Abstract
A preparative analytical method was developed to selectively remove (“chemically subtract”) a single compound from a complex mixture, such as a natural extract or fraction, in a single step. The proof of concept is demonstrated by the removal of pure benzoic acid (BA) from cranberry (Vaccinium macrocarpon Ait.) juice fractions that exhibit anti-adhesive effects vs. uropathogenic E. coli. Chemical subtraction of BA, representing a major constituent of the fractions, eliminates the potential in vitro interference of the bacteriostatic effect of BA on the E. coli anti-adherence action measured in bioassays. Upon BA removal, the anti-adherent activity of the fraction was fully retained, 36% inhibition of adherence in the parent fraction at 100 ug/mL increased to 58% in the BA-free active fraction. The method employs countercurrent chromatography (CCC) and operates loss-free for both the subtracted and the retained portions as only liquid-liquid partitioning is involved. While the high purity (97.47% by quantitative 1H NMR) of the subtracted BA confirms the selectivity of the method, one minor impurity was determined to be scopoletin by HR-ESI-MS and (q)HNMR and represents the first coumarin reported from cranberries. A general concept for the selective removal of phytoconstituents by CCC is presented, which has potential broad applicability in the biological evaluation of medicinal plant extracts and complex pharmaceutical preparations.
Keywords: Chemical subtraction; Selective removal, Countercurrent chromatography; CCC; Vaccinium macrocarpon Ait; Bacterial adhesion
1. Introduction
1.1. Cranberries as antibacterial agents
Cranberry juice (Vaccinium macrocarpon Ait., Ericaceae) is a popular dietary supplement used for the treatment of urinary tract infections (UTIs) [1-5]. This activity was originally believed to be due to acidification of urine, and/or increased excretion of the cranberry urinary metabolite hippuric acid [6, 7], although later research suggested these effects were not significant enough to account for observed bioactivity [8, 9]. Inhibition of E. coli adherence to uroepithelial cells [5, 10-14], rather than direct bacteriostatic or bactericidal activity, is currently believed to be the mechanism by which cranberry helps prevent and treat urinary tract infections.
In designing an assay to measure inhibition of E. coli adherence to a human uroepithelial cell line [15], it was determined that, at sufficiently low pH, the cranberry constituent benzoic acid and its urinary metabolite hippuric acid have bacteriostatic and/or bactericidal activity. The search for anti-adherent compounds was thus confounded by the presence of benzoic acid, which apparently killed the bacteria before they could be inhibited from adhering. Therefore, the need exists for a chromatographic method that removes benzoic acid and, at the same time, allows full recovery of the remaining compounds for further testing.
1.2. Determination of active principles
Various constituent metabolites are present in widely different quantities in plant extracts and other nature-derived pharmaceutical products. The initial characterization of these complex materials is usually done in terms of their major components. When done in parallel with in vitro or in vivo studies of the biological potency of crude and the fractionated material, (phyto)chemical analysis can be target towards the isolation of active principles through a process widely known as bioassay-guided fractionation (BGF, see [16] and refs therein). While an isolated active principle is the ultimate product of a BGF procedure, the active principle can equally be considered as having been removed (“subtracted”) from the active starting material. While isolation and subtraction are two sides of the same analytical coin seen from a chemical perspective, there are important differences when seen from a pharmaceutical and/or biological perspective, in particular when dealing with complex pharmaceuticals and their pharmacological effects. Thus, it often remains a challenge to isolate and characterize (all) active principles of a given material, in a quantitative fashion [16], without activity loss, when synergy is involved [17], and across the large dynamic range of the many constituents present
Due to the fact that irreversible adsorption cannot be ruled out, the use of analytical techniques that involve any solid support is not an option – in particular when detection involves biological endpoints, which often represent very sensitive assays. Another important consideration is that in many chromatographic separation methods, the presence of major components interferes with the subsequent detection, purification, and ultimate determination of the bioactivity for minor and micro components present in the same extract or fraction thereof. The quantity of a compound in an extract has no bearing on its relative bioactivity; in fact, it may be said that minor components are more likely to be active principles since they are much more numerous.
1.3. The liquid-liquid advantage of countercurrent separation
The use of countercurrent chromatography (CCC), a liquid-liquid partition-based methodology, in the chromatographic separation of natural extracts is an excellent method by which to surgically remove (subtract) a potentially disruptive major component from an extract in order to more closely examine the bioactivity of minor components. A major advantage of CCC results from the fact that both chromatographic phases are liquids; there is no chance for irreversible adsorption of metabolites to solid chromatographic media. This means that all of the analytes introduced into the column may be recovered. Countercurrent chromatographs, such as those that utilize the hydrodynamic principle of high-speed CCC (HSCCC) machines [18, 19], employ many mixing and settling steps corresponding to the number of coil turns and the motion of the centrifuge. Therefore, solute tailing is avoided due to the high surface area contact between the two immiscible phases. The straightforward scale-up capabilities of CCC (including HSCCC and CPC as current mainstream technologies) allow pilot experiments to be run as a precursor to high capacity separations. Furthermore, a solvent system can be chosen that will target desired analytes in a region of optimal resolution also known as the “sweet spot” of CCC [20]. Complex fractions constituted of metabolites of varying polarity are injected directing into the CCC without extensive preparation. The HSCCC procedure described in this work led to the discovery of novel coumaroyl iridoids and a depside from cranberries [21].
1.4. Purity of active constituents
Purity assessment is another important aspect of natural products chemistry [22]. By assessing the purity of the BA fraction, the effectiveness of the subtraction method may be evaluated. A subtracted fraction that contains compounds in addition to the major component may have removed some important active principle as well as the targeted compound. A poor separation method and/or excessive tailing of the major component would result in numerous impurities in the sample. Therefore, purity assays are key to the assessment of the selectivity of compound isolation and chemical subtraction.
2. Experimental
2.1 General Experimental Procedures
Diaion™ reverse-phase HP-20 resin was purchased from Sigma-Aldrich. All organic solvents were HPLC grade from Fisher Scientific. Water was deionized to 18 MΩ·cm at 25 °C through a Millipore Water system. The NMR spectra were recorded on a Bruker DRX 360 instrument.
2.2 Initial Vaccinium fractionation
A thirty-two liter volume of cranberry juice concentrate (equal to 6.4 kg dried cranberry juice (pH 2.5), Ocean Spray, Inc.) was fractionated over a polyaromatic adsorbent resin to remove water and the most polar constituents (e.g., sugars and polar organic acids). Retained material was eluted from the column with a step gradient of 100% deionized water, 20% methanol, 50% methanol, and finally 100% methanol, with 1 liter fractions collected throughout. The majority of the 100% methanol fractions, demonstrating positive anti-adhesion activity, were recombined (34.57 g). A portion (12.9 g) of the active fraction was subjected to HSCCC removal of benzoic acid (BA).
2.3. Anti-adhesion assay
The full details of this assay are described elsewhere [15] but, in brief, the procedure is as follows: Immortalized human uroepithelial T24 cells (ATCC HTB4) were grown to confluence in wells of a microplate. A urinary E. coli (ATCC 29194) isolate containing the uropathogenic papGII gene was grown on CFA agar, suspended in saline to 107 bacteria per mL, mixed with test cranberry fractions, and incubated with the T24 cells for 1 hour. Unadhered bacteria, media, and fractions or controls were rinsed off, fresh media was added to the microplate, and adherent bacteria were grown for 4 to 6 hours to a measurable optical density. Initial quantities of adherent bacteria (prior to the 4 to 6 hour incubation) were calculated using a standard growth curve produced in the same plate.
2.4. Countercurrent instrumentation
The countercurrent chromatography (CCC) instrumentation used in the present work was a high-speed CCC (HSCCC) apparatus, which consisted of a J-type instrument (Model CCC-1000; Pharma-Tech Research Corporation, Baltimore, MD). The centrifuge containing a self-balancing three-coil rotor (radius 7.5 cm), equipped with three 40 mL PTFE Teflon coil columns with an inner diameter (i.d.) of 0.8 mm for pilot experiments, or three 105 mL PTFE Teflon coil columns with a 1.6 mm i.d. for scale-up separation. In addition, the CCC system was equipped with a Lab-Alliance Series III digital single-piston solvent pump with a switchable solvent inlet valve, a Shimadzu SPD-10A VP UV-Vis detector with preparative flow cell, a Cole-Parmer modular paperless recorder model 80807−00, and a Foxy Jr. fraction collector (Isco, Inc.).
For the purpose of determining a suitable 2-phase CCC solvent system for the high-resolution separation of BA, the partition behavior of the target analyte was studied using commercially available BA (Sigma- Aldrich, Milwaukee, WI). Using the shake flask approach, BA was tested it in a number of solvent systems that have been described for the separation of compounds of similar nature (phenolic) and polarity [18, 23], In particular, solvent systems based on EtOAc-H2O, BuOH-H2O, CHCl3-H2O were considered [24]. The ternary solvent system of CHCl3:MeOH;H2O (10:7:5) was chosen based on the favorable K value of BA (2.7), which was calculated as the ratio of the amount of BA in the lower phase to the upper phase.
2.5. HSCCC separation
The HSCCC separation was performed as following: the coil columns was first entirely filled with the lower phase as stationary phase; after equilibrating the HSCCC coil columns at 995 rpm with pumping the mobile phase (upper phase), the sample (ca. 500 mg) dissolved into 2 ml equivolume mixture of the two phase solvents was injected into HSCCC instrument through a 2 ml sample loop for the pilot experiment. For the scale-up separation, 10 ml samples (average 1.18 g) were injected into HSCCC instrument through a 10 ml sample loop. The mobile phase was pumped into the HSCCC system in the tail-in head-out mode at 1.5 ml/min for the pilot experiment and 2.5 ml/min for scale-up separation. The eluates were collected at 5 min per test tube until the UV-Vis detector observed no additional peaks. The average stationary phase retention fractions (SF) were 0.56 for the pilot experiment and 0.52 for scale-up separation. Two parallel runs were conducted for the pilot experiments. For the scale-up separations, ten successive runs were carried out such that, right after the elution of the target analyte (BA), the mobile phase was switched to the former stationary phase while maintaining rotation. Under these extrusion conditions [25], the chromatographic run was finished within one column volume (Vtot= 120 mL; 260−380 mL in Fig.1, stages II and III according to [25]), leaving back a column filled with stationary phase and ready for equilibration and new injection.
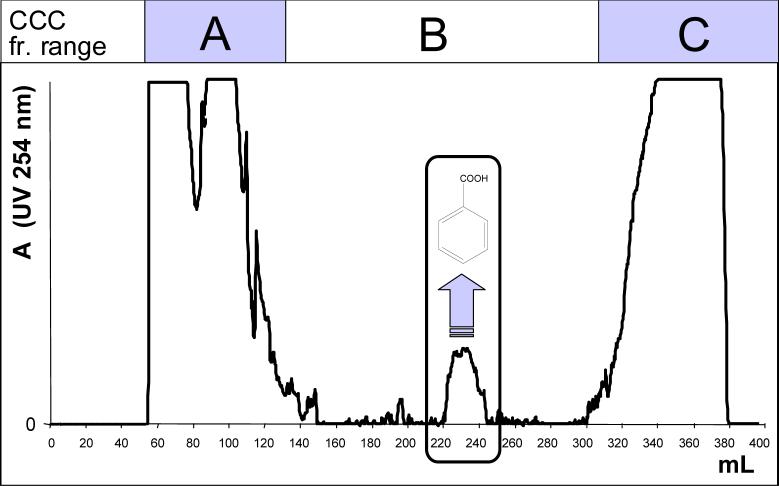
Fig. 1.
CCC separation of pre-fractionated cranberry extract using an HSCCC instrument and the 2-phase solvent system of chloroform:methanol:water (10:7:5). The upper aqueous phase was mobile with a flow rate of 1.5 mL/min from tail to head. The column exhibited a stationary phase retention ratio (Sf) of 0.53. The peak centered around 230 ml corresponds to the chemically subtracted benzoic acid which eluted in the B-range of fractions. In contrast, the bulk quantities of the accompanying phytoconstituents remain outside the elution window (B-fractions): while the polar anti-adherent phenolic constituents are concentrated in the A-fraction, the lipophilic components are sharply removed by pumping stationary phase (extrusion, stages II and III [25], 260−380 mL) to rapidly obtain the C-fraction (see [30] for the definition of CCC fraction ranges).
2.6. Analysis of HSCCC fractions and combination
All HSCCC fractions were analyzed by thin layer chromatography (TLC). Silica gel glass plates with thickness of 0.20 mm (Si GF254 Merck KGaA, Darmstadt, Germany) were used, solvent systems used for TLC development was CHCl3:MeOH (7:1). The compounds were first detected under UV light at 254 and 360 nm, then 5% H2SO4 in EtOH was sprayed on the plates as the visualization reagent, following by heating for 5 to 10 min. Based on the TLC chromatograms and the UV profiles from the HSCCC runs, all fractions from the ten HSCCC scale-up runs were finally combined to 7 fractions. Benzoic acid was present in the 5th fraction (collected fraction numbers: 53−65, combined from 10 separations on HSCCC), which was called the BA fraction.
2.7. Identification and impurity profiling of benzoic acid and scopoletin
The BA fraction from pilot experiment was dried thoroughly over P4O10 and characterized by NMR. The LC-MS analysis was performed on a Waters Alliance 2690 HPLC connected to a Micromass Q-TOF with a Discovery C18 2.1 × 100 mm column, particle size 5μm. The LC conditions were as follows: solvent A: 0.05% Acetic Acid in water, solvent B: MeOH. At the beginning, the column was equilibrated with 80% A, then a gradient increasing B from 20% to 90% in 30 min was applied. The flow rate was 0.2 ml/min, the column temperature 30 °C. The MS scan range was from m/z 100 to 500.
For NMR testing 15 mg were dissolved in 0.75 mL CDCl3 (99.8 % isotopic purity) in 5 mm NMR tubes. Chemical shifts [δ in ppm] were referenced to the residual proton signal of CDCl3 at 7.240 ppm, and the couplings constants [J] are given in Hz. For all NMR experiments including the qHNMR analysis, off-line data analysis was performed using the NUTS software package (Acorn NMR Inc, Livermore, CA).
For (im)purity profiling, an 1H NMR spectrum of the sample was measured with 128 scans to yield a spectrum suitable for a quantitative evaluation (qHNMR). Acquisition parameters were chosen in agreement with a quantitative NMR method recently reported [26, 27], with a precision of detection for minor compounds present at ca. 1% abundance to be better than 2%.
Data processing was performed according to a dossier [28, 29] developed to optimize NMR parameters for the quantitative assessment of natural products. The best line shape and signal to noise ratio was achieved with a Gaussian factor of 0.05 and a line broadening of 0.3. The digital resolution was increased by adding an equal number of zeros at the end of the FID data set (zero fill). To improve integration, the baseline of the FID was corrected, broad water as well as other –OH and exchangeable proton signals were eliminated by repeated simulation and subtraction from the uneven baseline, and, finally, a baseline flattening was applied by nth (n<10) order polynomial correction. The signal at 7.604 ppm of the main component benzoic acid served as a reference signal set to an arbitrary integral value of 100.
3. Results and discussion
3.1. Countercurrent separation
Reconstituted cranberry juice concentrate (pH 2.5) was initially fractionated on a solid phase column to remove water and the most polar constituents such as sugars. The resulting methanol fractions that showed positive activity in the E. coli cell adhesion assay were combined. In order to effectively remove the benzoic acid (BA) from this fraction with HSCCC, a solvent system with proper liquid-liquid partition coefficient (K) for BA was first identified. Usually, a suitable K value for CCC is 0.2 ≤ K ≤ 5 [19, 20, 24]. Therefore, in order to gain an optimal separation within the upper range of the high-resolution elution window of CCC (“sweet spot”) [20, 24], a solvent system with a K value (concentration of BA in the lower phase divided by its concentration the upper phase) close to 3 was targeted. After performing shake flask experiments with a number of well-tried solvent systems, the ternary system of CHCl3:MeOH;H2O (10:7:5) was chosen based on BA's favorable K value of 2.7 in this solvent system (see also Experimental).
Initially, two HSCCC experiments were done on a small-scale instrument (Vtot = 120 ml) in order to assure that the desired separation was feasible (Fig. 1). After the successful pilot experiments, scale-up HSCCC separations were employed with the same solvent system and instrument but with larger column (Vtot = 850 ml) and injection loop volumes.
The results of the countercurrent separation were analyzed by monitoring the UV absorption of the eluant at 220 and 275 nm as well as TLC of individual test tubes. All collected test tubes were combined into seven fractions. BA was confined to the fifth fraction (2.6 < KD < 3.4) that accounted for 4.9 g of the original 12.9 grams. The most polar fraction, (0 ≤ KD ≤ 0.3) with a mass of 5.6 g, was the most active fraction. The remaining five fractions accounted for 2.4 g of the total mass. This pattern of separation coincides with the “ABC” fractionation scheme recently described for the countercurrent separation of anti-tuberculosis ethnobotanicals [30]. In the current case, the A-fraction contains the polar active principles, the B-fraction contains the target subtraction compound, and the C-fraction consists of inactive lipophilic compounds (Fig. 1).
The initial methanol fraction prior to CCC separation was moderately active, with 36% inhibition of adherence of a uropathogenic strain of E. coli to human uroepithelial cells [15] at 100 μg/mL (p = 0.04). The most polar fraction from the CCC experiment retained this activity (58%, p = 0.03) at the same concentration. The fraction containing benzoic acid was also active (48%, p = 0.02), primarily or entirely due to bactericidal activity rather than inhibition of adherence.
3.2. Selectivity of the chemical subtraction process
In order to determine the accuracy and precision with which BA was removed from the complex anti-adhesive cranberry fraction, it was necessary to analyze the BA fraction for its identity and purity, respectively. As such, the purity of the subtracted BA (fraction) became a key measure of the overall selectivity of the chemical subtraction process. Given this aim, it was desirable to apply purity assays that are orthogonal to the chosen partition-based separation (subtraction) method. As such, LC-MS was chosen as it represents an adsorption-based chromatographic method, and quantitative 1H NMR (qHNMR) was selected as a highly independent, non-chromatographic assay.
The identity of the well-known main component BA was verified by comparison to a 1H NMR reference spectrum [31]. Three signals at 7.609 ppm [J = 7.5, 1.3 Hz; tt] for H-4, 7.470 ppm [J = 8.0, 7.3, 1.4; ddd] for H-3 and H-5, and at 8.117 ppm [J = 8.0, 1.9, 1,4, 0.3 dddd] were especially relevant. The high purity of the subtracted BA (97.47%) was determined by qHNMR and confirms the selectivity of the subtraction. Conversely, it also underlines the power of qNMR in assessing the purity of chemically subtracted fractions. Despite the fact that BA accounted for 38% (by mass) of the active fraction collected by reversed phase column chromatography, it was cleanly subtracted from the sample in one chromatographic step. The countercurrent separation also removed 19% (by mass) of inactive non-polar metabolites from the same fraction.
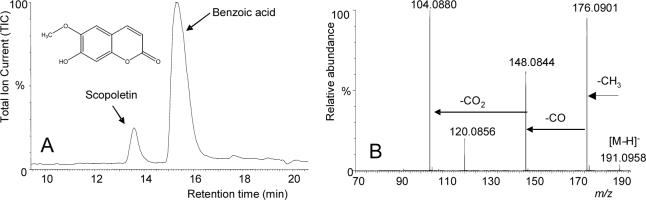
The LC-MS trace in Fig. 2A. shows a pronounced impurity eluting in front of the major BA peak. The ESI LC-MS analysis of this compound in negative mode (Fig. 2B.) determined the molecular formula of the impurity to be C10H8O4 on the basis of molecular ion m/z 191.0958. Structural information was also obtained from the fragmentation pattern. The m/z 176 fragment is produced by the loss of a methyl group from the methoxyl function in the compound. The m/z 148 fragment indicates subsequent loss of CO from the m/z 176 species, which indicates that there is at least one phenolic hydroxyl group present. An additional loss of 44 mass units to arrive at m/z 104.0880 indicates that there is a carboxylic acid or an ester in the compound. Based on this evidence, the impurity was deduced to be scopoletin, a known coumarin. This conclusion was confirmed by comparison of the MS-MS profile with that of commercial scopoletin reference material. In order to firmly establish the identity of scopoletin (7-hydroxy-6-methoxy-2H-chromen-2-one) with previous reports, the 1H NMR spectrum of the sample was compared with a scopoletin reference spectrum [31-33]. The structure could be verified by the presence of two doublets at 6.273 ppm [J = 9.5 Hz; H-2] and 6.922 ppm [J = 0.3 Hz; H-5] and two singlets at 6.850 and 3.959 ppm, assigned to H-8 and the methoxy group H3-9, respectively. The double doublet at 7.605 ppm [J = 9.5, 0.3 Hz] for H-3 was overlapped by the benzoic acid signal H-4. In summary, this is the first time that scopoletin has been reported from cranberries.
Fig. 2.
LC-MS purity analysis of the chemically subtracted benzoic acid (BA). The LC-MS total chromatogram (A) of the recombined B-fractions corresponding to BA (peak around ∼230 mL in Fig.1) exhibited the presence of only one abundant impurity, which was identified by negative mode high-resolution ES-MS (B) as scopoletin. Considering that quantitative NMR revealed scopoletin to be a very minor impurity (0.30%, see Tab. 1), this example also illustrates, how chromatographic impurity profiles have to be interpreted with due caution to account for potentially enormous differences in ionization/response rates.
While the two most abundant minor impurities (1.70 and 0.31%, respectively, of a total of 2.53% impurities) were BA analogues, which could be expected to produce critical pairs of separation, the finding of the structurally unrelated scopoletin, a coumarin, was unanticipated. Another important finding relates to the quantitation of this co-eluting minor impurity: while being unambiguously identified by HR-ESI-MS and 1H NMR, qHNMR analysis allowed quantitation of scopoletin without calibration, using the 100% integral method [22, 26]. Interestingly, scopoletin was proved to be a very minor impurity at a concentration of only 0.30%. While LC-MS was instrumental in dereplicating the structure of one of the minor impurities as scopoletin, this result provides a helpful illustration of the large variation observed with response factors in chromatographic detection (here: ionization potential; see Fig. 2). These observations are fully in line with the results of the TLC monitoring of the CCC fractionation, in which scopoletin was easily detected due to its fluorescence at 365 nm which commonly observed with coumarins.
3.3. General concept for chemical subtraction
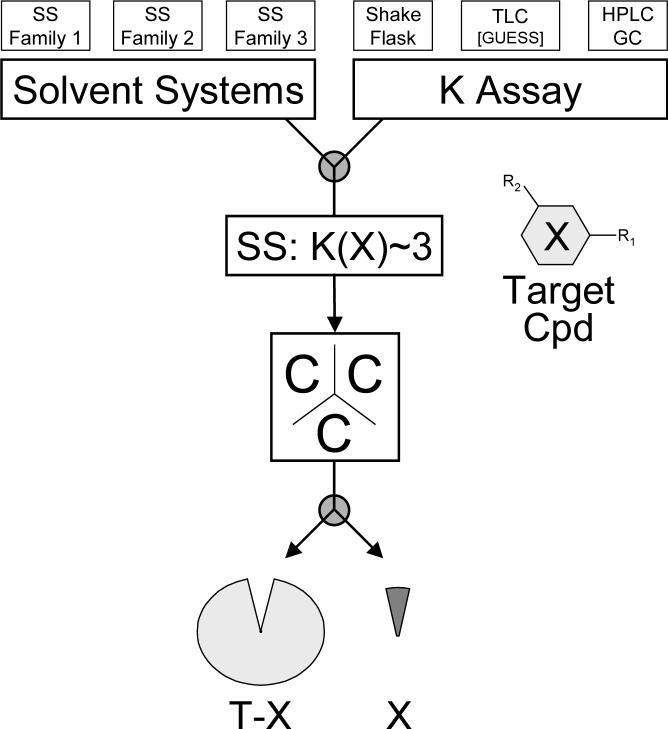
The principle of chemical subtraction, as exemplified for BA from cranberry extract, can be transferred to any analyte that is amenable to CCC separation. The general workflow is summarized in Fig. 3 and centers around the choice of an appropriate solvent system, in which the target analyte (X) has a suitable partition coefficient (K). It has been our practical observation that in elution-mode CCC the best resolution is obtained for analytes with K-values into the upper range of the resolution sweet spot. Accordingly, the recommended working range for CCC chemical subtraction is 1<K<5, with K∼3 being the recommended target value for HSCCC machines.
Fig. 3.
General workflow for the chemical subtraction of a target compound (X) from a total plant extract (T) or any other complex (natural) material: A suitable two-phase solvent system (SS) is selected from established or newly designed solvent system families. Utilizing an assay capable of measuring partition coefficients (K), the polarity of a selected SS family is then adjusted to match a suggested target values of K(X) ∼ 3. Subsequent HSCCC fractionation yields the target compound (X) at elution volumes that can be predicted using established CCC theory for elution [19, 37, 38] and extrusion [25, 39]. The surrounding fractions can be recombined to the chemically subtracted starting material, T-X (see discussion for further explanation and references).
There are two main aspects of choosing the best solvent system (Fig. 3): (i) determine the chemical composition of the two phases, which is typically composed of 2−5 volatile solvents and each constitutes a solvent system family [34]; (ii) within each family, adjust the polarity of a solvent system by variation of the specific proportions of the solvents, with the goal to match the desired target K for the target compound X, e.g., K(X) ∼ 3. A number of recent publications provide further guidance in the selection of appropriate solvent systems for know compounds and compound classes [16, 18, 23, 35, 36]. Furthermore, methods for the rational design of solvent system families [34] and their performance characteristics [20, 24] have recently been introduced that can be applied to previously studied classes of chemicals as well as to chemical entities that have no precedence in the CCC literature.
Once a suitable solvent system has been identified, CCC separation is performed by means of, e.g., HSCCC or centrifugal partition chromatography (CPC). The elution volume or time of the target peak can be readily calculated using well-established CCC theory [19, 37, 38]. In order to enhance throughput, single batch runs can stop elution once the target compound has been eluted from the column according to its K value and can take advantage of the liquid nature of the stationary phase by employing the recently developed and fully parameterized CCC extrusion methods, EECCC [25] and BECCC[39].
In order to assess the selectivity of different solvent system families for a given chemical subtraction problem, routine chromatographic methods such as TLC, HPLC, LC-MS/GC-MS, but also spectroscopic method such as qHNMR can be used. As a result of this project, the combination of qHNMR with a MS-hyphenated high-resolution chromatography is fit for the purpose of proving LC/MS subtraction selectivity and predicting the chances of success in a scaled-up CCC procedure.
4. Conclusions
Because countercurrent chromatography (CCC) is based on liquid-liquid partitioning only, it avoids the disadvantages of (selective) adsorption in solid-phase LC and allows full recovery of all analytes. This property of CCC is a prerequisite for the design of a method aimed at the selective removal of compounds (“chemical subtraction”), as both the subtracted and the retained portions remain unaffected in their chemical composition.
The presented CCC method establishes the concept of chemical subtraction of a target compound from a plant extract that interferes with (or acts in) a bioassay. The method works in a single-step and with high selectivity. From the target compound perspective, subtraction selectivity was 97.5%. Considering close BA analogues, which are likely to interfere the anti-adherence bioassay in a fashion similar to BA, selectivity was 99.5% (Tab. 1).
Table 1.
The quantitative 1H NMR (qNMR) purity profile and the high-purity (>97% by qHNMR) of the BA product prove the high selectivity of CCC separation, being capable of selectively subtracting BA from chemically complex starting material. From the pharmacological perspective of the anti-adhesive bioassay, BA and its close analogues were removed with 99.48% efficiency in a single separation step.
| Compound | % | Identity | Reference resonances(s) | Number of Hydrogens |
|---|---|---|---|---|
| 1 | 97.47 | benzoic acid | 7.604a | 1 |
| 2 | 1.70 | benzoic acid analogue | 7.429−7.567 | 2 |
| 3 | 0.31 | benzoic acid analogue | 2.384 | 1 |
| 4 | 0.30 | scopoletin | 3.950 | 3 |
| 5 | 0.29 | impurity | 0.811−0.929 | 3 |
| 6 | 0.15 | impurity | 3.511 | 3 |
| 7 | 0.04 | impurity | 1.031 | 2 |
| 8 | 0.02 | impurity | 2.705 | 6 |
The signal at 7.604 ppm of the main component benzoic acid served as a reference signal set to an arbitrary integral value of 100. Percentages based on the assumption that all compounds possess a molecular weight close to that of Benzoic Acid.
Besides the interesting observation that two structurally very different chemicals, BA and scopoletin, share (almost) identical partition behavior, it was shown that a combination of qHNMR and LC-MS analysis is capable of measuring the high degree of selectivity that justifies CCC's designation as a tool for chemical subtraction. The approach of using CCC for the chemical subtraction of single constituents from complex mixtures has potential broad applicability in the biological evaluation of natural products and other complex pharmaceutical preparations with regard to additive, synergistic, and/or “exclusive” effects. In addition, chemical subtraction by CCC could be very useful in other fields of pharmaceutical and biomedical analysis requiring clean preparative separations of undesired constituent, such as toxins, degradation products, or interfering bioactive compounds.
Acknowledgments
This research was generously supported by grant numbers P50 AT00155 and F3 AT00623 from the National Center for Complementary and Alternative Medicine (NCCAM), the Office of Dietary Supplements (ODS), the Office for Research on Women's Health (ORWH), and the National Institute of General Medical Sciences (NIGMS). The contents are solely the responsibility of the authors and do not necessarily represent the official views of NCCAM, ODS, ORWH or NIGMS. We also thank Ocean Spray Cranberries, Inc. for the cranberry juice concentrate used in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bennett J, Brown CM. J. Am. Pharm. Assoc. 2000;40:353–358. doi: 10.1016/s1086-5802(16)31082-8. [DOI] [PubMed] [Google Scholar]
- 2.Blumenthal M, Brinckmann J, Wollschlaeger B. The ABC clinical guide to herbs. American Botanical Council; Austin, Tex: 2003. [Google Scholar]
- 3.Avorn J, Monane M, Gurwitz JH, Glynn RJ, Choodnovskiy I, Lipsitz LA. JAMA. 1994;271:751–754. doi: 10.1001/jama.1994.03510340041031. [DOI] [PubMed] [Google Scholar]
- 4.Stothers L. Canadian Journal of Urology. 2002;9:1558–1562. [PubMed] [Google Scholar]
- 5.Valentova K, Stejskal D, Bednar P, Vostalova J, Cihalik C, Vecerova R, Koukalova D, Kolar M, Reichenbach R, Sknouril L, Ulrichova J, Simanek V. J. Agric. Food Chem. 2007;55:3217–3224. doi: 10.1021/jf0636014. [DOI] [PubMed] [Google Scholar]
- 6.Kinney AB, Blount M. Nursing Research. 1979;28:287–290. [PubMed] [Google Scholar]
- 7.Schultz A. Journal of Community Health and Nursing. 1984;1:159–169. doi: 10.1207/s15327655jchn0103_5. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt DR, Sobota AE. Microbios. 1988;55:173–181. [PubMed] [Google Scholar]
- 9.Sobota AE. J Urol. 1984;131:1013–1016. doi: 10.1016/s0022-5347(17)50751-x. [DOI] [PubMed] [Google Scholar]
- 10.Howell AB, Reed JD, Krueger CG, Winterbottom R, Cunningham DG, Leahy M. Phytochemistry. 2005;66:2281–2291. doi: 10.1016/j.phytochem.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 11.Howell AB, Vorsa N, Der Marderosian A, Foo LY. N. Engl. J. Med. 1998;339:1085–1086. doi: 10.1056/NEJM199810083391516. [DOI] [PubMed] [Google Scholar]
- 12.Habash MB, Van der Mei HC, Busscher HJ, Reid G. Can. J. Microbiol. 1999;45:691–694. doi: 10.1139/w99-065. [DOI] [PubMed] [Google Scholar]
- 13.Foo LY, Lu Y, Howell AB, Vorsa N. Phytochemistry. 2000;54:173–181. doi: 10.1016/s0031-9422(99)00573-7. [DOI] [PubMed] [Google Scholar]
- 14.Gupta K, Chou MY, Howell A, Wobbe C, Grady R, Stapleton AE. Journal of Urology. 2007;177:2357–2360. doi: 10.1016/j.juro.2007.01.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner A, Chen S-N, Joike MK, Pendland SL, Pauli GF, Farnsworth NR. J. Agric. Food Chem. 2005;53:8940–8947. doi: 10.1021/jf052035u. [DOI] [PubMed] [Google Scholar]
- 16.Garrard I. A systematic approach to the development of a countercurrent chromatography protocol with examples from polar, intermediate and nonpolar compounds., Institute for Bioenigneering. Brunel University; West London: 2005. [Google Scholar]
- 17.Inui T, Wang Y, Deng S, Smith D, Franzblau S, Pauli GF. J. Chromatogr. A. 2007;1151:211–215. doi: 10.1016/j.chroma.2007.01.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito Y. J. Chromatogr. A. 2005;1065:145–168. doi: 10.1016/j.chroma.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 19.Conway WD. Countercurrent chromatography : apparatus, theory, and applications. VCH; New York, N.Y.: 1990. [Google Scholar]
- 20.Friesen JB, Pauli GF. J. Liq. Chromatogr. Relat. Technol. 2005;28:2877–2808. [Google Scholar]
- 21.Turner A, Chen S-N, Nikolic D, van Breemen RB, Farnsworth NR, Pauli GF. J. Nat. Prod. 2007;70:253–258. doi: 10.1021/np060260f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pauli GF, Jaki B, Lankin D. J. Nat. Prod. 2007;70:589–595. doi: 10.1021/np060535r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hostettmann K, Hostettmann M, Marston A. Preparative chromatography techniques: applications in natural product isolation. Springer-Verlag; Berlin ; New York: 1998. [Google Scholar]
- 24.Friesen JB, Pauli GF. J. Agric. Food Chem. 2008;56 doi: 10.1021/jf072415a. in press. [DOI] [PubMed] [Google Scholar]
- 25.Berthod A, Friesen JB, Inui T, Pauli GF. Anal. Chem. 2007;79:3371–3382. doi: 10.1021/ac062397g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pauli GF. Phytochem. Anal. 2001;12:28–42. doi: 10.1002/1099-1565(200101/02)12:1<28::AID-PCA549>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 27.Pauli GF, Jaki B, Lankin D. J. Nat. Prod. 2005;68:133–149. doi: 10.1021/np0497301. [DOI] [PubMed] [Google Scholar]
- 28.Jaki B, Chadwick L, Chen S-N, Pauli GF. The pivotal role of spectral processing in the quantitative 1H NMR analysis of natural products.; The 46th Annual Meeting of the American Society of Pharmacognosy.; ASP, Corvallis (OR). 2005. [Google Scholar]
- 29.Pauli G, Jaki B, Lankin D, Walter JA, Burton I. Quantitative NMR of bioactive natural products. In: Colegate S, Molyneux RJ, editors. Bioactive Natural Products: Detection, Isolation, and Structural Determination. Taylor & Francis CRC Press; New York: 2007. [Google Scholar]
- 30.Inui T, Case R, Chou E, Soejarto D, Fong H, Franzblau S, Smith D, Pauli GF. J. Liq. Chromatogr. Relat. Technol. 2005;28:2017–2028. [Google Scholar]
- 31.Scott KN. J. Magn. Reson. 1970;2 :361–376. [Google Scholar]
- 32.Imai F, Itoh K, Kishibuchi N, Kinoshita T, Ushio S. Chemical & Pharmaceutical Bulletin. 1989;37:119–123. [Google Scholar]
- 33.Evans HBJ, Tarpley AR, Goldstein JH. J. Phys. Chem. 1968;72:2552–2556. [Google Scholar]
- 34.Friesen JB, Pauli GF. J. Chromatogr. A. 2007;1151:51–59. doi: 10.1016/j.chroma.2007.01.126. [DOI] [PubMed] [Google Scholar]
- 35.Garrard IJ, Janaway L, Fisher D. Journal of Liquid Chromatography & Related Technologies. 2007;30:151–163. [Google Scholar]
- 36.Marston A, Hostettmann K. J. Chromatogr. A. 2006;1112:181–194. doi: 10.1016/j.chroma.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 37.Berthod A. Countercurrent Chromatography: The Support-free Liquid Stationary Phase. Elsevier; Amsterdam ; Boston: 2002. [Google Scholar]
- 38.Ito Y. CRC Crit. Rev. Anal. Chem. 1986;17:65–143. [Google Scholar]
- 39.Lu Y, Pan Y, Berthod A. J. Chromatogr. A. 2007 in press. [Google Scholar]