Abstract
Background
Apoptosis is important for maintenance of tissue homeostasis and often dysregulated in cutaneous neoplasms. The apoptosis inhibitor survivin is expressed in melanoma and non-melanoma skin cancers and benign keratinocytic lesions. Its expression has not been studied in melanocytic nevi.
Objective
We determined the expression pattern of survivin in benign melanocytic nevi in comparison to markers of proliferation and apoptosis.
Methods
Six cases of each of the following melanocytic nevi were retrieved from a dermatopathology archive: compound dysplastic nevus, intradermal nevus, compound nevus, neurotized intradermal nevus, and Spitz nevus. Survivin expression was evaluated by in situ hybridization. Apoptotic and proliferation indices were calculated by counting immunoreactive cells in terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling and proliferating cell nuclear antigen immunostained sections, respectively.
Results
All nevi, regardless of histologic type, expressed survivin. Compound melanocytic lesions expressed survivin in both epidermal and dermal compartments. The apoptotic rate was low for dysplastic, compound, and Spitz nevi, and apoptotic cells were not identified in any neurotized nevus. The proliferative index was highest for Spitz nevi, while all other nevi demonstrated rare positive cells.
Conclusions
Survivin is consistently expressed in benign melanocytic lesions, while apoptotic cells are rarely identified, suggesting the dysregulation of apoptotic pathways with the accumulation of cells in these neoplasms.
Tissue homeostasis requires a tightly regulated program of cell division and cell death. Apoptosis, or programmed cell death, counters proliferation and is regulated by a complex interplay of pro-apoptotic and anti-apoptotic molecules.1 Dysregulation of apoptosis is a common feature of cancer2 and a likely factor in the development of both benign and malignant cutaneous neoplasms. Anti-apoptotic molecules are largely represented by Bcl-2 proteins (Bcl-2, Bcl-XL, and others) that restrain mitochondrial apoptosis3 and inhibitor of apoptosis (IAP) proteins (XIAP, c-IAP, and others) that generally function as caspase inhibitors.4
Examination of Bcl-2 family protein expression has been evaluated in benign and malignant melanocytic tumors but limited to Bcl-2. For example, melanocytes within the basal cell layer of the epidermis5,6 as well as benign melanocytic nevi5–7 and melanomas6–8 have been found to consistently express Bcl-2. However, Morales-Ducret et al.5 reported that within neurotized areas in nevi, Bcl-2 expression is much weaker or absent. With respect to IAP proteins, most are expressed in melanoma cells9 but have not been examined in melanocytic nevi.
Apoptotic cells can be identified in tissue samples by terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) immunostaining. Few apoptotic cells are found in malignant melanoma8,10 with increased number in Spitz nevi and the deep component of ordinary nevi.10 Other than these reports, little additional information is known regarding apoptosis within melanocytic lesions.
Proliferation has been studied in malignant and benign melanocytic neoplasms. Commonly used markers of cell proliferation include topoisomerase II-α, proliferating cell nuclear antigen (PCNA), and MIB1/Ki-67. Proliferation in junctional, intradermal, and compound nevi, including dysplastic nevi, ranges from only rare positive cells (<1% of total nevus cell population)11–13 to about 3% in dysplastic nevi.14 Spitz nevi are considerably more mitotically active than other melanocytic nevi, with reported rates of proliferation ranging from 713,14 to 40%.11,15 Malignant melanomas vary in proliferative activity from 20 to 40% of lesional cells.8,13–15
Survivin is an IAP family member that has been implicated as an inhibitor of apoptosis and a regulator of cell division.16 Unlike other IAP proteins, survivin appears to function as a regulator of mitochondrial apoptosis rather than as a caspase inhibitor.17–19 Survivin associates with the mitotic spindle in the G2/M phase of the cell cycle, and if disrupted, results in cell division defects and apoptosis.20 Survivin is not found in most normal tissues but is frequently expressed in human cancers.21 We have previously demonstrated that survivin is absent in normal skin22,23 but is present in melanoma8 and non-melanoma skin cancers,22 benign melanocytic nevi,8 and benign keratinocytic neoplasms.23
The goal of the present study was to determine the expression pattern of survivin in various nevus types, including atypical (dysplastic), neurotized intradermal, and Spitz nevi and to correlate expression with apoptotic and proliferation markers.
Materials and methods
Case selection
A computerized search was performed of the dermatopathology archive at the Department of Dermatology (University of Utah) between 1990 and 1998 to obtain specimens of compound dysplastic nevus, intradermal nevus, compound nevus, neurotized intradermal nevus, and Spitz nevus. The first 10–12 specimens that could be obtained of each diagnosis were examined for adequate tissue remaining in the block, and a total of six were studied. The dysplastic nevi contained both epidermal and dermal components and exhibited architectural disorder as reflected by fibroplasia of the papillary dermis and bridging of adjacent rete ridges by nests of nevomelanocytes. In all these lesions, there was cytologic atypia of individual melanocytes characterized by mild nuclear enlargement and finely granular pigmented cytoplasm. The architectural disorder and cytologic atypia was not severe, nor was there a pagetoid epidermal distribution or significant mitotic activity characteristic of melanoma. Of the six specimens of Spitz nevi, one displayed a junctional pattern, two were dermal, and the remaining three were compound. Three were from children (ages 2, 6, and 13) and three were from young adults (ages 24–30). All diagnoses were confirmed by a dermatopathologist (S.R.F) by review of hematoxylin and eosin-stained slides.
Immunohistochemistry
Immunohistochemical studies were carried out on formalin-fixed, paraffin-embedded 4-μm thick sections of tissue. Peroxidase-based techniques for PCNA and TUNEL were performed as described previously.8 For PCNA staining, a 1 : 100 dilution of antibody (BD Biosciences, San Diego, CA, USA) was used. Strong nuclear staining of lesional cells comparable to scattered keratinocyte nuclei within the basal layer of the epidermis (internal control) was considered positive. Up to 500 lesional cells were counted in both the epidermal and dermal compartments of each specimen. The PCNA proliferative index (PI) was defined as the number of positive-staining lesional cells divided by the total number of cells counted multiplied by 100 and averaged for each lesion type.
Survivin in situ hybridization
In situ hybridization for survivin utilizing a full-length human survivin antisense riboprobe was performed as described elsewhere.23 Normal skin, which does not express survivin,22,23 was used as a negative control. Survivin expression was revealed as dark blue diffuse granular cytoplasmic staining and was scored semi-quantitatively as absent (−), weak or focal (+), or strong (++).
Results
Nevomelanocyte proliferation
Firstly, nevus cell proliferation was assessed by PCNA immunohistochemical staining. The PI was calculated for the junctional and/or intradermal components of each lesion. The mean PI for each lesion type is summarized in Table 1, and representative examples of staining are shown in Figures 1A and B. The PI was highest for Spitz nevi (16.6% epidermal component and 11.3% dermal component), followed by dysplastic nevi (1.0% epidermal component and 0.4% dermal component). We did not observe differences in mitotic activity between Spitz lesions derived from adults and children. Compound nevi, intradermal nevi, and neurotized nevi demonstrated only rare staining of cells (compound nevi demonstrated 0% epidermal component, 0.4% dermal component; intradermal nevi 0.7%, and neurotized intradermal nevi 0.6%). In the neurotized nevi, the rare positively staining cells were within the superficial component of the dermal populace; no staining was seen in neurotized nevomelanocytes.
Table 1.
Summary of proliferating cell nuclear antigen (PCNA) immunostaining of melanocytic nevi
| Nevus type | Epidermal | Dermal |
|---|---|---|
| Dysplastic | 1.0 (%)* | 0.4 |
| Compound | 0.0 | 0.4 |
| Intradermal | no epidermal component | 0.7 |
| Neurotized intradermal | no epidermal component | 0.6† |
| Spitz | 16.6 | 11.3 |
Percent PCNA-positive cells (proliferation index).
Non-neurotized nevus cells (PCNA staining not observed in neurotized nevus cells).
Fig. 1.
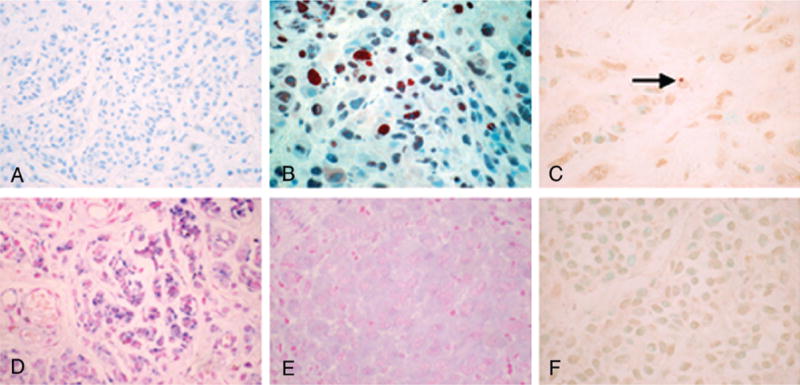
Representative proliferating cell nuclear antigen (PCNA), survivin, and terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) staining in nevi. A) PCNA staining of compound nevus without significant proliferative activity. B) PCNA staining of intradermal Spitz nevus cells, demonstrating a high proliferative activity. C) TUNEL staining of same Spitz nevus, showing a single apoptotic cell (arrow). D) Survivin in situ hybridization of compound nevus depicted in A), showing ++ cytoplasmic reactivity. E) Survivin in situ hybridization of Spitz nevus depicted in B), showing little to no cytoplasmic staining. F) TUNEL staining of a compound nevus demonstrating absence of staining. Original magnification for all photomicrographs was ×400.
Nevomelanocyte apoptosis
Next, apoptotic cells were identified by TUNEL immunostaining. Only rare positive cells were seen in the epidermal and dermal components of some dysplastic, compound, and Spitz nevi (Fig. 1C). Many nevi (particularly neurotized nevi) did not appear to contain any apoptotic cells (Fig. 1F), while two of the six compound nevi contained two TUNEL-positive cells per five ×400 fields in the dermal component.
Survivin expression
Survivin expression was identified by in situ hybridization, and the results are summarized in Table 2. All nevi, regardless of histologic type, expressed detectable levels of survivin. The dysplastic, compound, and Spitz nevi expressed survivin in both the epidermal and dermal compartments. We did not observe differences in survivin expression between Spitz lesions derived from adults and children. Dysplastic nevi showed strong cytoplasmic staining in nevomelanocytes of the epidermal and dermal compartments (four of six cases). While variable staining from focal to strong was observed in compound and intradermal nevi (Fig. 1D), survivin expression was generally weaker in Spitz nevi (Fig. 1E). Survivin expression was seen in both non-neurotized (six of six cases) and neurotized nevomelanocytes (four of six cases).
Table 2.
Survivin expression in melanocytic nevi
| Nevus type | Epidermal | Dermal |
|---|---|---|
| Dysplastic | + (2), ++ (4) | + (2), ++ (4) |
| Compound | + (5), ++ (1) | − (1), + (2), ++ (3) |
| Intradermal | NEC | + (4), ++ (2) |
| Neurotized | NEC | + (5), ++ (1)* |
| Spitz | NEC (2), − (1), + (3) | NDC (1), − (1), + (3), ++ (1) |
NEC, no epidermal component; NDC, no dermal component.
Staining and was scored semiquantitatively as absent (−), weak or focal (+), or strong (++). All lesions had detectable levels of survivin in the epidermal and/or dermal components.
Four of six lesions with expression in neurotized component.
Discussion
In this study, we examined the expression of the apoptotic inhibitor survivin in a spectrum of melanocytic nevi, and attempted to correlate expression with apoptotic and proliferative rates. We found that all nevi, regardless of histologic type, expressed survivin at detectable levels. Despite its previously demonstrated functional activities as an inhibitor of apoptosis and a promoter of mitotic progression,24 we did not observe a correlation between level of survivin expression and apoptotic or proliferative indices for any of the lesion types. In addition, despite its association with malignant phenotypes,21 survivin expression did not correlate with the degree of differentiation or cytologic atypia in nevomelanocytes.
The low mitotic rates of compound nevi and high proliferative rate in Spitz nevi reported here are consistent with earlier results obtained by others.11–14 Our finding of decreased survivin expression in Spitz nevi relative to compound nevi, however, is somewhat puzzling, as we would have anticipated that the increased mitotic rate and cytologic atypia of Spitzoid nevomelanocytes might be associated with increased survivin expression. Our finding of different levels of survivin expression in Spitz and normal nevi are consistent with recent studies by Bastian et al.25 utilizing comparative genomic hybridization demonstrating molecular differences between Spitz nevi and normal nevi. The lack of correlation between PI and survivin expression in these lesions is reminiscent of our findings in melanoma8 and keratinocytic neoplasms,23 and suggests that the survivin expression in nevi may be more important for control of apoptosis rather than proliferation.
In our previous investigation of survivin in melanoma, we found comparable expression in compound nevi but lack of expression in normal cultured melanocytes.8 In that study, we examined a panel of both invasive and metastatic melanoma lesions and found survivin expression to be consistently up-regulated in 13 of 15 invasive lesions and 15 of 15 metastatic lesions.8 Inhibition of survivin in melanoma cells led to spontaneous apoptosis and prevented tumor growth in vivo.26 These observations were consistent with its expression in premalignant (or precursor) keratinocytic neoplasms (actinic keratoses)22 and suggested to us that survivin expression may be an important and early step in the transformation from melanocyte to melanoma. In our previous study of keratinocytic lesions, we found survivin expression consistently up-regulated in both benign and malignant keratinocytic neoplasms and hyperplasias.23 It is interesting to consider our present findings in multiple nevus types in light of the natural history of nevus development and dissolution. Most nevi develop from a junctional proliferation of nevomelanocytes, which only rarely progress to melanoma; more commonly, there is a shift to a predominantly dermal component that over time acquires neuroid changes (neurotization) representing terminal differentiation, heralding its ultimate disappearance.27 The persistent expression of survivin in neurotized nevi indicate that in addition to its potential role in nevus generation, it may also be important for the long-term viability of nevomelanocytes.
All of the nevi examined in this study revealed very low rates of apoptosis. However, the use of conventional TUNEL staining to detect apoptotic cells in tissues is likely to underestimate the degree of apoptosis in situ, as apoptotic cells are rapidly cleared from tissues by phagocytic cells.28 Nevus growth, development, and persistence reflect a continual balance (or imbalance) between proliferation and apoptosis of nevomelanocytes. Small changes in either of the parameters can potentially have significant effects, as only a 5% difference in proliferation over cell death is sufficient to sustain tumor growth in animal models.29 For nevus growth, proliferation must predominate over apoptosis; but the persistence of established nevi necessarily requires a reduction in apoptosis as the rate of proliferation decreases. Alanko et al.30,31 have shown nevomelanocytes to be more resistant to apoptosis than normal melanocytes, and increased resistance to apoptosis in nevi is likely mediated by the expression of apoptotic inhibitors. It is likely that survivin is an important contributor to this increased resistance to apoptosis seen in nevomelanocytes, as it appears to be the only known inhibitor of apoptosis that is expressed in nevi but not normal cultured melanocytes.9
Acknowledgments
This work was funded in part by NIH grants K23RR17525 (S.R.F.), KO8AR48618 (D.G.), the Huntsman Cancer Foundation (D.G.), and a Fellowship-to-Faculty Transition Award from the University of Utah funded in part by the Howard Hughes Medical Institute (D.G.).
References
- 1.Reed JC. Mechanisms of apoptosis. Am J Pathol. 2000;157:1415. doi: 10.1016/S0002-9440(10)64779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reed JC. Dysregulation of apoptosis in cancer. J Clin Oncol. 1999;17:2941. doi: 10.1200/JCO.1999.17.9.2941. [DOI] [PubMed] [Google Scholar]
- 3.Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- 4.Liston P, Fong WG, Korneluk RG. The inhibitors of apoptosis: there is more to life than Bcl-2. Oncogene. 2003;22:8568. doi: 10.1038/sj.onc.1207101. [DOI] [PubMed] [Google Scholar]
- 5.Morales-Ducret CR, van de Rijn M, Smoller BR. Bcl-2 expression in melanocytic nevi. Insights into the biology of dermal maturation. Arch Dermatol. 1995;131:915. [PubMed] [Google Scholar]
- 6.Saenz-Santamaria MC, Reed JA, McNutt NS, Shea CR. Immunohistochemical expression of Bcl-2 in melanomas and intradermal nevi. J Cutan Pathol. 1994;21:393. doi: 10.1111/j.1600-0560.1994.tb00278.x. [DOI] [PubMed] [Google Scholar]
- 7.Cerroni L, Soyer HP, Kerl H. Bcl-2 protein expression in cutaneous malignant melanoma and benign melanocytic nevi. Am J Dermatopathol. 1995;17:7. doi: 10.1097/00000372-199502000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Grossman D, McNiff JM, Li F, Altieri DC. Expression and targeting of the apoptosis inhibitor, survivin, in human melanoma. J Invest Dermatol. 1999;113:1076. doi: 10.1046/j.1523-1747.1999.00776.x. [DOI] [PubMed] [Google Scholar]
- 9.Bowen AR, Hanks AN, Allen SM, Alexander A, Diedrich MJ, Grossman D. Apoptosis regulators and responses in human melanocytic and keratinocytic cells. J Invest Dermatol. 2003;120:48. doi: 10.1046/j.1523-1747.2003.12010.x. [DOI] [PubMed] [Google Scholar]
- 10.Sprecher E, Bergman R, Meilick A, et al. Apoptosis, Fas and Fas-ligand expression in melanocytic tumors. J Cutan Pathol. 1999;26:72. doi: 10.1111/j.1600-0560.1999.tb01805.x. [DOI] [PubMed] [Google Scholar]
- 11.Penneys N, Seigfried E, Nahass G, Vogler C. Expression of proliferating cell nuclear antigen in Spitz nevus. J Am Acad Dermatol. 1995;32:964. doi: 10.1016/0190-9622(95)91332-7. [DOI] [PubMed] [Google Scholar]
- 12.Florell SR, Boucher KM, Holden JA, et al. Failure to detect differences in proliferation status of nevi from CDKN2A mutation carriers and non-carriers. J Invest Dermatol. 2002;118:386. doi: 10.1046/j.1523-1747.2002.01659.x. [DOI] [PubMed] [Google Scholar]
- 13.Tu P, Miyauchi S, Miki Y. Proliferative activities in Spitz nevus compared with melanocytic nevus and malignant melanoma using expression of PCNA/cyclin and mitotic rate. Am J Dermatopathol. 1993;15:311. doi: 10.1097/00000372-199308000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Li LX, Crotty KA, McCarthy SW, Palmer AA, Kril JJ. A zonal comparison of MIB1-Ki67 immunoreactivity in benign and malignant melanocytic lesions. Am J Dermatopathol. 2000;22:489. doi: 10.1097/00000372-200012000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Niemann TH, Argenyi ZB. Immunohistochemical study of Spitz nevi and malignant melanoma with use of antibody to proliferating cell nuclear antigen. Am J Dermatopathol. 1993;15:441. doi: 10.1097/00000372-199310000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22:8581. doi: 10.1038/sj.onc.1207113. [DOI] [PubMed] [Google Scholar]
- 17.Blanc-Brude OP, Mesri M, Wall NR, Plescia J, Dohi T, Altieri DC. Therapeutic targeting of the survivin pathway in cancer: initiation of mitochondrial apoptosis and suppression of tumor-associated angiogenesis. Clin Cancer Res. 2003;9:2683. [PubMed] [Google Scholar]
- 18.Banks DP, Plescia J, Altieri DC, et al. Survivin does not inhibit caspase-3 activity. Blood. 2000;96:4002. [PubMed] [Google Scholar]
- 19.Liu T, Brouha B, Grossman D. Rapid induction of mitochondrial events and caspase-independent apoptosis in survivin-targeted melanoma cells. Oncogene. 2004;23:39. doi: 10.1038/sj.onc.1206978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li F, Ackermann EJ, Bennett CF, et al. Pleiotropic cell-division defects and apoptosis induced by interference with survivin function. Nat Cell Biol. 1999;1:461. doi: 10.1038/70242. [DOI] [PubMed] [Google Scholar]
- 21.Velculescu VE, Madden SL, Zhang L, et al. Analysis of human transcriptomes. Nat Genet. 1999;23:387. doi: 10.1038/70487. [DOI] [PubMed] [Google Scholar]
- 22.Grossman D, McNiff JM, Li F, Altieri DC. Expression of the apoptosis inhibitor, survivin, in nonmelanoma skin cancer and gene targeting in a keratinocyte cell line. Lab Invest. 1999;79:1121. [PubMed] [Google Scholar]
- 23.Bowen AR, Hanks AN, Murphy KJ, Florell SR, Grossman D. Proliferation, apoptosis, and survivin expression in keratinocytic neoplasms and hyperplasias. Am J Dermatopathol. 2004;26:177. doi: 10.1097/00000372-200406000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li F, Ambrosini G, Chu EY, et al. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature. 1998;396:580. doi: 10.1038/25141. [DOI] [PubMed] [Google Scholar]
- 25.Bastian BC, Olshen AB, LeBoit PE, Pinkel D. Classifying melanocytic tumors based on DNA copy number changes. Am J Pathol. 2003;163:1765. doi: 10.1016/S0002-9440(10)63536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grossman D, Kim PJ, Schechner JS, Altieri DC. Inhibition of melanoma tumor growth in vivo by survivin targeting. Proc Natl Acad Sci USA. 2001;98:635. doi: 10.1073/pnas.230450097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maize JC, Foster G. Age-related changes in melanocytic nevi. Clin Exp Dermatol. 1979;4:49. doi: 10.1111/j.1365-2230.1979.tb01590.x. [DOI] [PubMed] [Google Scholar]
- 28.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burns FJ, Vanderlaan M, Sivak A, Albert RE. Regression kinetics of mouse skin papillomas. Cancer Res. 1976;36:1422. [PubMed] [Google Scholar]
- 30.Alanko T, Rosenberg M, Saksela O. FGF expression allows nevus cells to survive in three-dimensional collagen gel under conditions that induce apoptosis in normal human melanocytes. J Invest Dermatol. 1999;113:111. doi: 10.1046/j.1523-1747.1999.00636.x. [DOI] [PubMed] [Google Scholar]
- 31.Alanko T, Saksela O. Transforming growth factor beta-1 induces apoptosis in normal melanocytes but not in nevus cells grown in type I collagen gel. J Invest Dermatol. 2000;115:286. doi: 10.1046/j.1523-1747.2000.00045.x. [DOI] [PubMed] [Google Scholar]


