Abstract
The tissue microarray is a recently-implemented, high-throughput technology for the analysis of molecular markers in oncology. This research tool permits the rapid assessment of a biomarker in thousands of tumor samples, using commonly available laboratory assays such as immunohistochemistry and in-situ hybridization. Although introduced less than a decade ago, the TMA has proven to be invaluable in the study of tumor biology, the development of diagnostic tests, and the investigation of oncological biomarkers. This review describes the impact of TMA-based research in clinical oncology and its potential future applications. Technical aspects of TMA construction, and the advantages and disadvantages inherent to this technology are also discussed.
Introduction
The tissue microarray (TMA) was first described by Kononen in 1998 (1), and represents a high-throughput technology for the assessment of histology-based laboratory tests, including immunohistochemistry and fluorescent in-situ hybridization (FISH). Small cylindrical cores are extracted from standard formalin-fixed, paraffin-embedded tissue and arranged in a matrix configuration within a recipient paraffin block; thereby facilitating rapid analysis of hundreds of patient samples by a surgical pathologist. Since the introduction of tissue microarrays, this technology has been applied to the study of tumor biology, the assessment of novel molecular biomarkers and laboratory quality assurance. The TMA also serves as an excellent validation and translation platform for other types of high-throughput molecular research.
Tissue microarray construction and analysis
The initial identification and collection of tumor samples represents the greatest portion of the work associated with TMA construction. Samples need to be identified based on their availability in sufficient numbers to address the proposed scientific or clinical question. For example, prognostic studies will require a large number of cases with long-term outcome data to provide adequate statistical power. Similarly, a study investigating a novel diagnostic biomarker may require the identification of histologically-related entities to assess a biomarker’s specificity. After archival tissue blocks are retrieved, a hematoxylin-and-eosin-stained slide needs to be reviewed by a pathologist to determine the best area of each tissue block from which to extract a core (Figure 1A).
Figure 1.
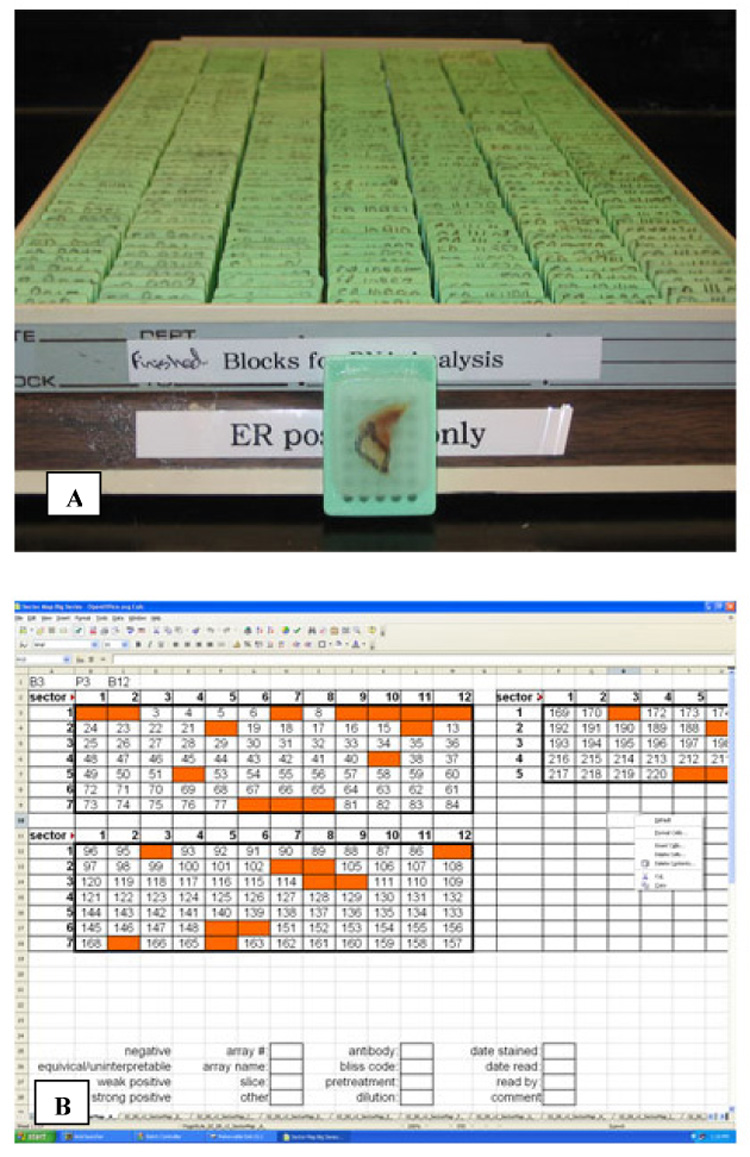
Tissue microarray planning. (A) Archival blocks are assembled and a surgical pathologist reviews the H&E slide for each case. The pathologist then circles the area of the block, localizing a representative tumor region from which a core will be extracted. (B) A sector map is designed; this is a grid that specifies a location within the TMA for each core sample. The sector map is then used to guide TMA construction and subsequent scoring, and it links biomarker scores to clinicopathological data on each case.
Prior to TMA construction a sector map is designed (Figure 1B). The sector map specifies a location within the TMA for each core sample, and it is used to guide both assembly and subsequent scoring. For the physical construction of the TMA, a tissue microarrayer is required and available commercially (e.g. Beecher Instruments, Sun Prairie, WI, USA; Figure 2A). Basic models include two hollow needles and a block holder that operates on a manual basis. Based on the planned sector map, a core is removed from a blank paraffin “recipient” block. The second needle is then used to remove a core of representative tissue from the donor block (Figure 2B). The tissue core is then inserted into the previously created hole in the recipient block (Figure 2C). This process relies on the operator to properly calibrate and maneuver the needles and blocks; more automated systems use a computer to guide the operator and trace the coordinates of the recipient block. These steps are repeated for each donor block that is to be incorporated into the TMA. The construction phase is relatively fast, and a typical project involving 200 cases may take two months for case identification and collection, but only two days for array building.
Figure 2.
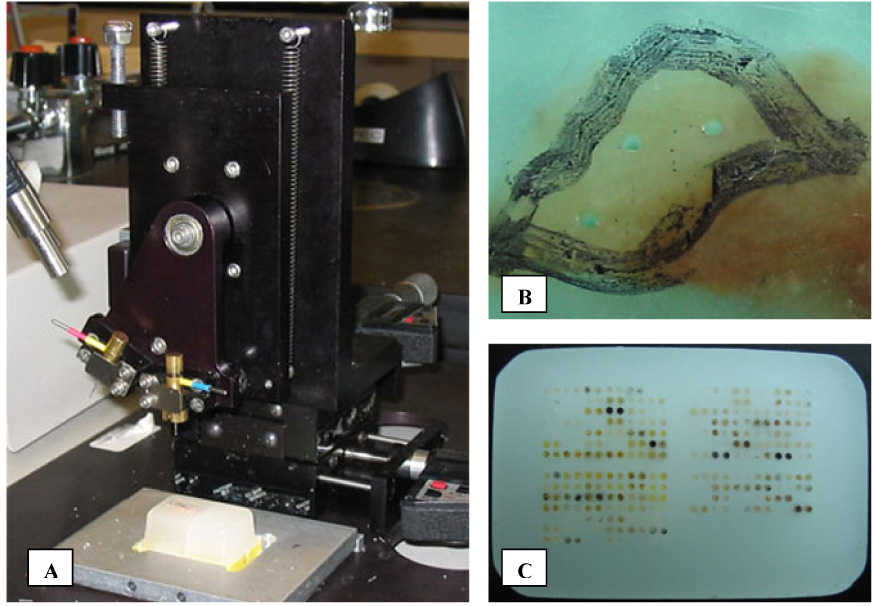
Tissue microarray construction and staining. (A) Beecher Instruments Microarrayer. The main components of the microarrayer are 2 hollow needles with stylets, a magnetic paraffin block holder, and positioning micrometers. (B) “Donor” block following extraction of triplicate 0.6 mm cores. The representative tumor region has been circled with a marker, and the cores were extracted from this area. (C) Completed TMA “recipient” block comprised of 300 cores.
Core sizes can range from 0.6 mm to 2.0 mm, with 0.6 mm most often used. The advantages of a smaller core include a lower incidence of lost cores during sectioning of the TMA, and a reduction in tissue material extracted from the donor block. With this size, at least 400 tissue cores can fit into a standard-sized recipient TMA block. For some heterogeneous lesions, such as Hodgkin lymphoma, a larger core may be preferable.
Sectioning of TMAs can be performed with a traditional microtome but this step requires an experienced histotechnologist. TMAs do not always section agreeably, particularly if multiple tissue types are included in the array. Each TMA section has significant value and commercial suppliers of TMAs charge well in excess of a hundred dollars per individual section (for arrays representing 50 – 100 cases, without clinical data). The number of sections that are available from one TMA block is dependent on the depth of the donor blocks, histotechnologist skill and the thickness of individual sections. The typical number of sections obtainable in practice ranges from 50 – 150. Immunohistochemistry or other applications can be performed on TMAs with minimal changes to standard protocols, although harsher antigen retrieval techniques or enzymatic digestions (e.g. deproteination for FISH) may cause some tissue to detach from the TMA slide and be lost to analysis.
Scoring of the TMA can be performed under light microscopy, or the TMA can be digitally scanned and displayed on a high resolution monitor (Figure 3A). During scoring, each core in the TMA is assessed by a surgical pathologist, and the result is recorded on the sector map Figure 3B and 3C). Scoring of the TMA is performed blinded to linked clinicopathological data, thereby reducing the potential for bias. As TMAs can include thousands of cases, scores from the sector map can then be merged with clinicopathological data with the aid of publicly available software, such as TMA-Deconvoluter(2).
Figure 3.
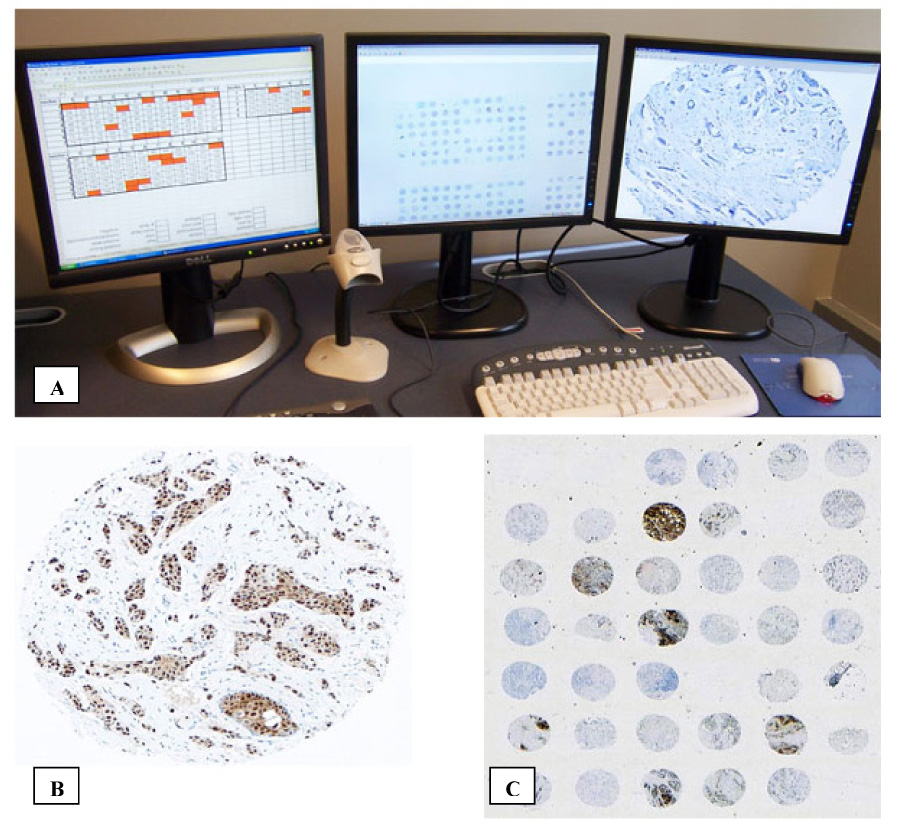
Scoring the TMA. (A) Example of a TMA scoring workstation with multiple displays. The TMA is best displayed concurrently at low magnification for orientation and unambiguous core assignment, and high magnification for scoring. (B) Sample of a TMA core under high magnification. This is a core from a breast adenocarcinoma that has been stained for estrogen receptors. (C) The TMA under low magnification.
Advantages of tissue microarrays
The staining of a few TMA sections in comparison to many more whole sections offers a clear benefit with respect to the use of laboratory reagents and technician time. As several research groups are now using TMAs representing in excess of a thousand tumors, this approach represents a major savings in scientific resources. In addition, there is also the benefit of decreased technical variability during the staining and interpretation process. The close proximity of cores also permits more rapid and consistent biomarker scoring by a surgical pathologist. In our experience, it requires approximately 15 working hours to score a single biomarker on a 4800 core tissue microarray series. Consequently, once construction of the TMA is completed, an entire suite of biomarker studies can be accomplished in a few weeks, as opposed to several months with whole sections.
In the field of molecular epidemiology, TMAs offer some distinct advantages over other high through-put molecular techniques, including DNA microarrays and proteomics. The source material for TMAs is formalin fixed, paraffin-embedded tissues, and this is the most common method of preserving surgical specimens. Many hospitals are required to retain archival tissue blocks for at least 20 years, and thus source material for TMAs is readily available and is often linked to long-term outcome data. In contrast, DNA microarray and proteomics methods generally require fresh-frozen tissue. As a result, studies using these techniques must draw on much more limited supplies of existing frozen samples, or be conducted prospectively with special tissue handling protocols.
TMAs are used for in-situ molecular techniques, the most common of which is immunohistochemistry. Most other techniques, including DNA microarray, Western Blot, sequencing and PCR require processing and homogenization of tissue. Consequently, experimental data is detached from its morphologic context; and any biomarker measurement will include contributions from stroma, vessels, inflammatory cells, and normal surrounding tissue in addition to tumor cells. For TMAs, the core is selected from a microscope-guided specific area of the source block, ensuring that representative areas of tumor are sampled and areas of normal tissue or necrosis are avoided. Furthermore, TMA interpretation is performed with morphologic correlation – that is, in visualizing a TMA core, the histology is intact and the surgical pathologist is able to ignore non-cancerous elements and assign a biomarker score based specifically on the tumor cells present.
Among translational research approaches, TMA technology raises few ethical concerns. The methodology does not require any modified or additional procedures to be performed on patients, core extraction can wait until after a final pathology diagnosis is rendered, and the source blocks are minimally altered and remain useable for whole section analysis in rare cases where there is a clinical need to go back to the blocks at a later date. Following TMA construction, patient identifiers can be reversibly (where clinical outcome updates are anticipated) or irreversibly stripped from the sector map database, to anonymize the series.
Tissue microarrays in the study of tumor biology
TMAs permit the rapid assessment of individual molecular markers on large patient cohorts. This approach complements molecular screening and discovery studies by confirming results on large numbers of primary tumor cases. TMAs have been so employed, for example, in the initial papers characterizing new breast cancer oncogenes such as EMSY (3) and alpha-basic crystallin(4), and the recently-identified prostate cancer fusion oncogene TMPRSS2:ERG(5).
TMAs are similarly useful in characterizing the immunohistochemical profile of cancer subtypes. For example, two studies have applied a large panel of antibodies to determine differences in the molecular profile of BRCA1/2 breast cancer as compared to sporadic breast cancers. They found that BRCA1 tumors were hormone receptor and HER2 negative, p53 positive, and expressed a specific set of cell cycle antigens(6, 7). There have also been studies that found new molecular markers in lower incidence cancers including nasopharyngeal cancer(8) and malignant melanoma(9). The discovery of new molecular markers provides some insight into the biochemical aberrations that lead to malignant progression, and in turn, this knowledge can contribute to the development of specific targeted therapies. In a prostate cancer study using TMAs, it was reported that there was co-expression of HIF, androgen receptor, and VEGF(10). Based on their results, the authors suggested that androgens may regulate VEGF levels (a key angiogenesis factor) through the activation of HIF, a transcription factor that regulates biological processes in response to hypoxia.
Progression TMAs are composed of cores demonstrating distinct stages of neoplasia (normal tissue, pre-invasive lesions, low and high grade invasive tumors, etc.) and they are also useful in the development of diagnostic assays. For some cancers, there is increasing evidence that screening can reduce cancer incidence and mortality (11, 12), suggesting that early detection and treatment can improve patient outcomes. Screening procedures, including mammography and endoscopy, will often diagnose pre-malignant conditions. Progression TMAs are used to determine the expression of a biomarker in different stages of neoplasia, and can be used to identify markers of malignant transformation. Recent studies have reported on the expression of PCNA to distinguish esophageal adenocarcinoma from Barrett’s esophagus(13), and have found that the loss of ANXII for prostatic epithelium is a marker for neoplasia(14).
Tissue microarrays for assessment of new diagnostic tools
In modern oncology, treatment decisions are critically dependent on accurate diagnostic pathology. Increasingly, molecular biomarkers are used in conjunction with conventional histology to improve diagnostic accuracy. Examples include the use of cytokeratin stain to localize micrometastases in sentinel lymph node biopsies for breast cancer, and the use of antibody panels to ascertain a tissue diagnosis for a metastasis of unknown primary origin. Primary unknown malignancies are particularly challenging as the biopsies obtained from metastatic sites are often poorly differentiated and difficult to diagnose based on histology alone, yet treatment options and prognosis could vary drastically depending on the exact diagnosis.
Large multi-tumor arrays can be used to estimate the diagnostic accuracy of a biomarker for a specific type of malignancy. One such study performed IHC analysis of FLI-1 on 4000+ tumors of various histologies. FLI-1 is a marker for Ewing family tumors but is also expressed in other small round cell tumors, and this study determined the sensitivity of FLI-1 to be 74% and the specificity 96%(15). Similarly, a large study of CD138 found that this glycoprotein, originally thought to be a specific marker of plasmacytic differentiation, was found to have widespread immunoreactivity in a 1700+ core multi-tumor array(16). Two recent TMA studies found p63, a marker of squamous differentiation, to be effective in distinguishing anal squamous cell carcinoma from low rectal adenocarcinoma(17), but in primary lung tumors it was found to stain both squamous cell and adenocarcinomas(18). Sarcomas and lymphomas are notoriously difficult to subtype by purely morphologic approaches, but new diagnostic biomarkers identified by gene expression profiling are being quickly tested, using TMA technology, against large numbers of histologically-similar neoplasms to determine their practical diagnostic value(19, 20).
Tissue microarrays for assessment of prognostic and predictive value
In the clinical practice of oncology, therapeutic decisions regarding adjuvant treatment are often based on a clinician’s estimate of recurrence risk, and the expected therapeutic gain from a specific treatment. More recently, clinicians have at their disposal prognostic models that are based on the retrospective analysis of large outcome databases. Examples include the Kattan nomograms for prostate cancer(21), and web-based prediction tool “Adjuvant! Online” for breast and colorectal carcinomas(22).
However, a significant drawback of these prognostic tools is that they rely upon the standard clinico-pathological features captured at the time of initial data collection. Thus, patient cohorts that have sufficiently mature outcome data for model-building often lack important biomarker information that would now be routinely collected. One example is the absence of HER2 status from the SEER data used in “Adjuvant! Online.” Of course, it is now known that HER2 status is an important prognostic and predictive biomarker in breast cancer. The ability to perform additional laboratory investigations on these large cohorts would be of great clinical value. Because TMA construction uses standard archival tissues, this technology is ideally suited for study or re-analysis of such valuable patient cohorts.
With DNA microarray technology, the expression of thousands of genes can be analyzed efficiently, and the pool of potentially useful prognostic biomarkers is rapidly increasing. However, high cost and limited tissue availability prevent the application of DNA microarray technologies to archival tissue libraries. The construction of a TMA requires only a small core from an archival block, and each core can then be used to assess multiple biomarkers – thus, this technology is appropriate for use on tissue banks with limited sample availability. The biomarker data acquired from TMA analysis can then be interpreted in the context of clinicopathological and outcome data for large patient cohorts. Furthermore, because TMA technology is based on widely available IHC and in situ hybridization techniques, results can be readily validated in other laboratories. However, one disadvantage of TMA studies is the need to assess each biomarker separately – thus, TMAs are not ideal for use as a discovery tool.
Many research groups are employing a two-step, discovery and validation approach to finding clinically useful biomarkers(23). The discovery phase involves the screening of a large number of molecular markers and finding those associated with clinically relevant endpoints – DNA microarrays are particularly useful for identifying potentially useful biomarkers. However, these studies are prone to false discovery as thousands of genes are assayed on relatively small patient cohorts (24). In the validation phase, where appropriate antibodies are available, the protein products of top candidate genes can be assayed in large, independent patient cohorts using TMAs. A study demonstrating the prognostic value of FoxP1 in diffuse large B-cell lymphoma exemplifies this approach(25).
DNA microarrays can also identify tumor subgroups based on gene expression; one example is the discovery of an intrinsic classification of breast cancer(26, 27). Gene expression profiling of a limited number of breast cancer tumors revealed 5 subtypes with prognostic relevance; these results have been reproduced in subsequent studies on independent patient cohorts. A panel of four immunostains derived from this profile was validated on TMAs, and was found to identify IHC-defined subgroups that were similar to the intrinsic classification, and had prognostic relevance(28).
In recent years, there have been a large number of studies that report on the prognostic value of individual or combinations of biomarkers, for particular cancer types. Studies include tumor sites for which there already exist strong prognostic models, such as breast and colorectal cancer, but also for malignancies in which prognosis is still based on TNM staging, including carcinoma of the head and neck(29, 30) and lung cancer (31, 32). For less common malignancies and for those where we lack good prognostic models, TMA technology has great potential for benefit. Examples that have particular relevance to the radiation oncologist are studies demonstrating that VEGF and Cox-2 are prognostic biomarkers in early stage laryngeal cancer treated with primary radiotherapy(33, 34).
In addition to the development of prognostic models, biomarker research is also being used to develop predictive tests. While there are tests for specific targeted therapies such as trastuzumab (for HER2 positive breast cancer) and imatinib (for c-kit positive gastrointestinal stromal tumors), there is also some evidence that molecular markers can predict for response to conventional adjuvant treatments(35, 36). Recently, a research group from the Royal Marsden assembled a large TMA comprised of patients enrolled in the ATAC study (Arimidex, Tamoxifen Alone, or in Combination), a randomized clinical trial that compared adjuvant tamoxifen to anastrozole for ER positive breast cancer. In the most recent published report of this study, anastrozole provided a statistically significant improvement in disease free survival (37). The purpose of the correlative TMA study is to discover molecular markers that predict for tamoxifen resistance or response to anastrozole(38); initial results suggest that the benefit of anastrozole over tamoxifen is independent of quantitative ER and PR expression, and Her2 status (39).
Tissue microarrays in quality control and clinical practice
While TMA studies are an important tool for the validation of novel biomarkers, most molecular epidemiology studies are derived from a single institution; and even if the tumor samples are collected from multiple sites, sample processing and data collection and analysis are performed centrally. There are multiple reports in the medical literature of conflicting results on the prognostic or predictive value of a novel biomarker. One example is Cyclin E in breast cancer, for which one research group reported strong prognostic value on multivariate analysis(40). Some studies confirmed these findings, but others failed to reproduce these results (41, 42). Inter-institution variations in laboratory technique, specimen handling, patient populations, and criteria used for immunostaining interpretation will certainly contribute to differences in results(43). While this presents some difficulties in biomarker research, inter-laboratory variations in a clinical test can profoundly affect patient care.
In order to effectively use a biomarker assay in the clinical setting, the test must be shown to be consistent and reproducible. TMAs can be applied in quality assurance studies to assess inter-laboratory variations. Unstained sections from a TMA can be sent to multiple laboratories; ideally, this TMA would consist of samples collected from a number of different institutions. Each laboratory then processes and scores the TMA section independently. The results from each laboratory can be assessed for concordance, and compared to whole section results. This process was recently performed for two important predictive biomarkers, HER2 in breast cancer (44) and CD117 (c-kit) in gastrointestinal stromal tumors(45) (Figure 4), and for a number of diagnostic biomarkers in diffuse large B-cell lymphoma(46).
Figure 4.
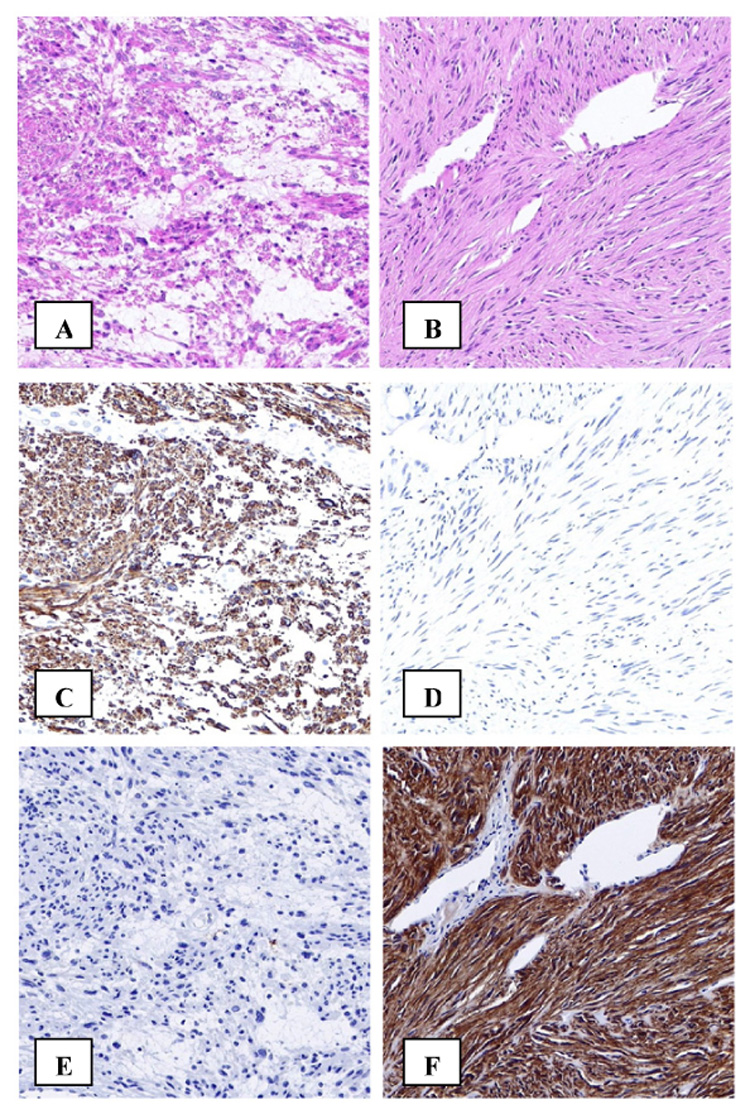
Immunohistochemistry can improve pathological diagnosis. Immunohistochemistry plays an important role in differentiating between gastrointestinal leiomyosarcoma (A) and gastrointestinal stromal tumor (GIST) (B). Desmin immunostaining is positive in leiomyosarcoma (C) but negative in GIST (D). Conversely C-Kit immunostaining is negative in leiomyosarcoma (E) and positive in GIST (F).
TMAs can also be used to improve existing biomarker assays. For example, a recent study compared the diagnostic effectiveness of two antibodies in determining estrogen receptor status in breast cancer (47). Both antibodies were applied to a large 4000+ case TMA, and the results of staining were compared to the original biochemical test for ER that was performed at the time of diagnosis. While maintaining specificity and equal positive predictive value, the more recently developed rabbit monoclonal antibody (SP1) was determined to be 8% more sensitive than the common clinically-used mouse monoclonal antibody (1D5) (Figure 5).
Figure 5.
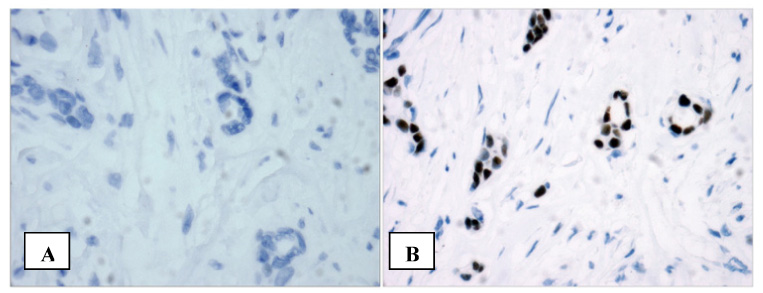
TMAs in laboratory medicine. Using a large tissue microarray, 2 estrogen receptor (ER) antibodies were assessed on more than 4000 invasive breast cancer tumors. It was found that the novel SP1 antibody was 8% more sensitive and equally specific as compared to the more commonly used 1D5 antibody. This breast cancer tumor would be classified ER negative with 1D5 (A) but is strongly positive with SP1 (B).
While TMAs have an important role in research and quality assurance, they are not generally used in clinical laboratory testing. However, for certain common biomarker assays, such as ER and HER2 for breast cancer, TMAs present a more cost-efficient approach to routine testing. Two studies have reported high concordance between TMA scores and whole section results for HER2 testing (48, 49). Furthermore, a centre in Italy reported on a study using TMAs in ER, PR and HER2 testing on breast cancer, they found that TMA results were concordant with whole section results, and were effective in reducing reagent use, and technician and pathologists’ time(50).
Validation of tissue microarray analysis
The most frequent criticism of TMA technology relates to the small size of each tissue core – there is concern that due to tumor heterogeneity, biomarker scores obtained from small TMA cores will not accurately reflect scores obtained from whole sections(43, 51, 52). It should be noted, however, that although whole section analysis is a “gold standard” for in-situ tests, whole sections themselves represent just a small portion of a tumor; and indeed core needle, punch and bite biopsies used for primary diagnosis often do not contain much more diagnostic tissue than a TMA core. The concern of tumor inhomogeneity is partially addressed by visualizing the tumor region from which a TMA core is extracted; in addition, numerous studies have now been published validating the use of TMAs in various tumor sites, including breast(53), lung(54), and colorectal carcinomas(55). Using one to four cores per source block most TMA validation studies demonstrate good concordance between biomarker scores obtained from TMA and those obtained from whole section. Indeed, whole sections can be overinterpreted, especially for biomarkers with focal staining (53). In general, the biomarkers of true clinical value will display relatively uniform staining in tumors, and TMAs are certainly adequate in such cases.
A significant technical problem associated with TMA studies is tissue loss during sectioning, transfer, and staining (56). Some reports estimate that the rate of core loss is 10–30% due to technical causes alone. Furthermore, due to variations in preparation and storage of source blocks, tissue quality can also affect biomarker results. One study demonstrated loss in antigen immunoreactivity with increasing time between sample preparation and staining (56). It is reasonable to assume that all TMAs will contain a fraction of non-reactive cores that will be uninterpretable or false negatives during analysis.
The combination of sampling error during core extraction, core loss during slide preparation, and non-reactive cores leads to missing biomarker data, and under-estimation of the true incidence of a molecular marker. Missing biomarker data may necessitate the exclusion of cases and a consequent loss of statistical power during analysis. This is especially a problem for studies involving multiple biomarkers, where most statistical analyses require complete biomarker data for each case. Both the issues of missing data and under-estimation of biomarker incidence can be partly addressed by extracting multiple cores from each source block.
During TMA construction, the number of cores extracted per source block must be decided. One study addressed this issue by constructing four replicate tissue microarrays from 553 breast cancer specimens(53). For ER, the estimated incidence of positive cases from using a single core was almost identical to the incidence obtained from whole section analysis. In contrast, for PR, analysis of any one of the replicate arrays underestimated the incidence. However, if data from additional replicate arrays were combined, the TMA incidence for PR approached that obtained from whole section staining. With respect to missing data points, analysis of a single TMA was associated with 25–30% uninterpretable cores, combined analysis of three TMAs reduced data loss to 5–7%, and there was a marginal additional benefit from using all four replicate TMAs.
Of course, the use of multiple cores can rapidly increase the workload associated with TMA preparation and biomarker scoring, especially in large series. Despite improved data retention with the use of multiple cores per case, the authors of the previous study found that analysis of a single TMA (one core per case) produced the same prognostic effect for ER and PR as analysis of whole section staining. They conclude that in sufficiently large patient cohorts, use of single core TMAs can reproduce important clinical associations. This is a particularly important conclusion as it underscores the intent of most TMA studies: to discover or confirm important clinicopathological associations. As these studies are surveying entire patient cohorts, loss of some individual data points is acceptable. In most cases, false negatives and lost cores will occur randomly, and statistical analyses can easily be performed on cases with missing biomarker data to exclude systematic bias.
Conclusions and future directions
The most important step in initiating a TMA study is the identification of appropriate patient cohorts with accessible archival tissue materials. Prognostic studies require patient data that is thorough, complete, and includes long term follow-up. Diagnostic studies may require locating source blocks from multiple tumor types and tissues in various stages of malignant progression.
In laboratory medicine, TMAs already play an important role in quality assurance and the characterization of new antibodies for IHC. There have now been numerous large molecular epidemiology studies using TMAs, but to this point, the impact to clinical oncology has been relatively limited. Prior to clinical application, biomarkers still need to be validated in multiple studies by independent groups. Although this process could be lengthy, it has potential to significantly affect prognostic estimates and clinical practice.
The next, critical development in cancer therapeutics will be predictive assays and targeted therapies. The potential of TMA technology is now more widely recognized and source blocks are being prospectively collected during clinical studies, eliminating the need to conduct tedious searches for archival tissue blocks. Indeed, planned construction of TMAs from surgical materials involved in cancer trials can greatly facilitate subsequent correlative science projects. The tissue microarray is a powerful tool in translational research, and its potential extends beyond oncology – it is an accessible, cost-efficient, and reliable means for the assessment of molecular markers in a clinically practical format.
Acknowledgements
David Voduc and Torsten O. Nielsen are supported by the National Institutes of Health Strategic Partnering to Evaluate Cancer Signatures program. Torsten O. Nielsen is a scholar of the Michael Smith Foundation for Health Research. The Genetic Pathology Evaluation Centre is supported by an unrestricted educational grant from sanofi-aventis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
David Voduc, Dept. of Radiation Oncology, British Columbia Cancer Agency, Vancouver, British Columbia, Canada.
Challayne Kenney, Genetic Pathology Evaluation Centre, Vancouver, British Columbia, Canada.
Torsten O. Nielsen, Pathology, University of British Columbia, Vancouver, British Columbia, Canada.
References
- 1.Kononen J, Bubendorf L, Kallioniemi A. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 2.Liu CL, Montgomery KD, Natkunam Y. TMA-Combiner, a simple software tool to permit analysis of replicate cores on tissue microarrays. Mod Pathol. 2005;18:1641–1648. doi: 10.1038/modpathol.3800491. [DOI] [PubMed] [Google Scholar]
- 3.Hughes-Davies L, Huntsman D, Ruas M. EMSY links the BRCA2 pathway to sporadic breast and ovarian cancer. Cell. 2003;115:523–535. doi: 10.1016/s0092-8674(03)00930-9. [DOI] [PubMed] [Google Scholar]
- 4.Moyano JV, Evans JR, Chen F. AlphaB-crystallin is a novel oncoprotein that predicts poor clinical outcome in breast cancer. J Clin Invest. 2006;116:261–270. doi: 10.1172/JCI25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomlins SA, Rhodes DR, Perner S. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 6.Eerola H, Heikkila P, Tamminen A. Histopathological features of breast tumours in BRCA1, BRCA2 and mutation-negative breast cancer families. Breast Cancer Res. 2005;7:R93–R100. doi: 10.1186/bcr953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palacios J, Honrado E, Osorio A. Phenotypic characterization of BRCA1 and BRCA2 tumors based in a tissue microarray study with 37 immunohistochemical markers. Breast Cancer Res Treat. 2005;90:5–14. doi: 10.1007/s10549-004-1536-0. [DOI] [PubMed] [Google Scholar]
- 8.Fan SQ, Ma J, Zhou J. Differential expression of Epstein-Barr virus-encoded RNA and several tumor-related genes in various types of nasopharyngeal epithelial lesions and nasopharyngeal carcinoma using tissue microarray analysis. Hum Pathol. 2006;37:593–605. doi: 10.1016/j.humpath.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Alonso SR, Ortiz P, Pollan M. Progression in cutaneous malignant melanoma is associated with distinct expression profiles: a tissue microarray-based study. Am J Pathol. 2004;164:193–203. doi: 10.1016/s0002-9440(10)63110-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boddy JL, Fox SB, Han C. The androgen receptor is significantly associated with vascular endothelial growth factor and hypoxia sensing via hypoxia-inducible factors HIF-1a, HIF-2a, and the prolyl hydroxylases in human prostate cancer. Clin Cancer Res. 2005;11:7658–7663. doi: 10.1158/1078-0432.CCR-05-0460. [DOI] [PubMed] [Google Scholar]
- 11.Hawk ET, Levin B. Colorectal cancer prevention. J Clin Oncol. 2005;23:378–391. doi: 10.1200/JCO.2005.08.097. [DOI] [PubMed] [Google Scholar]
- 12.Kerlikowske K, Grady D, Rubin SM. Efficacy of screening mammography. A meta-analysis. JAMA. 1995;273:149–154. [PubMed] [Google Scholar]
- 13.Hammoud ZT, Badve S, Saxena R. A novel biomarker for the detection of esophageal adenocarcinoma. J Thorac Cardiovasc Surg. 2007;133:82–87. doi: 10.1016/j.jtcvs.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Stewart JM, Fleshner N, Cole H. Comparison of Annexin II, p63 and AMACR immunoreactivity in prostatic tissue: A tissue microarray study. J Clin Pathol. 2006 doi: 10.1136/jcp.2006.040808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mhawech-Fauceglia P, Herrmann F, Bshara W. FLI-1 Expression in 4323 malignant and benign tumours: a multiple tumour tissue microarray analysis using polyclonal antibody. J Clin Pathol. 2006 doi: 10.1136/jcp.2006.039230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kambham N, Kong C, Longacre TA. Utility of syndecan-1 (CD138) expression in the diagnosis of undifferentiated malignant neoplasms: a tissue microarray study of 1,754 cases. Appl Immunohistochem Mol Morphol. 2005;13:304–310. doi: 10.1097/01.pai.0000159773.50905.7b. [DOI] [PubMed] [Google Scholar]
- 17.Owens SR, Greenson JK. Immunohistochemical staining for p63 is useful in the diagnosis of anal squamous cell carcinomas. Am J Surg Pathol. 2007;31:285–290. doi: 10.1097/01.pas.0000213362.10756.d3. [DOI] [PubMed] [Google Scholar]
- 18.Au NH, Gown AM, Cheang M. P63 expression in lung carcinoma: a tissue microarray study of 408 cases. Appl Immunohistochem Mol Morphol. 2004;12:240–247. doi: 10.1097/00129039-200409000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Hans CP, Weisenburger DD, Greiner TC. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 20.Terry J, Saito T, Subramanian S. TLE1 as a diagnostic immunohistochemical marker for synovial sarcoma emerging from gene expression profiling studies. Am J Surg Pathol. 2007;31:240–246. doi: 10.1097/01.pas.0000213330.71745.39. [DOI] [PubMed] [Google Scholar]
- 21.Stephenson AJ, Kattan MW. Nomograms for prostate cancer. BJU Int. 2006;98:39–46. doi: 10.1111/j.1464-410X.2006.06173.x. [DOI] [PubMed] [Google Scholar]
- 22.Ravdin PM, Siminoff LA, Davis GJ. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001;19:980–991. doi: 10.1200/JCO.2001.19.4.980. [DOI] [PubMed] [Google Scholar]
- 23.van de Rijn M, Gilks CB. Applications of microarrays to histopathology. Histopathology. 2004;44:97–108. doi: 10.1111/j.1365-2559.2004.01766.x. [DOI] [PubMed] [Google Scholar]
- 24.Ransohoff DF. Rules of evidence for cancer molecular-marker discovery and validation. Nat Rev Cancer. 2004;4:309–314. doi: 10.1038/nrc1322. [DOI] [PubMed] [Google Scholar]
- 25.Banham AH, Connors JM, Brown PJ. Expression of the FOXP1 transcription factor is strongly associated with inferior survival in patients with diffuse large B-cell lymphoma. Clin Cancer Res. 2005;11:1065–1072. [PubMed] [Google Scholar]
- 26.Sorlie T, Tibshirani R, Parker J. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perou CM, Sorlie T, Eisen MB. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen TO, Hsu FD, Jensen K. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 29.Psyrri A, Yu Z, Weinberger PM. Quantitative determination of nuclear and cytoplasmic epidermal growth factor receptor expression in oropharyngeal squamous cell cancer by using automated quantitative analysis. Clin Cancer Res. 2005;11:5856–5862. doi: 10.1158/1078-0432.CCR-05-0420. [DOI] [PubMed] [Google Scholar]
- 30.Yu Z, Weinberger PM, Haffty BG. Cyclin d1 is a valuable prognostic marker in oropharyngeal squamous cell carcinoma. Clin Cancer Res. 2005;11:1160–1166. [PubMed] [Google Scholar]
- 31.Deeb G, Wang J, Ramnath N. Altered E-cadherin and epidermal growth factor receptor expressions are associated with patient survival in lung cancer: a study utilizing high-density tissue microarray and immunohistochemistry. Mod Pathol. 2004;17:430–439. doi: 10.1038/modpathol.3800041. [DOI] [PubMed] [Google Scholar]
- 32.Tan D, Li Q, Deeb G. Thyroid transcription factor-1 expression prevalence and its clinical implications in non-small cell lung cancer: a high-throughput tissue microarray and immunohistochemistry study. Hum Pathol. 2003;34:597–604. doi: 10.1016/s0046-8177(03)00180-1. [DOI] [PubMed] [Google Scholar]
- 33.Parikh RR, Yang Q, Haffty BG. Prognostic significance of vascular endothelial growth factor protein levels in T1-2 N0 laryngeal cancer treated with primary radiation therapy. Cancer. 2007;109:566–573. doi: 10.1002/cncr.22432. [DOI] [PubMed] [Google Scholar]
- 34.Cho EI, Kowalski DP, Sasaki CT. Tissue microarray analysis reveals prognostic significance of COX-2 expression for local relapse in T1-2N0 larynx cancer treated with primary radiation therapy. Laryngoscope. 2004;114:2001–2008. doi: 10.1097/01.mlg.0000147936.67379.e7. [DOI] [PubMed] [Google Scholar]
- 35.Paik S, Tang G, Shak S. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 36.Paik S, Shak S, Tang G. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 37.Howell A, Cuzick J, Baum M. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 38.Dowsett M. Biomarker investigations from the ATAC trial: the role of TA01. Breast Cancer Res Treat. 2004;87 Suppl 1:S11–S18. doi: 10.1007/s10549-004-1578-3. [DOI] [PubMed] [Google Scholar]
- 39.Dowsett M, Allred DC. Relationship between quantitative ER and PgR and Her2 status with recurrence in the ATAC trial. Presented at the 29th Annual San Antonio Breast Cancer Symposium; December 14–17; San Antonio, Texas. 2006. [Google Scholar]
- 40.Keyomarsi K, Tucker SL, Buchholz TA. Cyclin E and survival in patients with breast cancer. N Engl J Med. 2002;347:1566–1575. doi: 10.1056/NEJMoa021153. [DOI] [PubMed] [Google Scholar]
- 41.Porter PL, Barlow WE, Yeh IT. p27(Kip1) and cyclin E expression and breast cancer survival after treatment with adjuvant chemotherapy. J Natl Cancer Inst. 2006;98:1723–1731. doi: 10.1093/jnci/djj467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L, Shao ZM. Cyclin e expression and prognosis in breast cancer patients: a meta-analysis of published studies. Cancer Invest. 2006;24:581–587. doi: 10.1080/07357900600894799. [DOI] [PubMed] [Google Scholar]
- 43.Bubendorf L, Nocito A, Moch H. Tissue microarray (TMA) technology: miniaturized pathology archives for high-throughput in situ studies. J Pathol. 2001;195:72–79. doi: 10.1002/path.893. [DOI] [PubMed] [Google Scholar]
- 44.Fitzgibbons PL, Murphy DA, Dorfman DM. Interlaboratory comparison of immunohistochemical testing for HER2: results of the 2004 and 2005 College of American Pathologists HER2 Immunohistochemistry Tissue Microarray Survey. Arch Pathol Lab Med. 2006;130:1440–1445. doi: 10.5858/2006-130-1440-ICOITF. [DOI] [PubMed] [Google Scholar]
- 45.Dorfman DM, Bui MM, Tubbs RR. The CD117 immunohistochemistry tissue microarray survey for quality assurance and interlaboratory comparison: a College of American Pathologists Cell Markers Committee Study. Arch Pathol Lab Med. 2006;130:779–782. doi: 10.5858/2006-130-779-TCITMS. [DOI] [PubMed] [Google Scholar]
- 46.de Jong D, Rosenwald A, Chhanabhai M. Immunohistochemical prognostic markers in diffuse large B-cell lymphoma: validation of tissue microarray as a prerequisite for broad clinical applications--a study from the Lunenburg Lymphoma Biomarker Consortium. J Clin Oncol. 2007;25:805–812. doi: 10.1200/JCO.2006.09.4490. [DOI] [PubMed] [Google Scholar]
- 47.Cheang MC, Treaba DO, Speers CH. Immunohistochemical detection using the new rabbit monoclonal antibody SP1 of estrogen receptor in breast cancer is superior to mouse monoclonal antibody 1D5 in predicting survival. J Clin Oncol. 2006;24:5637–5644. doi: 10.1200/JCO.2005.05.4155. [DOI] [PubMed] [Google Scholar]
- 48.Selvarajan S, Tan SY, Sii LH. c-erbB-2 (HER-2/neu) immunohistochemistry in invasive breast cancer: is there concordance between standard sections and tissue microarrays? Pathology. 2006;38:316–320. doi: 10.1080/00313020600820872. [DOI] [PubMed] [Google Scholar]
- 49.O'Grady A, Flahavan CM, Kay EW. HER-2 analysis in tissue microarrays of archival human breast cancer: comparison of immunohistochemistry and fluorescence in situ hybridization. Appl Immunohistochem Mol Morphol. 2001;11:177–182. doi: 10.1097/00129039-200306000-00016. [DOI] [PubMed] [Google Scholar]
- 50.Sapino A, Marchio C, Senetta R. Routine assessment of prognostic factors in breast cancer using a multicore tissue microarray procedure. Virchows Arch. 2006;449:288–296. doi: 10.1007/s00428-006-0233-2. [DOI] [PubMed] [Google Scholar]
- 51.Horvath L, Henshall S. The application of tissue microarrays to cancer research. Pathology. 2001;33:125–129. doi: 10.1080/003130201200338791. [DOI] [PubMed] [Google Scholar]
- 52.Simon R, Sauter G. Tissue microarrays for miniaturized high-throughput molecular profiling of tumors. Exp Hematol. 2002;30:1365–1372. doi: 10.1016/s0301-472x(02)00965-7. [DOI] [PubMed] [Google Scholar]
- 53.Torhorst J, Bucher C, Kononen J. Tissue microarrays for rapid linking of molecular changes to clinical endpoints. Am J Pathol. 2001;159:2249–2256. doi: 10.1016/S0002-9440(10)63075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leversha MA, Fielding P, Watson S. Expression of p53, pRB, and p16 in lung tumours: a validation study on tissue microarrays. J Pathol. 2003;200:610–619. doi: 10.1002/path.1374. [DOI] [PubMed] [Google Scholar]
- 55.Jourdan F, Sebbagh N, Comperat E. Tissue microarray technology: validation in colorectal carcinoma and analysis of p53, hMLH1, and hMSH2 immunohistochemical expression. Virchows Arch. 2003;443:115–121. doi: 10.1007/s00428-003-0833-z. [DOI] [PubMed] [Google Scholar]
- 56.Hoos A, Cordon-Cardo C. Tissue microarray profiling of cancer specimens and cell lines: opportunities and limitations. Lab Invest. 2001;81:1331–1338. doi: 10.1038/labinvest.3780347. [DOI] [PubMed] [Google Scholar]
- 57.Mirlacher M, Kasper M, Storz M. Influence of slide aging on results of translational research studies using immunohistochemistry. Mod Pathol. 2004;17:1414–1420. doi: 10.1038/modpathol.3800208. [DOI] [PubMed] [Google Scholar]


