for buffer equilibration and change.
The second-dimension electrophoresis was made according to ref. 14, with modifications. The separating gel (16.5% T) with linear gradient from 2 to 6% of the cross-linker contained 1 M urea. No urea was present in the stacking gel (4% T, 3% C). A spacer gel between stacking and separating gels was omitted. The cathode buffer contained 0.3% SDS and 0.08% mercaptoacetic acid. The second-dimension gels were fixed with 15% formaldehyde in 60% methanol and stained with Coomassie blue G-250 in 15% formaldehyde.
Extraction of Protein from the Y Spot.
Two-dimensional electrophoresis gels used as a source of protein Y for sequencing were fixed with 50% methanol in 10% acetic acid, and then stained with Coomassie blue G-250 solution in water. The Y spots were cut out from several gels and homogenized in extraction buffer (8% SDS/50 mM Tris-HCl, pH20 6.8/2% (vol/vol) 2-mercaptoethanol). After 30-min incubation at 37°C the homogenate was sonicated for 15 min and left overnight at room temperature. The gel suspension was then filtered and the protein Y was precipitated with 8 volumes of acetone in the presence of 200 μg of glycogen added as a carrier to the filtrate. The final precipitate contained about 1 μg of protein Y.
N-Terminal Sequence Determination.
Sequencing of the protein was performed in automatic gas phase sequencer (Applied Biosystems model 477A) equipped with HPLC system 120A from the same manufacturer. After extraction from gels, protein Y was first subjected to SDS/PAGE in a 16.5% T, 3% C gel (14), then transferred onto Immobilon-Psq transfer membrane according to Matsudaira (15) in the transfer buffer containing 50 mM Na3BO3 (pH20 8.0), 20% methanol, and 0.02% (vol/vol) 2-mercaptoethanol.
Isolation and Purification of Protein Y.
Frozen E. coli MRE-600 cell paste was thawed, suspended in equal volume of buffer A (20 mM MgCl2/40 mM Tris-HCl, pH20 7.6/200 mM NH4Cl/0.1 mM Na2EDTA/1 mM DTT) and disrupted in a French press. DNase was added to the homogenate up to 0.5 μg/ml, and the mixture was incubated at 4°C for 20 min. Debris was removed by centrifugation for 40 min at 23,000 × g. Ribosomes were pelleted from the cell extract for 1.5 hr at 184,000 × g. The pellet was resuspended in buffer A and pelleted again. The procedure was repeated one more time. Then ribosomes were resuspended in dissociation buffer (10 mM MgCl2/40 mM Tris-HCl, pH20 7.6/400 mM NaCl/0.1 mM Na2EDTA/1 mM DTT) and spun down by centrifugation for 2 hr at 265,000 × g. The supernatant was collected and dialyzed against buffer B (50 mM NaCl/40 mM Tris-HCl/pH15 7.3/15 mM 2-mercaptoethanol). The dialyzed sample was subjected to chromatography in DEAE-Sepharose Fast Flow column (5 × 10 cm) equilibrated with buffer B. Isocratic elution with the same buffer was carried out at the rate of about 2.8 ml/min. Fractions containing protein Y were combined and dialyzed against buffer C [1 M (NH4)2SO4/40 mM Tris-HCl, pH15 7.3/15 mM 2-mercaptoethanol]. The dialyzed sample was applied on Butyl-Toyopearl 650S column (1.5 × 5 cm) equilibrated with buffer C. Purified protein Y was eluted with buffer C at the rate of about 2 ml/min and concentrated in Centriprep-10 (Amicon) centrifugal concentrator. Concentrated preparation was dialyzed against storage buffer [1 mM MgCl2/20 mM Tris-HCl, pH15 7.3/100 mM NH4Cl/0.1 mM Na2EDTA/1 mM DTT/5% (wt/vol) glycerol], concentrated on Centriprep-10 once again, clarified by centrifugation for 30 min at 23,000 × g and stored frozen at −80°C.
Binding of Protein Y to Ribosomal Subunits.
In the binding assay the ribosomes were used after four washings with 1 M NH4Cl. Samples contained 0.8 μM ribosomes or ribosomal subunits in 300 μl of buffer D (20 mM Tris-HCl, pH20 7.6/100 mM NH4Cl/0.1 mM Na2EDTA/1 mM DTT) and 1 μM protein Y. Concentration of MgCl2 was either 10 or 1 mM. Samples were incubated for 15 min at 37°C, then centrifuged in a Centricon-100 (Amicon) centrifugal concentrator at 1000 × g for 1 hr. Filtrates were collected, precipitated with seven volumes of acetone in the presence of 150 μg of E. coli tRNA as a carrier, and analyzed by SDS/PAGE in a 10% T, 3% C polyacrylamide gel system (14).
Hot Tritium Bombardment of the 30S Subunit–Protein Y Complex.
Complex of the 30S subunit with protein Y was formed as in the binding assay. The samples contained 1.2 nmol of the 30S subunit and 1.2 nmol of protein Y in 1 ml of buffer D with 10 mM MgCl2. Some samples were supplemented with 1.5 nmol of the 50S subunit. Labeling procedure conditions were the same as in ref. 9. Incorporation of tritium into ribosomal proteins was analyzed by two-dimensional polyacrylamide/urea/SDS electrophoresis with subsequent Coomassie blue G-250 staining. Gel pieces containing individual proteins were solubilized with hydrogen peroxide (9), and the radioactivity was counted in a liquid scintillation spectrometer.
Determination of 70S Ribosomes Dissociation by Light Scattering.
The Mg2+ dependence of dissociation of ribosomes in the presence of protein Y was determined by light scattering at 400 nm in a Shimadzu spectrofluorophotometer (model RF-5301PC). Measurements were carried out in a microcell with a light pass of 10 mm, and the emission monochromator was set at a band pass of 1.5 nm.
Analytical Ultracentrifugation.
Sedimentation analysis was carried out in the AN-D rotor of a Beckman model E ultracentrifuge equipped with schlieren optics; analytical runs were conducted at 20°C and 40,000 rpm.
Results
Identification of Protein Y.
Determination of N-terminal amino acid sequence was performed with protein Y samples extracted from gels of two-dimensional electrophoresis of total ribosomal protein. Sixteen amino acid residues were determined as a result of 17 sequencing steps. As follows from searching in SwissProt database (http://expasy.hcuge.ch/sprot/sprot-top.html), the polypeptide sequence YFIA ECOLI (accession P11285) is identical with the determined part of protein Y sequence. The alignment of the determined N-terminal amino acid sequence of protein Y with the sequence deduced from yfia is presented below, as follows:
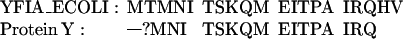 |
Thus, it can be concluded that the polypeptide Y is encoded by the ORF yfia that is referred to as “unidentified reading frame 1 (URF1)” (16) and located between 16S rRNA gene and the phe operon in the E. coli chromosome. The ORF should code for a protein of 113 amino acid residues with a molecular mass of 12,785 Da.
Isolation and Purification of Protein Y.
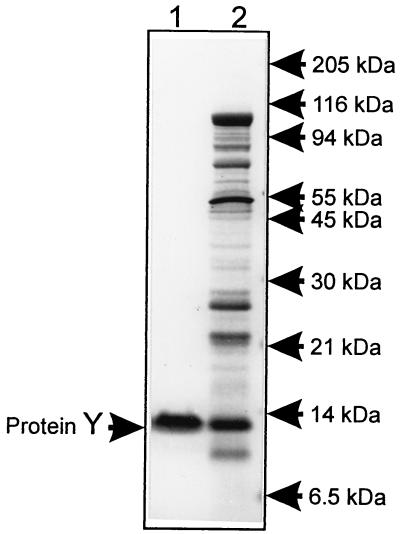
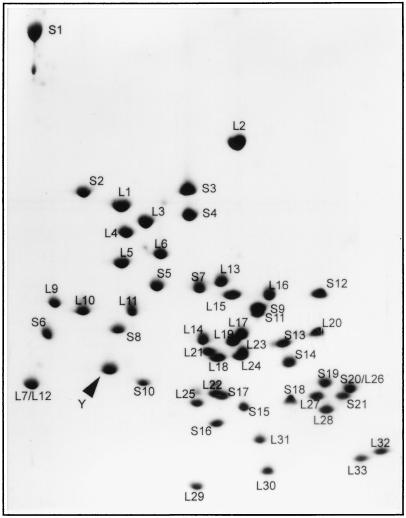
The purification procedure was based on the assumption that protein Y is located on the ribosome interface (9) and could be extracted from the ribosome by high salt treatment of dissociated ribosomes. To remove proteins loosely bound with ribosomes, crude ribosomes were washed twice with buffer containing 20 mM Mg2+ where ribosomes remained associated. The treatment of ribosomes with 400 mM NaCl resulted in dissociation of ribosomes and washing off of protein Y. Two steps of column chromatography allowed the purification of protein Y with >90% purity (Fig. 1). Two-dimensional coelectrophoresis of the purified product with ribosomal proteins has demonstrated its identity with the protein migrating as the Y spot (Fig. 2).
Figure 1.
SDS/PAGE analysis of purified protein Y. The gel (10% T, 3% C) was stained with Coomassie blue. Lanes: 1, protein Y purified by two-stage column chromatography of the ribosome wash; 2, proteins washed from ribosomes with the dissociation buffer containing 400 mM NaCl.
Figure 2.
Separation of individual ribosomal proteins by two-dimensional gel polyacrylamide/urea/SDS electrophoresis. Photograph of Coomassie-stained gel containing 110 μg of total ribosomal protein and ≈5 μg of isolated and purified protein Y (its position is pointed).
Mass Spectrometry of Protein Y.
Mass spectrum was recorded in a matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometer vision 2000 from Thermo BioAnalysis (Santa Fe, NM). A single component was detected with molecular mass of 12,640 Da (data not shown). Taking into account the absence of N-terminal methionine residue in the protein sequence (see the alignment above), the deduced molecular mass of the protein should be 12,654 Da, which is in a good agreement with the MS result.
Binding of Protein Y to Ribosomal Particles.
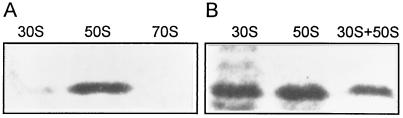
To determine which ribosomal subunit has an affinity to protein Y, an ultrafiltration binding test has been applied. Suspensions of ribosomes or isolated ribosomal subunits were filtered through the membrane of a Centricon-100 concentrator with 100-kDa cutoff. The membrane retains ribosomal subunits but allows protein Y to pass. Analysis of filtrates by SDS/PAGE clearly showed that protein Y was associated with the 30S subunit and the 70S ribosome but not with the 50S subunit (Fig. 3A). The test also showed that protein Y binding depends on Mg2+ concentration. When the binding reaction was carried out in the presence of 1 mM MgCl2, association of protein Y was detected neither with 30S subunits nor with 70S ribosomes (Fig. 3B).
Figure 3.
Binding of protein Y to ribosomal particles. SDS/PAGE of filtrates passed through the membrane with 100-kDa cutoff. The binding test was performed in buffer D containing 10 mM MgCl2 (A) and 1 mM MgCl2 (B). Type of the ribosomal particle used for binding is indicated above corresponding gel lane. Shown are photographs of Coomassie-stained gels.
Exposure of Protein Y on the Surface of the 30S Subunit and the 70S Ribosome.
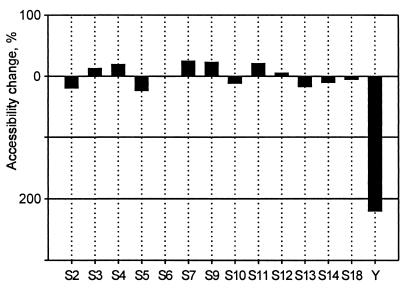
Complex of protein Y with the 30S subunit in 10 mM Mg2+ was subjected to the hot tritium bombardment without the 50S subunit and in the presence of the large subunit that associated with the small one with the formation of the 70S ribosome. Analysis of tritium incorporation into proteins showed a significant shielding effect of the 50S subunit on the exposure of protein Y on the 30S subunit surface. The association of the 30S subunit with the 50S subunit resulted in a more than 3-fold decrease of protein Y accessibility, whereas accessibility of other proteins of the small subunit did not change significantly in the same experiment (Fig. 4). This result is in accordance with the previously published data on the absence of the canonical ribosomal proteins on the surfaces of the intersubunit contact (5, 7, 9). At the same time, the protein Y is proven to be localized at the subunit interface.
Figure 4.
Change in protein accessibility as a result of the association of 30S and 50S subunits. The complexes of the 30S subunit with protein Y were labeled with the use of hot tritium bombardment technique before and after the association with the large subunit. Differences in accessibility of individual 30S proteins are shown as percentage of their accessibility in the associated ribosome state (70S), relative to the dissociated ribosome state (30S+50S). Bars plotted below the “zero” line show a decrease in accessibility of a corresponding protein on the 50S subunit attachment.
Effect of Protein Y on Ribosome Dissociation.
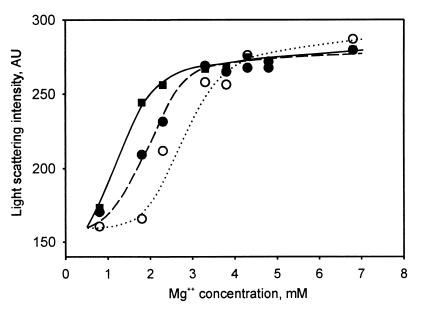
The location of protein Y at the 30S subunit surface contacting the 50S subunit suggested that the protein could affect association of ribosomal subunits. To check the suggestion, Mg2+ dependence of dissociation of ribosomes was recorded by light scattering at 400 nm. The result of the measurements is presented in Fig. 5. It is seen that in the range of Mg2+ concentrations from 6.8 to 4 mM the intensity of scattered light remained unchanged (and equal to that in 10 mM Mg2+, data not shown), thus indicating the associated (70S) state of the ribosome. At Mg2+ concentration below 1 mM, where only ribosomal subunits were present in the assay mixture, the scattered light intensity was at the lower level corresponding to the dissociated (50S+30S) state of the ribosome. As follows from the intensity value, about half of ribosomes were dissociated at 2.8 mM Mg2+. The addition of protein Y in equimolar ratio to ribosomes resulted in a significant increase of 70S ribosome fraction at this Mg2+ concentration. The effect was more pronounced at 3-fold excesses of the protein over ribosomes: about 50% of ribosomes remained associated even at 1.3 mM Mg2+.
Figure 5.
Mg2+ dependence of ribosome dissociation determined by light scattering at 400 nm. Each sample contained 0.8 μM ribosomes in 150 μl of buffer D. Concentration of MgCl2 in the samples ranged from 0.8 to 6.8 mM. Scattered light intensity is shown in arbitrary units (AU). Samples contained 0.8 μM protein Y (●, dashed line), 2.4 μM protein Y (■, solid line), or no protein Y (○, dotted line).
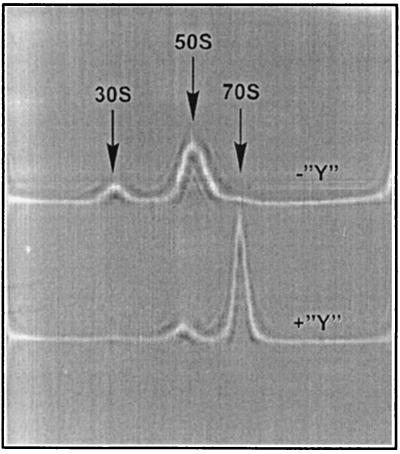
A similar result was obtained by sedimentation analysis of ribosomes in the presence of protein Y. Four-fold molar excess of protein Y over ribosomes resulted in virtually complete association of ribosomal subunits at 1.8 mM Mg2+ (Fig. 6).
Figure 6.
Sedimentation analysis of protein Y effect on dissociation of the 70S ribosome. Samples contained either 1.25 μM ribosomes without protein Y (the upper pattern, “−Y”) or 1.25 μM ribosomes with 5 μM protein Y (the lower pattern, “+Y”) in 400 μl of buffer D containing 1.8 mM MgCl2. Shown are schlieren optics recording; positions of ribosomal particles are indicated by arrows.
Protein Y Homologues.
The program psi-blast from the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov./cgi-bin/BLAST/nph-psi blast/) was used to find the homologous sequences. Among complete eubacterial genomes available by now in GenBank, the sequences having significant homology levels with yfia are found in the following organisms: Aquifex aeolicus (gene “aq1603”), Bacillus subtilis (gene “yvyD”), Borrelia burgdorferi (gene “BB0449”), Chlamidophyla pneumoniae (gene “yvyD”), Haemophilus influenzae (gene “hi0257”), Mycobacterium tuberculosis (“gene Rv3241c”), Rickettsia prowazekii (gene “yhbH”), Synechocystis sp. (gene “lrtA”), Thermotoga maritima (gene “TM1607”) and E. coli (genes “yfia” and “RP5M”).
Other homologues found among proteins from SwissProt database are RP5M AZOVI from Azotobacter vinelandii, RP5M KLEPN from Klebsiella pneumoniae, RP5M SALTY from Salmonella typhimurium, RP5M PSEPU from Pseudomonas putida, RP5M ALCEU from Alcaligenes eutrophus, RP5M RHIME from Rhizobium meliloti, RP5M BRAJA from Bradyrhizobium japonicum, RP5M ACICA from Acinetobacter calcoaceticus, RP5M THIFE from Thiobacillus ferrooxidans, YSEA STACA from Staphylococcus carnosus, and RR30 SPIOL from chloroplasts of Spinacia oleracea. Functions of most of them are unknown. The sequences called RP5M were supposed to be transcription modulator protein of σ54. The gene “lrtA” from Synechocystis sp. encodes a light-repressed transcript (17). The homologue of protein Y found in chloroplasts of Spinacia oleracea is a ribosomal protein of the 30S subunit, called PSrp-1 (18) or S30.
Discussion
The main result of this work is that a previously undiscovered protein has been found in the E. coli ribosomes. The protein is encoded by the ORF called yfia. Previously the protein encoded by yfia was identified on two-dimensional electrophoretic map of total E. coli protein (19), but its localization in the cell was not determined. A 30S subunit-associated protein having some homology with the E. coli protein Y was isolated earlier from total ribosomal protein of spinach chloroplasts (18); the authors, however, concluded that the protein is unique for chloroplasts.
The protein Y seems to belong to a family present in a number of bacterial species. In particular, the sequences homologous to yfia can be found in 10 of 15 complete genomes of eubacteria, which has by now been presented in GenBank. However, no homologues are seen in the genomes of Chlamydia trachomatis, Helicobacter pylori, Treponema pallidum, Mycoplasma pneumoniae, and Mycoplasma genitalium, as well as in archaebacteria. Proteins of this family seem to be much less conservative as compared with the canonical ribosomal proteins.
The protein Y can be detected only in the ribosomal fraction and not in the ribosome-free supernatant of the E. coli extract (our unpublished observations). This fact makes the protein related to ribosomal proteins. At the same time, our preliminary data indicate that the molar ratio of protein Y to ribosomes is approximately one-third. Hence, the protein is less abundant than ribosomes and thus should be considered rather as an auxiliary protein factor serving the ribosome only at some specific phases of its functional cycle.
The amount of protein Y recovered by gel electrophoresis was found to vary significantly in different preparations of ribosomes (5, 7). It is likely that the variations are caused by the relatively loose binding of the protein with the ribosome, especially at low Mg2+ concentrations. The Mg2+ dependence of protein Y binding to the ribosome explains also its absence in preparations of ribosomal subunits, because dissociation of ribosomes by lowering concentration of Mg2+ is a necessary step in all protocols of the subunit isolation.
The most interesting feature of the protein Y is its apparent intersubunit position in the ribosome. Although the protein binding site is on the surface of the isolated small (30S) subunit, the association of subunits results in hiding the protein from the ribosome surface. This situation is unique for exposed ribosomal proteins, none of which is found at the subunit interface and hidden by the association with a partner subunit (9).
The interface localization of the protein Y in the bacterial ribosome suggests its possible influence on the association of ribosomal subunits. Indeed, as shown in the experiments presented here, the protein stabilizes the ribosome against dissociation. The opposite effect is displayed by the initiation factor IF3 whose binding to the 30S subunit prevents its association with the 50S subunit (20). The functional significance of the association effect of the protein Y is not clear yet. One can speculate about possible role of the protein Y in the initiation phase of translation where the reassociation of ribosomal subunits follows the binding of mRNA and initiator F-Met-tRNA and completes the initiation.
Acknowledgments
We thank S. A. Spirin for the database computer analysis, A. V. Golubtsov and A. Kommer for helpful suggestions and comments, P. M. Kolossov for participation in some experiments, and A. N. Turkin for technical assistance. This work was supported by grants 99-04-48087 and 96-15-97999 from the Russian Foundation for Fundamental Research and by the Russian Academy of Sciences.
Abbreviations
- % T
concentration of acrylamide plus methylenebisacrilamide in gels
- % C
methylenebisacrilamide percentage of the total acrylamide present
References
- 1.Goldanskii V I, Kashirin I A, Shishkov A V, Baratova L A, Grebenshchikov N I. J Mol Biol. 1988;201:567–574. doi: 10.1016/0022-2836(88)90638-9. [DOI] [PubMed] [Google Scholar]
- 2.Baratova L A, Grebenshchikov N I, Dobrov E N, Gedrovich A V, Kashirin I A, Shishkov A V, Efimov A V, Jarvekulg L, Radavsky Y L, Saarma M. Virology. 1992;188:175–180. doi: 10.1016/0042-6822(92)90747-d. [DOI] [PubMed] [Google Scholar]
- 3.Baratova L A, Grebenshchikov N I, Shishkov A V, Kashirin I A, Radavsky J L, Jarvekulg L, Saarma M. J Gen Virol. 1992;73:229–235. doi: 10.1099/0022-1317-73-2-229. [DOI] [PubMed] [Google Scholar]
- 4.Tsetlin V I, Alyonycheva T N, Shemyakin V V, Neiman L A, Ivanov V T. Eur J Biochem. 1988;178:123–129. doi: 10.1111/j.1432-1033.1988.tb14437.x. [DOI] [PubMed] [Google Scholar]
- 5.Yusupov M M, Spirin A S. FEBS Lett. 1986;197:229–233. doi: 10.1016/0014-5793(86)80332-5. [DOI] [PubMed] [Google Scholar]
- 6.Kolb V A, Kommer A, Spirin A S. Dokl Akad Nauk SSSR. 1987;296:1497–1501. [PubMed] [Google Scholar]
- 7.Yusupov M M, Spirin A S. Methods Enzymol. 1988;164:426–439. doi: 10.1016/s0076-6879(88)64059-6. [DOI] [PubMed] [Google Scholar]
- 8.Spirin A S, Agafonov D E, Kolb V A, Kommer A. Biokhimiya (Biochem Moscow) 1996;61:1366–1368. [PubMed] [Google Scholar]
- 9.Agafonov D E, Kolb V A, Spirin A S. Proc Natl Acad Sci USA. 1997;94:12892–12897. doi: 10.1073/pnas.94.24.12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staehelin T, Maglott D, Monro R E. Cold Spring Harbor Symp Quant Biol. 1969;34:39–48. doi: 10.1101/sqb.1969.034.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Gavrilova L P, Kostiashkina O E, Koteliansky V E, Rutkevitch N M, Spirin A S. J Mol Biol. 1976;101:537–552. doi: 10.1016/0022-2836(76)90243-6. [DOI] [PubMed] [Google Scholar]
- 12.Hardy S J S, Kurland C G, Voynow P, Mora G. Biochemistry. 1969;8:2897–2905. doi: 10.1021/bi00835a031. [DOI] [PubMed] [Google Scholar]
- 13.Madjar J-J, Michel S, Cozzone A J, Reboud J-P. Anal Biochem. 1979;92:174–182. doi: 10.1016/0003-2697(79)90641-9. [DOI] [PubMed] [Google Scholar]
- 14.Schägger H, von Jagow G. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 15.Matsudaira P. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 16.Hudson G S, Davidson B E. J Mol Biol. 1984;180:1023–1051. doi: 10.1016/0022-2836(84)90269-9. [DOI] [PubMed] [Google Scholar]
- 17.Tan X, Varughese M, Widger W R. J Biol Chem. 1994;269:20905–20912. [PubMed] [Google Scholar]
- 18.Johnson C H, Kruft V, Subramanian A R. J Biol Chem. 1990;265:12790–12795. [PubMed] [Google Scholar]
- 19.Link A J, Robison K, Church G M. Electrophoresis. 1997;18:1259–1313. doi: 10.1002/elps.1150180807. [DOI] [PubMed] [Google Scholar]
- 20.Subramanian A R, Davis B D. Nat New Biol. 1971;228:1254–1268. [Google Scholar]