SUMMARY
In Xenopus, mesoderm induction by endoderm at the blastula stage is well documented, but the molecular nature of the endogenous inductive signals remains unknown. The carboxy-terminal fragment of Cerberus, designated Cer-S, provides a specific secreted antagonist of mesoderm-inducing Xenopus Nodal-Related (Xnr) factors. Cer-S does not inhibit signalling by other mesoderm inducers such as Activin, Derrière, Vg1 and BMP4, nor by the neural inducer Xnr3. In the present study we show that Cer-S blocks the induction of both dorsal and ventral mesoderm in animal-vegetal Nieuwkoop-type recombinants. During blastula stages Xnr1, Xnr2 and Xnr4 are expressed in a dorsal to ventral gradient in endodermal cells. Dose-response experiments using cer-S mRNA injections support the existence of an endogenous activity gradient of Xnrs. Xnr expression at blastula can be activated by the vegetal determinants VegT and Vg1 acting in synergy with dorsal β-catenin. The data support a modified model for mesoderm induction in Xenopus, in which mesoderm induction is mediated by a gradient of multiple Nodal-related signals released by endoderm at the blastula stage.
Keywords: Mesoderm induction, Nodal, Xnr, Cerberus, TGF-β, Derrière, Activin, VegT, β-catenin, Vg1, Cer-S
INTRODUCTION
The amphibian embryo provides a much utilized model system to study the early phases of embryonic patterning. The pioneering work of Nieuwkoop, Slack and colleagues has led to the current three-signal model of mesoderm induction and patterning (reviewed by Nieuwkoop, 1973; Slack, 1991a; Kessler and Melton, 1994; Heasman, 1997). Using recombinants of blastula endodermal and ectodermal explants, it was shown that mesoderm is generated via inductive signals from endoderm (Nieuwkoop, 1969; Slack, 1991b). The endoderm is thought to release two signals: first, a uniform or ventral endodermal signal that induces ventral mesoderm such as lateral plate, mesenchyme and blood and, second, a signal emanating from dorsal endoderm that induces dorsal organizer tissue in the overlying mesoderm (Nieuwkoop, 1969, 1973; Boterenbrood and Nieuwkoop, 1973; Harland and Gerhart, 1997; Heasman, 1997). The third signal in this model, also called the horizontal signal, emanates from dorsal organizer tissue during gastrulation and is able to induce the differentiation of dorsal histotypes such as notochord, somites and kidney in ventral mesodermal cells (Smith and Slack, 1983; Harland and Gerhart, 1997; Heasman, 1997). A number of molecules secreted by Spemann’s organizer are thought to participate in this third signal (De Robertis et al., 1997; Harland and Gerhart, 1997).
A recent advance in the field has been the realization that the generation of mesoderm-inducing signals by endoderm is dependent on the activity of maternally encoded transcriptional regulators. Vegetal explants depleted of β-catenin mRNA by antisense oligodeoxynucleotides are unable to release Nieuwkoop’s dorsal signal (Heasman et al., 1994). In a recent study, it was shown that the signal initiated by the β-catenin pathway is not released until after the midblastula transition (Wylie et al., 1996). This finding, based on the use of heterochronic animal-vegetal conjugates, is important for the present analysis because it demonstrated that, contrary to the previously held view, mesoderm induction may be mediated by zygotically expressed genes (Heasman, 1997). Oligonucleotide depletion experiments have also shown that VegT, a transcription factor maternally stored in the vegetal hemisphere of the Xenopus egg, is required for the release of both the ventral and dorsal mesoderm-inducing signals by vegetal explants (Zhang et al., 1998).
Despite intensive efforts, the molecules mediating the first two inductive signals of the Xenopus model remain unknown. Many growth factors with mesoderm-inducing activity have been identified to date, such as FGF, Activin, Vg1, Xenopus Nodal-related factors (Xnrs) and Derrière (Slack, 1991a; Smith, 1995; Thomsen and Melton, 1993; Jones et al., 1995; Sun et al., 1999). There is evidence suggesting that neither Activin nor FGF are likely to be endogenous mesoderm inducers released by endoderm, since mesoderm induction could not be blocked by Follistatin (an Activin and BMP inhibitor) nor by FGF blocking antibodies (Slack, 1991b). Interestingly, Activin applied at increasing doses to blastula animal cap cells is able to induce the entire range of mesodermal derivatives, with ventral cell types at low and dorsal ones at high doses (Green and Smith, 1990; Green et al., 1992; Smith, 1995). Other known mesoderm-inducing factors of the transforming growth factor beta (TGF-β) superfamily - Vg1, Xnrs and Derrière - share with Activin this ability to induce both ventral and dorsal mesoderm.
In mouse and zebrafish, genetic studies strongly suggest that Nodal signalling pathways play a central role in mesoderm formation (Zhou et al., 1993; Conlon et al., 1994; Waldtrip et al., 1998; Nomura and Li, 1998; Song et al., 1999; Sampath et al., 1998; Rebagliati et al., 1998; Feldman et al., 1998; Gritsman et al., 1999). In Xenopus, four Xnr genes have been identified to date. Three of them, Xnr1, Xnr2 and Xnr4 are mesoderm-inducing factors (Jones et al., 1995; Joseph and Melton, 1997; Osada and Wright, 1999). Xnr3, despite sharing sequence similarities with other Xnrs, has very different activities since it lacks mesoderm-inducing capacity, acts as a neural inducer, and is under different regulatory control (Smith et al., 1995; Ecochard et al., 1995; Hansen et al., 1997; McKendry et al., 1997). The existence of three mesoderm-inducing Xnr genes, and possibly additional ones not yet identified, makes Xenopus loss-of-function studies difficult.
Cerberus is a head-inducing secreted factor (Bouwmeester et al., 1996) that acts as a multifunctional antagonist of Nodal, BMP (Bone Morphogenetic Proteins) and Wnt signals (Hsu et al., 1998; Piccolo et al., 1999). A carboxy-terminal fragment of Cerberus, called Cerberus-short (Cer-S), lacks the anti-Wnt and anti-BMP activities but retains full anti-Xnr1 activity (Piccolo et al., 1999). In biochemical studies, Cer-S was found to bind Xnr1, but not Activin nor Vg1 proteins (Piccolo et al., 1999).
In the present study we use the cer-S reagent as a specific inhibitor of Xnr signals. In Nieuwkoop animal-vegetal recombinants, cer-S was able to block mesoderm induction by both dorsal and ventral endodermal fragments. It was also found that endogenous Xnr1, Xnr2 and Xnr4 are expressed zygotically in blastula endoderm in a graded fashion with a maximum in dorsal endoderm, i.e., at the right time and place to mediate mesoderm induction. The expression of Xnr1, Xnr2 and Xnr4 transcripts starts at midblastula and can be activated synergistically by the vegetally localized determinants VegT and Vg1 (Zhang et al., 1998; Joseph and Melton, 1998) acting together with the dorsal determinant β-catenin (Heasman et al., 1994; Schneider et al., 1996). These results lead us to propose that the first two signals of the Xenopus three-signal model could be provided by a gradient of Nodal-related signals released by the endoderm during the blastula stage.
MATERIALS AND METHODS
Synthetic mRNAs
The plasmids pCS2-cer-S, pCS2-Xnr1, pCS2-Xnr2, pCS2-A-Vg1, pCS2-A-mouse-nodal, pCS2-tALK-4, pCS2-derrière and pCS2-β-catenin were linearized with NotI, and pCS2-VegT-EnR and pCS2-Sia-EnR were linearized with SacII; all were transcribed with SP6 RNA polymerase (Piccolo et al., 1999). pcDNA-ΔN-XTcf-3 was linearized with XbaI and transcribed with T7 polymerase. ΔN-XTcf-3 and derrière plasmids were gifts from Drs H. Clevers and H. L. Sive respectively. pCS2-En-VegT was constructed essentially as described by Horb and Thomsen (1997) and pCS2-tAlk-4 was constructed as described by Chang et al. (1997).
Embryo manipulations
Xenopus embryos obtained by in vitro fertilization were maintained in 0.1× Barth medium and staged according to Nieuwkoop and Faber (1994). RNA injections were performed at the 4-cell stage. At stage 8, vegetal explants were dissected in 1× Barth medium and allowed to heal for 10 minutes. Animal caps were cut in CMFM/LCMR 1:1 (Piccolo et al., 1999) and placed on top of vegetal explants in Terasaki plates (Nunc) for 2 hours before being either processed by RT-PCR or transferred into 0.3× Barth medium until stage 36 for histological analysis. For cycloheximide (CHX) treatment, caps were cut at stage 8, pretreated 30 minutes with CHX (25 μg/ml), treated for 90 minutes with 4 nM Vg1 protein (Piccolo et al., 1999) and CHX (25 μg/ml) and then processed for RT-PCR. This treatment with CHX inhibited [35S]methionine and [35S]cysteine incorporation into proteins by 94%. In situ hybridization was performed on hemisectioned embryos in order to visualize endodermal cells as described by Belo et al. (1997; http://www.lifesci.ucla.edu/hhmi/derobertis/index.html).
RT-PCR analysis
Primer sets used for RT-PCR are detailed below. Activin βB, 288 bp, forward 5′-CGGATCCAGTTTTACATTGAC-3′, reverse 5′-CGAATTCTGCAGCACGAGTTC-3′, 30 cycles. α-Actin, 252 bp, forward 5′-TCCCTGTACGCTTCTGGTCGTA-3′, reverse 5′-TCTCAAAGTCCAAAGCCACATA-3′, 20 cycles. Brachyury (XBra), 319 bp, forward 5′-GCTGGAAGTATGTGAATGGAG-3′, reverse 5′-TTAAGTGCTGTAATCTCTTCA-3′, 25 cycles. chordin (chd), 267 bp, forward 5′-CCTCCAATCCAAGACTCCAGCAG-3′, reverse 5′-GGAGGAGGAGGAGCTTTGGGACAAG-3′, 25 cycles. derrière, 178 bp, forward 5′-GACAGCAAGATGAACAGGAA-3′, reverse 5′-CTACAAATGATCGATTGCCT-3′, 25 cycles. Dickkopf1 (Dkk1), 255 bp, forward 5′-CACCAAGCACAGGAGGAA-3′, reverse 5′-TCAGGGAAGACCAGAGCA-3′, 25 cycles. Elongation Factor α (EF1α), 221 bp, forward 5′-CCTGAACCACCCAGGCCAGATTGGTG-3′, reverse 5′-GAGGGTAGTCAGAGAAGCTCTCCACG-3′, 20 cycles. follistatin, 230 bp, forward 5′-CAGTGCAGCGCTGGAAAGAAAT-3′, reverse 5′-TGCGTTGCGGTAATTCACTTAC-3′, 30 cycles. Frzb1, 200 bp, forward 5′-GGAGATGCAGACTCCTCTGTCA-3′, reverse 5′-GACCACTGAATGTAGCCAGGAC-3′, 25 cycles. goosecoid (gsc), 305 bp, forward 5′-CACACAAAGTCGCAGAGTCTC-3′, reverse 5′-GGAGAGCAGAAGTTGGGGCCA-3′, 25 cycles. NCAM, 138 bp, forward 5′-GCGGGTACCTTCTAATAGTCA C-3′, reverse 5′-GGCTTGGCTGTGGTTCTGAAGG-3′, 25 cycles. noggin, 281 bp, forward 5′-AGTTGCAGATGTGGCTCT-3′, reverse 5′-AGTCCAAGAGTCTCAGCA-3′, 30 cycles. Ornithine decarboxylase (ODC), 228 bp, forward 5′-CAGCTAGCTGTGGTGTGG-3′, reverse 5′-CAACATGGAAACTCACACC-3′, 25 cycles. Siamois (sia), 205 bp, forward 5′-AAGATAACTGGCATTCCTGAGC-3′, reverse 5′-GGTAGGGCTGTGTATTTGAAGG-3′, 25 cycles. Vg1, 160 bp, forward 5′-ATGCCTATTGCTTCTATTTGC-3′, reverse 5′-GGTTTACGATGGTTTCACTCA-3′, 25 cycles. Xnr1, 190 bp, forward 5′-AAGTCAAGTCCTCTGCCAAC-3′, reverse 5′-AGAGGTTTCCCATTTTCGAC-3′, 25 cycles. Xnr2, 211 bp, forward 5′-TAAGGGCTGAGGTTGAAGAAG-3′, reverse, 5′-CGGGGTCTTCTGGTATCTGTC-3′, 25 cycles. Xnr3, 219 bp, forward 5′-CGAGTGCAAGAAGGTGGACA-3′, reverse 5′-ATCTTCATGGGGACACAG GA-3′, 25 cycles. Xnr4, 300 bp, forward 5′-GAAATGGAGGTGATGGTAGAC-3′, reverse, 5′-GACCATCATCACTATCTGCTG-3′, 25 cycles.
RESULTS
Cer-S is an inhibitor of Xnrs and of organizer formation
The starting point for this investigation was the observation that cer-S mRNA microinjection blocked the formation of the dorsal lip and the expression of goosecoid and other Spemann organizer markers (Fig. 1A-D and G, lanes 1 and 2). Previous biochemical work had shown that the Cer-S secreted protein bound to Xnr1 protein (but not to other mesoderm inducing factors such as Activin, Vg1 and BMP4) and inhibited its activity in the subnanomolar range (Piccolo et al., 1999). To further test the specificity of the cer-S construct when injected as synthetic mRNA into embryos, we made use of an assay involving the formation of ectopic blastopore lips (Lustig et al., 1996). Various TGF-β family mRNAs were carefully titrated and the amount of mRNA required to induce ectopic blastopore lips after microinjection into a single animal blastomere at the 8-cell stage was determined. As shown in Fig. 2A to D’, the activities of activin (Green et al., 1992; Gurdon et al., 1995), derrière (Sun et al., 1999) and A-Vg1 (Piccolo et al., 1999; Thomsen and Melton, 1993) mRNAs were unaffected by co-injection of cer-S mRNA, whereas Xnr1 was blocked. In addition to Xnr1 (50 pg), Xnr2 (150 pg), Xnr4 (50 pg) and mouse Nodal (50 pg) mRNAs (Jones et al., 1995; Zhou et al., 1993; Joseph and Melton, 1997; Conlon et al., 1994) were also blocked in this assay (data not shown), demonstrating that cer-S is an inhibitor of multiple Xnrs. The differences in response observed between Xnrs and other TGF-β factors was not due to differences in the amount of mRNA injected, as all mRNAs were tritrated to the minimal dose required to induce ectopic lips. Furthermore, 150 pg of Xnr1 or of Xnr4 were still inhibited by co-injection of 150 pg cer-S mRNA, whereas derrière mRNA (150 pg) was not affected by the same dose of cer-S mRNA (Fig. 2 and data not shown).
Fig. 1.
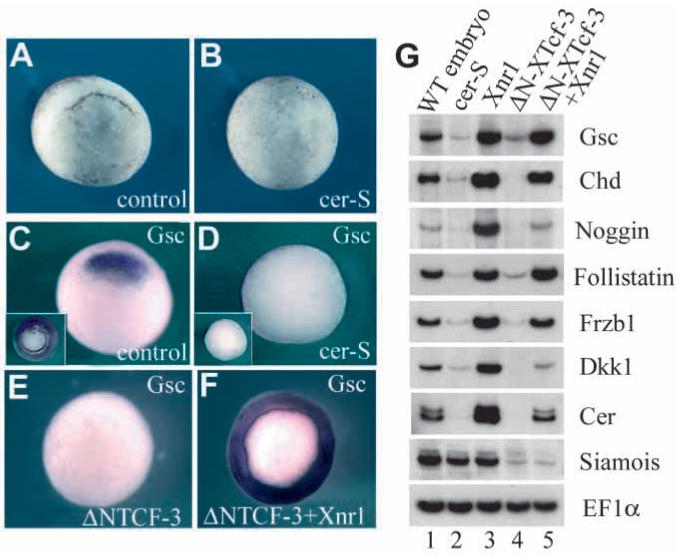
Cer-S, a secreted inhibitor of Nodal-related factors, inhibits formation of Spemann’s organizer. (A,B) cer-S mRNA (radial injection of 150 pg into each blastomere at the 4-cell stage) blocks dorsal lip formation. (C,D) gsc expression is blocked by cer-S, even after LiCl treatment that expands gsc expression to the entire mesoderm (insets). (E,F) ΔN-XTcf-3 mRNA (800 pg radially) inhibits goosecoid expression, and co-injection of Xnr1 (50 pg) restores it in the entire marginal zone. (G) RT-PCR analysis of Spemann organizer markers at stage 10. Lane 1: whole embryos. Lane 2: radial injection of cer-S (600 pg total) represses organizer genes. Lane 3: radial injection of Xnr1 mRNA (50 pg) upregulates organizer markers. Lane 4: the β-catenin pathway antagonist ΔN-XTcf-3 (800 pg) inhibits organizer markers, which are rescued by co-injection with 50 pg Xnr1 (lane 5). Xnr1 acts downstream of, or in parallel to, the β-catenin pathway. Siamois (Lemaire et al., 1995) is regulated by the β-catenin pathway (Brannon et al., 1997) independently of Xnr signals.
Fig. 2.
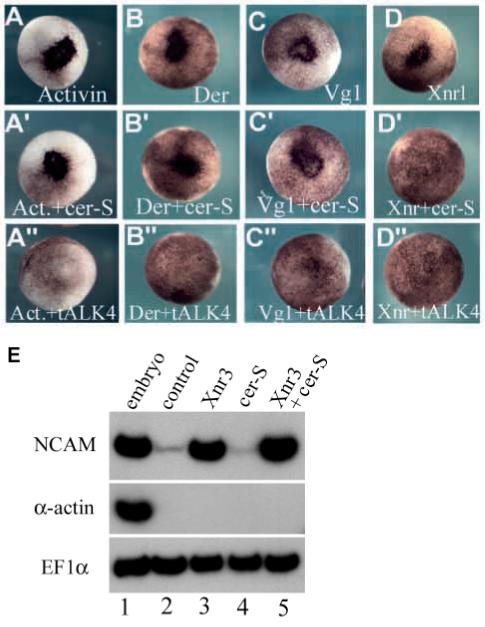
Cer-S inhibits Xnrs but not activin, derrière, Vg1 and Xnr3. Co-injection of cer-S mRNA (150 pg into a single animal blastomere) inhibited ectopic blastopore lip formation by Xnr1 (50 pg, D’), but not by activin (30 pg), derrière (150 pg) or A-Vg1 (50 pg) mRNA (A’-D’). Xnr2 mRNA (150 pg) was also inhibited (data not shown). Although the doses used for each TGF-β mRNA differed, they all were titrated to elicit comparable biological responses. tALK4 (800 pg) mRNA blocked all TGF-β mesoderm inducers tested (A”-D”). The ectopic blastopore lips are seen as a darker area in the injected animal cap region due to the apical constrictions of bottle cells (Lustig et al., 1996). (E) Xnr3 (1.2 ng) is a neural (NCAM) but not mesodermal (α-actin) inducer in microinjected animal caps (lanes 2 and 3). cer-S mRNA (600 pg) does not inhibit Xnr3 activity in animal caps (lanes 4 and 5). EF1α was used as a loading control.
Xnr3 is a gene related to Xnrs that functions as a neural inducer (Hansen et al., 1997). The finding that cer-S mRNA does not inhibit neural induction by Xnr3 (Fig. 2E, compare lanes 3 and 5) strongly supports the view that cer-S is a specific antagonist of mesoderm-inducing Nodal-related factors. In addition, the inability of cer-S mRNA (600 pg) to induce N-CAM in animal caps (Fig. 2E, lane 4) is in agreement with the previous finding (Piccolo et al., 1999) that cer-S is devoid of anti-BMP activity. Although cer-S mRNA does not block signalling by the known TGF-β mesoderm inducers - activin, derrière, Vg1 and BMP4 - it is currently not possible to rule out the existence of an as yet undiscovered TGF-β mesoderm inducer that might be inhibited by cer-S. Keeping this caveat in mind, in this study cer-S mRNA is considered a specific anti-Xnr reagent.
The cer-S injection experiments (Fig. 1A-D and G, lanes 1 and 2) indicated that Nodal-related signals are required for the formation of the Spemann organizer at the gastrula stage. In the reciprocal experiment, radial injection of Xnr1 mRNA into each blastomere of 4-cell embryos was sufficient to increase the expression of the organizer marker genes goosecoid, chd, noggin, follistatin, Frzb1, Dkk1 and cerberus (Fig. 1G, lane 3). Since organizer formation requires an active β-catenin pathway (Heasman, 1997), we next asked whether Xnr1 was able to rescue organizer tissue formation when this pathway was blocked. Microinjection of a specific inhibitor of the β-catenin pathway, ΔN-XTcf-3 (Molenaar et al., 1996), blocked organizer formation, which could be rescued by co-injection of Xnr1 mRNA (Fig. 1E,F and G, lanes 4 and 5).
These results suggest that Xnr signals are necessary and sufficient for formation of Spemann organizer tissue in the Xenopus gastrula. The ability of Xnr1 mRNA to rescue organizer tissue in embryos injected with ΔN-XTcf-3 further suggests that Xnrs act downstream or in parallel of β-catenin, mediating some of its biological activities. Results from genetic and microinjection experiments in zebrafish are consistent with this possibility (Fekany et al., 1999; Feldman et al., 1998).
Cer-S blocks mesoderm induction in Nieuwkoop recombinants
In Xenopus, it is well established that Spemann organizer tissue is induced by dorsal endoderm. The classical approach to study this inductive event is the Nieuwkoop animal-vegetal conjugate. We therefore used this experimental paradigm (Nieuwkoop, 1969; Wylie et al., 1996) to investigate the nature of the endogenous mesoderm-inducing signals (Fig. 3A).
Fig. 3.
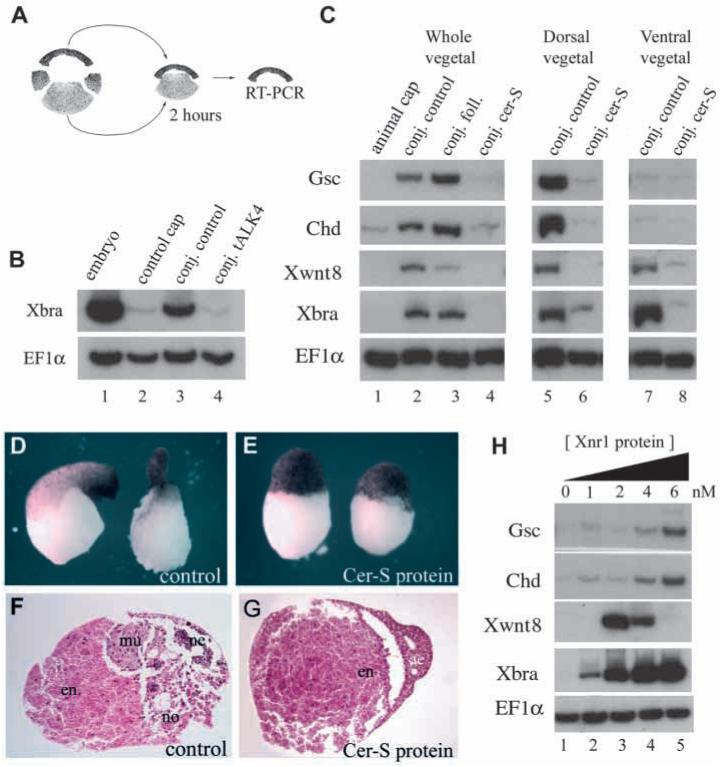
The endogenous mesoderm-inducing signals are blocked by Cer-S in Nieuwkoop animal-vegetal conjugates. (A) Experimental design. (B) Microinjection of tALK4 mRNA (500 pg into each animal blastomere at 8-cell stage) blocks the response of animal caps to endogenous mesoderm-inducing signals (compare lanes 3 and 4). Caps were in contact with endoderm for 2 hours and are compared to control animal caps incubated without endoderm (lane 2). EF1α is a control for RNA recovery. (C) Lanes 1-4, Nieuwkoop recombinants of uninjected animal caps with vegetal pole explants injected with follistatin (2 ng) or cer-S (600 pg) mRNA. Note in lane 4 that cer-S blocks dorsal (gsc, chd), ventral (Xwnt-8) and pan-mesodermal (Xbra) markers, whereas in lane 3 follistatin mRNA has only a slight dorsalizing effect (total conjugates n=45, two experiments). This amount of follistatin mRNA was sufficient to abolish the activity of activin mRNA in co-injection assays (not shown). Lane 5, dorsal endoderm (Nieuwkoop center) induces preferentially the organizer markers gsc and chd (n=16, three independent experiments). Lane 7, ventral endoderm induces ventral markers Xwnt-8 and the pan-mesodermal marker Xbra (n=17). Lanes 6 and 8, cer-S mRNA in the endodermal fragment prevents both dorsal and ventral mesoderm inductions (n=15 each). Conjugates were prepared between stage 8 and 8.5 and harvested for RNA after two hours. (D-G) External and histological morphology of vegetal fragments conjugated in the presence of control conditioned medium or of 20 nM Cer-S (Piccolo et al., 1999) protein and cultured until stage 36. Note that sections of the control contain muscle (mu), notochord (no) and some neural tissue (ne), whereas in the protein-treated sample the animal cap remains as atypical epidermis (ae) and endoderm (en) (n=26, three independent experiments). (H) Animal caps treated for two hours with control oocyte conditioned medium (lane 1) or with increasing doses (lanes 2-5) of Xnr1 protein (Piccolo et al., 1999). Increasing concentrations of Xnr1 protein induce first ventral and then dorsal mesodermal markers, producing thresholds after 2 hours in culture.
We first asked whether a TGF-β-like signal secreted by endoderm is required for mesoderm induction. To this end, a truncated activin type IB receptor (Chang et al., 1997), tALK4, was expressed in the animal cap cells that receive the signal. As shown in Fig. 3B, tALK4 mRNA blocked induction of the pan-mesodermal marker Xbra by the endogenous endodermal signal. This implicated a requirement for TGF-β signalling in Nieuwkoop conjugates after only 2 hours of contact, but did not distinguish which factor was involved, since tALK4 was able to block signalling by the mesoderm inducing factors activin, derrière, A-Vg1 and Xnr1 (Fig. 2A” to D”).
We next tested whether the endodermal signal required Nodal-related factors by microinjecting cer-S mRNA into the vegetal pole of early embryos. Endodermal explants from these embryos were prepared at stage 8 to 8.5, recombined with uninjected animal caps and analyzed only after 2 hours of contact with vegetal explants; i.e., during the period in which mesoderm induction occurs in vivo (Wylie et al., 1996). PCR was carried out in the animal cap fragments as described by Wylie et al. (1996); as a control for accuracy of the dissections, vegetal fragments were analyzed for the mesodermal markers Xbra and Xwnt8, which were not expressed in either uninjected or cer-S mRNA-injected vegetal explants (not shown). It was found that in these Nieuwkoop recombinants cer-S inhibited not only the induction of the organizer markers goosecoid and chd, but also the ventral marker Xwnt8 and the pan-mesodermal marker Xbra (Fig. 3C, compare lanes 2 and 4). As a negative control we used follistatin mRNA (Fig. 3C, lane 3), an inhibitor of Activin and BMPs (Harland and Gerhart, 1997), which failed to prevent mesoderm induction in agreement with previous work (Slack, 1991b).
Since dorsal and ventral endoderm have different inductive activities (Boterenbrood and Nieuwkoop, 1973), we extended these results using dorsal-vegetal or ventral-vegetal endodermal explants, which induced predominantly dorsal or ventral mesoderm, respectively, in animal cap recombinants (Fig. 3C, lanes 5 and 7). Expression of cer-S mRNA in endoderm resulted in the inhibition of mesoderm induction by both dorsal and ventral endodermal fragments (Fig. 3C, lanes 6 and 8).
As the endogenous mesoderm-inducing signal is released at the blastula stage (Wylie et al., 1996), we tested whether Cer-S protein could block mesoderm induction when added at this time. Animal-vegetal explants were recombined in the presence of 20 nM Cer-S protein (Piccolo et al., 1999) at midblastula (stage 8). The resulting conjugates failed to form either dorsal or ventral mesoderm, with the animal cap differentiating as epidermis and the vegetal fragment as endoderm (Fig. 3D-G).
In the reciprocal gain-of-function experiment, Xnr1 protein was added to stage 8 animal caps and incubated for 2 hours. At low concentrations (2 nM) Xnr1 protein induced ventral mesoderm and at higher doses (6 nM) dorsal mesoderm, producing sharp thresholds after only two hours of incubation (Fig. 3H, lanes 3-5). These results confirm and extend previous work of Jones et al. (1995), who used injected Xnr2 mRNA. The loss- and gain-of-function experiments presented here indicate that Nodal-related signals are necessary and sufficient for the induction of both dorsal and ventral mesoderm at the blastula stage.
Graded expression of Xnrs in blastula endoderm
To determine whether Xnrs are expressed at the right time and place to mediate endogenous inductive activities during the blastula stage, we re-examined their expression patterns. Using RT-PCR analysis, Xnr1, Xnr2 and Xnr4 were found to be expressed after midblastula, starting to accumulate at the same time as early zygotic genes transcribed at the midblastula transition (Fig. 4A) such as siamois (Lemaire et al., 1995; Brannon et al., 1997) and Xnr3 (Hansen et al., 1997; McKendry et al., 1997) that are direct targets of early β-catenin signalling. Organizer-specific markers such as goosecoid start to be expressed after Xnrs (Fig. 4A). At stage 9, when mesoderm induction takes place, embryo dissections showed that Xnr1, Xnr2 and Xnr4 transcripts were enriched in the dorsal half and in the vegetal region of the embryo (Fig. 4B). Previously, Xnr expression was detected in blastula endoderm, but was thought to be uniform (Jones et al., 1995; Yasuo and Lemaire, 1999; Clements et al., 1999). Our results suggested a possible dorsal to ventral gradient composed of multiple Nodal-related factors in the endoderm of the blastula.
Fig. 4.
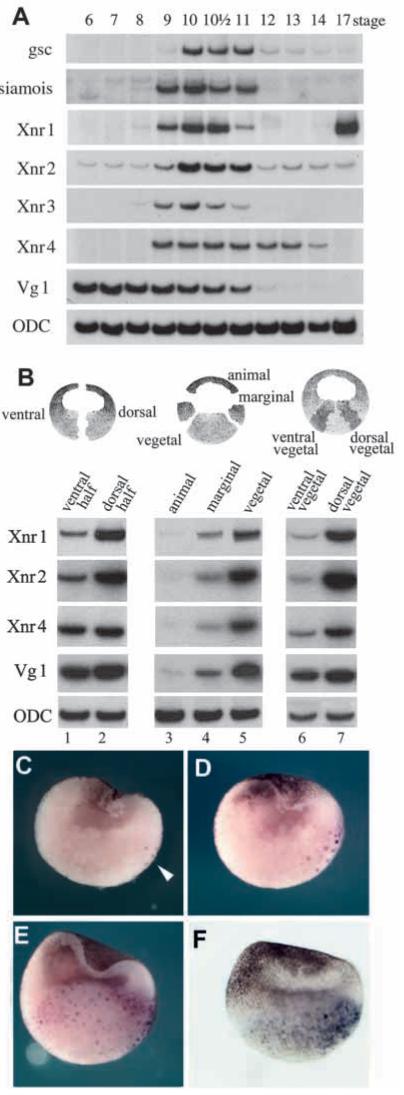
Endogenous Xnrs are expressed at the right time and place to function as mesoderm inducers. (A) Time course of gene expression analyzed by RT-PCR at various developmental stages (Nieuwkoop and Faber, 1994). The mesoderm inducers Xnr1, Xnr2 and Xnr4 start zygotic expression at the same time as Siamois and Xnr3 (which are expressed immediately after midblastula and are direct targets of β-catenin regulation). ODC is used as a loading control. (B) Dissections of embryos at stage 9 showing that Xnr1, Xnr2 and Xnr4 are expressed in the endoderm and at higher levels dorsally than ventrally. Vg1 is expressed uniformly in the vegetal pole. (C-F) Xnr1 in situ hybridizations of blastula stage embryos showing a gradient of expression in endoderm. (C) Stage 8 embryo showing a few nuclei stained in the dorsal vegetal mass (arrowhead). (D) Stage 8.5 blastula embryo in which Xnr1 expression has expanded into neighboring vegetal cells. (E) Stage 9 blastula embryo displaying graded Xnr1 expression throughout the embryonic endoderm. (F) External view of a stage 9 embryo cleared in Murray’s solution in order to visualize Xnr1 staining in the vegetal hemisphere. In this embryo, the ventral side, with its more pigmented animal cap, can be clearly distinguished from the less pigmented dorsal side. Note that Xnr1 expression on the dorsal side is of longer duration, in addition to reaching higher levels than in ventral endoderm.
To confirm the existence of graded Xnr signals in the endoderm of the blastula, the expression of Xnr1 was re-examined using a more sensitive in situ hybridization procedure, in which the embryos are fixed and hemisectioned facilitating the penetration of the probe into endodermal cells. As can be seen in Fig. 4C, Xnr1 expression started at midblastula in superficial large yolky endodermal cells, on one side of the embryo. Using regularly cleaving pigmented embryos with distinct dorsoventral polarity, we established that these cells were located in the dorsal side. The expressing cells correspond to the superficial cells in which nuclear translocation of β-catenin was first discovered by Schneider et al. (1996). At stage 8.5, Xnr1 transcripts expanded to deeper neighboring cells (Fig. 4D). At stage 9, Xnr1 expression was detected throughout the vegetal mass, while still displaying a dorsal to ventral gradient expression (Fig. 4E). This graded expression at stage 9 was also seen in external views of embryos rendered transparent by treatment with Murray’s solution (Fig. 4F). By the gastrula stage Xnr1 transcripts became undetectable in the endoderm and were found instead in the dorsal marginal zone as described previously (Jones et al., 1995 and data not shown).
We conclude that Xnrs are expressed at the correct time and place to participate in mesoderm induction by endoderm. In the case of Xnr1, the in situ hybridizations suggest that a gradient of activity could be established not only by increased mRNA levels on the dorsal side, but also by the longer duration of its expression in dorsal endoderm.
cer-S blocks Xbra expression in a dose-dependent way
To test a possible gradient of Xnr activity, we examined the response of the mesodermal ring of Xbra expression to increasing doses of cer-S. Vegetal injection of cer-S mRNA into each blastomere at the 4-cell stage (Fig. 5A) caused a dose-dependent reduction of the extent of Xbra expression in the marginal zone at the gastrula stage (Fig. 5B-F). At the highest concentrations (150 pg per blastomere) Xbra expression was abolished. This experimental design follows on the footsteps of Thisse and Thisse (1999), who applied it to the inhibition of zebrafish mesoderm formation by antivin, a TGF-β type molecule that can block both activin and nodal signalling via interactions with activin receptors (Meno et al., 1999). Using lacZ mRNA as a lineage tracer, it was found that at intermediate doses Xbra is inhibited in the ventral side of the embryo (Fig. 4F). Since low doses inhibit ventrally and high doses dorsally, these results strongly support the idea that a dorsal-ventral gradient of Xnr activity exists in vivo.
Fig. 5.
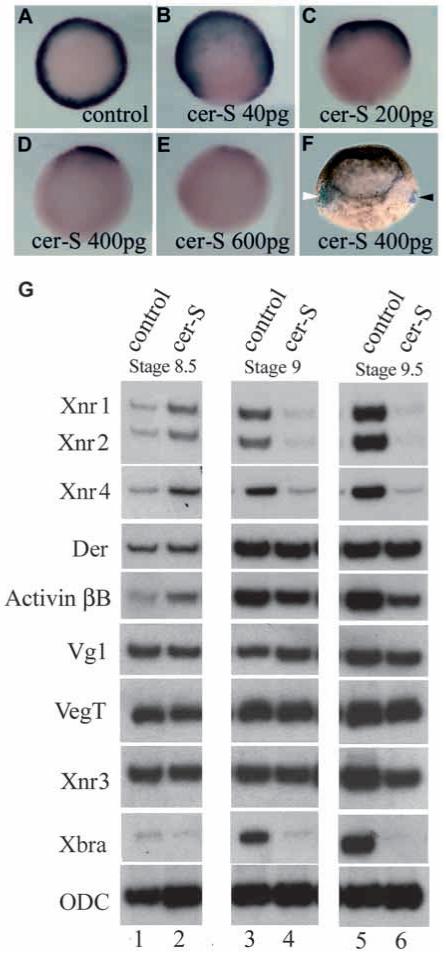
Injections of cer-S mRNA dose-dependently reduce Xbra expression in gastrula embryos. Embryos are injected radially in the vegetal pole at the 4 cell stage, then processed for Xbra in situ staining at stage 10.5. (A) Control uninjected embryo, Xbra is expressed as a mesodermal ring. (B-E) Embryos injected with increasing amounts of cer-S mRNA, showing graded reduction of the Xbra expression domain. (F) Embryos injected vegetally with 400 pg of cer-S mRNA at the 4-cell stage and with lacZ lineage tracer mRNA into blastomere C4 at the 32-cell stage. In this lateral view the white arrowhead indicates lacZ in the ventral side (note that the pigment in the animal cap also marks the ventral side) and the black arrowhead points to the expression of Xbra transcripts on the dorsal side (n=51). (G) RT-PCR analysis of Xenopus embryos injected with 600 pg of cer-S mRNA. RNAs were harvested from uninjected controls or cer-S-injected embryos at one-hour intervals at stages 8.5 (lanes 1, 2), 9.0 (lanes 3, 4) and 9.5 (lanes 5, 6). Xnr1, 2 and 4 transcripts are initially not inhibited by cer-S (lanes 1, 2), but are decreased at later stages (a positive feedback loop for Nodal-related gene expression has been described; Meno et al., 1999). Importantly, the levels of derrière, Vg1, VegT and Xnr3 remained unchanged, and activin βB was only partially decreased. Note that cer-S mRNA inhibited the initial expression of Xbra and that cer-S can inhibit Xbra transcriptional activation even in the presence of derrière, activin and Vg1 transcripts.
Recent studies involving the dissociation and reaggregation of Xenopus embryos have shown that some aspects of endoderm development require cell-cell interactions (Yasuo and Lemaire, 1999; Clements et al., 1999). To test whether cer-S mRNA affected the post-midblastula expression of known TGF-β mesoderm-inducing candidates, we analyzed embryos injected radially with 150 pg cer-S mRNA. As shown in Fig. 5G, the initial expression of Xnr1, Xnr2 and Xnr4 was not inhibited by cer-S at stage 8.5, but was decreased at later blastula stages. This inhibition can be explained by the positive feedback loop proposed for Nodal-related genes in zebrafish (Meno et al., 1999). Importantly, the expression of derrière (Sun et al., 1999) was not affected, and activin βB (Dohrmann et al., 1993) was only partially decreased by cer-S mRNA injection. Transcript levels of VegT, Vg1 and Xnr3 were not affected, whereas initial expression of Xbra at blastula stages was blocked by cer-S mRNA (Fig. 5G).
These microinjection experiments show a dose-dependent inhibition of Xbra by cer-S in intact embryos. It is noteworthy that the block in mesoderm formation takes place in the presence of continued expression of the putative mesoderm-inducing mRNAs derrière, activin and Vg1 at the blastula stage (Fig. 5G). The results indicate that mesoderm induction in Xenopus requires an endogenous activity gradient of Xnrs.
The maternal determinants β-catenin, VegT and Vg1 regulate Xnr expression
Oligonucleotide depletion experiments have demonstrated that two maternal mRNAs, β-catenin and VegT, are required for the production of zygotic mesoderm-inducing signals in Nieuwkoop conjugates (Wylie et al., 1996; Zhang et al., 1998). To investigate whether these intracellular molecules function as upstream regulators of Xnr expression at the blastula stage, we performed the gain- and loss-of-function analyses shown in Fig. 6. At stage 9, expression of Xnr1 in whole embryos was increased by β-catenin mRNA and decreased by dominant-negative ΔN-XTcf-3 mRNA injections (Fig. 6A, lanes 4-6). This requirement of the β-catenin pathway for Xnr expression was transient and was no longer seen at stage 10 (Fig. 6A, lanes 7-9), indicating the existence of additional, β-catenin independent, regulatory mechanisms.
Fig. 6.
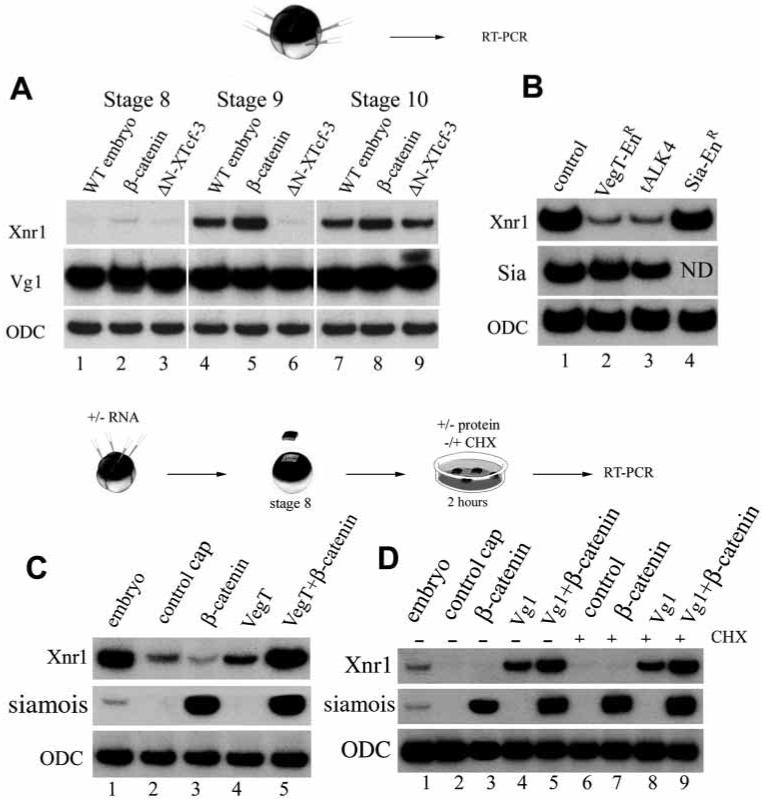
Zygotic expression of Xnr1 is regulated by β-catenin, VegT and Vg1. (A) Xnr1 is upregulated by β-catenin (200 pg) and inhibited by ΔN-XTcf-3 mRNA (800 pg) at stage 9 (lanes 4-6) in radially injected embryos. At stage 10, however, Xnr1 transcripts are expressed even in the presence of ΔN-XTcf-3; this is consistent with the formation of ventral mesoderm in these ventralized embryos. Maternal Vg1 is not affected. (B) Whole embryos injected radially at 4-cell stage with 800 pg VegT-EnR mRNA (lane 2), 2 ng of tALK4 (lane 3), or 120 pg of Sia-EnR (lane 4) and analyzed by RT-PCR at stage 9. Xnr1 requires VegT activity and TGF-β-like signalling for expression at the blastula stage. Xnr1 expression at blastula is not dependent on Siamois activity since it is not blocked by Sia-EnR mRNA. (C) Animal cap experiments showing that VegT mRNA (200 pg) can weakly induce Xnr1 and synergizes with β-catenin (100 pg) mRNA (lanes 4 and 5). β-catenin mRNA on its own is unable to induce Xnr1 in animal caps (lane 3). (D) Treatment of animal caps with 4 nM Vg1 protein at stage 8 for 2 hours induces Xnr1, and β-catenin (100 pg mRNA) enhances this induction (lanes 2-5). The same response was obtained after blocking protein synthesis with cycloheximide (lanes 6-9), indicating that Xnr1 is a primary response gene to Vg1 protein. Although only results for Xnr1 transcripts are shown, Xnr2 and Xnr4 were also analyzed in all samples of A, C and D with comparable results (data not shown).
Injection of a dominant-negative construct consisting of the engrailed (En) repressor domain fused to the VegT transcription factor (Horb and Thomsen, 1997), VegT-EnR, repressed expression of Xnr1, Xnr2 and Xnr4 in stage 9 embryos (Fig. 6B, lanes 1 and 2 and data not shown). This suggested a requirement for VegT in the regulation of Xnr expression. In animal cap explants, overexpression of VegT (but not of β-catenin alone) led to weak expression of Xnr1 at stage 9 (Fig. 6C). This induction was potentiated by co-injection of β-catenin and VegT mRNAs (Fig. 6C, lanes 4 and 5), suggesting that VegT and β-catenin act synergistically.
Injection of tALK4 mRNA (Chang et al., 1997) into whole embryos revealed that zygotic expression of Xnr1, Xnr2 and Xnr4 at the blastula stage also requires a TGF-β-like signal (Fig. 6B, lane 3 and data not shown). The best endogenous candidate molecule for this signal is Vg1, whose mRNA is maternal and localized (like VegT) uniformly in the vegetal pole of the egg (Kessler and Melton, 1994). Mature Vg1 protein secreted by microinjected oocytes (Piccolo et al., 1999) was added to stage 8 animal caps for 2 hours. During this period, Xnr1 mRNA was induced by Vg1 protein and this response was potentiated in β-catenin-injected explants (Fig. 6D, lanes 4 and 5). Xnr1 induction was a primary response to Vg1 protein, since it also took place in the presence of 25 μg/ml cycloheximide (Fig. 6D, lanes 8 and 9), which in these experiments inhibited protein synthesis by 94%. The results suggest that vegetally localized cytoplasmic determinants such as VegT and Vg1 may generate a gradient of expression of mesoderm-inducing Xnrs in blastula endoderm by synergizing, directly or indirectly, with the dorsal determinant β-catenin.
DISCUSSION
The cerberus-short reagent provides an antagonist of multiple Xnrs, which was used here to study the mechanism of mesoderm induction in Xenopus. We find, first, that Cer-S either as injected mRNA or as soluble protein added at the blastula stage is able to block both the dorsal and the ventral mesoderm inducing signals released by endodermal explants. Second, endogenous Xnrs are expressed during blastula in a graded fashion in endoderm. Xnr1 expression starts on the dorsal side at midblastula and from there expands to the rest of the endoderm (Fig. 4C-E). Thus, dorsal endoderm (also known as the Nieuwkoop center) expresses Xnr1 not only at higher levels but also for a longer period of time than ventral endoderm during the blastula stage. Third, microinjection of cer-S mRNA into embryos causes a dose-dependent inhibition of Xbra expression in embryos in the presence of endogenous derrière, activin and Vg1 mRNAs (Fig. 5). Since ventral Xbra is blocked at low doses and dorsal expression at high doses of cer-S mRNA, this result is consistent with the requirement of an Xnr gradient of activity for induction of mesoderm. Fourth, maternal determinants such as VegT, Vg1 and β-catenin can cooperate in the zygotic activation of Xnr expression at the blastula stage.
These results suggest that the classical 3-signal model (Slack, 1991a; Heasman, 1997) for mesoderm induction in Xenopus could be modified in the way shown in Fig. 7. Maternal activities such as dorsal β-catenin and vegetal VegT and Vg1 cooperate to set up a zygotic dorsal to ventral gradient in the endoderm composed of multiple Xnrs at stage 9, when mesoderm induction takes place. At high Nodal-related concentrations, which require a functional β-catenin pathway in the dorsal side of the embryo, the Spemann organizer (expressing genes such as chordin, noggin and Frzb1) is induced in overlying cells by early gastrula. In the ventral side, VegT and Vg1 would lead to the production of lower levels of Nodal-related signals, and ventral mesoderm (expressing genes such as Xwnt8 and BMP4) would be induced. Similarly, in embryos ventralized by UV irradiation (Heasman, 1997) or by ΔN-XTcf-3 (Molenaar et al., 1996, Fig. 6A), the uniformly distributed VegT and Vg1 products would produce low levels of Xnrs sufficient to induce ventral mesoderm at the gastrula stage.
Fig. 7.
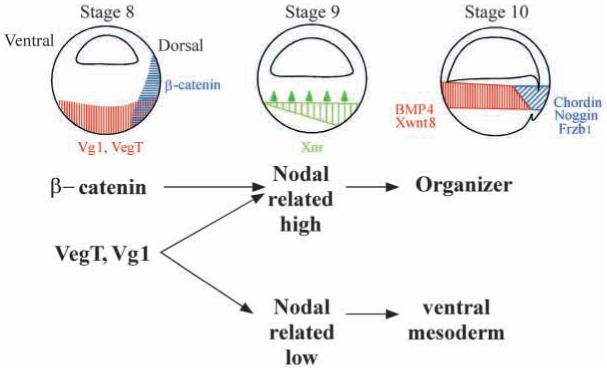
Model of mesoderm induction at the blastula stage by a dorsal to ventral gradient composed of multiple Nodal-related genes expressed in endoderm. This simplified model concerns the nature of the endodermal signals, but does not address the function of important regulators such as β-catenin and siamois in the marginal zone of the embryo. The model has predictive value as it may help explain how molecules as dissimilar as β-catenin, Vg1, Xnr1 and Xnr2, noggin and chordin mRNAs might rescue dorsal development via a sequential pathway when overexpressed in UV-ventralized embryos.
A particularly attractive aspect of the model in Fig. 7 is that it may help explain a long-standing puzzle in Xenopus embryology. A surprisingly large number of microinjected molecules are able to rescue, often completely, the UV ventralized phenotype that results from interfering with cortical rotation of the fertilized egg (Heasman, 1997). The UV-rescuing gene products include such diverse molecules as β-catenin (and other members of this signalling pathway; Harland and Gerhart, 1997), Vg1 (Thomsen and Melton, 1993), Xnr1 and Xnr2 (Jones et al., 1995), noggin (Smith and Harland, 1992) and chordin (Sasai et al., 1994). Although one can argue that each of these diverse genes acts via different redundant pathways, their common UV-rescue activity may be easier to unravel if considered as part of a cascade of sequential gene activations. In this view, overexpression of β-catenin or Vg1 would lead to high levels of Xnr expression in blastula endoderm, which in turn would mediate the induction of Spemann organizer in overlying cells, activing genes such as noggin and chordin that execute dorsal patterning at the gastrula stage (Fig. 7).
This sequential model of gene activation must be considered an oversimplification of the in vivo situation. It is likely that multiple signalling pathways synergize to pattern the gastrula. For example, our model does not take into account the role that β-catenin and its target genes siamois and Xtwn (Lemaire et al., 1995; Laurent et al., 1997) may have in the marginal zone itself. It is known that the promoter of the homeobox gene goosecoid, which is active in dorsal mesoderm (Steinbeisser et al., 1993), contains Siamois and Xtwn binding sites (Watabe et al., 1995; Laurent et al., 1997) in addition to a TGF-β responsive element. However, in embryos in which the function of β-catenin and expression of siamois are blocked by the inhibitor ΔN-XTcf-3, overexpression of Xnr1 mRNA is sufficient to activate goosecoid and other organizer markers (Fig. 1). Furthermore, in animal cap experiments Xnr1 protein is able to induce ventral and dorsal mesodermal markers with sharp thresholds in the low nanomolar range (Fig. 3H). Since Activin, Vg1 and Derrière are not inhibited by Cer-S, the present experiments do not address whether these molecules may cooperate with Xnrs in mesoderm patterning in vivo.
There is ample genetic support for a critical role of Nodal-related molecules in mesoderm formation in many vertebrates. In mouse, mutations in the gene nodal result in embryos severely deficient in mesodermal tissues (Zhou et al., 1993; Conlon et al., 1994). It has been argued that, because some mutant embryos contain patches of Brachyury expression, mouse Nodal is involved in the maintenance rather than in the initiation of mesoderm induction (Conlon et al., 1994). In Xenopus, the present results suggest a requirement for Nodal-related signalling in the initial mesoderm induction by endoderm. In zebrafish, two Nodal-related genes, cyclops and squint have been identified to date (Sampath et al., 1998; Erter et al., 1998; Feldman et al., 1998; Rebagliati et al., 1998). Both mutations affect axial mesoderm and in double mutants the effects are synergistic, leading to the loss of goosecoid expression in the organizer and lack of head and trunk mesoderm (Feldman et al., 1998). In cyclops;squint double homozygotes a horseshoe of Brachyury expression persists, as is the case in Xenopus embryos injected with intermediate doses of cer-S mRNA. In Xenopus, high doses of cer-S can block Xbra expression. This difference between the two species might be explained by residual Xnr signals in the zebrafish mutants, or by subtle differences in the transcriptional control of Brachyury. For example, it has been reported that overexpression of antivin abolishes mesodermal expression of snail1 and eve1 in conditions in which some Brachyury expression persists in injected zebrafish embryos (Thisse and Thisse, 1999).
In Xenopus, a proteolytic cleavage mutant of Xnr2 was shown to act as a dominant-negative agent in Xenopus, leading to inhibition of dorso-anterior endodermal markers and partial inhibition of mesodermal markers (Osada and Wright, 1999). Since the dominant-negative Xnr2 construct is active only in injected cells, the distribution of microinjected mRNA could play a role in the differences seen with the stronger effects reported here for cer-S mRNA. Cer-S is an antagonist of multiple Nodal-related signals that is secreted into the extracellular space and may achieve a more uniform distribution, causing, at high levels, a complete loss of mesoderm induction. It should be mentioned that Jones et al. (1996) reported that Xnr2 can only signal at short range in animal cap explants and that this range was increased in constructs in which processing and secretion was enhanced. Our results imply that Xnrs can diffuse from vegetal explants to animal caps recombinants. It is possible that endogenous Xnrs in endoderm are more efficiently processed, secreted and transported than Xnr2 expressed ectopically in animal caps.
The model of Xnr-mediated mesoderm induction in Xenopus (Fig. 7) is strongly supported by a recent study carried out by Kofron et al. (1999). In VegT-depleted embryos the induction of mesoderm is inhibited, and can be rescued by expression of Xnr1 mRNA in vegetal fragments of Nieuwkoop conjugates. In addition, they demonstrated that VegT response sites exist in the Xnr1 promoter.
It is noteworthy that studies using increasing concentrations of Activin had previously demonstrated that graded doses of a TGF-β family member were sufficient to induce and pattern ventral and dorsal mesoderm (Green and Smith, 1990; Green et al., 1992). The results presented here suggest that in Xenopus such a gradient of mesoderm-inducing factors can be provided by multiple Nodal-related signals expressed in the endoderm at the blastula stage.
Acknowledgments
We are indebted to Drs A. Clevers, D. Kessler, D. Melton, H. Sive and C. V. E. Wright for generous gifts of plasmids. We thank C. V. E. Wright, E. Delot, H. Hing, T. Hummel, G. Monteillet-Agius, I. Salecker, D. Schmucker and members of our laboratory for critically reviewing the manuscript, and A. Cuellar for technical assistance. E. A. was an ARC fellow and an HHMI Associate. M. O. was supported by DFG and HFSPO and O. W. by FFWF and HFSPO. The work was supported by grant R37 HD-21502-14 from the NIH. E. M. D. R. is an HHMI Investigator.
REFERENCES
- Belo JA, Bouwmeester T, Leyns L, Kertesz N, Gallo M, Follettie M, De Robertis EM. Cerberus-like is a secreted factor with neuralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mech. Dev. 1997;68:45–57. doi: 10.1016/s0925-4773(97)00125-1. [DOI] [PubMed] [Google Scholar]
- Bouwmeester T, Kim SH, Sasai Y, Lu B, De Robertis EM. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann’s organizer. Nature. 1996;382:595–601. doi: 10.1038/382595a0. [DOI] [PubMed] [Google Scholar]
- Boterenbrood EC, Nieuwkoop PD. The formation of the mesoderm in urodelean amphibians. V. Its regional induction by the endoderm. Wilhelm Roux’ Arch. Dev. Biol. 1973;173:319–332. doi: 10.1007/BF00575837. [DOI] [PubMed] [Google Scholar]
- Brannon M, Gomperts M, Sumoy L, Moon RT, Kimelman D. A beta-catenin/XTcf-3 complex binds to the siamois promoter to regulate dorsal axis specification in Xenopus. Genes Dev. 1997;11:2359–2370. doi: 10.1101/gad.11.18.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Wilson PA, Mathews LS, Hemmati-Brivanlou A. A Xenopus type I activin receptor mediates mesodermal but not neural specification during embryogenesis. Development. 1997;124:827–837. doi: 10.1242/dev.124.4.827. [DOI] [PubMed] [Google Scholar]
- Clements D, Friday RV, Woodland HR. Mode of action of VegT in mesoderm and endoderm formation. Development. 1999;126:4903–4911. doi: 10.1242/dev.126.21.4903. [DOI] [PubMed] [Google Scholar]
- Conlon FL, Lyons KM, Takaesu N, Barth KS, Kispert A, Herrmann B, Robertson EJ. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development. 1994;120:1919–1928. doi: 10.1242/dev.120.7.1919. [DOI] [PubMed] [Google Scholar]
- De Robertis EM, Kim SH, Leyns L, Piccolo S, Bachiller D, Agius E, Belo JA, Yamamoto A, Hainski-Brosseau A, Brizuela B, Wessely O, Lu B, Bouwmeester T. Patterning by genes expressed in Spemann’s organizer. Cold Spring Harbor Symp. Quant. Biol. 1997;62:169–175. [PubMed] [Google Scholar]
- Dohrmann CE, Hemmati-Brivanlou A, Thomsen GJ, Fields A, Woolf TM, Melton DA. Expression of activin mRNA during early development in Xenopus laevis. Dev. Biol. 1993;157:474–483. doi: 10.1006/dbio.1993.1150. [DOI] [PubMed] [Google Scholar]
- Ecochard V, Cayrol C, Foulquier F, Zaraisky A, Duprat AM. A novel TGF-β-like gene, fugacin, specifically expressed in the Spemann organizer of Xenopus. Dev. Biol. 1995;172:699–703. doi: 10.1006/dbio.1995.8052. [DOI] [PubMed] [Google Scholar]
- Erter CE, Solnica-Krezel L, Wright CV. Zebrafish nodal-related 2 encodes an early mesendodermal inducer signaling from the extraembryonic yolk syncytial layer. Dev. Biol. 1998;204:361–372. doi: 10.1006/dbio.1998.9097. [DOI] [PubMed] [Google Scholar]
- Fekany K, Yamanaka Y, Leung TC, Sirotkin HI, Topczewski J, Gates MA, Hibi M, Renucci A, Stemple D, Radbill A, Schier AF, Driever W, Hirano T, Talbot WS, Solnica-Krezel L. The zebrafish bozozok locus encodes Dharma, a homeodomain protein essential for induction of gastrula organizer and dorsoanterior embryonic structures. Development. 1999;126:1427–1438. doi: 10.1242/dev.126.7.1427. [DOI] [PubMed] [Google Scholar]
- Feldman B, Gates MA, Egan ES, Dougan ST, Rennebeck G, Sirotkin HI, Schier AF, Talbot WS. Zebrafish organizer development and germ-layer formation require nodal-related signals. Nature. 1998;395:181–185. doi: 10.1038/26013. [DOI] [PubMed] [Google Scholar]
- Green JBA, Smith JC. Graded changes in dose of a Xenopus activin A homologue elicit stepwise transitions in embryonic cell fate. Nature. 1990;347:391–394. doi: 10.1038/347391a0. [DOI] [PubMed] [Google Scholar]
- Green JBA, New HV, Smith JC. Responses of embryonic Xenopus cells to activin and FGF are separated by multiple dose thresholds and correspond to distinct axes of the mesoderm. Cell. 1992;71:731–739. doi: 10.1016/0092-8674(92)90550-v. [DOI] [PubMed] [Google Scholar]
- Gritsman K, Zhang J, Cheng S, Heckscher E, Talbot WS, Schier AF. The EGF-CFC protein one-eyed pinhead is essential for Nodal signaling. Cell. 1999;97:121–132. doi: 10.1016/s0092-8674(00)80720-5. [DOI] [PubMed] [Google Scholar]
- Gurdon JB, Mitchell A, Mahony D. Direct and continuous assessment by cells of their position in a morphogen gradient. Nature. 1995;376:520–521. doi: 10.1038/376520a0. [DOI] [PubMed] [Google Scholar]
- Hansen CS, Marion CD, Steele K, George S, Smith WC. Direct neural induction and selective inhibition of mesoderm and epidermis inducers by Xnr3. Development. 1997;124:483–492. doi: 10.1242/dev.124.2.483. [DOI] [PubMed] [Google Scholar]
- Harland R, Gerhart J. Formation and function of Spemann’s organizer. Ann. Rev. Cell. Dev. Biol. 1997;13:611–667. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- Heasman J. Patterning the Xenopus blastula. Development. 1997;124:4179–4191. doi: 10.1242/dev.124.21.4179. [DOI] [PubMed] [Google Scholar]
- Heasman J, Crawford A, Goldstone K, Garner-Hamrick P, Gumbiner B, McCrea P, Kintner C, Noro CY, Wylie C. Overexpression of cadherins and underexpression of β-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell. 1994;79:791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Horb ME, Thomsen GH. A vegetally localized T-box transcription factor in Xenopus eggs specifies mesoderm and endoderm and is essential for embryonic mesoderm formation. Development. 1997;124:1689–1698. doi: 10.1242/dev.124.9.1689. [DOI] [PubMed] [Google Scholar]
- Hsu DR, Economides AN, Wang X, Eimon PM, Harland RM. The Xenopus dorsalizing factor gremlin identifies a novel family of secreted proteins that antagonize BMP activities. Mol. Cell. 1998;1:673–683. doi: 10.1016/s1097-2765(00)80067-2. [DOI] [PubMed] [Google Scholar]
- Jones CM, Kuehn MR, Hogan BLM, Smith JC, Wright CVE. Nodal-related signals induce axial mesoderm and dorsalize mesoderm during gastrulation. Development. 1995;121:3651–3662. doi: 10.1242/dev.121.11.3651. [DOI] [PubMed] [Google Scholar]
- Jones CM, Armes N, Smith JC. Signalling by TGF-β family members: short-range effects of Xnr-2 and BMP-4 contrast with the long-range effects of activin. Curr. Biol. 1996;6:1468–1475. doi: 10.1016/s0960-9822(96)00751-8. [DOI] [PubMed] [Google Scholar]
- Joseph EM, Melton DA. Xnr4: A Xenopus Nodal-related gene expressed in the Spemann organizer. Dev. Biol. 1997;184:367–372. doi: 10.1006/dbio.1997.8510. [DOI] [PubMed] [Google Scholar]
- Joseph EM, Melton DA. Mutant Vg1 ligands disrupt endoderm and mesoderm formation in Xenopus embryos. Development. 1998;125:2677–2685. doi: 10.1242/dev.125.14.2677. [DOI] [PubMed] [Google Scholar]
- Kessler DS, Melton DA. Vertebrate embryonic induction: mesodermal and neural patterning. Science. 1994;266:596–604. doi: 10.1126/science.7939714. [DOI] [PubMed] [Google Scholar]
- Kofron M, Demel T, Xanthos J, Lohr J, Sun B, Sive H, Osada SI, Wright C, Wylie C, Heasman J. Mesoderm induction in Xenopus is a zygotic event regulated by maternal VegT via TGFβ growth factors. Development. 1999;126:5759–5770. doi: 10.1242/dev.126.24.5759. [DOI] [PubMed] [Google Scholar]
- Laurent MN, Blitz IL, Hashimoto C, Rothbächer U, Cho KWY. The Xenopus homeobox gene Twin mediates Wnt induction of Goosecoid in establishment of Spemann’s organizer. Development. 1997;124:4905–4916. doi: 10.1242/dev.124.23.4905. [DOI] [PubMed] [Google Scholar]
- Lemaire P, Garrett N, Gurdon JB. Expression cloning of Siamois, a Xenopus homeobox gene expressed in dorsal-vegetal cells of blastulae and able to induce a complete secondary axis. Cell. 1995;81:85–94. doi: 10.1016/0092-8674(95)90373-9. [DOI] [PubMed] [Google Scholar]
- Lustig KD, Kroll KL, Sun EE, Kirschner MW. Expression cloning of a Xenopus T-related gene (Xombi) involved in mesodermal patterning and blastopore lip formation. Development. 1996;122:4001–4012. doi: 10.1242/dev.122.12.4001. [DOI] [PubMed] [Google Scholar]
- McKendry R, Hsu SC, Harland RM, Grosschedl R. LEF-1/TCF proteins mediate Wnt-inducible transcription from the Xenopus nodal-related promoter. Dev. Biol. 1997;192:420–431. doi: 10.1006/dbio.1997.8797. [DOI] [PubMed] [Google Scholar]
- Meno C, Gritsman K, Ohishi S, Ohfuji Y, Heckscher E, Mochida K, Shimono A, Kondoh H, Talbot WS, Robertson EJ, Schier AF, Hamada H. Mouse Lefty2 and zebrafish Antivin are feedback inhibitors of Nodal signaling during vertebrate gastrulation. Mol. Cell. 1999;4:287–298. doi: 10.1016/s1097-2765(00)80331-7. [DOI] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destrée O, Clevers H. XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD. The formation of the mesoderm in Urodelean amphibians. I. Induction by the endoderm. Roux’ Arch. f. Entw. Mech. 1969;162:34–373. doi: 10.1007/BF00578701. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD. The “organization center” of the amphibian embryo: its origin, spatial organization and morphogenetic action. Adv. Morphogen. 1973;10:1–39. doi: 10.1016/b978-0-12-028610-2.50005-8. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis. Garland Publishing, Inc.; New York: 1994. [Google Scholar]
- Nomura M, Li E. Smad2 role in mesoderm formation, left-right patterning and craniofacial development. Nature. 1998;393:786–790. doi: 10.1038/31693. [DOI] [PubMed] [Google Scholar]
- Osada SI, Wright CVE. Xenopus nodal-related signaling is essential for mesendodermal patterning during early embryogenesis. Development. 1999;126:3229–3240. doi: 10.1242/dev.126.14.3229. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Leyns L, Bhattacharyya S, Grunz H, Bouwmeester T, De Robertis EM. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature. 1999;397:707–710. doi: 10.1038/17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebagliati MR, Toyama R, Haffter P, Dawid IB. cyclops encodes a nodal-related factor involved in midline signaling. Proc. Natl. Acad. Sci. 1998;95:9932–9937. doi: 10.1073/pnas.95.17.9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath K, Rubinstein AL, Cheng AM, Liang JO, Fekany K, Solnica-Krezel L, Korzh V, Halpern ME, Wright CVE. Induction of the zebrafish ventral brain and floorplate requires cyclops/nodal signalling. Nature. 1998;395:185–189. doi: 10.1038/26020. [DOI] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994;79:779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Steinbeisser H, Warga RM, Hausen P. β-catenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mech. Dev. 1996;57:191–198. doi: 10.1016/0925-4773(96)00546-1. [DOI] [PubMed] [Google Scholar]
- Slack JMW. From egg to embryo. Cambridge University Press; Cambridge, UK: 1991a. [Google Scholar]
- Slack JMW. The nature of the mesoderm-inducing signal in Xenopus: a transfilter induction study. Development. 1991b;113:661–669. doi: 10.1242/dev.113.2.661. [DOI] [PubMed] [Google Scholar]
- Smith JC. Mesoderm-inducing factors and mesodermal patterning. Curr. Opin. Cell Biol. 1995;7:856–861. doi: 10.1016/0955-0674(95)80070-0. [DOI] [PubMed] [Google Scholar]
- Smith JC, Slack JMW. Dorsalization and neural induction: properties of the organizer in Xenopus laevis. J. Embryol. Exp. Morphol. 1983;78:299–317. [PubMed] [Google Scholar]
- Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Smith WC, McKendry R, Ribisi S, Jr., Harland RM. A nodal-related gene defines a physical and functional domain within the Spemann organizer. Cell. 1995;82:37–46. doi: 10.1016/0092-8674(95)90050-0. [DOI] [PubMed] [Google Scholar]
- Song J, Oh SP, Schrewe H, Nomura M, Lei H, Okano M, Gridley T, Li E. The type II activin receptors are essential for egg cylinder growth, gastrulation, and rostral head development in mice. Dev. Biol. 1999;213:157–169. doi: 10.1006/dbio.1999.9370. [DOI] [PubMed] [Google Scholar]
- Steinbeisser H, De Robertis EM, Ku M, Kessler DS, Melton DA. Xenopus axis formation: induction of goosecoid by injected Xwnt-8 and activin mRNA. Development. 1993;118:499–507. doi: 10.1242/dev.118.2.499. [DOI] [PubMed] [Google Scholar]
- Sun BI, Bush SM, Collins-Racie LA, LaVallie ER, DiBlasio-Smith EA, Wolfman NM, McCoy JM, Sive HL. derrière: a TGF-β family member required for posterior development in Xenopus. Development. 1999;126:1467–1482. doi: 10.1242/dev.126.7.1467. [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B. Antivin, a novel and divergent member of the TGFβ superfamily, negatively regulates mesoderm induction. Development. 1999;126:229–240. doi: 10.1242/dev.126.2.229. [DOI] [PubMed] [Google Scholar]
- Thomsen GH, Melton DA. Processed Vg1 protein is an axial mesoderm inducer in Xenopus. Cell. 1993;74:433–441. doi: 10.1016/0092-8674(93)80045-g. [DOI] [PubMed] [Google Scholar]
- Waldrip WR, Bikoff EK, Hoodless PA, Wrana JL, Robertson EJ. Smad2 signaling in extraembryonic tissues determines anterior-posterior polarity of the early mouse embryo. Cell. 1998;92:797–808. doi: 10.1016/s0092-8674(00)81407-5. [DOI] [PubMed] [Google Scholar]
- Watabe T, Kim S, Candia A, Rothbächer U, Hashimoto C, Inoue K, Cho KWY. Molecular mechanisms of Spemann’s organizer formation: conserved growth factor synergy between Xenopus and mouse. Genes Dev. 1995;9:3038–3050. doi: 10.1101/gad.9.24.3038. [DOI] [PubMed] [Google Scholar]
- Wylie C, Kofron M, Payne C, Anderson R, Hosobuchi M, Joseph E, Heasman J. Maternal β-catenin establishes a ‘dorsal signal’ in early Xenopus embryos. Development. 1996;122:2987–2996. doi: 10.1242/dev.122.10.2987. [DOI] [PubMed] [Google Scholar]
- Yasuo H, Lemaire P. A two-step model for the fate determination of presumptive endodermal blastomeres in Xenopus embryos. Curr. Biol. 1999;9:869–879. doi: 10.1016/s0960-9822(99)80391-1. [DOI] [PubMed] [Google Scholar]
- Zhang J, Houston DW, King ML, Payne C, Wylie C, Heasman J. The role of maternal VegT in establishing the primary germ layers in Xenopus embryos. Cell. 1998;94:515–524. doi: 10.1016/s0092-8674(00)81592-5. [DOI] [PubMed] [Google Scholar]
- Zhou X, Sasaki H, Lowe L, Hogan BLM, Kuehn MR. Nodal is a novel TGF-β-like gene expressed in the mouse node during gastrulation. Nature. 1993;361:543–547. doi: 10.1038/361543a0. [DOI] [PubMed] [Google Scholar]


