Abstract
Behavioral and physiological changes were studied following prolonged exposure to social competition in pairs of non food-deprived rats competing daily for a limited supply of graham cracker crumbs. Stable dominant-subordinate relationships developed in most pairs, as measured by feeding time, which were maintained over a 5–6-week study period. In other behavioral tests, subordinates demonstrated a decreased latency to immobility in the forced swim test compared with dominants, but no difference in locomotor activity. Subordinates had increased bladder size, decreased adrenal gland size, and a 35% reduction of hippocampus cell proliferation compared with the dominant member. Therefore, prolonged social competition, based on restricted access to palatable substances, produced hierarchies among individuals that were associated with differences in behavior, physiology and hippocampal cell proliferation.
Keywords: Social Stress, Depression, Forced Swim Test, Neurogenesis
1. Introduction
Prolonged exposure to stress produces detrimental behavioral and biological consequences that contribute to the development of neuropsychiatric disorders [20,21]. In animal models, chronic exposure to stress impairs learning [40], produces cognitive deficits [11,7] and decreases preference for sucrose, which has been interpreted as a form of anhedonia [50]. Along with genetic vulnerability, chronic stress is a major contributor to the development of depression [20]. Although numerous studies have examined the effects of chronic stress on animals in the laboratory, most have used unnatural stressors such as electric shock, restraint or forced swimming. Social stressors, however, are believed to play a special role in the onset of depression in people [1,6], although they may be difficult to study under controlled circumstances. Therefore, controlled studies of social stress present during interactions between animals in social hierarchies, may aid in the understanding of the role of social stress in contributing to stress-related psychiatric disorders, such as depression or anxiety.
A variety of methods has been used to study the biological effects of social stress in various species of animals, ranging from natural ethological studies to the development of laboratory models [46]. Naturally occurring hierarchies have been documented in the wild by primate ethologists [38, 39], and one type of social stress studied under controlled laboratory conditions derives from the establishment of hierarchies among conspecifics. Social hierarchies in groups of rats have been examined using the ‘hidden burrow’ paradigm. In this design, a group of male and female rats are housed together, and a hierarchy is formed between the male rats as they compete for access to food, water and female rats [2,3]. Over time, subordinate rats in this paradigm lose weight, become sick, and develop deficient immune responses relative to dominant members. Social competition between conspecifics has also been studied in the laboratory when two or more deprived animals compete repeatedly in daily sessions for limited access for food [14,15,29]. In some of the groups, a dominant-subordinate relationship emerges where one of the rats consistently defeat their counterpart(s). Once a hierarchical relationship is established, the subordinate animal will concede to the dominant animal in the absence of a physical attack to avoid repeated physical encounters with the stronger conspecific.
Exposure to stress produces numerous physiological effects that can result in long-term deleterious effects on behavior, including morphological changes in key brain regions. Some of the evidence for these effects comes from studies of social interaction in the tree shrew, which are aggressively territorial [13]. Subordinate male tree shrews exposed repeatedly to a dominant member develop numerous detrimental behavioral and physiological effects, including a decrease in cell proliferation in the dentate gyrus of the hippocampus [17]. This effect was prevented by co-administration of the antidepressant clomipramine [49]. In the “hidden burrow” paradigm, where rats live in established colonies, dominant rats had increased cell survival, but not proliferation, compared to subordinate rats [24]. Therefore, differences in cell proliferation/cell survival can be measured between dominant and subordinate members of social hierarchies, and the hippocampus is a sensitive site for reflecting the effects of chronic social stress on neuroplasticity. These cellular effects could model the development of mood disorders, such as depression, if impaired affective responses derive from the experience of subordination or defeat that arise in response to prolonged social conflict [35,43]. However, stress from food deprivation and wounds from fighting could also contribute to morphological indices of stress in subordinate animals.
The current experiments examined the impact of daily competition for limited access to food among male rats on behavior and neuroplasticity using a new paradigm. A key feature of the procedure is that competition for a palatable food (graham cracker crumbs) avoided the use of food deprivation or other physical stress, allowing the consequences of the competition to reflect more of the effects on psychological variables accompanying social competition. Social hierarchies were rapidly established by competition for a limited supply of graham cracker crumbs. Although competition produced some initial physical contact between members, there were no displays of aggression or severe physical attacks throughout weeks of competition. In other words, rats developed reliable social hierarchies by competing for a palatable food they wanted but did not need for physical sustenance. During assessment of the stability of their behavior, differences between dominant and subordinate rats were examined in behavioral tests such as the forced swim test, sucrose consumption and locomotor activity. The forced swim test is a model of antidepressant-like activity, which is also sensitive to the effects of prior stress [5,27]. Examination of cell proliferation in the dentate gyrus of the hippocampus revealed substantial differences in cell proliferation between dominant and subordinate members arising from prolonged exposure to social competition. Additional control studies examined the effects of graham cracker consumption in the absence of competition on cell proliferation, as well as the effects of competition on physiological endpoints such as bladder and adrenal gland weights, as well as corticosterone levels.
2. Materials and Methods
2.1. Subjects
Male Sprague Dawley rats weighing approximately 250 g on arrival (Charles River; Wilmington, MA) were housed in pairs in a temperature-controlled facility maintained on a 12:12 light/dark cycle. Rats were given free access to food and water throughout the experiment. The rats were handled and weighed three times per week throughout the experiment. All phases of the experiment were carried out Monday through Friday. The studies were carried out in accordance with the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health and were reviewed by the University of Pennsylvania Institutional Animal Care and Use Committee.
2.2. Establishment of Competition, Behavioral Testing and Cell Proliferation
2.2.1 Eating graham cracker crumbs
Rats were housed in the animal facility for 6 days prior to initiating experimental sessions. Each rat (N = 22) was given individual access to 3 g of graham cracker crumbs (Nabisco Products) for 30 min/day. Sessions were conducted between 10:00 and 16:00 h. The rats were placed in a round testing chamber made of clear plastic (dimensions: 34 cm in height × 28 cm in diameter), with a small plastic dish (2.5 cm in diameter) containing the graham cracker crumbs secured to the bottom of the chamber. At the end of each session, the remaining food was weighed and recorded. This phase of the study lasted for a total of 10 d.
2.2.2. Establishment of competition hierarchy
Social competition pairs were formed based on body weight and average amount of food consumed at the end of the acclimation phase. Rats were not paired with their home cage mates. Over a 6-week period, each pair competed for access to graham crackers during daily 10 min sessions. The graham cracker crumbs were placed inside a 5 ml container attached to a small plastic opening that was secured to the bottom of the testing chamber against the side. The small diameter of the plastic opening (5 cm in diameter) ensured that only one rat had access to the food at a time. Each session was recorded on videotape. From reviewing the videotapes, the amount of time that each rat spent eating was timed and the percentage of time spent eating during each session was calculated. The development of a dominant-subordinate relationship was defined as when one rat ate a minimum average of 70% of the time over a 3-d period.
2.2.3. Behavioral tests
During weeks four, five and six of competition, behavioral tests were administered that correlate with affective state and motor performance, and scores were compared between dominant and subordinate rats. The behavioral tests were administered on the first day of the week, and competition did not occur on the days that the tests took place.
During week four, the rats were exposed to the forced swimming test (FST) for 10 min. Rats initially actively try to escape from the cylinder, but eventually develop behavioral immobility. The FST is a widely used test of antidepressant-like behavior, which reduces the duration of immobility. An increase in immobility during this test has been associated with increased vulnerability for stress-induced depression in a number of models, such as chronic stress [8]. Rats were placed in a glass cylinder (44 cm in height, 20 cm in diameter) filled with water (30 cm high, 20–23°C) from which they could not escape. The FST session was video taped from above the cylinder, and scored for the presence of immobility, as previously described [10]. Since the rats became immobile quickly, only the first 5 min were compared between the subjects in order to measure differences in the latency to become immobile.
During week five, reactivity to a sucrose solution was examined. The rats were placed in individual cages, and given access to a 10% sucrose solution for 30 min/day for 3 days. The amount of sucrose consumed was recorded.
During the sixth week, the rats were placed in an open (40 cm × 40 cm) field for 30 min. The total distance traveled was recorded by an automated video tracking system (San Diego Instruments; San Diego CA).
2.2.4. Measurement of cell proliferation
Following the six weeks of competition, cell proliferation was measured in the dentate gyrus of the hippocampus, as previously described [31]. Each rat received a single injection of the thymidine analog bromodeoxyuridine (BrdU; 100 mg/kg; i.p.; Roche Applied Sciences; Indianapolis, IN) before the final competition session. Two hours after the injection, the rats were euthanized with an overdose of sodium pentobarbital (100 mg/kg; i.p.), and perfused with 0.1 M PBS and followed by 4% paraformaldehyde. The brains were quickly removed, stored in 4% paraformaldehyde overnight, then transferred to 30% sucrose in 1M PBS, and stored at 4°C until sectioning. The hippocampus was cut in 35 μm sections, and mounted on slides. After the slides had dried, immunohistochemistry was performed to detect BrdU labeled cells. Sections were heated in 0.1 M citric acid (~90°C) for 15 min, followed by treatment with 0.1% trypsin/CaCl for 10 min. Next, the sections were incubated with 2N HCL for 30 min, followed by 1 h blocking in normal goat serum (Vector Laboratories; Burlingame, CA), and overnight incubation in the primary antibody at room temperature (1:100; Becton Dickinson; Bridgeport, NJ). Eighteen hours later, the sections were incubated for 1 h with the secondary antibody (1:200; Sigma) followed by incubation in avidin-biotin complex (Vector Laboratories). Finally, the cells were visualized with DAB (Vector Laboratories), and counter stained with neutral red (ICN Biomedicals; Costa Mesa, CA). The number of BrdU-labeled cells in the subgranular zone of the hippocampus was counted in every ninth section by two separate viewers blind to the treatment condition of the animals. The mean value between viewers was used. A cell was considered to be in the subgranular zone if it was within three cells of the granular cell layer.
2.3. Replication of Competition Hierarchy and Cell Proliferation
A second group of rats (N = 24) was trained in the social competition paradigm, as described in Experiment 1. After acclimation to eating the graham cracker crumbs was established, the rats competed in daily sessions for 5 consecutive weeks. At the end of the experiment, cell proliferation was measured in all rats, as described above. On the basis of their performance during the final week of the study, rats from stable hierarchies were categorized as either dominant or subordinate rats. In addition, rat pairs that did not form stable hierarchies were also categorized separately. To rule out the possibility that the behavioral tests in Experiment 1 may have affected cell proliferation, no behavioral tests were performed in Experiment 2.
2.4. Control for Graham Cracker Consumption
A third group of rats (N = 16) was used to test for the effects of graham cracker consumption on cell proliferation. After a one-week acclimation period, each rat was placed in the competition cylinder for 30 min/day for a total of 4 weeks. Half of the rats were given 5 g of graham cracker crumbs in the container, and the amount eaten was recorded. The control rats were placed in the containers with no graham cracker crumbs. At the end of the four weeks, the rats were given an injection of BrdU (100 mg/kg; i.p.) and sacrificed 2 h later. Cell proliferation in the dentate gyrus was measured as described above.
2.5. Physiological Differences between Dominant and Subordinate Rats
A fourth group of rats (N = 40) were run through the social competition paradigm as described above. In this group rats competed against their cagemates during the competition phase. After five weeks of competition, 8 pairs of rats that reached the competition hierarchy were sacrificed 1 h after the last competition session. Sixty minutes after the last competition session, the bladder and adrenal glands were removed and weighed, and their average weight was adjusted for body weight. In addition, trunk blood was collected from each animal in order to measure corticosterone levels. Briefly, plasma was separated by centrifugation and stored at 20°C. Plasma corticosterone was measured by radioimmunoassay using a commercially available kit (ICN Biomedicals, Inc; Costa Mesa, CA; USA). Corticosterone levels are presented as ng/ml, and adjusted for body weight.
2.6. Statistics
Social competition was examined by measuring the duration of feeding of each rat during the behavior sessions. Feeding duration times were combined at weekly intervals and graphed as a percentage of the total time spent eating for each pair. Differences between dominant and subordinate rats in the forced swim test and locomotor activity were compared using Student’s t-test, two-tailed. The total number of BrdU-labeled cells in the hippocampus were determined and compared between hierarchical groups using Student’s t-test or analysis of variance.
3. Results
3.1. Establishment of Competition, Behavioral Testing and Cell Proliferation
3.1.1. Establishment of competition
The results of the competition session are shown in Fig. 1. The mean time spent eating for the dominant and subordinate rats is shown for the 6 weeks of competition. Of the 11 pairs tested, 8 established a social hierarchy, in which one rat spent at least 70% of the time for three consecutive days. Once established, the hierarchy remained stable throughout the 6 weeks of competition. The behavioral testing during weeks four, five and six did not appear to disrupt the stability of the hierarchy. The 3 pairs of rats that did not establish a social hierarchy were not tested.
Fig. 1.
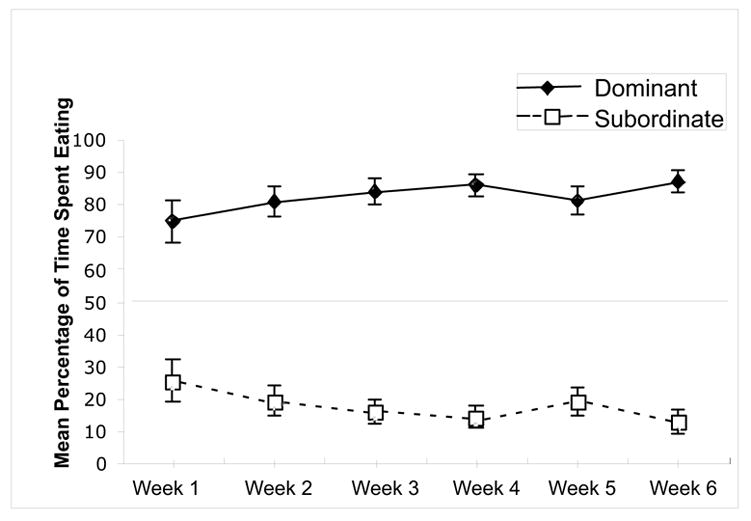
Percentage of time spent eating for the dominant and subordinate rats during the six weeks of social competition. Pairs of rats competed for 5 min/day for access to 3 g of graham cracker crumbs. The time spent eating for each rat was recorded. Of the original 11 pairs, 8 reached the criterion for a dominant/subordinate relationship where one rat consistently ate for at least 70% of the time over a 3 d period.
3.1.2. Forced swim test
The mean frequency of immobility for the first 5 min of the FST for the dominant and subordinate rats is shown in Fig. 2. Overall, the rats reached immobility quickly due to their body weight at the time of testing (~450 g, N = 16). However, the subordinate rats developed immobility more rapidly than dominant rats during the first 5 min of the test. Repeated measures ANOVA on the first five minutes of testing revealed that there was a significant effect for time, F (4,56) = 180.59, p < .0001, hierarchy level, F (1,14) = 11.45, p < .005, as well as a significant interaction, F (4,56) = 6.26, p < .003. Post hoc tests indicated that the subordinate rats showed significantly more immobility counts during the second minute of swimming, p < .03. These data show that the development of immobility was faster for the subordinate than the dominant rats.
Fig. 2.
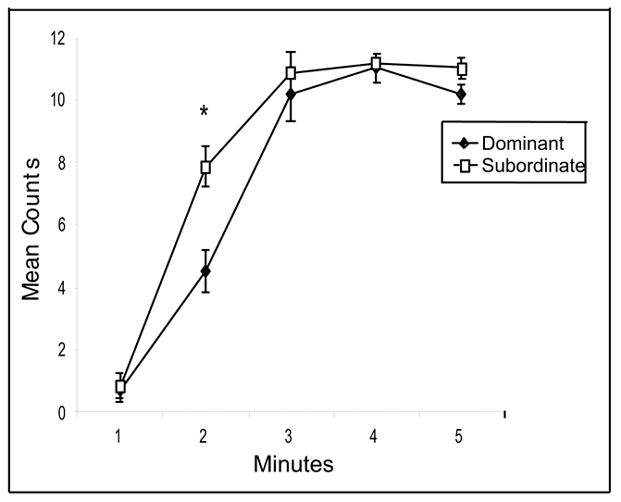
Immobility counts during the first five minutes of the forced swim test for the dominant and subordinate rats. During week 4, the rats were individually placed in containers of water (30 inches deep) for 10 minutes. The sessions were videotaped and scored for the presence of immobility, swimming and climbing [10]. The test was broken down into one-minute intervals. * indicates p < .03.
3.1.3. Locomotor activity and sucrose consumption
The mean distance traveled for the dominant and subordinate rats during the 30-min exposure to the open field is shown in Fig. 3. There was no significant difference between the dominant and subordinate rats in distance traveled, t (14) = 0.37, p > .05, showing that the differences in hierarchy were not due to differences in locomotor activity. In addition, there were no differences in sucrose consumption between the dominant and subordinate rats (data not shown).
Fig. 3.
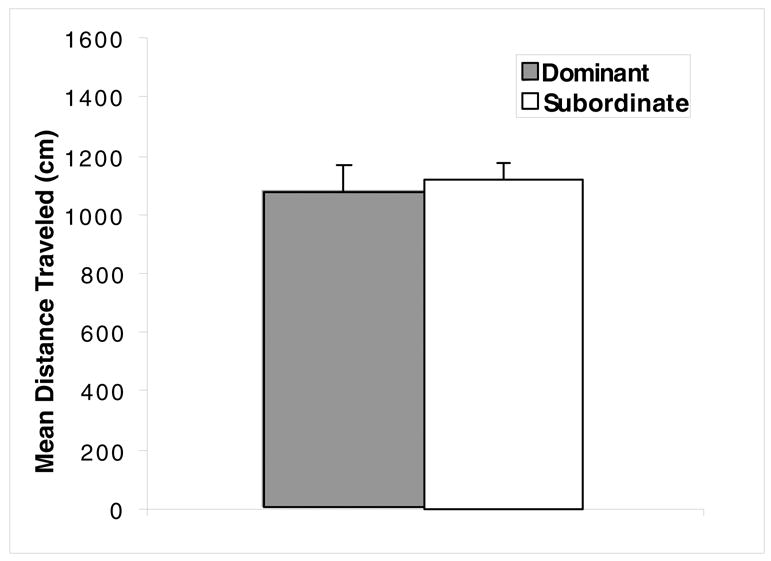
Locomotor activity for the dominant and subordinate rats during the 30 min exposure to the open field during week 6. Each rat was individually placed in the open field, and the total distance traveled (cm) was recorded by a digital tracking system. There were no significant differences between dominant and subordinates in locomotor activity.
3.1.4. Cell proliferation
The mean number of BrdU-positive cells counted in the subgranular zone for dominant and subordinate rats is shown in Fig. 4. Dominant rats showed a significantly greater number of BrdU-positive cells by ~35% over subordinate rats, t (14) = 3.83, p < .002.
Fig. 4.
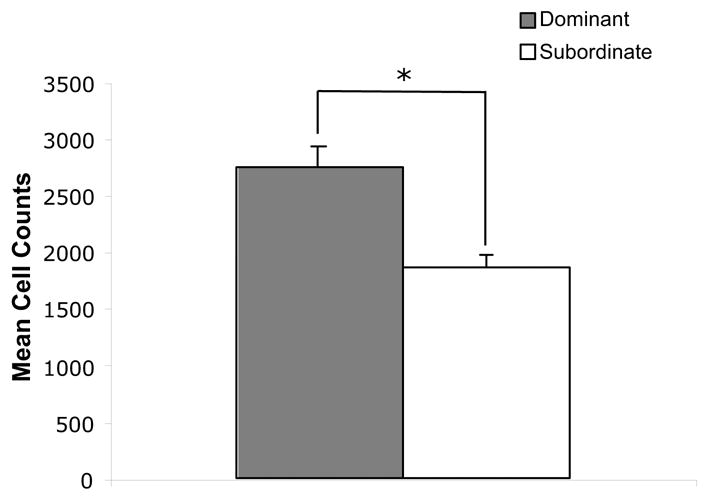
Cell proliferation in the subventricular zone of the dentate gyrus in the dominant and subordinate rats. Two hours after administration of BrdU, the rats were sacrificed, and immunohistochemistry was used to detect BrdU labeled cells in the dentate gyrus. The mean number of BrdU labeled cells were counted for each rat by two separate investigators, and the counts were averaged together.
* indicates p < .02.
3.2. Replication of Competition Hierarchy and Cell Proliferation
Of the 12 pairs tested, 8 consistently adopted a dominant-subordinate hierarchy, defined as the dominant rat eating for at least 70% of the total time. The mean percentage of time spent eating for the dominant and subordinate rats that established a hierarchy is shown in Fig. 5a across the 5 weeks of competition. As seen in Experiment 1, differences in eating time were established within the first week, and remained stable for each of the pairs over the 5 weeks of competition. In addition, there were no significant weight differences between the dominant and subordinate rats at either the first day of competition, t(14) = 0.25, p < .05, or the last day of competition, t(14) = 0.63 (data not shown).
Fig. 5.
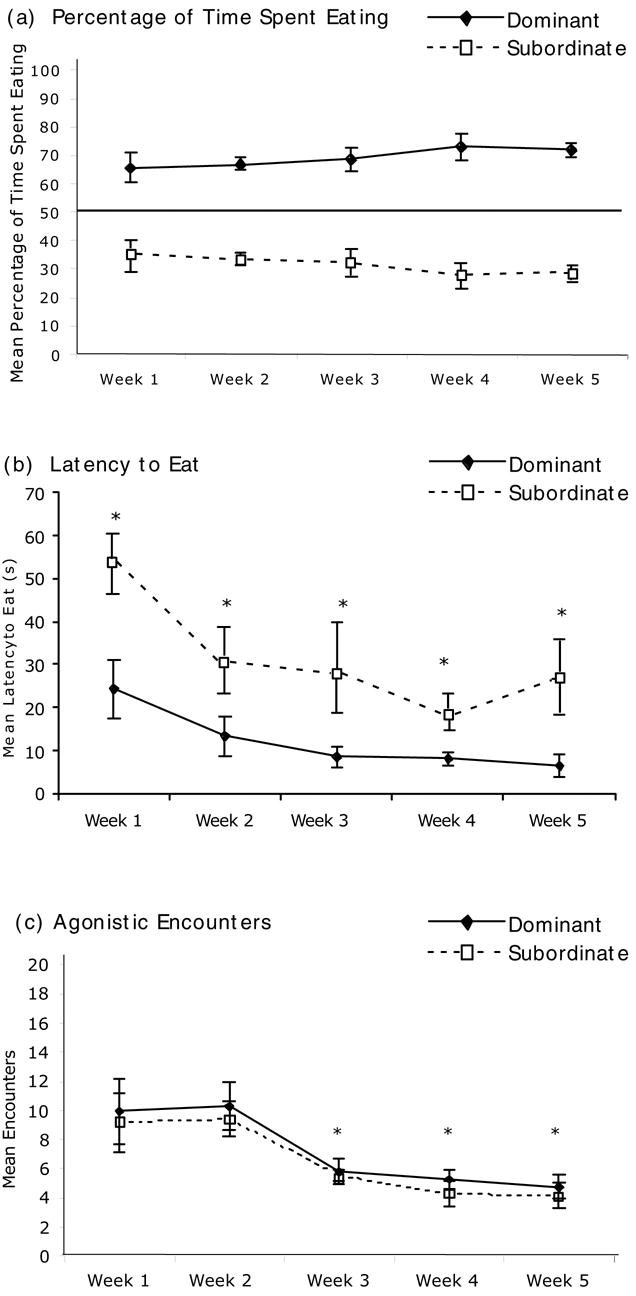
(a) Percentage of time spent eating for the dominant and subordinate rats during the five weeks of social competition in the second experiment. Pairs of rats competed for 10 min/day for access to 3g of graham cracker crumbs. The time spent eating for each rat was recorded. Of the original 12 pairs, 8 reached the criterion for a dominant/subordinate relationship where one rat consistently ate for at least 70% of the time. (b) Latency to eat for the dominant and subordinate rats during the last day of each week. * indicates that there was a significant difference between the dominant and subordinate groups. (c) Agonistic encounters between the dominant and subordinate groups during the last day of each week. Agonistic encounters were defined as an
Fig. 5b shows the latency to eat during the final test day of each week for the dominant and subordinate rats. Repeated measure ANOVA revealed that there was a significant effect for hierarchy ranking, F (1,14) = 15.96, p < .002, and over weeks, F (4, 56) = 7.05, p < .002. Post hoc tests revealed that during the last day of each week, the dominant rats had a significantly lower latency to eat compared to the subordinate rats.
In addition, the agonistic interactions between the dominant and subordinate rats were recorded during the last test day of each week (Fig. 5c). Agonistic interactions were defined as bouts of physical contact initiated by one of the rats that usually took the form of mounting, pushing, biting or pinning. Although there was no significant difference in number of agonistic encounters between the rats according to social hierarchy, F (1,14) = 0.65, p > .05, there was a significant effect for week, F (4,56) = 8.59, p < .001. Post hoc tests revealed that the number of significant interactions between the dominant and subordinate rats decreased significantly during weeks 3, 4 and 5 compared to weeks 1 and 2.
Table 1 shows an expanded view for different measures of the behavior of the dominant and subordinate rats during the establishment of competition during the first week of the study. A significant difference in percentage of time spent eating was established quickly during the first week. In addition, a significant difference between the dominant and subordinate rats in latency to eat emerged on day 5 of week 1, t(14) = 3.23, p < .01. However, the number of agonistic interactions remained constant during the first week.
Table 1.
Behavioral Measures of Social Competition During the First Week of Competition
| Behavioral Measure | Group | Day 1 | Day 3 | Day 5 |
|---|---|---|---|---|
| Time Spent Eating (%) | Dominant:
Subordinate: |
61.41 ± 7.79
38.59 ± 7.79 |
67.16 ± 6.13
32.84 ± 6.13 |
71.98 ± 5.65
28.02 ± 5.65 |
| Latency to Eat (s) | Dominant:
Subordinate: |
101.29 ± 13.87
91.13 ± 22.43 |
29.50 ± 5.12
37.88 ± 9.02 |
24.38* ± 6.93
53.38 ± 5.71 |
| Agonistic Encounters | Dominant:
Subordinate: |
14.50 ± 2.98
10.88 ± 1.88 |
11.88 ± 1.74
10.75 ± 1.41 |
9.88 ± 2.20
9.13 ± 2.01 |
During the first week, the hierarchy was formed quickly, with the dominant rats spent a higher percentage of time eating compared to the subordinate rats. In addition, the number of agonistic encounters remained stable throughout the week. However, by the last day of week 1, the dominant rats showed a significantly lower latency to eat compared to the subordinate rats. Asterisk (*) indicates value of dominant animal differed significantly from subordinate animal, p < .05.
In summary, these data show that dominant and subordinate roles emerged rapidly between pairs during the first days of competition that would be maintained for weeks. The emergence of dominant behavior was accompanied by agonistic interactions (pushing and pinning) between pairs throughout the first week that did not decrease until week 3. After week 3, dominant rats would consume the graham cracker crumbs and not be challenged by subordinate rats.
3.2.1. Cell proliferation
The mean cell proliferation for the dominant and subordinate rats, as well as the rats that did not adopt a stable hierarchy (n = 8) is shown in Fig. 6. Due to problems with the perfusion and sectioning, only 7 of the 8 dominant rats were examined for cell proliferation. There was a significant overall difference in cell proliferation between the three groups, F (2, 20) = 4.15, p < .03. Post hoc tests indicated that the mean cell proliferation for the dominant rats was ~50% higher than that of the subordinate rats, p < .02. There were no significant differences between the uncategorized rats and either the dominant or subordinate rats.
Fig. 6.
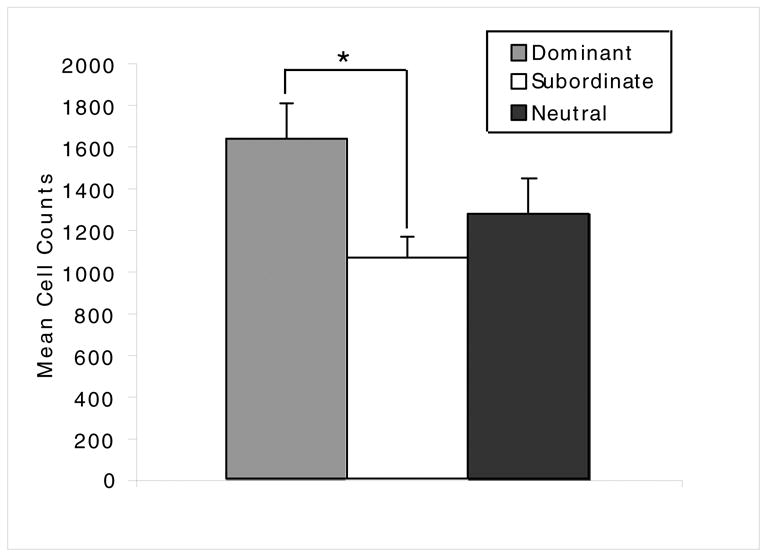
Cell proliferation in the subventricular zone of the dentate gyrus in the dominant, subordinate rats and neutral rats. Neutral rats competed but did not form dominant subordinate hierarchies. Two hours after administration of BrdU, the rats were sacrificed, and immunohistochemistry was used to detect BrdU labeled cells in the dentate gyrus. The mean number of BrdU labeled cells were counted for each rat by two separate investigators, and the counts were averaged together.
* indicates p < .02.
3.3. Graham Cracker Control Experiment
The mean cell proliferation for rats given access to graham crackers crumbs for four weeks and their controls is shown in Fig. 7. One of the subjects in the graham cracker group could not be used due to complications from the perfusion. There was no significant difference in cell proliferation in the dentate gyrus between the two groups, t (13) = 1.06, p > .05. Therefore, differences in cell proliferation between the dominant and subordinate rats were not due to differences in consumption of graham cracker crumbs.
Fig. 7.
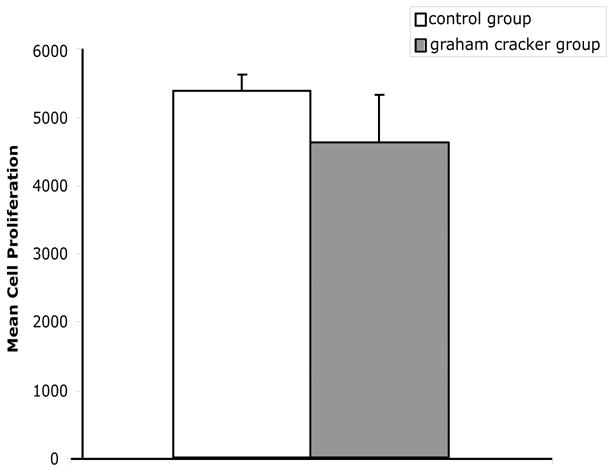
Consumption of graham cracker crumbs does not lead to an increase in cell proliferation in the dentate gyrus. Two groups of rats were placed individually in the competition containers for 30 min/day for a total of four weeks. Half of the rats were given access to 5 g of graham cracker crumbs, and the other half were placed in the containers with no graham cracker crumbs. At the end of the experiment, the rats were given an injection of BrdU and perfused 2 h later. Cell proliferation was measured in the dentate gyrus of the hippocampus.
3.4. Physiological Differences between Dominant and Subordinate Rats
The results of the percentage of time spent eating and seconds spent eating during the last week of competition, as well as the physiological and hormonal measures for the dominant and subordinate rats are shown in Table 2. As was the case in the first two social competition experiments, the dominant rats spent approximately 70% of the time eating during the last week of competition. Although there were no significant differences in corticosterone level, t(14) = 0.67, p > .05, there were significant differences in bladder weight t(14) = 1.13, p < .03, and adrenal weight, t(13) = 2.40, p < .03, with subordinate rats having higher bladder weights and lower adrenal gland weights.
Table 2.
Behavioral and Physiological Measures for Dominant and Subordinate Rats
| Hierarchy | Dominant Rats | Subordinate Rats |
|---|---|---|
| % of Time Spent Eating | 74.95 ± 4.36 | 25.05 ± 4.36 |
| Time Spent Eating (sec) | 95.57 ± 16.95 | 35.05 ± 6.90 |
| Bladder Weight (mg/g) | 0.383 ± 0.035* | 0.468 ± 0.030 |
| Adrenal Gland Weight (mg/g) | 0.066 ± 0.004* | 0.054 ± 0.002 |
| Corticosterone Level (ng/ml) | 0.082 ± 0.031 | 0.056 ± 0.020 |
After five weeks of competition, the dominant and subordinate rats were sacrificed, and the bladders and adrenal glands were removed and weighed. Blood samples were also taken, and corticosterone was measured by radioimmuno assay. All measures were adjusted for body weight. Asterisk (*) indicates value of dominant animal differed significantly from the subordinate animal, p < .035.
4. Discussion
Pairs of rats were allowed to develop a stable social hierarchy by repeatedly competing for access to a palatable food in a neutral test cage. Although other laboratory methods have been used to study competitive behavior in rats, such as competition for food in deprived rats [29], dominance hierarchies in colonies [24], or colony behavior in the rat “hidden burrow system” [3], this procedure bears salient differences from the other methods. Because they competed for food they desired but did not need for physical sustenance, these rats developed a social hierarchy under non-deprived conditions, unlike prior tests for food competition [29]. Brief periods of physical contact accompanied the outset of competition when the hierarchical relationship was being established. However, the competitive behavior was not based on outright aggression or physical wounding of one of the members of the pair, as is common in the “hidden burrow system” [3]. The experiments yielded a high number of participating animals, relative to these other models, with approximately 70% of the pairs reaching the criterion for establishment of a hierarchical relationship. The dominant-subordinate relationship formed during the first week of competing and remained stable for at least 6 weeks with exposure to daily test sessions.
An important finding in the present study was that rats demonstrated differences in cell proliferation in the dentate gyrus of the hippocampus according to social status, with dominant rats showing 35–50% higher levels of cell proliferation. The difference in cell proliferation produced by social competition was replicated in the second experiment, and was not due to increased consumption of graham cracker crumbs by the dominant rats. These results correspond with other studies where aggressive interactions associated with psychosocial stress were shown to decrease cell proliferation in subordinate animals. Specifically, exposure of tree shrews to an acute aggressive encounter for 1 hour decreased cell proliferation in the hippocampus of the subordinate members [17,49]. In the rat “hidden burrow” system, dominant rats demonstrated increased cell survival in the hippocampus over a 3-week study period, compared to subordinate rats [24]. However, hippocampal cell proliferation did not differ between dominant and subordinate rats, possibly because the rats were exposed to the social colony for only 3 d before cell proliferation was measured. Although agonistic interactions in the social competition procedure were associated with the emergence of dominant and subordinate roles within the first week of competition, they were relatively mild in the form of pushing contacts. Such interactions stopped after 3 weeks when subordinates no longer challenged dominant rats for the food. Furthermore, because the present social competition procedure allowed rats to develop stable hierarchies for 5 or 6 weeks, it is possible that exposure to social interactions for varying periods of time affects different components of hippocampal neurogenesis, cell proliferation, neuronal differentiation and survival.
In addition to differences in cell proliferation, dominant and subordinate rats also displayed differences in adrenal gland and bladder weights. The adrenal gland plays a significant role in the stress response system by releasing corticosterone and other hormones into the bloodstream [23] and adrenal hypertropy is considered a sign of chronic or severe stress [41] Increased adrenal size has been reported in subordinate animals subject to social defeat [36] and chronic mild stress [16]. Therefore, it was surprising that subordinate rats in the social competition model in the present study had consistently smaller adrenal weights. However, in another model of competition, the visible burrow system, no differences were detected in adrenal weight between dominant and subordinates [45]. Moreover, a subset of subordinate rats in this model had remarkably low corticosterone responses to stress. These findings suggest a complex impact of social competition on the hypothalamic-pituitary-adrenal axis.
The finding that bladder weight was greater in subordinate rats in the social competition model is consistent with previous reports of bladder hypertrophy in subordinate mice that have established a social hierarchy [9,28]. Although the mechanism for this has yet to be elucidated, this could involve corticotropin releasing factor (CRF), a peptide that is integral to the stress response. CRF-containing neurons in Barrington’s nucleus (the pontine micturition center) innervate lumbosacral preganglionic parasympathetic neurons that elicit bladder contraction in response to distention [47,48]. Evidence suggests that CRF is inhibitory in this pathway [22,34] and a history of stress increases CRF mRNA expression in Barrington’s nucleus neurons [18,19]. These data demonstrate that social competition induces enduring visceral, as well as behavioral, effects.
The use of laboratory models of competition allowed control of the specific conditions and exposure to social stress. Although contesting for food among food-deprived rats has commonly been used as a model of social competition, rats in the current study were not food deprived. Since food deprivation has been shown to increase the generation of newborn cells [25], measures of cell proliferation in the rats, as well as their behavior, would have been influenced by the heightened stress of food deprivation. Another advantage of the current procedure to establish and maintain dominance hierarchies is that social competition emphasized a more psychological component in the maintenance of social behavior, as opposed to the use of threats of physical attacks or aggression to maintain social dominance. Thus, the detrimental impact on hippocampal cytogenesis by the failure to obtain an available palatable reward demonstrates a morphological impact of the psychological components of social competition. Competition for other naturalistic rewards, such as access to females, may produce similar effects. The psychological context of the presentation of stress has already been emphasized to have an important impact on hippocampal neuroplasticity. For example, presentations of electric shock produced a more lasting detrimental impact on hippocampal cell proliferation when delivered under inescapable circumstances (i.e., learned helplessness) than as escapable shock [30].
Interest in cell proliferation in the dentate gyrus is a focal point of research examining the morphological effects of stress that may be related to the onset and treatment of mood disorders, such as depression. Since it has been proposed that the onset of depression in humans is associated more commonly with nonviolent social interactions in the family or workplace as opposed to physical stress [6,21], paradigms that utilize social stressors to establish the context of dominance-subordinate hierarchies may help identify morphological differences between dominant and subordinate animals that can elucidate important biological underpinnings associated with depression. Chronic stress decreases cell proliferation in the hippocampus, whereas chronic administration of antidepressants produces the opposite effects [12]. In addition, postmortem and imaging studies of depressed patients revealed that people suffering from depression have smaller hippocampal volumes, which may be related, in part, to a decrease in cell proliferation [42]. Although the function of hippocampal neurogenesis is unknown, studies have suggested that newborn cells participate in learning or in responses to anxiety [26,37]. Therefore, the differences in cell proliferation seen in the present social competition paradigm add further evidence for a role of social stress in regulating behavior and mood disorders. Although social status and subordinate behaviors are correlated with reduced cell proliferation, their precise physiological relationship is uncertain. In the present study, the differences in cell proliferation between the dominant and subordinate rats could be caused by prolonged exposure to the competition. However, preexisting differences could also have made the rats vulnerable to becoming subordinate or dominant. Additional studies where neurogenesis is measured in dominant and subordinate rats that have been reassigned from varying behavioral histories would provide systematic information about both predisposing and performance factors that contribute to the morphological effects of stress.
Exposure of rats to social competition was associated with behavioral changes in the forced swim test, where the subordinate rats in the hierarchy showed a decreased latency to become immobile. The decreased latency to become immobile in the forced swim test is consistent with other reports examining the role of stress in the forced swim test and other models of antidepressant-like activity. When rats are exposed to the forced swim test a second time, they become immobile more rapidly, and this effect is reversed by administration of antidepressants [8]. In addition, prior stress, genetic history or diseases comorbid with depression hasten the development of immobility for rats in the forced swim test [33]. Therefore, the decreased latency to become immobile seen in the subordinate rats is consistent with a greater vulnerability to depressive behavior mediating the effects of stress in the forced swim test. These results correspond to other studies which show that rats exposed to chronic stress in the resident intruder paradigm showed higher immobility time when tested in the forced swim test [36], giving further evidence to the relationship between stress and immobility in this paradigm. However, there were no differences between the dominant and subordinate rats in consumption of sucrose or in locomotor activity.
Competition among conspecifics has been studied in a number of species from rodents to primates. In addition to competing for food, laboratory paradigms have examined competition for access to receptive mates. For example, in the hidden burrow system [3], male rats are placed in the colony and must compete for access to a limited number of female rats. In this paradigm, the competition for mating, in addition to the competition for food and water, results in heightened aggressive encounters. Among primates in the wild, competition for numerous resources, including females, leads to increases in stress indices, which are often experienced by all members of the hierarchy [38]. Although it is often assumed that the subordinate members are the most stressed, the complexity of the hierarchy leads to varied results. For example, when the hierarchy is in a state of flux, and the dominant primate must defend their rank, the dominant member often experiences the highest amount of stress [39]. Therefore, competition for mates, in addition to food and water, leads to complex stressful interactions.
Social competition has been suggested to play a role in the evolutionary origin of mood disorders such as depression [35,43,44]. According to this theory, the establishment of the hierarchy comes about when one animal gains access to a limited resource desired by others. Once an animal learns that they cannot compete against a dominant conspecific, it displays signs of subordination in order to avoid physical harm. It has been proposed that depression involves, in part, an exaggerated and persistent display of the evolved subordinate signs in humans. Subordinate animals also show many of the same symptoms as patients suffering from depression, such as weight loss, dysregulation of the HPA axis, and increased anxiety [3,4,32,46]. Additionally, studies have demonstrated that chronic administration of antidepressants reverses the subordinate behavior seen in animal models of competition [14,29], giving more credibility to the relationship between subordinate behavior and depression.
In summary, these data show that repeated social competition for palatable food without violent aggressive interactions between pairs of rats is associated with behavioral and neural changes. Social competition is a laboratory model of the social stress that would be expected to emerge after naturalistic encounters between rodents in the wild. Future research will examine further behavioral and biological changes that occur after exposure to the competition, as well as the role of antidepressants in reversing these effects.
Acknowledgments
The authors thank Dr. Jessica Malberg, Wyeth Neuroscience Research, Princeton, NJ for her assistance in developing the methods for measuring cell proliferation, Dr. Anita Bechtholt and Dr. Andre Curtis for their assistance with the dissections, and Dave Flannigan for running the corticosterone RIA. The authors also thank Dr. Robert Seyfarth, Department of Psychology at the University of Pennsylvania, for helpful discussions on social competition in animals. This research was supported by an unrestricted grant to the University of Pennsylvania from Wyeth Neuroscience Research and by the National Cooperative Drug Discovery Group grant MH 72832.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bjorkqvist K. Social defeat as a stressor in humans. Physiol Behav. 2001;73:435–442. doi: 10.1016/s0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- 2.Blanchard DC, Cholvanich P, Blanchard RJ, Clow DW, Hammer R, Rowlett JK, Bardo MT. Serotonin, but not dopamine, metabolites are increased in selected brain regions of subordinate male rats in a colony environment. Brain Res. 1991:568. doi: 10.1016/0006-8993(91)91379-f. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen B, Sakai RR. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 1995;20:117–134. doi: 10.1016/0306-4530(94)e0045-b. [DOI] [PubMed] [Google Scholar]
- 4.Blanchard RJ, McKittrick CR, Blanchard DC. Animal models of social stress: Effects on behavior and brain neurochemistry. Physiol Behav. 2001;73:261–271. doi: 10.1016/s0031-9384(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 5.Borsini F, Lecci A, Sessarago A, Frassine R, Meli A. Discovery of antidepressant activity by forced swim test may depend on pre-exposure of rats to a stressful situation. Psychopharmacology. 1989;97:183–188. doi: 10.1007/BF00442247. [DOI] [PubMed] [Google Scholar]
- 6.Brown GW, Prudo R. Psychiatric disorder in a rural and an urban environment: 1. Aetiology of depression Psychol Med. 1981;11:581–599. doi: 10.1017/s0033291700052880. [DOI] [PubMed] [Google Scholar]
- 7.Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- 8.Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified forced swim test. Neurosci Biobehav Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science. 1973;182:939–41. doi: 10.1126/science.182.4115.939. [DOI] [PubMed] [Google Scholar]
- 10.Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology. 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- 11.Dhabhar FS. Stress-induced enhancement of cell-mediated immunity. Ann NY Acad Sci. 1998;840:359–272. doi: 10.1111/j.1749-6632.1998.tb09575.x. [DOI] [PubMed] [Google Scholar]
- 12.Duman RS, Malberg J, Nakagawa S. Regulation of adult neurogenesis by psychotropic drugs and stress. J Pharmacol Exp Ther. 2001;299:401–407. [PubMed] [Google Scholar]
- 13.Fuchs E, Czeh B, Flugge G. Examining the novel concepts of the pathophysiology of depression in the chronic psychosocial stress paradigm in the tree shrews. Behav Pharmacol. 2004;15:315–325. doi: 10.1097/00008877-200409000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Gentsch C, Lichtsteiner M, Freer H. Competition for sucrose-pellets in triads of male Wistar rats: effects of three serotonergic drugs. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:639–651. doi: 10.1016/0278-5846(88)90009-7. [DOI] [PubMed] [Google Scholar]
- 15.Gentsch C, Lichtsteiner M, Freer H. Competition for sucrose-pellets in triads of male Wistar rats: effects of acute and subchronic chlordiazepoxide. Psychopharmacology. 1990;100:530–534. doi: 10.1007/BF02244007. [DOI] [PubMed] [Google Scholar]
- 16.Gouirand AM, Matuszewich L. The effects of chronic unpredictable stress on male rats in the water maze. Physiol Behav. 2005;86:21–31. doi: 10.1016/j.physbeh.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 17.Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew in regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imaki T, Nahan JL, Rivier C, Sawchenko PE, Vale W. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neurosci. 1991;11:585–99. doi: 10.1523/JNEUROSCI.11-03-00585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imaki T, Vale W, Sawchenko PE. Regulation of corticotropin-releasing factor mRNA in neuroendocrine and autonomic neurons by osmotic stimulation and volume loading. Neuroendocrinology. 1992;56:633–40. doi: 10.1159/000126286. [DOI] [PubMed] [Google Scholar]
- 20.Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of depression. Am J Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- 21.Kessler RC. The effects of stressful life events on depression. Ann Rev Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- 22.Kiddoo DA, Valentino RJ, Zderic S, Ganesh A, Leiser SC, Hale L, Grigoriadis DE. Impact of state of arousal and stress neuropeptides on urodynamic function in freely moving rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1697–R1706. doi: 10.1152/ajpregu.00742.2005. [DOI] [PubMed] [Google Scholar]
- 23.Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848:141–52. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- 24.Kozorovitskiy Y, Gould E. Dominance hierarchy influences adult neurogenesis in the dentate gyrus. J Neurosci. 2004;24:6755–6759. doi: 10.1523/JNEUROSCI.0345-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J, Duan W, Long JM, Ingram DK, Mattson MP. Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. J Mol Med. 2000;15:99–108. doi: 10.1385/JMN:15:2:99. [DOI] [PubMed] [Google Scholar]
- 26.Leuner B, Mendolia-Loffredo S, Kozorovitskiy Y, Samburg D, Gould E, Shors TJ. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J Neurosci. 2004;24:7477–81. doi: 10.1523/JNEUROSCI.0204-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Lumley LA, Sipos ML, Charles RC, Charles RF, Meyerhoff JL. Social stress effects on territorial marking and ultrasonic vocalizations in mice. Physiol Behav. 1999;67:769–75. doi: 10.1016/s0031-9384(99)00131-6. [DOI] [PubMed] [Google Scholar]
- 29.Malatynska E, Goldenberg R, Shuck L, Haque A, Zamecki P, Crites G, Schindler N, Knapp RJ. Reduction of submissive behavior in rats: A test for antidepressant drug activity. Pharmacology. 2000;64:8–17. doi: 10.1159/000056145. [DOI] [PubMed] [Google Scholar]
- 30.Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–1571. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- 31.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez M, Calvo-Torrent A, Pico-Alfonso MA. Social defeat and subordination as models of social stress in laboratory rodents: A review. Aggr Behav. 1998;24:241–256. [Google Scholar]
- 33.Molina VA, Heyser CJ, Spear LP. Chronic variable stress or chronic morphine facilitates immobility in a forced swim test: reversal by naloxone. Psychopharmacology. 1994;114:433–440. doi: 10.1007/BF02249333. [DOI] [PubMed] [Google Scholar]
- 34.Pavcovich LA, Valentino RJ. Central regulation of micturition in the rat the corticotropin-releasing hormone from Barrington’s nucleus. Neurosci Lett. 1995;196:185–8. doi: 10.1016/0304-3940(95)11873-u. [DOI] [PubMed] [Google Scholar]
- 35.Price J, Sloman L, Gardner R, Gilbert P, Rohde P. The social competition hypothesis of depression. Brit J Psychiatry. 1994;164:309–315. doi: 10.1192/bjp.164.3.309. [DOI] [PubMed] [Google Scholar]
- 36.Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: Impact of chronic social stress. Behav Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 38.Sapolsky RM. Testicular function, social rank and personality among wild baboons. Psychoneuroendocrinology. 1991;16:281–293. doi: 10.1016/0306-4530(91)90015-l. [DOI] [PubMed] [Google Scholar]
- 39.Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- 40.Seligman S, Biegley G. Learned helplessness in the rat. J Comp Physiol Psychol. 1975;88:534–541. doi: 10.1037/h0076430. [DOI] [PubMed] [Google Scholar]
- 41.Selye HA. Syndrome produced by diverse nocuous agents. J Nature. 1936:138. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- 42.Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sloman L. How the involuntary defeat strategy relates to depression. In: Sloman Gilbert P, editor. Subordination and Defeat: An Evolutionary Approach to Mood Disorders and Their Therapy. Mahway, N.J: Lawrence Erlbaum Associates; 2000. pp. 47–70. [Google Scholar]
- 44.Sloman L, Gilbert P, Hasey G. Evolved mechanisms in depression: the role and interaction of attachment and social rank in depression. J Affec Disord. 2003;74:107–121. doi: 10.1016/s0165-0327(02)00116-7. [DOI] [PubMed] [Google Scholar]
- 45.Tamashiro KL, Nguyen MM, Fujikawa T, Xu T, Yun Ma L, Woods SC, Sakai RR. Metabolic and endocrine consequences of social stress in a visible burrow system. Physiol Behav. 2004;80:683–93. doi: 10.1016/j.physbeh.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 46.Tamashiro KLK, Nguyen MMN, Sakai RR. Social Stress: From rodents to primates. Fron Neuroendocrinol. 2005;26:27–40. doi: 10.1016/j.yfrne.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Valentino RJ, Page ME, Luppi PH, Zhu Y, Van Bockstaele E, Aston-Jones G. Evidence for widespread afferents to Barrington’s nucleus, a brainstem region rich in corticotropin-releasing hormone neurons. Neuroscience. 1994;62:125–43. doi: 10.1016/0306-4522(94)90320-4. [DOI] [PubMed] [Google Scholar]
- 48.Valentino RJ, Pavcovich LA, Hirata H. Evidence for corticotropin-releasing hormone projections from Barrington’s nucleus to the periaqueductal gray and dorsal motor nucleus of the vagus in the rat. J Comp Neurol. 1995;363:402–22. doi: 10.1002/cne.903630306. [DOI] [PubMed] [Google Scholar]
- 49.Van der Hart MG, Czeh B, de Biurrun MT, Watanabe T, Natt O, Fram J, Fuchs E. Substance P receptor antagonist and clomipramine prevent stress-induced alterations in cerebral metabolites, cytogenesis in the dentate gyrus and hippocampal volume. Mol Psychiatry. 2002;7:933–941. doi: 10.1038/sj.mp.4001130. [DOI] [PubMed] [Google Scholar]
- 50.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology. 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]


