Abstract
Background
There is a continuing interest in defining the incidence, prevalence and burden of otitis media (OM) in the individual and population for purposes of assigning “risk factors”. Often overlooked in past studies are the contributions of cold-like illnesses (CLIs) and sampling interval to those estimates.
Objective
Describe the incidence of symptomatic (AOM) and asymptomatic (OME) OM, the prevalence of OM, the contribution of CLI incidence, burden and other OM “risk factors” to the incidence and burden of OM, and the effect of sampling interval on those measures in children.
Methods
148 children (74 male; 131 white, aged 1.0–8.6 years) were followed from November 1 to April 30 by weekly pneumatic otoscopy to diagnose OM presence/absence and by daily parental diary to assign CLI episodes. Data for previously identified OM “risk factors” were collected on 127. Results were summarized using standard measures of incidence, prevalence and burden, and multiple-regression techniques were used to identify OM “risk factors”.
Results
The basal OM prevalence was 20% with peaks in December and March and the temporal pattern was correlated with CLI prevalence. The incidence of OME (per 27232 child-days) was 317, AOM was 74 and CLI was 456. The seasonal pattern of AOM and OME incidences tracked and was correlated with that for CLIs. New OM episodes were usually of short duration (≤7 days in 40%, ≤4 weeks in 75–90%) and the usual OM burden was low (median=12%). OM and breastfeeding histories and CLI incidence/prevalence were significant predictors of OME and AOM incidence and OM burden. Longer sampling intervals were less efficient in capturing AOM and OME durations and incidences, but not OM burden.
Conclusions
These results demonstrate a high incidence and prevalence of OM, most OM episodes were of short duration and longer sampling intervals introduced biases into some parameter estimates. There was a significant relationship between OM and CLI incidence, prevalence and burden suggesting that CLI experience should be controlled for in assessing independent “risk factors” for AOM and OME.
Keywords: Otitis Media, Incidence, Prevalence, Burden
INTRODUCTION
Otitis media (OM) is defined as inflammation of the middle ear (ME) mucosa, and is often clinically subclassified based on initial presentation with (acute OM [AOM]) or without (OM with effusion [OME]) concurrent signs/symptoms thought to be associated with in situ infection of the ME mucosa. There has been a long held interest in defining the incidence and prevalence of OM in different populations for purposes of abstracting “risk factors” that circumscribe groups most likely to include affected individuals and in defining the OM burden in individuals for purposes of identifying complications and sequelae of the disease 1.
With the exception of a possible hearing loss that may or may not be appreciated by the child or parent, OME is usually not associated with a signal identifier 1, 2, and consequently, the most common method for estimating OME prevalence is population screening. However, the validity of estimates of OME incidence and episode duration depends largely on the inter-assessment interval since longer intervals can assign resolution with reacquisition of the disease to a single episode 3, 4. Few past studies included the short interval assessments required for accurate estimation of those parameters and, with some exceptions 5, those that did had a short period of follow-up 6–8.
In contrast, AOM presumably is associated with a discernable signal identifier making incidence estimates easier 9. However, because some of the signs/symptoms diagnostic for AOM overlap those for a cold-like illness (CLI) which is most often the appreciable expression of a viral upper respiratory tract infection (vURI) and a known precipitant of OM 10–13, clinical certainty with respect to assignment to this subcategory is undermined. Also, many studies of AOM incidence abstracted the reported presentations from large data bases representing multiple health care providers 14–17, and given the variable the skills of the contributing physicians 18, the quality of those data is uncertain.
Largely ignored in past longitudinal and cross-sectional studies of OM incidence, prevalence and burden is the contribution of CLIs despite the fact that numerous past studies show that the majority of OM episodes are a complication of a CLI and identified “risk factors” for OM also predispose individuals to CLIs 8, 10–13. Here, we define the incidence, prevalence, duration and burden of OM episodes and of CLIs in a large, unselected cohort of children followed through the typical CLI months of November through April by pneumatic otoscopy at intervals of approximately 1 week and by daily parental diary for CLI signs. We explore the validity of certain “risk factors” for predicting the incidence and burden of CLIs and OM in the children, define the temporal relationship between the incidences of CLIs and OM in the population and evaluate the effect of inter-assessment interval on estimating OM incidence, duration and burden.
MATERIALS AND METHODS
The data for this report were abstracted from those available for the first 4 years of our ongoing, 5-year study entitled “Role of Virus and Genetic Susceptibility in Otitis Media”. Each year, families from the local communities of 2 study sites (Charlottesville, Pittsburgh) with at least 2 children aged 1 to 5 years were recruited by advertisement for participation. Exclusion criteria included the presence in either child of a serious medical condition, a medical condition that predisposes to persistent or recurrent OM, a non-intact or structurally abnormal tympanic membrane, a pre-existing sensorineural hearing loss, or an inability to cooperate sufficiently with the examination and test procedures. After affirmation of willingness to participate and acquisition of written informed consent, families were entered into the study in October and followed through April of the respective study year. The two index children that satisfied the enrollment criteria for the family and any older sib less than 10 years who provided assent were followed. Families were reimbursed $100/month ($125/month if more than 2 children) for their participation. The study protocol was approved by the Institutional Review Boards at the University of Pittsburgh and the University of Virginia.
The purposes of the present analyses are to determine for the study population and for each child the prevalence, incidence, duration and burden of OM episodes, the incidence, prevalence and burden of CLI episodes and the risk factors that predispose to OM. Because of the variable entry times during the month of October, this report focuses on the period between November 1 and April 30 of each year. To maximize the temporal resolution of the analyses so as to approximate that of the planned observation interval, the data for the enrolled subjects were screened for completeness with respect to compliance with the planned otologic examinations (1/7 days). Those with a minimum of 22 bilateral otoscopic examinations (an average examination frequency of 1/8.3 days) were included in the data set.
Parents evaluated their children’s status daily with respect to the presence/absence of a CLI and the presence/absence of 7 signs (i.e. runny nose, nasal congestion, sore throat, cough, fever, irritability and earache) characteristic of a CLI. Data were analyzed using an algorithm developed and validated in a subset of these children that operates on the parent-recorded sign elements for each child and assigns a cold-day if the parental report for that day included two or more of the signs, runny nose, nasal congestion and cough, or if the parental report included runny nose or nasal congestion given that the previous day was assigned by algorithm to a cold-day. A CLI episode was defined as a string of consecutive cold-days of at least 3 days in duration with onset defined by the first observation of a cold-day and termination as the first non-cold-day in a sequence of at least 5 consecutive non-cold-days. For each child, the CLI data were examined to enumerate CLI incidence (episodes/child/study period) and burden (total number of cold-days/total study days). For the population, CLI incidence was calculated as new episodes/7days/population and CLI prevalence was defined as the percentage of affected children/population/day.
Bilateral pneumatic otoscopy on enrolled children was scheduled at approximately weekly intervals during an “in home visit” (Pittsburgh) or a study clinic visit (Virginia). At each observation time, both ears were examined by a validated otoscopist 19 and classified as to the presence/absence of OM based on ratings of the tympanic membrane with respect to visibility, condition, position, appearance, color, vascularity, light reflex and mobility. A positive diagnosis for OM was made when ME effusion (with or without air/fluid level) was observed irrespective of the presence or absence of concurrent signs of ME infection. AOM was diagnosed by the presence of OM with concurrent signs of ME infection including parental report of ear pulling, otalgia, irritability and fussiness and otoscopic signs of erythema and/or white opacification (other than from scarring) of the tympanic membrane, bulging or fullness of the tympanic membrane, or otorrhea from a perforation of a previously intact tympanic membrane. Supplemental data for AOM diagnosis provided by the child’s physician were also used to assign an OM episode to AOM. OME was defined as OM episodes without concurrent signs of infection. Episodes of AOM but not OME were treated empirically with antibiotics. Because otoscopy was not necessarily done at a time when the child first presented with otologic symptoms and data from the child’s primary care physician were not available for most illnesses, subclassification of OM was biased to OME assignment.
Otoscopic data were coded for the left and right ears as OME present/absent (0=absent, 1=present) and AOM present/absent (0=absent, 2=present). Between otoscopic assessments, daily OM assignment for each ear was made based upon that at the preceding visit to yield a string of observations for each day of the study period. A new OME episode was defined as a sequence of 1’s preceded and followed by a sequence of 0’s and the duration of the episode was defined by counting the number of days for the episode. An episode of AOM was defined as a newly introduced string of 2’s irrespective of preceding and subsequent observations. In calculating AOM duration all days with OM following a diagnosed day with AOM were included. In most analyses, the child (either or both ears affected=OM) was considered to be the unit of measure. From these data, OME and AOM incidences and OM prevalence were calculated for the population, the duration of each episode enumerated, and the OME and AOM incidences (episodes/study period) and OM burden (days affected/total days) were calculated for each child.
To explore the effects of inter-observation interval (1 vs 4 weeks) on OM incidence, duration and burden in the children, the OM data set was reduced to OM assignments for the first and then every 28th day of the study period. Daily OM assignments were reconstructed as described above and the various parameters calculated. The differences between parameter estimates for the two sampling intervals were compared using a paired Student’s t test.
For enrolled children, general demographic information including age, race and sex were collected and, in a subset (i.e. all subjects enrolled at Pittsburgh in Years 1–4 and subjects enrolled at Virginia in Years 2–4), the mother completed a general history that included information related to typical “risk factors” associated with OM. Factors included were past history of OM in the child (yes/no), number of children in the family (number), past history of frequent CLIs (yes/no), a 4-point measure of breast feeding history for the child (0=none, 1 = 1-<2 months, 2 = 2-<4 months and 3=>4months), the daily environment of the child (1=home with parent/guardian, 2=day care/preschool/school), the child’s exposure to tobacco smoke (yes/no) and a 6-point measure of the occupation of the primary wage earner.
The analysis of the potential “risk factors” for OM was done using a multistage procedure. First, the contribution of subject characteristics (age, race and sex) to the various predictors and outcomes was evaluated using stepwise or logistic regression as applicable (See Table I for a listing of significant predictors). Because age and race influenced some of the “risk factors”, the latter were adjusted for these effects using regression. Then, CLI burden and incidence were entered into a multivariate regression equation to predict AOM and OME incidence and total and bilateral OM burden, and residualized values for the four OM variables were calculated to control for effects of CLI burden and incidence. Finally, the subject characteristics and adjusted “risk factors” were entered into stepwise regression equations to predict CLI incidence and burden and the 4 residualized OM measures.
TABLE I.
AGE, RACE, SEX EFFECTS ON PREDICTOR AND OUTCOME VARIABLES
| Variable | Predictor | Coef | R2 | T-Value Z-Value* | P-Level |
|---|---|---|---|---|---|
| OM History* | None | ||||
| History Frequent Colds* | None | ||||
| Children in Family | Age | 0.26 | 0.07 | 2.99 | <.01 |
| Parent Occupation | Race | 0.31 | 0.09 | 3.51 | <.01 |
| Daily Environment* | Age | 0.62 | 0.16 | 3.973 | <.01 |
| Breastfeeding* | Age | −0.40 | 0.06 | −2.39 | 0.01 |
| Race | −2.81 | 0.15 | −3.42 | <.01 | |
| Passive Smoke Exposure* | Race | 2.54 | 0.20 | 3.05 | <.01 |
| Cold Burden | Age | −0.24 | 0.06 | −2.93 | <.01 |
| Cold Incidence | Age | −0.24 | 0.06 | −2.89 | <.01 |
| OME Incidence | Age | −0.17 | 0.03 | −2.13 | 0.03 |
| AOM Incidence | Age | −0.15 | 0.02 | −1.83 | 0.07 |
| OM Burden | Age | −0.15 | 0.02 | −1.80 | 0.07 |
| OM Burden (Bilateral) | None |
Analysis by Logistic Regression vs Stepwise Multiple Regression
RESULTS
A total of 242 children were entered into the study and 148 (61.2%) qualified on the basis of the established criterion. They were distributed as: 74 male; 131 white, 9 black, 8 other or mixed race, and ranged in age from 1.0 to 8.6 years (avg ± std=3.9 ± 1.6 years). One hundred twenty-seven mothers completed the intake history with respect to known “risk factors” for their child. Of these, 39 (30.7%) reported a known history of OM in the child; 103 (81.1%) reported that the child was breast-fed for at least 1 month; 18 (14.2%) reported that the child was exposed to passive tobacco smoke; 75 (59.1%) reported that the child was in day care/preschool/school, 69 (54%), 44 (35 %), 11 (9%), 0, 2 and 1 reported the number of children in the family to be 2, 3, 4, 5, 6 and 7, respectively, and 75 (59.1%), 15 (11.8%), 20 (15.7%), 6 (4.7%), 4 (3.1%) and 1 (0.8%) reported the occupation of the primary wage earner as executive/professional, small business/salesman, skilled worker, clerical/retail sales, semi-skilled worker and welfare/AFDC, respectively. As shown in Table I, some of these “risk factors” were conditioned by the age and race, but not sex, of the child. For example, parent occupational status (whites > blacks), positive breastfeeding history (whites > blacks) and positive passive tobacco smoke exposure (whites < blacks) were predicted by race, while the number of children in the family (older age>), daily environment (older age > out of home), and breastfeeding history (older age<) were predicted by age of the child. Consequently, these “risk factors” were adjusted for those effects as discussed above. Table I also shows that the majority of outcome measures reflecting CLI and OM experience were predicted by age, but not sex and race.
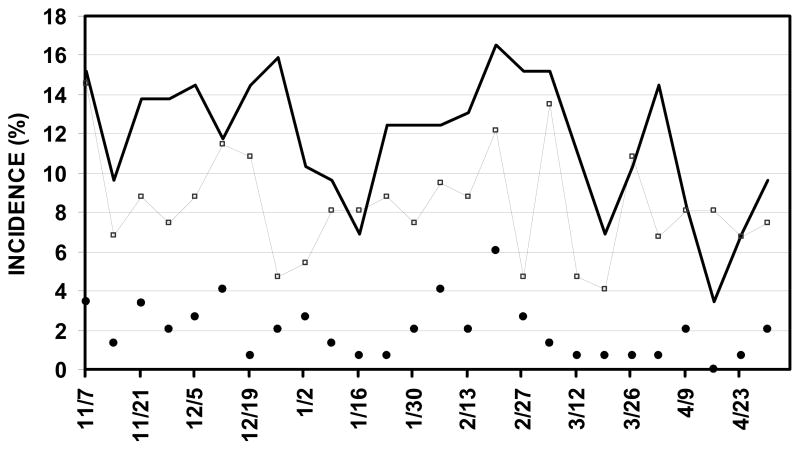
The overall incidence of new OME episodes (unilateral or bilateral) in the population was 317 episodes per 27232 child-days (.012/child-day), of new AOM was 74 episodes (0.003/child-day) and of CLIs was 456 (.017/child-day). The seasonal incidences of AOM, OME and CLIs in the population defined as the percent of subjects with new episodes per 7 days are shown in Figure 1. The incidences of AOM and OME tracked those of a CLI. Peak values for these measures occurred at similar times in mid-December and mid-February but the seasonal distributions showed a high variability. Regression of OME and AOM incidence on CLI incidence over time yielded Pearson product moment correlation coefficients of .27 (F ratio=3.02, P=.09) and .23 (F ratio=9.6, P<.01), respectively.
Figure 1.
Incidence of AOM (closed circles, unilateral or bilateral), OME (open squares, unilateral or bilateral) and CLIs (solid line) in the study population as a function of study week.
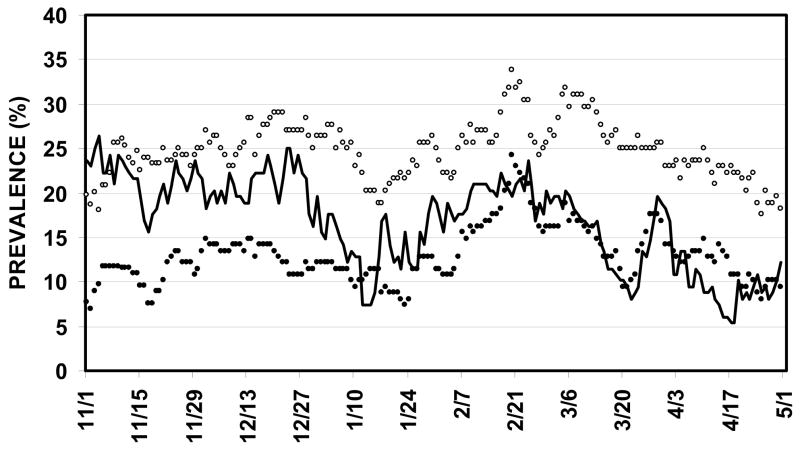
The daily prevalence of OM, bilateral OM and CLI in the population (by subject) is shown as a function of time in Figure 2 for the interval between November 1 and April 30. For persons, the OM prevalence floor was approximately 20% with peaks approaching 30% in mid December and 35% in early March. The bilateral OM prevalence floor was much less at approximately 10% with peaks at 15 and 25% in mid December and early March. The CLI prevalence mapped closely to the OM prevalence rates, varying between 5 and 25%. Regression of OM and bilateral OM prevalence on CLI prevalence over time yielded Pearson product moment correlation coefficients of .25 (F ratio=34.4, P<.01) and .18 (F ratio=17.5, P<.01), respectively.
Figure 2.
Prevalence of OM (open circles), bilateral OM (closed circles) and CLIs (sold line) in the study population as a function of study week.
Figure 3 shows the duration of new AOM and OME episodes for the left and right ears in the population as a cumulative distribution function of episodes by duration in weeks. For both ears, the OME and AOM durations were both less than or equal to 7 days in approximately 40% of the episodes and less than or equal to 4 weeks in between 75 and 90% of the episodes. The remaining 10 to 25% of the episodes showed highly variable durations that ranged from 5 to 27 weeks. The difference between average OME (16.9 ± 19.8 days) and AOM (22.4 ± 26.4 days) durations was significant (Student’s T=-1.99, P=.047)
Figure 3.
Cumulative percent of left (lines) and right (symbols) AOM (dashed line, open circle) and OME (solid line, open squares) episodes as a function of episode duration.
Most past studies estimated OM burden, incidence and duration using observation intervals of 28 days or longer as contrasted with the weekly observation format used here. To explore possible biases attributable to greater observation intervals, the AOM and OME incidences and durations and the OM and bilateral OM burdens for this data set were reconstructed from observations made every 28 days as discussed in the Methods section. A summary of the results for comparison of the 7-day versus 28-day intervals is given in Table II. Those data show that the estimates for the 7-day observation interval of AOM and OME incidence were significantly less and the AOM and OME durations significantly greater than those for the 28-day interval. Of interest, there were no between-group differences in the estimates of either OM or bilateral OM burden. Indeed, the values of the burden estimates for the 28-day assessment interval were linearly related and highly correlated with those for the 7-day assessment interval (OM: slope=0.97, intercept=0.01, r2 = 93, P<.01; bilateral OM: slope=0.95, intercept=0.00, r2=.89, P<.01).
TABLE II.
COMPARISON OF OM PARAMETERS FOR 7 AND 28 DAY INTERVALS
| 7-DAY | 28-DAY | |||||||
|---|---|---|---|---|---|---|---|---|
| PARAMETER | AVG | STD | AVG | STD | T-value | P-level | ||
| OME | INCIDENCE1 | 2.1 | 2.0 | 0.9 | 0.9 | 10.5 | <.01 | |
| DURATION2 | LEFT EAR | 16.7 | 18.3 | 44.9 | 29.4 | −9.9 | <.01 | |
| DURATION | RIGHT EAR | 17.1 | 21.4 | 39.8 | 23.5 | −8.7 | <.01 | |
| AOM | INCIDENCE | 2.1 | 2.0 | 0.9 | 0.9 | 10.5 | <.01 | |
| DURATION | LEFT EAR | 23.5 | 26.4 | 33.3 | 11.3 | −4.2 | <.01 | |
| DURATION | RIGHT EAR | 21.2 | 26.3 | 31.1 | 9.1 | −4.3 | <.01 | |
| OM | BURDEN3 | TOTAL | 25.0 | 28.6 | 24.9 | 28.9 | −0.2 | NS |
| BILATERAL | 13.1 | 21.1 | 12.5 | 21.3 | −1.1 | NS | ||
Incidence measured as episodes/individual/study period
Duration measured in days
Burden measured as %affected days/total days
Using the subset of 127 children with data for “risk factors” and the procedures outlined in the Methods section, an evaluation of the “risk factors” contributing to CLI incidence and burden, OME and AOM incidence and OM and bilateral OM burden was done. Table III summarizes these results for significant predictors. Specifically, age was the only predictor of CLI incidence (>age = lesser incidence), and age (>age = lesser burden), a positive history of frequent colds (+ history > burden) and occupational status (> status > burden) were significant predictors of CLI burden. CLI incidence was a significant positive predictor of OME incidence, OM burden and bilateral OM burden, and CLI prevalence was a significant positive predictor AOM incidence. A positive history for OM was a significant predictor of OME and AOM incidences and OM burden and positive breastfeeding history was a significant positive predictor of OME incidence. The variance in the OM variables explained by the CLI parameters ranged from 4 to 10%, and with OM history, the explained variance ranged from 8 to 20%.
TABLE III.
RISK FACTOR EFFECTS ON CLI AND OM PARAMETERS
| Variable | Predictor | Coef | R2 | T-Value | P-level |
|---|---|---|---|---|---|
| CLI Incidence | Age | −0.20 | 0.04 | −2.19 | 0.03 |
| CLI Burden | Age | −0.18 | 0.03 | −2.06 | 0.04 |
| History Frequent Colds | 0.29 | 0.08 | 3.20 | <.01 | |
| Occupational Status | 0.20 | 0.04 | 2.19 | 0.03 | |
| AOM Incidence | CLI Burden | 0.31 | 0.10 | 3.90 | <.01 |
| +OM History | 0.32 | 0.10 | 3.58 | <.01 | |
| OME Incidence | CLI Incidence | 0.21 | 0.04 | 2.51 | 0.01 |
| +OM History | 0.19 | 0.04 | 2.17 | 0.03 | |
| +Breast Feeding | 0.22 | 0.05 | 2.42 | 0.02 | |
| OM Burden | CLI Incidence | 0.24 | 0.06 | 3.00 | <.01 |
| +OM History | 0.21 | 0.05 | 2.37 | 0.02 | |
| OM Burden (bi) | CLI Incidence | 0.31 | 0.10 | 3.88 | <.01 |
DISCUSSION
Despite the large number of publications describing the incidence, prevalence and “risk factors” for OM, synthesis of the available data for generalization is made difficult by cultural factors that affect the underlying distribution of potential “risk factors” among countries and by the wide variation in study formats used to obtain the relevant parameter estimates 20. Indeed, different studies used different disease definitions (e.g. AOM, OME, ME effusion), methods of assessment (e.g. symptom presentation, record review, questionnaires for AOM; tympanometry, otoscopy, acoustic reflex for OME), temporal durations (e.g. retrospective or prospective, long-term or short-period, longitudinal or single point sampling), population characteristics (e.g. ethnic identity, age-specific range, study setting) and “risk factor” sets for which data were collected. This diversity compromises the utility of meta-analytic techniques for summarizing data to yield generalized conclusions 21, 22.
An ideal study format would include longitudinal follow-up on a large group of unselected children from infancy through childhood using high frequency sampling, unambiguous definitions of disease presence/absence, accepted methods for disease identification and concurrent assessment of CLIs and other proposed “risk factors” for OM. No published study has achieved this degree of rigor and the present study is no exception. For example, our study used a cross-sectional rather than longitudinal design with respect to age and followed children in different age groups closely for only the 9 months of the typical “CLI” season.
Our data for OM incidence showed a seasonal pattern that temporally tracked CLI incidence and, as expected, mapped closely to that for OM prevalence. CLI incidence was significantly correlated with AOM incidence over time and the correlation with OME incidence approached significance. For reasons associated with the fixed sampling times, the fact that we required a concurrent otoscopic sign and symptom for AOM assignment and the fact that natural history studies show that uncomplicated AOM progresses to an expression diagnostically indistinguishable from OME 29, our OM assignments are biased to OME and only 19% of OM episodes were assigned to AOM. Consequently, the incidence of AOM was less in our study when compared to previous studies and, because of the detections of short duration OME episodes, the incidence of OME was much greater 4, 9, 29. For our children aged 1 to <2, 2 to <3, 3 to <4, 4 to <5 and >5 years, the average AOM incidence in this study was 0.82±.98, 0.46±.90, 0.31±.58, 0.56±.72 and 0.37±.69 episodes/study period/child and the average OME incidence was 2.6 ± 2.3, 2.1 ± 1.9, 2.3 ± 2.1, 2.3 ± 2.1 and 1.3 ± 1.6 episodes/study period/child, respectively.
Because AOM progresses to an expression not different from OME 29, we presented prevalence data for OM (OME+OM) and separated affected individuals (all OM) from bilaterally affected individuals. The point prevalences for OM and bilateral OM varied over the study period with the highest prevalence documented in mid-December and late February/early March. Both measures were significantly correlated to CLI prevalence over time. A number of earlier studies reported OM prevalence at different ages in different populations. For example, in a longitudinal study of Danish children, the point prevalence of a type B tympanogram was 13% at 1 year of age, 11–18% at ages 2–5 years and 7% at ages 6 and 7 years 23. In 86 Black children followed longitudinally using biweekly assessments, the estimated point prevalences for bilateral and all OM were 12 and 23% at 24 to 30 months of age and 4% and 12% at 54 to 60 months of age 24. In a longitudinal study of 1290 British children assessed by tympanometry at 8, 12, 18, 25, 31, 37 and 43 months of age, the point prevalence of bilateral OM decreased with age, from 24.6% at 8 months to 11.9% at 43 months 25. The prevalence of OM by type B tympanograms in German children 0 to 2 years of age varied between 44 and 21% 26. In 3013 Chinese children aged 3–6 years screened for OM by tympanometry and otoscopy, the point prevalences were 11.3% for 3-year-olds, 12.4% for 4-year-olds, 11.8% for 5-year-olds, and 6.1% for 6-year-olds 27. One longitudinal study of OM in 2253 American infants followed until age 2 years reported an average OM prevalence of 20.4% in the first year of life and 16.6% in the second year of life 28. In our study, the average OM point prevalence was 32%, 26%, 24%, 25% and 18% for children ages 1 to <2, 2 to <3, 3 to <4, 4 to <5 and >5 years, respectively. The prevalence floor for those age groups was 18%, 12%, 11%, 13% and 7%, and the prevalence ceiling was 50%, 42%, 40%, 41% and 30%, respectively. Comparing our data with those previously reported show a higher average point prevalence at each age, but the estimates reported by others lie between our measured prevalence floor and ceiling.
Our data show that approximately 40% of OM (both AOM and OME) episodes resolved by 1 week and approximately 75–90% resolved by 1 month. There was a small, but statistically significant difference in time to resolution that favored shorter OME duration. Few past studies had the required resolution to detect short duration episodes. However, one study of 40 children aged 2–6 years followed longitudinally by daily tympanometry and weekly otoscopy through the typical CLI season reported 67 OM episodes defined by type B tympanograms with an average duration of 20.5 days (range=3–129 days). Of the 67 episodes 16 (24%), 6 (9%) and 1(1.5%) lasted longer than 30, 60 and 90 days, respectively 30. A second study that followed children daily with tympanometry for 1 month reported that only 8 of 43 (19%) new OM episodes lasted longer than 26 days 6. The results for these studies agree well with ours and support the validity of short duration OM as the most frequent temporal expression.
Despite the publication of a recent study that reported no differences in developmental outcomes measured at various ages up to 9–11 years between children with persistent OME before age 3 years treated with early versus delayed tympanostomy tubes 31, there remains an interest in the long term complications and sequelae of the disease 32. Such analyses require that the disease experience of individual subjects be well characterized. However, most data available to address this are obtained using sampling formats with rather long inter-assessment intervals (≥28 days). To determine the effect of sampling interval on the various measures of an individual’s OM experience, we simulated the data for a 28-day assessment interval and compared the results to those for the 7-day interval used in this study. The results of that analysis documented significant biases to longer estimates of OME and AOM durations and to lesser estimates of OME and AOM incidence for the longer inter-assessment interval. These biases will impact estimates of AOM and OME incidence for group assignment based on those measures and, perhaps, estimated cure rates for clinical trials. However, and importantly, there was no difference between assessment intervals in either the OM or bilateral OM burdens. Since burden is a summary measure of time with ME effusion, our analysis suggests that inter-assessment intervals as long as 28-days can adequately capture an individual’s ME effusion experience and thus be used to reliably assign individuals to different groups defined by that measure.
The literature suggests a large number of both fixed (e.g. genetic) and modifiable (e.g. breast feeding) “risk factors” that predispose to OM 33. Factors like young age and day-care attendance are clearly established but others like allergy and race are less so, and the contribution of the various “risk factors” to OM may be both nested and age-dependent 28. Our analyses document factor nesting as exemplified by the observation that some of the included OM “risk factors” were, in turn, dependent on the age and race of the subjects. The effect of race on tobacco smoke exposure and breastfeeding history is consistent with the aforementioned effect of cultural factors that can skew the distribution of “risk factors” in study populations 20. Factor nesting may also explain the loss of a significant age effect on the OM variables after controlling for the CLI parameters. There, older age was associated with lesser CLI incidence and prevalence, factors that were positive predictors of OM incidence and burden. Here, we attempted to control for such nesting and thereby determine the independent contribution of age, race, sex, CLI measures and a subset of the previously identified “risk factors” to OM incidence and burden. Our analysis showed that CLI experience was the major predictor of AOM and OME incidence and OM burden, and that a positive history of OM predicted 3 of the 4 OM measures. Together, these variables explained between 8 and 20% of the variance in the OM variables. The only other significant predictor of OM experience (OME incidence) was a positive history of breastfeeding and unlike the effects of CLIs and OM history, this effect is directionally opposed to reported that for many past studies 33. While the majority of those studies focused on AOM and some reports suggested that the breastfeeding effect is age sensitive and relatively small 28, a similar but non-significant effect of breastfeeding was noted for the other OM variables in our study (.10>P>.05) and the effect was robust to alternative analytic approaches.
It should be noted that our analyses do not discount contributions of other “risk factors” to OM expression such as daily environment since the sample size was relatively small and the analytic methods used are sensitive to the underlying distributions within and between variables. None-the-less, given that most OM episodes are temporally related to an existing CLI 8, 12, that many of the OM “risk factors” predict CLI incidence and prevalence and, as shown by our results, that the incidence and prevalence of OM track those for a CLI, we believe that data for CLIs need be included in any analyses that focus on identifying independent “risk factors” for AOM, OME or OM.
Acknowledgments
Supported in Part by NIH grant DC005832. The authors thank Harriette Wheatley, Ellen Reynolds and Margaretha Casselbrant for assisting with the otoscopic examinations and James Seroky, Julianne Banks, Allison Cullen-Doyle and Brendan Cullen Doyle for assisting with data entry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marchant CD, Shurin PA, Turczyk VA, Wasikowski DE, Tutihasi MA, Kinney SE. Course and outcome of otitis media in early infancy: a prospective study. J Pediatr. 1984;104:826–31. doi: 10.1016/s0022-3476(84)80475-8. [DOI] [PubMed] [Google Scholar]
- 2.Odabasi O, Basak O, Basak S, Mutlu C, Erpek G. Middle ear pathology in day-care centre children. Fam Pract. 1998;15:332–5. doi: 10.1093/fampra/15.4.332. [DOI] [PubMed] [Google Scholar]
- 3.Williamson IG, Dunleavey J, Bain J, Robinson D. The natural history of otitis media with effusion--a three-year study of the incidence and prevalence of abnormal tympanograms in four South West Hampshire infant and first schools. J Laryngol Otol. 1994;108:930–4. doi: 10.1017/s0022215100128567. [DOI] [PubMed] [Google Scholar]
- 4.Hogan SC, Stratford KJ, Moore DR. Duration and recurrence of otitis media with effusion in children from birth to 3 years: prospective study using monthly otoscopy and tympanometry. BMJ. 1997;314:350–3. doi: 10.1136/bmj.314.7077.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeisel SA, Roberts JE, Burchinal M, Neebe E, Henderson FW. A longitudinal study of risk factors for otitis media in African American children. Matern Child Health J. 2002;6:189–93. doi: 10.1023/a:1019730213505. [DOI] [PubMed] [Google Scholar]
- 6.Elbrønd O, Birch L. Daily impedance audiometric screening of children in a day-care institution. Auris Nasus Larynx. 1985;12(Suppl 1):S216–8. doi: 10.1016/s0385-8146(85)80154-1. [DOI] [PubMed] [Google Scholar]
- 7.Møller H, Tos M. Point and period prevalence of otitis media with effusion evaluated by daily tympanometry. J Laryngol Otol. 1990;104:937–41. doi: 10.1017/s0022215100114422. [DOI] [PubMed] [Google Scholar]
- 8.Moody SA, Alper CM, Doyle WJ. Daily tympanometry in children during the cold season: association of otitis media with upper respiratory tract infections. Int J Pediatr Otorhinolaryngol. 1998;45:143–50. doi: 10.1016/s0165-5876(98)00103-7. [DOI] [PubMed] [Google Scholar]
- 9.Harsten G, Prellner K, Heldrup J, Kalm O, Kornfalt R. Recurrent acute otitis media. A prospective study of children during the first three years of life. Acta Otolaryngol. 1989;107:111–9. doi: 10.3109/00016488909127487. [DOI] [PubMed] [Google Scholar]
- 10.Vesa S, Kleemola M, Blomqvist S, Takala A, Kilpi T, Hovi T. Epidemiology of documented viral respiratory infections and acute otitis media in a cohort of children followed from two to twenty-four months of age. Pediatr Infect Dis J. 2001;20:574–81. doi: 10.1097/00006454-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Winther B, Alper CM, Mandel EM, Doyle WJ, Hendley JO. Temporal relationships between colds, upper respiratory viruses detected by polymerase chain reaction, and otitis media in young children followed through a typical cold season. Pediatrics. 2007;119:1069–75. doi: 10.1542/peds.2006-3294. [DOI] [PubMed] [Google Scholar]
- 12.Winther B, Doyle WJ, Alper CM. A high prevalence of new onset otitis media during parent diagnosed common colds. Int J Pediatr Otorhinolaryngol. 2006;70:1725–30. doi: 10.1016/j.ijporl.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Revai K, Dobbs LA, Nair S, Patel JA, Grady JJ, Chonmaitree T. Incidence of acute otitis media and sinusitis complicating upper respiratory tract infection: the effect of age. Pediatrics. 2007;119:e1408–12. doi: 10.1542/peds.2006-2881. [DOI] [PubMed] [Google Scholar]
- 14.Pukander J, Luotonen J, Sipila M, Timonen M, Karma P. Incidence of acute otitis media. Acta Otolaryngol. 1982;93:447–53. doi: 10.3109/00016488209130903. [DOI] [PubMed] [Google Scholar]
- 15.Lundgren K, Ingvarsson L. Epidemiology of acute otitis media in children. Scand J Infect Dis Suppl. 1983;39:19–25. [PubMed] [Google Scholar]
- 16.Lanphear BP, Byrd RS, Auinger P, Hall CB. Increasing prevalence of recurrent otitis media among children in the United States. Pediatrics. 1997;99:E1. doi: 10.1542/peds.99.3.e1. [DOI] [PubMed] [Google Scholar]
- 17.Kvestad E, Kvaerner KJ, Roysamb E, Tambs K, Harris JR, Magnus P. Recurrent otitis media and tonsillitis: common disease predisposition. Int J Pediatr Otorhinolaryngol. 2006;70:1561–8. doi: 10.1016/j.ijporl.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Takata GS, Chan LS, Morphew T, Mangione-Smith R, Morton SC, Shekelle P. Evidence assessment of the accuracy of methods of diagnosing middle ear effusion in children with otitis media with effusion. Pediatrics. 2003;112:1379–87. doi: 10.1542/peds.112.6.1379. [DOI] [PubMed] [Google Scholar]
- 19.Kaleida PH, Stool SE. Assessment of otoscopists’ accuracy regarding middle-ear effusion Otoscopic validation. Am J Dis Child. 1992;146:433–5. doi: 10.1001/archpedi.1992.02160160053013. [DOI] [PubMed] [Google Scholar]
- 20.Rovers MM, de Kok IM, Schilder AG. Risk factors for otitis media: an international perspective. Int J Pediatr Otorhinolaryngol. 2006;70:1251–6. doi: 10.1016/j.ijporl.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Lubianca Neto JF, Hemb L, Silva DB. Systematic literature review of modifiable risk factors for recurrent acute otitis media in childhood. J Pediatr (Rio J) 2006;82:87–96. doi: 10.2223/JPED.1453. [DOI] [PubMed] [Google Scholar]
- 22.Uhari M, Mantysaari K, Niemela M. A meta-analytic review of the risk factors for acute otitis media. Clin Infect Dis. 1996;22:1079–83. doi: 10.1093/clinids/22.6.1079. [DOI] [PubMed] [Google Scholar]
- 23.Tos M. Epidemiology and spontaneous improvement of secretory otitis. Acta Otorhinolaryngol Belg. 1983;37:31–43. [PubMed] [Google Scholar]
- 24.Zeisel SA, Roberts JE, Neebe EC, Riggins R, Jr, Henderson FW. A longitudinal study of otitis media with effusion among 2- to 5-year-old African-American children in child care. Pediatrics. 1999;103:15–9. doi: 10.1542/peds.103.1.15. [DOI] [PubMed] [Google Scholar]
- 25.Dewey C, Midgeley E, Maw R. The relationship between otitis media with effusion and contact with other children in a british cohort studied from 8 months to 3 1/2 years. The ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. Int J Pediatr Otorhinolaryngol. 2000;55:33–45. doi: 10.1016/s0165-5876(00)00377-3. [DOI] [PubMed] [Google Scholar]
- 26.Engel J, Anteunis L, Chenault M, Marres E. Otoscopic findings in relation to tympanometry during infancy. Eur Arch Otorhinolaryngol. 2000;257:366–71. doi: 10.1007/s004050000239. [DOI] [PubMed] [Google Scholar]
- 27.Chen CH, Lin CJ, Hwang YH, Ku CJ. Epidemiology of otitis media in Chinese children. Clin Otolaryngol Allied Sci. 2003;28:442–5. doi: 10.1046/j.1365-2273.2003.00741.x. [DOI] [PubMed] [Google Scholar]
- 28.Paradise JL, Rockette HE, Colborn DK, Bernard BS, Smith CG, Kurs-Lasky M, et al. Otitis media in 2253 Pittsburgh-area infants: prevalence and risk factors during the first two years of life. Pediatrics. 1997;99:318–33. doi: 10.1542/peds.99.3.318. [DOI] [PubMed] [Google Scholar]
- 29.Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160:83–94. doi: 10.1093/infdis/160.1.83. [DOI] [PubMed] [Google Scholar]
- 30.Antonio SM, Don D, Doyle WJ, Alper CM. Daily home tympanometry to study the pathogenesis of otitis media. Pediatr Infect Dis J. 2002;21:882–5. doi: 10.1097/00006454-200209000-00022. [DOI] [PubMed] [Google Scholar]
- 31.Paradise JL, Feldman HM, Campbell TF, Dollaghan CA, Rockette HE, Pitcairn DL, et al. Tympanostomy tubes and developmental outcomes at 9 to 11 years of age. N Engl J Med. 2007;356:248–61. doi: 10.1056/NEJMoa062980. [DOI] [PubMed] [Google Scholar]
- 32.Roberts J, Hunter L, Gravel J, Rosenfeld R, Berman S, Haggard M, et al. Otitis media, hearing loss, and language learning: controversies and current research. J Dev Behav Pediatr. 2004;25:110–22. doi: 10.1097/00004703-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Daly KA, Rovers MM, Hoffman HJ, Uhari M, Casselbrant ML, Zielhuis G, et al. Recent advances in otitis media. 1 Epidemiology, natural history, and risk factors. Ann Otol Rhinol Laryngol Suppl. 2005;194:8–15. doi: 10.1177/00034894051140s104. [DOI] [PubMed] [Google Scholar]