Abstract
Dorsal-ventral patterning in vertebrate and Drosophila embryos requires a conserved system of extracellular proteins to generate a positional information gradient. The components involved include bone morphogenetic proteins (BMP/Dpp), a BMP antagonist (Chordin/Short gastrulation; Chd/Sog) and a secreted metalloproteinase (Xolloid/Tolloid) that cleaves Chd/Sog. Here we describe Xenopus Twisted gastrulation (xTsg), another member of this signalling pathway. xTsg is expressed ventrally as part of the BMP-4 synexpression group and encodes a secreted BMP-binding protein that is a BMP signalling agonist. The data suggest a molecular mechanism by which xTsg dislodges latent BMPs bound to Chordin BMP-binding fragments generated by Xolloid cleavage, providing a permissive signal that allows high BMP signalling in the embryo. Drosophila Tsg also binds BMPs and is expressed dorsally, supporting the proposal that the dorsal-ventral axis was inverted in the course of animal evolution.
Dorsal-ventral patterning in vertebrates is regulated by a gradient of BMP activity. BMPs are expressed relatively uniformly in a wide area of the gastrulating Xenopus embryo and the gradient is thought to be generated by the localized secretion of BMP antagonists, such as Chordin and Noggin, by the dorsal lip or Spemann’s organizer1,2. A further level of regulation is introduced by a secreted zinc metalloproteinase, Xolloid, which cleaves inactive Chordin-BMP complexes, resulting in the reactivation of BMP signalling in the embryo3,4. This model of dorsal-ventral patterning has been validated by genetic studies in zebrafish5-10. Chordin contains four cysteine-rich (CR) domains of about 70 amino acids each, and the Xolloid cleavage sites are located at conserved aspartic acid residues just downstream of CR1 and CR3 (refs 3, 11). Individual cysteine-rich domains, in particular CR1 and CR3, bind BMP, albeit with a 10-fold lower affinity than full-length Chordin12. Microinjection of CR1 or CR3 messenger RNA results in dorsalization and induction of secondary axes in Xenopus embryos. Thus, even after cleavage by Xolloid, the Chordin fragments can still inhibit BMP signalling12. This observation indicated that additional factors might be required to release BMP from the Chordin fragments generated by Xolloid to reactivate BMP signalling through its cognate receptor. In Drosophila, seven zygotic genes have been proposed to regulate dorsal-ventral patterning1,13. Among them, decapentaplegic (dpp) and screw (scw) encode BMP homologues that promote dorsal cell fates such as amnioserosa and inhibit development of the ventral central nervous system13-16. The chordin homologue short gastrulation (sog) is expressed ventrally and promotes central nervous system development17-19.
The phenotype of sog loss-of-function mutants is intriguing: as expected for a Dpp/Scw antagonist, ventral structures are lost but, in addition, the amnioserosa is reduced. This result is paradoxical, as the amnioserosa is the dorsal-most tissue and therefore Sog, a BMP antagonist, is required for maximal BMP signalling20-23. A model proposed to explain the role of Sog in promoting peak Dpp activity suggests that Sog-BMP complexes may permit the diffusion of BMPs originating from more ventral regions, which are then released dorsally by the proteolytic activity of Tolloid21. The recent demonstration that BMPs remain bound to individual cysteine-rich domains, which remain intact in the Chordin proteolytic products12, makes this interpretation unlikely, unless an additional factor that releases BMP from the cysteine-rich modules is proposed. The twisted gastrulation gene encodes a secreted protein that is specifically required for the differentiation of amnioserosa cells in Drosophila24,25 and is a candidate for such a factor.
Here we show that the Xenopus homologue of twisted gastrulation (xTsg) shares sequence similarities with the cysteine-rich domains of Chordin, and is part of the BMP synexpression group26. Biochemical studies show that xTsg and Drosophila dTsg directly bind BMPs with dissociation constants in the low nanomolar range. In microinjection experiments, xTsg mRNA behaves as an agonist of BMP signalling, ventralizing the Xenopus embryo. xTsg competes efficiently with CR1 for binding to BMP and can bind full-length Chordin, forming a ternary complex containing Chordin, BMP and xTsg in vitro. The dorsalizing activity of CR1 is readily competed by wild-type xTsg, and is greatly potentiated by reducing endogenous xTsg activity. The results indicate that xTsg is involved in dorsal-ventral patterning, permitting peak BMP signalling by antagonizing the residual anti-BMP activity of the cleavage products of Chordin.
xTsg is expressed in ventral-most tissues
We isolated a full-length xTsg complementary DNA by using a human expressed sequence tag (EST) to probe a Xenopus gastrula library. The cDNA encodes a protein sharing 41% amino-acid identity with Drosophila Tsg (dTsg), 89% identity with the partial human Tsg sequence and 94% identity with a mouse EST. The xTsg sequence contains a signal peptide, as expected for a secreted protein, and two conserved domains containing multiple cysteines at its amino and carboxy termini (Fig. 1a). Whole-mount in situ hybridization and polymerase chain reaction with reverse transcription (RT-PCR) showed that abundant xTsg maternal transcripts are distributed throughout the animal half of the embryo during cleavage stages (Fig. 1b, and data not shown). At the late gastrula stage, maternal transcripts decrease and zygotic transcripts appear specifically in the ventral region of the embryo (Fig. 1c). After neurulation, xTsg transcripts surround ventrally the closed blastopore slit and the neural tube (Fig. 1d). At the tailbud stage, xTsg transcripts are detected in the postanal region, heart and dorsal eye (Fig. 1e, f) and closely mimic the expression patterns of BMP-4 and BAMBI27,28 (Fig. 1e-j). The postanal region of xTsg expression derives from the ventral-most tissue29 of the gastrula embryo, as illustrated by the transplantation experiment shown in Fig. 1k and l. We conclude that xTsg is part of the BMP-4 synexpression group26 and is expressed in the ventral pole of the embryo.
Figure 1.
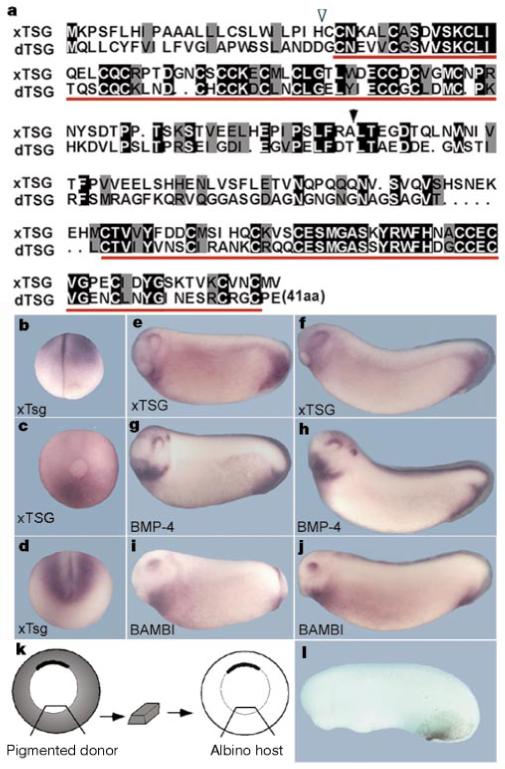
xTsg shares two conserved regions with dTsg and is co-expressed with BMP-4 and BAMBI. a, Alignment of xTsg and dTsg. The potential cleavage site for the signal peptide (open arrowhead), the conserved regions (red bars) and the position at which the N-terminal and C-terminal fragments were divided (black arrowhead) are indicated. b-f, Whole-mount in situ analysis of xTsg expression. b, Four-cell stage; c, late gastrula (posterior view); d, stage 14. e, f, At stages 20 and 24, xTsg expression marks the postanal region, the heart and the dorsal eye. g, h, BMP-4 and i, j, BAMBI expression at stages 20 and 24, respectively. k, Experimental design of an isotopic and isochronic transplant of the ventral-most tissue at early gastrula (stage 10½) from a pigmented donor to an albino host (n = 11). l, The ventral pigmented transplanted tissue populates the perianal region of the early tailbud embryo.
xTsg has ventralizing activity
Microinjection of xTsg mRNA into each blastomere of the four-cell embryo resulted in the reduction of dorso-anterior structures (Fig. 2a). This phenotype was reminiscent of the chordin zebrafish mutant5, and of Xenopus embryos microinjected with Xolloid mRNA3,30 or with low doses of BMP-4 mRNA31. Molecular marker analyses showed that dorsal ectoderm (Sox-2) and dorsal mesoderm (MyoD and Shh) are reduced and that ventral tissues (BMP-4) are expanded in xTsg-injected embryos (Fig. 2b-e). We conclude from these results that xTsg has ventralizing activity.
Figure 2.
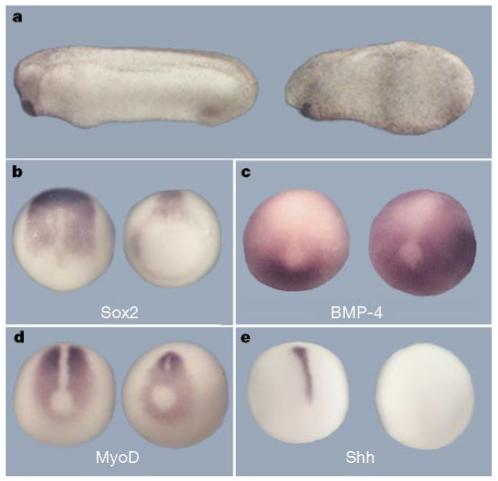
Microinjection of xTsg mRNA leads to ventralization of the embryo. a, Control embryo (left) and embryo with xTsg mRNA microinjected into each animal cell at the eight-cell stage (500 pg total; right), leading to the reduction of dorso-anterior structures. b-e, In situ analysis of late gastrulae microinjected with xTsg mRNA (500 pg per blastomere). Injected embryos (right) and uninjected controls (left) in dorsal view. b, Sox2; c, BMP-4; d, MyoD; e, Shh. The changes in gene expression are characteristic of ventralization in Xenopus3.
To determine whether xTsg functions in the BMP pathway, epistatic analyses were performed by injecting xTsg mRNA into a ventral blastomere together with a dominant-negative BMP receptor (tBR) or extracellular BMP antagonists (noggin, chordin and CR1). xTsg had no effect on the formation of secondary axes by tBR, indicating that it may ventralize the embryo upstream of the BMP receptor (Fig. 3b). To test whether xTsg could antagonize the dorsalizing activity of the proteolytic fragments of Chordin, we generated a construct containing the N-terminal CR1 domain and terminating at the Xolloid cleavage site11. CR1 mRNA induced secondary axes (although at 32-fold higher molar concentrations than those required for full-length chordin mRNA, Fig. 3g), which were blocked by co-injection of xTsg (Fig. 3h). Xolloid mRNA was able to block the activity of full-length chordin, as shown previously3, but had no effect on secondary axes induced by the CR1 construct (Fig. 3f, i). This indicates that xTsg efficiently antagonizes high doses of CR1 downstream of Xolloid cleavage. The ability of xTsg to inhibit full-length Chordin mRNA (Fig. 3d, e) presumably results from the activity of uniformly expressed Xolloid proteases in the early embryo30. That the ventralizing activity of xTsg requires Xolloid cleavage is confirmed by the observation that a CR1 construct containing 80 additional amino acids downstream of the first Xolloid site could not be antagonized by co-injection of either xTsg or Xolloid mRNAs (unpublished observations). The ventralizing activity of xTsg appears to be specific for the Chordin/BMP pathway, as inhibition of BMP by noggin was not affected by co-injection of xTsg mRNA (Fig. 3c). These epistatic studies are consistent with a model in which xTsg would ventralize the embryo by antagonizing the residual anti-BMP activity of Chordin proteolytic cleavage products (Fig. 3j).
Figure 3.
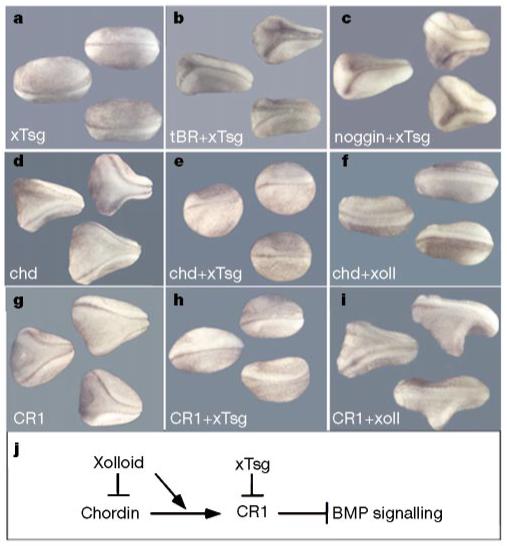
xTsg blocks secondary axis formation by CR1 downstream of Chordin cleavage by Xolloid. a, Microinjection of 250 pg xTsg mRNA. b,c, The induction of secondary axes by 200 pg tBR (dominant-negative BMP receptor; b) or 5 pg noggin mRNA (c) is not affected by co-injection of 250 pg xTsg mRNA. d, Injection of 10 pg chordin mRNA induces the formation of secondary axes that are inhibited by co-injection of 250 pg xTsg mRNA (e) or 200 pg Xolloid mRNA (f). g, Secondary axes induced by injections of 80 pg CR1 mRNA (a 32-fold molar excess compared with full-length chordin mRNA) are inhibited by co-injection of 250 pg xTsg mRNA (h) but not by co-injection of 200 pg Xolloid mRNA (i). Similar results were obtained in at least three independent experiments, with n = 22-52 embryos per mRNA combination. All embryos were injected once ventrally at the eight-cell stage. j, Summary of the epistatic studies. Xolloid inhibits Chordin activity but not that of CR1, and xTsg can block CR1 function. These epistatic analyses place the xTsg ventralizing activity downstream of Xolloid cleavage and upstream of the receptor.
xTsg is a BMP-binding protein
When the N-terminal domain of xTsg was compared with the cysteine-rich domains of Chordin, we noticed sequence similarities (Fig. 4a). As cysteine-rich domains are BMP-binding modules12, we tested whether epitope-tagged xTsg secreted by transfected 293T cells could bind BMP-4. xTsg did bind to BMP-4 in solution, and this interaction was specific as it could be competed by BMP-2 but not by a 10-fold excess of platelet-derived growth factor, epidermal growth factor, Activin or transforming growth factor (TGF)-β1 (Fig. 4b). We used chemical crosslinking with disuccinimidyl suberate (DSS) to determine whether this molecular interaction was direct. After separation of the complexes under reducing conditions, haemagglutinin (HA)-tagged xTsg (1 nM) formed mostly dimers and a small amount of monomers (Fig. 4c, lane 1). In the presence of 1 nM BMP-4, the xTsg dimers shifted to a molecular mass consistent with the binding of one BMP-4 dimer (Fig. 4c, lanes 2 and 5). Using an immunoprecipitation assay, the apparent dissociation constant for equilibrium binding of BMP-4 to xTsg (Kd) was determined by Scatchard analysis and found to be about 2.5 nM for both Xenopus and Drosophila Tsg (Fig. 4d and data not shown). To map the BMP binding domain, we generated constructs consisting of the conserved domains of xTsg and dTsg separated at the sites indicated in Fig. 1a and prepared secreted proteins. As shown in Fig. 5a and b, the BMP-binding activity resided in the N-terminal region of xTsg and dTsg, that is, in the domain that shares sequence similarities with the BMP-binding modules of Chordin. We conclude that xTsg is a secreted BMP-binding protein that functions in embryos as an agonist of BMP signalling. The other secreted BMP-binding proteins identified, such as Chordin, Noggin, Follistatin and members of the Cerberus family, are antagonists of BMP activity2,32-34.
Figure 4.
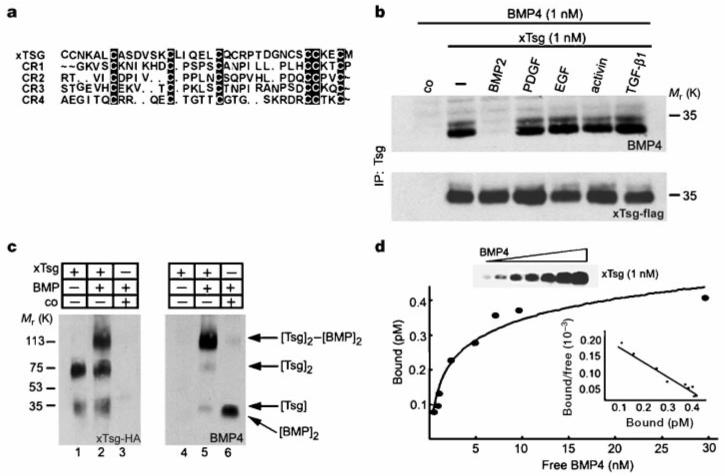
xTsg binds to BMP specifically and directly. a, Sequence similarities between Chordin cysteine-rich modules and the N-terminal domain of xTsg. b, Western blot analyses of BMP-4 (1.0 nM) bound to xTsg (1.0 nM) after immunoprecipitation by xTsg in the presence or absence of a 10-fold excess of various growth factors. The control (co) lanes in this and Figs 5-7 contain conditioned medium from mock-transfected 293T cells. To measure the recovery of xTsg after immunoprecipitation, the same membrane was stripped and probed for xTsg protein (bottom). c, Crosslinking analysis of xTsg-BMP-4 complexes with DSS. Left, immunoblot probed for xTsg. Right, the same membrane probed for BMP-4 after stripping. d, Equilibrium binding of increasing concentrations of BMP-4 (0.2-30 nM) to 1 nM Tsg. Binding was for 3 h at 4 °C, and bound and free BMP-4 were separated by immunoprecipitation; crosslinking agents were not used. The amount of bound BMP-4 was determined as described12. Each data point was in duplicate, and two independent experiments were performed for each protein. Scatchard analyses of the dTsg data gave an apparent Kd of 2.5 nM. The same results and Kd were obtained for the xTsg protein as illustrated by the anti-BMP-4 immunoblot (inset and data not shown).
Figure 5.
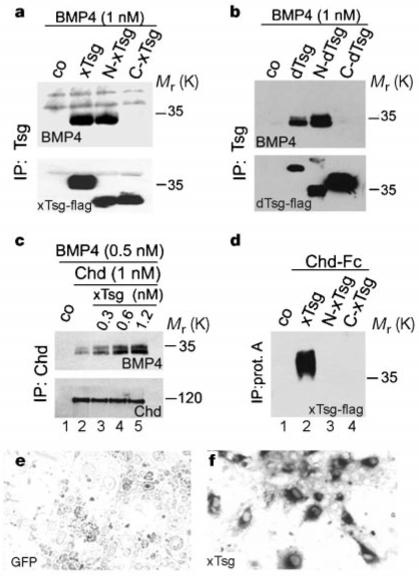
The Tsg N-terminal domain is sufficient to interact with BMP-4, but not with Chordin. a,b, Western blot analyses of BMP-4 (1 nM) bound to 1 nM of Xenopus or Drosophila Tsg. As a loading control, the same membranes were stripped and probed against Tsg (bottom). c, Western blot analyses of BMP-4 (0.5 nM) bound by 1 nM Chordin (lane 2) in the presence of 0.3, 0.6 and 1.2 nM xTsg (lanes 3, 4 and 5, respectively). The presence of xTsg increases the binding of BMP-4 to Chordin about fourfold (quantification by Phosphoimager, not shown). Bottom, same membrane probed with anti-Chordin antibody. d, Immunoprecipitation with protein A-sepharose of a mixture of 1 nM Chordin-Fc fusion protein with 1 nM xTsg, N-xTsg and C-xTsg. e,f, The Chordin-xTsg interaction was confirmed by binding to COS cells transfected with green fluorescent protein (negative control) or with xTsg cDNA, which were permeabilized and stained with a Chordin-alkaline phosphatase fusion protein.
xTsg competes with CR-1 to bind BMP
We next tested whether Chordin and xTsg could compete for BMP binding. Full-length Chordin (1 nM) was immunoprecipitated in the presence of 0.5 nM BMP-4 and increasing amounts of xTsg protein. Instead of competing for the limited amounts of BMP-4, xTsg stimulated binding of BMP-4 to full-length Chordin (Fig. 5c, lanes 2-5). Further analyses showed that xTsg itself could bind to Chordin even in the absence of added BMP (Fig. 5d, lane 2). This binding was confirmed using a cell-culture assay in which permeabilized cells transfected with xTsg cDNA were stained with a Chordin-alkaline phosphatase fusion protein35,36 (Fig. 5e, f). The binding of xTsg to Chordin required intact xTsg, as neither the N-nor the C-terminal fragments sufficed for the interaction (Fig. 5d, lanes 3 and 4). The observation that the N terminus is unable to bind Chordin but can still bind BMP-4 suggests that these two interactions occur through different sites.
Crosslinking experiments with DSS showed that xTsg stimulates the binding of BMP-4 to full-length Chordin by forming a trimolecular complex with a relative molecular mass of about 220,000 (Mr 220K) (Fig. 6a, lane 3). This shift is consistent with the crosslinking of one dimer of BMP-4 and one dimer of xTsg per Chordin monomer, as depicted in Fig. 6b. To investigate the molecular mechanism by which xTsg can antagonize the activity of CR1, equimolar amounts (1 nM) of BMP-4, CR1 and xTsg were incubated for 1 h at room temperature before crosslinking with DSS. When CR1 and BMP-4 were incubated together, a complex of Mr 50K was formed, corresponding to the binding of a CR1 monomer to a BMP dimer (Fig. 6a, compare lanes 1 and 4). Incubation of xTsg and BMP-4 resulted in the formation of a complex of about 100K, corresponding to a dimer of xTsg bound to a dimer of BMP-4 (Fig. 6a, lane 6). Remarkably, when CR1, BMP-4 and xTsg were incubated together, only the [xTsg]2-[BMP]2 complex was formed (Fig. 6a, lane 5). Furthermore, when BMP-4 and CR1 were preincubated for 1 h, BMP-4 could still be dislodged from these preformed complexes by addition of xTsg (not shown). We conclude from these results that, in contrast to full-length Chordin, CR1 bound to BMP-4 is released in the presence of xTsg, resulting in a binary complex of BMP-4 and xTsg (Fig. 6c).
Figure 6.
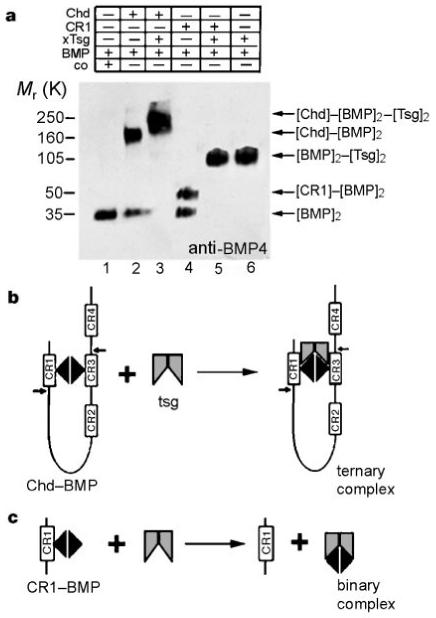
xTsg competes for binding of BMP-4 with CR1 but not with full-length Chordin. Crosslinking analyses were performed using DSS. a, Lane 1, BMP-4 (1 nM). 1 nM Chd and 1 nM BMP-4 form a complex of about 150K (lane 2) that shifts to ∼220K in the presence of 1 nM xTsg (lane 3). When 1 nM CR1 was crosslinked to BMP-4 a 50K complex was detected (lane 4). When xTsg was added a complex of around 100K was produced exclusively (lane 5), which was identical to the [BMP]2-[xTsg]2 complex (lane 6). b, c, Diagrams summarizing the crosslinking results. The small arrows in Chordin indicate the Xolloid cleavage sites.
Endogenous xTsg antagonizes CR1 activity
To investigate the effect of loss of function of endogenous xTsg, we used two complementary approaches. A construct secreting the C-terminal conserved domain (dn-xTsg) was found to have dominant-negative effects (see below) and double-stranded xTsg RNA (RNAi) was also found to interfere with endogenous xTsg function. RNAi potently and specifically inhibits gene expression in a number of species37-39. xTsg RNAi (100 pg) was injected into each animal blastomere at the eight-cell stage together with epitope-tagged xTsg and CR1 mRNAs. As shown in the inset of Fig. 7h, RNAi inhibited the expression of secreted xTsg but not of CR1 protein, indicating that RNAi is effective and specific in Xenopus. Microinjection of dn-xTsg or of RNAi resulted in abnormal development of the postanal region, in particular loss of the ventral fin and shortening of the tail which were only evident at swimming tadpole stages (data not shown). At earlier stages of development, only mild defects in the perianal region were observed (Fig. 7d, e); these minimal early phenotypes may be due to the strong maternal contribution of xTsg (Fig. 1b). However, both dn-xTsg mRNA and RNAi greatly potentiated the dorsalizing effects of low doses of CR1 mRNA injected into all blastomeres of four-cell embryos (compare Fig. 7a, f and h). These effects could be partially rescued by co-injection of wild-type xTsg mRNA, although the rescue was more effective for dn-xTsg (Fig. 7f, g) than for xTsg RNAi (Fig. 7h, i).
Figure 7.
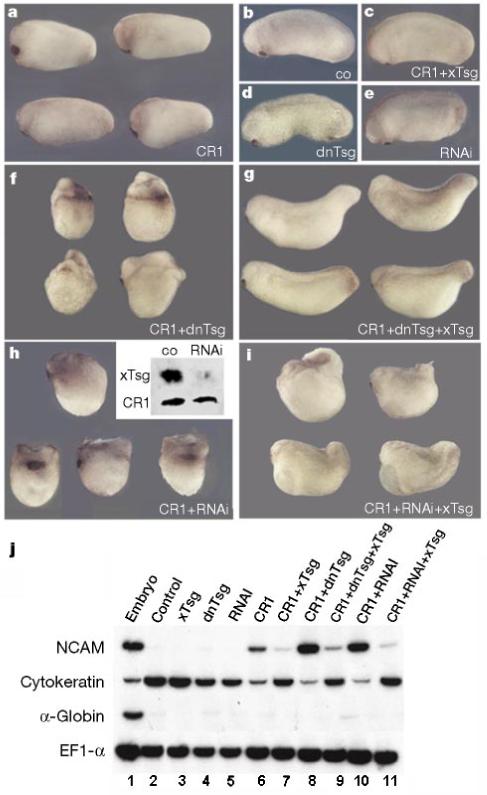
Loss of function of endogenous xTsg potentiates the activity of CR1 mRNA. Four-cell embryos were injected into each cell in the marginal region and cultured to stage 25. a, Injection of CR1 results in slight dorsalization compared with control uninjected embryos (b). c, Co-injection of xTsg and CR1 mRNA prevents dorsalization by CR1. Injection of dn-xTsg (d) or RNAi (e) disrupts the development of the perianal region leading to shortening of the tail at later stages (not shown); at these early tadpole stages the phenotype was minimal. Co-injection of CR1 and dn-xTsg mRNA (f) or RNAi (h) leads to strong synergistic dorsalization of the embryos, which can be partially rescued by co-injecting full-length xTsg mRNA (g, i). h, Inset, co-injection of HA-tagged xTsg mRNA and Myc-tagged CR1 mRNA with or without xTsg RNAi. Ectodermal explants were dissociated3, incubated for 14 h at 16 °C, and secreted proteins analysed by western blot. j, RT-PCR analysis of stage 25 ectodermal explants of embryos injected at the eight-cell stage. NCAM induction reflects inhibition of BMP signalling by CR1 (lane 6), which is potentiated by co-injection of dn-xTsg or RNAi (lanes 8 and 10). The induction of NCAM by CR1, as well as its potentiation by co-injection of dn-xTsg or RNAi, was inhibited by co-injection of wild-type xTsg (lanes 7, 9, 11). The epidermal marker gene cytokeratin is regulated reciprocally by the injected RNAs and serves as a reporter of endogenous BMP signals. α-Globin was used as a mesodermal marker and EF1-α as loading control. The amounts of mRNA per blastomere injected were CR1, 20 pg; xTsg, 200 pg; dn-xTsg, 100 pg; and RNAi, 100 pg.
The results could be confirmed using molecular markers in ectodermal explants. As can be seen in Fig. 7j, dn-xTsg and xTsg RNAi potentiated the induction of the neural marker NCAM, as well as the inhibition of the epidermal marker cytokeratin, by CR1 mRNA (lanes 6, 8 and 10). The neuralizing effect of CR1, but not that of tBR, could be reversed by xTsg mRNA (lanes 6 and 7, and data not shown). The strong neuralizing effects observed in the presence of dn-xTsg or RNAi could also be reversed by full-length xTsg mRNA (lanes 9 and 11). These loss-of-function experiments indicate that endogenous levels of xTsg activity are sufficient to inhibit the dorsalizing effects of microinjected CR1.
Discussion
The Xenopus homologue of dTsg is a member of the BMP-4 synexpression group26, and is transcribed in the ventral-most region of the embryo. Overexpression of this secreted protein ventralizes the embryo through a molecular mechanism functioning upstream of the BMP receptor (Fig. 3b). Epistasis experiments support a model in which xTsg would promote BMP signalling downstream of Chordin cleavage by the metalloproteinase Xolloid. Chordin is abundantly secreted by the dorsal pole of the embryo, reaching concentrations of 6-12 nM in the extracellular space of Spemann’s organizer40. Chordin binds BMPs through cysteine-rich modules12. Xolloid cleaves Chordin downstream of the two cysteine-rich domains that have the highest affinity for BMP binding3,11,12. These proteolytic digestion products retain residual BMP-binding activity and can still inhibit the interaction between BMP and its cognate receptor12. Our results show that xTsg binds BMP directly in the low nanomolar range and will compete effectively with a cysteine-rich module for binding to BMP, leading to the formation of xTsg-BMP complexes that permit BMP signalling (Figs 2, 3 and 6). In this way, xTsg would promote BMP activity by dislodging the growth factor from an inhibitor in the extracellular space. Interfering with endogenous xTsg by two independent approaches, dn-xTsg and RNAi, greatly increased the ability of CR1 to inhibit BMP. xTsg mRNA did not cooperate with BMP-4 mRNA in the induction of the target gene Xvent-1 (ref. 26) in animal cap explants, nor did xTsg facilitate the binding of BMP-4 to recombinant BMP receptor IA (ref. 12) in biochemical assays (data not shown). This indicates that in the absence of Chordin cleavage products xTsg does not increase BMP signalling. Unlike CR1, the binding of full-length Chordin to BMP-4 is enhanced by xTsg, leading to the formation of a ternary complex that can be crosslinked in vitro; perhaps xTsg results in more efficient cleavage of Chordin by Xolloid.
The initial impetus to investigate whether xTsg bound BMP was provided by the observation that the N-terminal conserved domain shared amino-acid similarities with Chordin cysteine-rich repeats. This similarity was found to be functionally significant, as this domain contains the BMP-binding activity of xTsg. Cysteine-rich modules of the Chordin type are found in many extracellular proteins such as von Willebrand factor, thrombospondin, Nel and fibrillar procollagens. The cysteine-rich domain in procollagen IIA binds BMP and TGF-β1 (refs 12, 41) and is responsible for the dorsalizing activity of procollagen IIA in Xenopus12. It is therefore possible that the molecular mechanism described here for xTsg may provide a more general paradigm for signalling in the extracellular space. Similar functions in the release of latent growth factors bound to cysteine-rich domains could be executed by other secreted proteins that share structural similarities with the N-terminal repeat of xTsg, such as members of the connective tissue growth factor (CTGF) and insulin-like growth factor-binding protein (IGFBP) families24,42. In addition, the Cripto and Oep (one-eyed pinhead) proteins, which provide a permissive function for signalling by nodal TGF-β family members in mouse and zebrafish43,44, share sequence similarities to xTsg in their conserved EGF-like domains (data not shown).
In Drosophila, loss of function of dTsg results in the loss of amnioserosa, which requires maximal amounts of Dpp/Scw signalling14-16, but does not affect the rest of the dorsal-ventral pattern24. Overexpression of dTsg driven by a promoter expressed in the ventral-most region of the embryo rescues dorsal amnioserosa formation in a dTsg mutant background, indicating that the protein can diffuse throughout the embryo25. As dTsg does not affect other dorsal-ventral fates in Drosophila, it was thought to provide a permissive signal required for amnioserosa differentiation after cells have been exposed to peak levels of Dpp/Scw signalling24,25. Our results show that Drosophila Tsg can bind BMPs through its N-terminal domain (Fig. 5b). Drosophila Sog, although not yet demonstrated to bind Dpp/Scw/BMP in biochemical studies, is cleaved by Tolloid at three sites in the presence of BMP45. Two of these cleavage sites are located at similar positions to those in Xenopus Chordin3. We propose that dTsg can act only in the amnioserosa because its function is to release active Dpp/Scw from the cleavage fragments of Sog by Tolloid. Peak signalling would be mediated by Dpp/Scw originating in more ventral regions and reaching the dorsal region by diffusion as a complex with Sog21. Release would occur only in dorsal-most regions in which maximal levels of dTsg, Tolloid, and fragments of Sog bound to BMP are prevalent. In more ventral regions, full-length free Sog would be in excess21 and would inhibit any released BMPs. This would be particularly true in the presence of dTsg in dorso-lateral regions, in view of the observation that xTsg increases the binding of BMP to full-length Chordin (Fig. 5c). This model has the attraction of explaining the paradoxical effects of Sog, which inhibits Dpp/Scw signalling in ventral ectoderm but promotes it in the most dorsal regions of the embryo20,46. By introducing dTsg as an additional factor that permits the release of Dpp/Scw from inactive complexes in the amnioserosa, it is not necessary to invoke positive (that is, non-inhibitory) signalling effects of Sog proteolytic cleavage products to account for the Tolloid-dependent long-range effects of Sog diffusion on peak Dpp signalling21-23.
One of the great surprises of developmental genetics has been the evolutionary conservation of molecular mechanisms. The best known example is the discovery that conserved sets of Hox genes determine the anterior-posterior axis in all bilateral animals47,48. The activities and expression patterns of Dpp/BMP-4, Sog/Chordin and Tolloid/Xolloid have led to the view that the dorsal-ventral axis has been inverted in the course of evolution1,18. We now show that Drosophila Tsg, a gene expressed in the dorsal-most blastoderm, has a vertebrate homologue expressed in the ventral-most tissues of the Xenopus embryo. xTsg is part of an intricate extracellular signalling pathway in which the two poles of the dorsal-ventral pattern are defined by sources of secreted Sog/Chordin and dTsg/xTsg in opposite sides of the embryo. The results are consistent with the idea that the ventral side of the arthropod is homologous to the dorsal side of the vertebrate, as proposed by Geoffroy Saint-Hilaire49.
Note added in proof
As this work was being reviewed, it was reported50 that alternative Sog processing may generate protein forms with increased BMP inhibitory activity. In the light of our data, some of those findings might be explained by the formation of trimolecular complexes consisting of Tsg, Dpp and Sog fragments that are insensitive to Tolloid cleavage.
Methods
DNA constructs
We screened a Xenopus gastrula cDNA library with the coding sequence of the hTsg EST; a full-length clone was isolated, sequenced and subcloned in the expression vector pCS2+. xTsg and dTsg were epitope-tagged by PCR either using a 3′ primer including an HA tag before the stop codon or by amplification of xTsg or dTsg subclones lacking the leader sequence and cloning into an expression vector including the chordin signal peptide and a Flag tag sequence (pchd-Flag, constructed by S. Piccolo). Pchd-Flag was used to generate N-xTsg, C-xTsg, N-dTsg and C-dTsg. The Xenopus pCS2-CR1 construct was generated by PCR from pCS2-chordin3 using a 5′ vector primer and a 3′ primer including Chordin sequences up to Asn 146, followed by a Myc tag.
Protein binding and crosslinking
Proteins were obtained by transient transfection of 293T cells (xTsg, dTsg) or S2 cells (Ch-dAP, Chd-Fc). Immunoprecipitations and equilibrium binding assays were performed as described12. For crosslinking40, we incubated protein mixtures containing BMP-4 (R&D Systems) for 1 h at room temperature in 80 μl of PBS; disuccinimidyl suberate (Pierce) was then added to a final concentration of 0.85 mM and incubated at room temperature for a further 30 min. The reaction was stopped by adding 4 μl 1 M Tris-HCl (pH 7.4) and samples were electrophoresed in SDS gels under reducing conditions. For secretion-trap cell staining35, COS-7 cells were transiently transfected and after two days fixed (1.8% paraformaldehyde for 15 min), permeabilized (0.1% TritonX-100 for 15 min), and incubated for 1 h at room temperature with Chordin-alkaline phosphatase fusion protein. Embryo manipulations and RNA analyses were as described12.
Acknowledgements
We thank J. Fessler, L. Zipursky and members of our laboratory for comments on the manuscript, and U. Tran and A. Cuellar for technical assistance. M.O. and J.L. are HFSPO and Pew postdoctoral fellows, respectively. This work was supported by the NIH and the Howard Hughes Medical Institute.
The accession number for xTsg is AF245221. Human and mouse Tsg homologues are encoded by ESTs AA486291 and AW258143.
References
- 1.De Robertis EM, Sasai Y. A common plan for dorsoventral patterning in Bilateria. Nature. 1996;380:37–40. doi: 10.1038/380037a0. [DOI] [PubMed] [Google Scholar]
- 2.Harland R, Gerhart J. Formation and function of Spemann’s organizer. Annu. Rev. Cell. Dev. Biol. 1997;13:611–667. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- 3.Piccolo S, et al. Cleavage of Chordin by Xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann organizer activity. Cell. 1997;91:407–416. doi: 10.1016/s0092-8674(00)80424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinmaster G. Reprolysins and astacins. Science. 1998;279:336–337. doi: 10.1126/science.279.5349.336. [DOI] [PubMed] [Google Scholar]
- 5.Schulte-Merker S, Lee KJ, McMahon AP, Hammerschmidt M. The zebrafish organizer requires chordino. Nature. 1997;387:862–863. doi: 10.1038/43092. [DOI] [PubMed] [Google Scholar]
- 6.Kishimoto Y, Lee KH, Zon L, Hammerschmidt M, Schulte-Merker S. The molecular nature of zebrafish swirl: BMP2 function is essential during early dorsoventral patterning. Development. 1997;124:4457–4466. doi: 10.1242/dev.124.22.4457. [DOI] [PubMed] [Google Scholar]
- 7.Dick A, et al. Essential role of Bmp7 (snailhouse) and its prodomain in dorsoventral patterning of the zebrafish embryo. Development. 2000;127:343–354. doi: 10.1242/dev.127.2.343. [DOI] [PubMed] [Google Scholar]
- 8.Schmid B, et al. Equivalent genetic roles for bmp7/snailhouse and bmp2b/swirl in dorsoventral pattern formation. Development. 2000;127:957–967. doi: 10.1242/dev.127.5.957. [DOI] [PubMed] [Google Scholar]
- 9.Hild M, et al. The smad5 mutation somitabun blocks Bmp2b signaling during early dorsoventral patterning of the zebrafish embryo. Development. 1999;126:2149–2159. doi: 10.1242/dev.126.10.2149. [DOI] [PubMed] [Google Scholar]
- 10.Connors SA, Trout J, Ekker M, Mullins MC. The role of tolloid/mini fin in dorsoventral pattern formation of the zebrafish embryo. Development. 1999;126:3119–3130. doi: 10.1242/dev.126.14.3119. [DOI] [PubMed] [Google Scholar]
- 11.Scott IC, et al. Mammalian BMP-1/Tolloid-related metalloproteinases, including novel family member mammalian Tolloid-like 2, have differential enzymatic activities and distributions of expression relevant to patterning and skeletogenesis. Dev. Biol. 1999;213:283–300. doi: 10.1006/dbio.1999.9383. [DOI] [PubMed] [Google Scholar]
- 12.Larraín J, et al. BMP-binding modules in Chordin: a model for signalling regulation in the extracellular space. Development. 2000;127:821–830. doi: 10.1242/dev.127.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson EL, Anderson KV. Localized enhancement and repression of the activity of the TGF-beta family member, decapentaplegic, is necessary for dorsal-ventral pattern formation in the Drosophila embryo. Development. 1992;114:583–597. doi: 10.1242/dev.114.3.583. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson EL, Anderson KV. Decapentaplegic acts as a morphogen to organize dorsal-ventral pattern in the Drosophila embryo. Cell. 1992;71:451–461. doi: 10.1016/0092-8674(92)90514-d. [DOI] [PubMed] [Google Scholar]
- 15.Neul JL, Ferguson EL. Spatially restricted activation of the SAX receptor by SCW modulates DPP/TKV signaling in Drosophila dorsal-ventral patterning. Cell. 1998;95:483–494. doi: 10.1016/s0092-8674(00)81616-5. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen M, Park S, Marques G, Arora K. Interpretation of a BMP activity gradient in Drosophila embryos depends on synergistic signaling by two type I receptors, SAX and TKV. Cell. 1998;95:495–506. doi: 10.1016/s0092-8674(00)81617-7. [DOI] [PubMed] [Google Scholar]
- 17.François V, Solloway M, O’Neill JW, Emery J, Bier E. Dorsal-ventral patterning of the Drosophila embryo depends on a putative negative growth factor encoded by the short gastrulation gene. Genes Dev. 1994;8:2602–2616. doi: 10.1101/gad.8.21.2602. [DOI] [PubMed] [Google Scholar]
- 18.Holley SA, et al. A conserved system for dorsal-ventral patterning in insects and vertebrates involving sog and chordin. Nature. 1995;376:249–253. doi: 10.1038/376249a0. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt J, François V, Bier E, Kimelman D. Drosophila short gastrulation induces an ectopic axis in Xenopus: evidence for conserved mechanisms of dorsal-ventral patterning. Development. 1995;121:4319–4328. doi: 10.1242/dev.121.12.4319. [DOI] [PubMed] [Google Scholar]
- 20.Zusman SB, Sweeton D, Wieschaus EF. Short gastrulation, a mutation causing delays in stage-specific cell shape changes during gastrulation in Drosophila melanogaster. Dev. Biol. 1988;129:417–427. doi: 10.1016/0012-1606(88)90389-2. [DOI] [PubMed] [Google Scholar]
- 21.Holley SA, et al. The Xenopus dorsalizing factor noggin ventralizes Drosophila embryos by preventing DPP from activating its receptor. Cell. 1996;86:607–617. doi: 10.1016/s0092-8674(00)80134-8. [DOI] [PubMed] [Google Scholar]
- 22.Bier E. A unity of opposites. Nature. 1999;398:375–376. doi: 10.1038/18778. [DOI] [PubMed] [Google Scholar]
- 23.Ashe HL, Levine M. Local inhibition and long-range enhancement of Dpp signal transduction by Sog. Nature. 1999;398:427–431. doi: 10.1038/18892. [DOI] [PubMed] [Google Scholar]
- 24.Mason ED, Konrad KD, Webb CD, Marsh JL. Dorsal midline fate in Drosophila embryos requires twisted gastrulation, a gene encoding a secreted protein related to human connective tissue growth factor. Genes Dev. 1994;8:1489–1501. doi: 10.1101/gad.8.13.1489. [DOI] [PubMed] [Google Scholar]
- 25.Mason ED, Williams S, Grotendorst GR, Marsh JL. Combinatorial signaling by Twisted gastrulation and Decapentaplegic. Mech. Dev. 1997;64:61–75. doi: 10.1016/s0925-4773(97)00049-x. [DOI] [PubMed] [Google Scholar]
- 26.Niehrs C, Pollet N. Synexpression groups in eukaryotes. Nature. 1999;402:483–487. doi: 10.1038/990025. [DOI] [PubMed] [Google Scholar]
- 27.Fainsod A, Steinbeisser H, De Robertis EM. On the function of BMP-4 in patterning the marginal zone of the Xenopus embryo. EMBO J. 1994;13:5015–5025. doi: 10.1002/j.1460-2075.1994.tb06830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onichtchouk D, et al. Silencing of TGF-beta signalling by the pseudoreceptor BAMBI. Nature. 1999;401:480–485. doi: 10.1038/46794. [DOI] [PubMed] [Google Scholar]
- 29.Green JB, Smith JC. Graded changes in dose of a Xenopus activin A homologue elicit stepwise transitions in embryonic cell fate. Nature. 1990;347:391–394. doi: 10.1038/347391a0. [DOI] [PubMed] [Google Scholar]
- 30.Goodman SA, et al. BMP1-related metalloproteinases promote the development of ventral mesoderm in early Xenopus embryos. Dev. Biol. 1998;195:144–157. doi: 10.1006/dbio.1997.8840. [DOI] [PubMed] [Google Scholar]
- 31.Jones CM, Lyons KM, Lapan PM, Wright CVE, Hogan BLM. DVR-4 (Bone Morphogenetic Protein-4) as a posterior-ventralizing factor in Xenopus mesoderm induction. Development. 1992;115:639–647. doi: 10.1242/dev.115.2.639. [DOI] [PubMed] [Google Scholar]
- 32.Hsu DR, Economides AN, Wang X, Eimon PM, Harland RM. The Xenopus dorsalizing factor Gremlin identifies a novel family of secreted proteins that antagonize BMP activities. Mol. Cell. 1998;1:673–683. doi: 10.1016/s1097-2765(00)80067-2. [DOI] [PubMed] [Google Scholar]
- 33.Piccolo S, et al. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature. 1999;397:707–710. doi: 10.1038/17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez Esteban C, et al. The novel Cer-like protein Caronte mediates the establishment of embryonic left-right asymmetry. Nature. 1999;401:243–251. doi: 10.1038/45738. [DOI] [PubMed] [Google Scholar]
- 35.Davis S, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 36.Flanagan JG, Leder P. The kit ligand: a cell surface molecule altered in steel mutant fibroblasts. Cell. 1990;63:185–194. doi: 10.1016/0092-8674(90)90299-t. [DOI] [PubMed] [Google Scholar]
- 37.Fire A, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 38.Tuschl T, Zamore PD, Lehmann R, Bartel DP, Sharp PA. Targeted mRNA degradation by double-stranded RNA in vitro. Genes Dev. 1999;13:3191–3197. doi: 10.1101/gad.13.24.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bosher JM, Labouesse M. RNA interference: genetic wand and genetic watchdog. Nature Cell Biol. 2000;2:31–36. doi: 10.1038/35000102. [DOI] [PubMed] [Google Scholar]
- 40.Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu Y, Oganesian A, Keene DR, Sandell LJ. Type IIA procollagen containing the cysteine-rich amino propeptide is deposited in the extracellular matrix of prechondrogenic tissue and binds to TGF-β1 and BMP-2. J. Cell Biol. 1999;144:1069–1080. doi: 10.1083/jcb.144.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grotendorst GR. Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts. Cytokine Growth Factor Rev. 1997;8:171–179. doi: 10.1016/s1359-6101(97)00010-5. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, Talbot WS, Schier AF. Positional cloning identifies zebrafish one-eyed pinhead as a permissive EGF-related ligand required during gastrulation. Cell. 1998;92:241–251. doi: 10.1016/s0092-8674(00)80918-6. [DOI] [PubMed] [Google Scholar]
- 44.Schier AF, Shen MM. Nodal signalling in vertebrate development. Nature. 2000;403:385–389. doi: 10.1038/35000126. [DOI] [PubMed] [Google Scholar]
- 45.Marques G, et al. Production of a DPP activity gradient in the early Drosophila embryo through the opposing actions of the SOG and TLD proteins. Cell. 1997;91:417–426. doi: 10.1016/s0092-8674(00)80425-0. [DOI] [PubMed] [Google Scholar]
- 46.Biehs B, François V, Bier E. The Drosophila short gastrulation gene prevents Dpp from autoactivating and suppressing neurogenesis in the neuroectoderm. Genes Dev. 1996;10:2922–2934. doi: 10.1101/gad.10.22.2922. [DOI] [PubMed] [Google Scholar]
- 47.De Robertis EM. In: Guidebook of Homeobox Genes. Duboule D, editor. IRL; Oxford: 1994. pp. 11–23. [Google Scholar]
- 48.Knoll AH, Carroll SB. Early animal evolution: emerging views from comparative biology and geology. Science. 1999;284:2129–2137. doi: 10.1126/science.284.5423.2129. [DOI] [PubMed] [Google Scholar]
- 49.Geoffroy Saint-Hilaire E. Considérations générales sur la vertèbre. Mém. Mus. Hist. Nat. 1822;9:89–119. [Google Scholar]
- 50.Yu, et al. Processing of the Drosophila Sog protein creates a novel BMP inhibitory activity. Development. 2000;127:2143–2154. doi: 10.1242/dev.127.10.2143. [DOI] [PubMed] [Google Scholar]


