SUMMARY
Embryos have the ability to self-regulate and regenerate normal structures after being sectioned in half. How is such a morphogenetic field established? We discovered that quadruple knockdown of ADMP and BMP2/4/7 in Xenopus embryos eliminates self-regulation, causing ubiquitous neural induction throughout the ectoderm. ADMP transcription in the Spemann organizer is activated at low BMP levels. When ventral BMP2/4/7 signals are depleted, Admp expression increases, allowing for self-regulation. ADMP has BMP-like activity and signals via the ALK-2 receptor. It is unable to signal dorsally because of inhibition by Chordin. The ventral BMP antagonists Sizzled and Bambi further refine the pattern. By transplanting dorsal or ventral wild-type grafts into ADMP/BMP2/4/7-depleted hosts, we demonstrate that both poles serve as signaling centers that can induce histotypic differentiation over considerable distances. We conclude that dorsal and ventral BMP signals and their extracellular antagonists expressed under opposing transcriptional regulation provide a molecular mechanism for embryonic self-regulation.
INTRODUCTION
How do embryos reliably generate a perfect tissue and body pattern time after time? Experimental embryology has shown that embryonic regions with equivalent developmental potential, or morphogenetic fields, have the remarkable property of “regulating” to re-form a normal structure after experimental perturbations (Harrison, 1918; Huxley and de Beer, 1934; Spemann, 1938). One striking example of this self-regulation is the ligature experiment of Hans Spemann, in which he used a baby-hair loop to divide a cleaving amphibian embryo into two halves (Spemann, 1903). The half lacking the dorsal blastopore lip developed into a “bauchstück,” or belly-piece, consisting of only ventral tissues with no axial structures, whereas the dorsal half developed into a perfectly well-proportioned half-sized embryo containing both dorsal and ventral tissues. Another example of self-regulation is provided by the chick embryo: subdividing the blastoderm into fragments can result in multiple axes when cultured in isolation (Spratt and Hass, 1960; Joubin and Stern, 1999; Bertocchini et al., 2004). Strikingly, one mammal, the armadillo (Dasypus novemcinctus), can naturally regulate to generate four genetically identical embryos from a single blastocyst during normal reproduction (Enders, 2002). In humans, it is thought that the most frequent type of identical twins arises from single blastocysts in which the inner cell mass is split in two (Sadler, 2004). Despite long-standing interest, the molecular mechanisms underlying the capacity of any morphogenetic field to self-regulate remain one of the unsolved mysteries of developmental biology.
A new framework for understanding how the embryo self-regulates is provided by the recent proposal that the gastrula Xenopus embryo contains a dorsal and a ventral signaling center. The dorsal center, or Spemann organizer, is a source of secreted BMP antagonists (such as Chordin, Noggin, Follistatin, and Cerberus) and Wnt antagonists (such as Dkk, Frzb, sFRP2, and Crescent). At the opposite pole, a ventral center develops in which yet another subset of extracellular regulators—such as the BMP pathway components Xolloid-related (Xlr), Twisted Gastrulation (Tsg), Crossveinless-2 (CV-2), Sizzled, and Bambi—are expressed at gastrula (reviewed in De Robertis and Kuroda, 2004). Expression of ventral-center genes parallels that of the Bmp4 gene (Niehrs and Pollet, 1999) and is prominently marked by the expression of Sizzled/ogon (Collavin and Kirschner, 2003) and Bambi (Onichtchouk et al., 1999).
Genetic and molecular studies in zebrafish and Xenopus have shown that BMPs and their secreted antagonists are important regulators of embryonic dorsoventral (DV) patterning (De Robertis and Kuroda, 2004; Schier and Talbot, 2005). In particular, much evidence suggests that BMP inhibition is required for central nervous system (CNS) formation in Xenopus (Harland, 2000; Oelgeschläger et al., 2003a; Kuroda et al., 2004; Khokha et al., 2005). Nevertheless, a recent loss-of-function study of the three main ventral BMPs in Xenopus revealed that even triple BMP2/4/7 knockdowns still retained substantial DV polarity (Reversade et al., 2005).
In the present study, we bisected Xenopus embryos into dorsal and ventral halves at the blastula stage and found that inhibition of BMP4/7 signaling was able to induce neural tissue in ventral half-embryos but was without effect in dorsal halves. An appealing candidate to ensure ventral development in the dorsal halves was the anti-dorsalizing morphogenetic protein (ADMP), a member of the BMP family known to have ventralizing activity despite being expressed in the dorsal gastrula organizing center of Xenopus, zebrafish, and chick embryos (Moos et al., 1995; Joubin and Stern, 1999; Dosch and Niehrs, 2000; Lele et al., 2001; Willot et al., 2002). Using morpholino oligomer (MO) knockdown, we now demonstrate that transcriptional upregulation of Admp plays a key role in compensating for the depletion of ventrally expressed Bmp2/4/7 since Admp expression is activated by low BMP levels. In the embryo, ADMP activity is blocked on the dorsal side by the secreted BMP antagonist Chordin, which prevents ADMP from binding to its cognate receptor ALK-2. This inhibition is reversed when Chordin is cleaved by the Xolloid-related (Xlr) metalloproteinase (Piccolo et al., 1997; Larrain et al., 2001; Dale et al., 2002), which is expressed ventrally. Strikingly, when ventral and dorsal BMP signals were simultaneously inactivated in embryos by the quadruple depletion of BMP2, BMP4, BMP7, and ADMP, self-regulation was lost, causing the entire ectoderm to become neural tissue. Transplantation of ventral and dorsal centers into BMP2/4/7/ADMP-depleted hosts demonstrated that both centers can act as a source of BMP signals, restoring epidermal patterning over considerable distances. We conclude that the DV embryonic morphogenetic field of the Xenopus embryo is established in part by BMP signals and extracellular BMP antagonists—such as Chordin, Sizzled, and Bambi—expressed at the dorsal and ventral poles of the embryo, which are under opposite transcriptional regulation.
RESULTS
BMP4 and BMP7 Are Only Required in Ventral Half-Embryos
An embryological assay that separates the dorsal and ventral centers was developed. When embryos with strong DV polarity (Klein, 1987) were bisected at blastula, the ventral half, which is more pigmented, developed into rounded belly-pieces consisting of only ventral tissues, while the dorsal half gave rise to a well-proportioned half-sized embryo (Figures 1A-1D). We first tested the effects of depletion of BMP4/7 because, in most embryonic assays, triple depletion of BMP2/4/7 gave similar phenotypes to those of double BMP4/7 signals (Reversade et al., 2005). Depletion of BMP4/7 signals reproducibly caused striking phenotypes in ventral halves (Figures 1D and 1F-1H). Neural tissue is normally lacking in ventral halves, but after BMP4/7 depletion, strong elongation and CNS differentiation, marked by Sox2 expression, were observed (Figure 1F). We were surprised to find, however, that, in dorsal half-embryos, knockdown of BMP4/7 was virtually without effect (compare Figures 1C and 1E). This suggested that an additional ventralizing signal present in the dorsal side might compensate for the lack of BMP4/7.
Figure 1. An Assay for Self-Regulative Development: DV Patterning of Dorsal Half-Embryos Is Independent of BMP4/7 Signals.

(A) Embryos were injected with a mixture of Bmp4/7 MOs (12 ng each) and bisected at blastula stage (n = 15 or more per experimental set).
(B) Uninjected control sibling.
(C) Dorsal halves self-regulate.
(D) Ventral halves develop into a ventralized belly-piece lacking all CNS.
(E) Dorsal half injected with Bmp4/7 MOs self-regulates pattern.
(F) Ventral halves lacking BMP4/7 activity elongate and differentiate Sox2-positive CNS.
(G and H) Multiple ventral half-embryos illustrate reproducibility of the assay.
ADMP Is Required in Dorsal Half-Embryos
The BMP-like growth factor ADMP (Moos et al., 1995; Dosch and Niehrs, 2000) provided an excellent candidate for this signal (Figure 2A). This prompted us to re-examine its expression and function during Xenopus development. At blastula and gastrula stages, Admp was expressed dorsally in cells closely associated with the region of Chordin expression, and injection of Xenopus Admp mRNA activated expression of known BMP target genes (Figure 2B; see also Figures S1 and S2 in the Supplemental Data available with this article online).
Figure 2. Admp Expression Is Triggered by Low BMP Signaling, and Admp MO Dorsalizes the Embryo.

(A) The Xenopus ventral and dorsal centers.
(B) Admp is expressed dorsally (stage 12).
(C) Chordin protein injection in the blastula cavity (5 ng) leads to upregulation of Admp expression.
(D) Admp MO targeting both Xenopus laevis Admp pseudoalleles.
(E) Xenopus Admp translation is specifically inhibited by Admp MO but not by control MO of random sequence.
(F) ADMP causes phosphorylation of endogenous Smad1 in Xenopus embryos, which is blocked by Chordin protein injection
(G and H) Admp MO-injected embryos (12 ng total) are dorsalized.
(I) zAdmp mRNA (75 pg total) ventralizes the Xenopus embryo.
(J) Admp MO rescued by zAdmp mRNA.
To study the function of ADMP in vivo, we designed an antisense MO targeting both Xenopus laevis Admp pseudoalleles found in the EST databases (Figures 2E and 2F). Microinjection of Admp MO into each blastomere at the four-cell stage resulted in embryos showing signs of decreased BMP signaling, such as impaired Sizzled expression in the ventral side and increased Six3 expression in the forebrain and eyes (Figures 2G and 2H). Importantly, all defects associated with loss of endogenous ADMP activity could be rescued by microinjection of synthetic mRNA encoding zebrafish Admp, which lacks the sequences targeted by Admp MO (Figures 2H-2J; Willot et al., 2002).
We next asked whether ADMP was required for the self-regulation observed in dorsal half-embryos (Figure 3). Knockdown of ADMP was without effect on the development of ventral halves (Figures 3D and 3F). Instead, dorsal halves depleted of ADMP had greatly expanded neural tissue marked by Sox2 expression (compare Figures 3C and 3E). We conclude that self-regulation in dorsal half-embryos requires ADMP signals originating from Spemann’s organizer.
Figure 3. Self-Regulative DV Patterning in Dorsal Half-Embryos Requires ADMP and Has the Opposite Transcriptional Regulation of BMP4 and Ventral BMP Antagonists.
(A) Experimental design (n = 28 or more per experimental set).
(B) Sox2 expression in uninjected siblings.
(C) Control blastula dorsal halves self-regulate.
(D) The ventral half forms belly-pieces lacking CNS.
(E) In dorsal halves injected with Admp MO, much of the ectoderm becomes CNS.
(F) Ventral half-embryos are unaffected by Admp MO.
(G) RT-PCR analysis of whole embryos at stage 11 after injection of Chordin (1 and 5 ng) or BMP4 (0.5 and 2.5 ng) protein (in a volume of 40 nl) into the blastocoele cavity at stage 8.
(H) Sox2 expression in control embryos at stage 20. Inset illustrates the dorsal and ventral centers marked by Chordin and Sizzled at stage 12.
(I) Coinjection of Bambi and Sizzled MOs ventralizes the embryo.
(J) A single dorsal injection of xAdmp mRNA (50 pg) into wild-type embryo decreases anterior CNS differentiation and increases Sizzled expression (inset).
(K) Depletion of Bambi and Sizzled greatly sensitizes the embryo to the effects of Admp mRNA.
(L) Model of regulatory interactions in the Xenopus embryonic field. Green lines indicate pro-BMP effects; red lines indicate anti-BMP effects.
Admp and Bmp4 Are under Opposite Transcriptional Regulation
ADMP and BMP4 share similar sequence and ventralizing activity yet have diametrically opposed expression patterns. This poses a conundrum as to why embryos have two distinct sources of BMP signals at opposite poles, when in principle one should suffice. Microinjection of Chordin protein or knockdown of BMP4/7 activity increased Admp expression (Figure 2C and data not shown), indicating that expression of Admp might be negatively regulated by BMP signaling.
As shown in Figure 3G, we tested this directly by analyzing transcript levels of ventral and dorsal marker genes in embryos in conditions of either high or low BMP signaling, using injections of recombinant BMP4 or Chordin protein into the blastocoele cavity (Figure 3G). RT-PCR analyses of whole embryos at midgastrula (stage 11) showed that transcription of dorsal genes such as Chordin and Admp was inhibited by high BMP levels (Figure 3G, lanes 3 and 5). When BMP signaling levels were decreased by injecting increasing amounts of Chordin protein, Admp expression was upregulated (Figure 3G, lane 1). Conversely, expression of Bmp4 was positively regulated by BMP signaling (Figure 3G). High levels of BMP4 activated transcription of the ventral-center genes Sizzled and Bambi (Figure 3G, lane 5). In situ hybridizations confirmed an expansion of the ventral center marked by Bambi or Sizzled at the expense of the Spemann organizer marked by Admp or Chordin (Figure S3).
Bambi encodes a natural dominant-negative BMP receptor (Onichtchouk et al., 1999), and Sizzled/Ogon is a negative-feedback inhibitor of BMP signaling (Yabe et al., 2003). To show that, at high BMP levels, Bambi and Sizzled function as BMP antagonists in the ventral center, we knocked down these genes. Both Bambi (this study) and Sizzled (Collavin and Kirschner, 2003) MO caused ventralization on their own (data not shown). Bambi and Sizzled MO synergized when coinjected, expanding the ventral center and greatly reducing neural tissue (Figures 3H and 3I). Knockdown of these ventral-center genes sensitized the embryo to a single injection of Admp mRNA on the dorsal side, causing the complete loss of CNS (Figures 3J and 3K). This experiment shows that Bambi and Sizzled function as bona fide BMP antagonists in the ventral center.
We conclude that a key difference between ventral BMPs (e.g., BMP4) and ADMP, which have similar ventralizing activity, resides in their opposite transcriptional regulation by the BMP signaling pathway itself. In this “seesaw-like” type of self-regulation, Admp expression is turned on by low BMP signaling levels (Figure 3L and Figure S7). The opposite is true for Bmp4, which is positively regulated by BMP signals. High BMP levels increase expression of the BMP antagonists Bambi and Sizzled, which serve as feedback inhibitors of BMP signaling in the ventral side. When Admp/Chordin expression goes down, ventral Bmp4/Sizzled/Bambi expression goes up, and vice versa. This provides the embryo with an elegant yet simple way of adjusting and maintaining BMP signaling levels in a developing morphogenetic field (Figure 3L).
Inhibition of ADMP Binding to ALK-2 Receptor by Chordin/Tsg Is Released by Xlr
Xenopus ADMP promotes ventral development, and this is achieved, as is the case for other BMPs, through the phosphorylation of the transcription factor Smad1 (Figure 2F) without affecting the Smad2, Erk, or β-catenin pathways (Figure S4). Chordin protein injection blocked ADMP-induced Smad1 phosphorylation in embryos (Figure 2F, lane 4), suggesting that Chordin might repress ADMP in vivo.
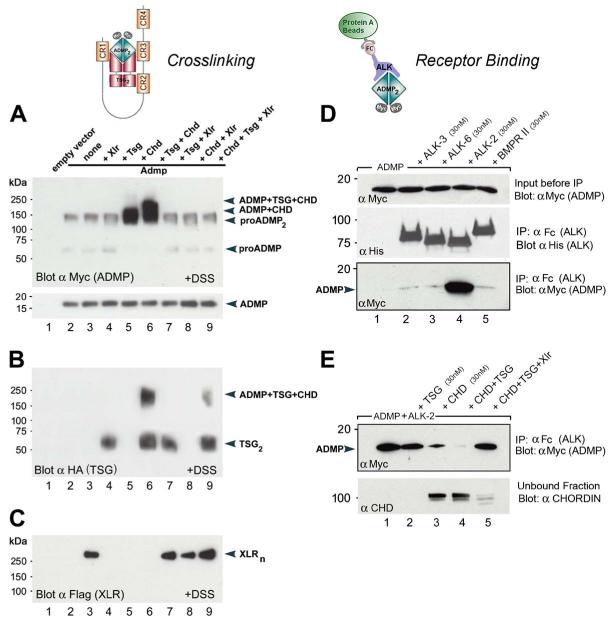
To test for a direct biochemical interaction between Chordin, ADMP, and cofactors of the Chordin pathway, crosslinking experiments were performed with supernatants of transfected 293T cell cocultures (Figures 4A-4C). The results showed that Myc-tagged ADMP protein (Willot et al., 2002) could be detected in a high-molecular weight complex when chemically crosslinked (by disuccinimidyl suberate) in solution to Chordin alone or to Chordin and Tsg together (Figure 4A, lanes 5 and 6). Moreover, formation of these complexes was inhibited by the metalloproteinase Xlr (Figures 4A and 4B, lanes 8 and 9), which cleaves Chordin. The binding of ADMP, Chordin, and Tsg was confirmed by immunoprecipitation in the absence of crosslinker (Figure S5).
Figure 4. ADMP Binds to Chordin and Tsg, and Cleavage by Xolloid-Related Metalloproteinase Restores Binding to ALK-2 Receptor.
Supernatants of cocultures of 293T cells were used for crosslinking experiments (A-C). Pure recombinant proteins from R&D Systems were used for receptor binding assays (D and E).
(A) ADMP protein forms a binary complex with Chordin (lane 5) or a ternary complex with Chordin and Tsg (lane 6), which can be inactivated by Xolloid-related (lanes 8 and 9).
(B) Tsg protein forms a molecular complex with ADMP and Chordin proteins (lanes 6 and 9).
(C) Crosslinked Xlr enzyme runs as a discrete oligomer (Xlrn).
(D) ADMP binds specifically to the type I receptor ALK2 (lane 4) but not to BMPR-1a (ALK-3), BMPR-1b (ALK-6), or BMPR-II (lanes 2, 3, and 5). Other panels show loading controls.
(E) ADMP binding to ALK-2 is partially blocked by Chordin (top panel, lane 3) and completely inhibited by Chordin and Tsg (lane 4). Importantly, cleavage of Chordin by the metalloproteinase Xlr restores ADMP binding to ALK-2 (lane 5).
We next investigated whether Chordin and Tsg block the binding of ADMP to its cognate receptor. Previous work of Dosch and Niehrs (2000) had shown that ADMP signaling could not be inhibited by tBR, a truncated ALK-3 BMP receptor. Immunoprecipitation studies using chimeric human BMP receptors (BMPR) fused to the Fc fragment of IgG confirmed that ADMP does not bind to BMPR-Ia (ALK-3), BMPR-Ib (ALK-6), or BMPR-II (Figure 4D). Instead, we found specific binding to ALK-2 (Figure 4D, lane 4), a different type I BMPR (Macias-Silva et al., 1998). Using this ALK-2-Fc fusion protein, we were able to show that Chordin decreased ADMP binding to its receptor, while the combination of Chordin and Tsg blocked it (Figure 4E). Importantly, addition of Xlr metalloproteinase cleaved Chordin and restored ADMP binding to ALK-2 (Figure 4E, lanes 4 and 5). These biochemical results show that ADMP is subjected to extracellular regulation by Chordin, Tsg, and Xlr, which regulate ADMP binding to its dedicated ALK-2 cell-surface receptor.
ADMP and Chordin Interact in the Embryo
We next asked whether ADMP is regulated by Chordin in vivo (Figure 5). Admp MO-injected embryos were dorsalized, with expanded Otx2 and decreased Sizzled expression (Figure 5B), indicating reduced BMP signaling. Conversely, Chd MO-injected embryos were ventralized (Figure 5C), with an expanded Sizzled expression domain and reduced head structures (Oelgeschläger et al., 2003a). When ADMP and Chordin activity were depleted simultaneously, Chordin/Admp morphants were virtually indistinguishable from wild-type embryos (Figure 5D), demonstrating that an inhibitory interaction between ADMP and Chordin does take place in the embryo. To further test this interaction in a gain-of-function situation, embryos were injected with doses of Xenopus Admp mRNA sufficient to eliminate CNS formation, which could be restored by a single dorsal injection of Chordin mRNA (Figures 5E and 5F). In addition, ventral injection of Chordin mRNA, which induced partial secondary axes, was potentiated in ADMP-depleted embryos, causing complete secondary axes with two eyes marked by Six3 (Figures 5G and 5H). Moreover, depletion of ADMP rendered the embryo hypersensitive to forebrain expansion induced by a low dose of Chordin (Figure S6). Finally, classical Spemann organizer grafts performed at stage 10¾ (midgastrula), which induce trunks with no eyes or cyclopic eyes, were able to induce complete head structures containing two eyes when the donor organizer had been depleted of ADMP (Figures 5I and 5J). Taken together, these loss- and gain-of-function experiments strongly suggest that Chordin inhibits ADMP in vivo.
Figure 5. ADMP and Chordin Interact In Vivo.

(A-D) Epistasis experiment in embryos injected with Admp MO, Chordin MO, or a mixture of both (n = 23 or more per experimental set).
(E) Injection of xAdmp mRNA (25 pg in each blastomere at the four-cell stage) abolishes CNS formation (absence of Sox2 expression).
(F) A single dorsal injection of Chordin mRNA (50 pg) rescues CNS formation in embryos ventralized by Admp mRNA injection.
(G) Secondary axes (marked by Sox2, n = 37) induced by a single ventral injection of Chordin mRNA (50 pg) lack eye and forebrain tissues (marked by Six3, inset).
(H) In ADMP-depleted embryos, Chordin mRNA induces complete secondary axes including eye and forebrain tissues (n = 21).
(I) Spemann organizer grafts at midgastrula (stage 10¾ ) induce trunks with no eyes (n = 9) or cyclopic eyes (n = 4).
(J) ADMP-depleted Spemann organizer grafts transplanted at midgastrula (stage 10¾ ) induce complete head structures with two eyes (in 7 of 13 grafts).
Quadruple Knockdown of ADMP/BMP2/4/7 Is Sufficient for Neural Induction
To determine whether a self-regulating field is indeed established by the opposite regulation of ventral BMPs and dorsal ADMP, we inhibited both BMP sources simultaneously by coinjecting Bmp2, Bmp4, Bmp7, and Admp MOs (Figure 6). This experiment produced a remarkable result. Knockdown of ADMP or of BMP2/4/7 caused CNS expansion, but embryos still retained DV patterning (Figures 6A-6C). Strikingly, quadruple ADMP/BMP2/4/7 knockdown resulted in radially dorsalized embryos in which the entire ectoderm was converted into CNS, as demonstrated by the panneural marker Sox2 (Figure 6D).
Figure 6. Quadruple Knockdown of BMP2/4/7 and ADMP Results in Ubiquitous CNS Formation In Vivo.

(A) CNS marked by Sox2 in control embryos; inset shows frontal view (DAI = 5.2).
(B) Knockdown of ADMP moderately increases Sox2 expression in anterior CNS (inset) (DAI = 6.2).
(C) Triple knockdown of BMP2/4/7 results in embryos with a neural plate approximately 5 times the width of that of control siblings (transversal section shown in inset).
(D) Quadruple inactivation of BMP2/4/7 and ADMP (9 ng total each) results in radially dorsalized embryos and ubiquitous neural differentiation in the ectoderm (DAI = 9.7; n = 59).
(E) Cytokeratin expression marks epidermal cells on the surface of control embryos.
(F) Expression of epidermal Cytokeratin is abolished in Bmp2/4/7/Admp morphants at stage 16.
(G and H) BMP2/4/7/ADMP-deficient embryos express radial Otx2, Rx2a, and Krox20.
(I) Quadruple Chordin/Noggin/Follistatin/Cerberus MO-injected embryos are strongly ventralized (DAI = 2.3) but retain posterior CNS (compare to [K]).
(J) ADMP is held inactive by forming a stable inhibitory ternary complex with Chordin and Tsg. DN-Xlr inhibits Xolloid-related (Xlr), preventing the proteolytic cleavage of Chordin at two specific sites.
(K) Sox2 in wild-type embryos.
(L) Injection of DN-Xlr mRNA (400 pg) broadens Sox2 expression.
(M) Simultaneous knockdown of BMP2/4/7/ADMP and of Chordin/Noggin/Follistatin/Cerberus display ubiquitous neural induction, indicating that BMP ligands are epistatic to their secreted antagonists.
(N) Blastocoele injection (40 nl) of Noggin-Fc (0.5 μM) or Chordin (1 μM) protein causes complete neuralization of ectoderm.
(O) BMP2/4/7-depleted embryos retain nonneural ectoderm.
(P) Ubiquitous neural differentiation in BMP2/4/7-depleted embryos injected with DN-Xlr mRNA; this experiment suggests that Chordin cleavage by Xlr is required for ADMP-mediated epidermal differentiation.
This ubiquitous induction of neural fate in the ectoderm (Figure 6D, inset) was accompanied by the reciprocal loss of epidermal fate, as shown by the absence of Cytokeratin expression (Figures 6E and 6F). If any of the four MOs was omitted, neuralization was incomplete and some epidermal differentiation remained. With the exception of an extruding extension that contained the blastopore at its tip (corresponding to the spinal-cord region), the CNS induced by quadruple knockdowns consisted mostly of radial brain tissues, as evidenced by the circumferential expression of the fore- and midbrain marker Otx2, the telencephalic marker Rx2a, and the hindbrain marker Krox20 (Figures 6G and 6H). We conclude that ventral (BMP2/4/7) and dorsal (ADMP) BMPs are simultaneously required for regulative DV patterning. Quadruple inhibition of ADMP/BMP2/4/7 signaling is sufficient for the entire ectoderm to acquire neural fate at the expense of the epidermal lineage. This strongly supports the view that inhibition of BMP signaling is sufficient for neural induction in vivo and that BMP signaling establishes a self-regulating morphogenetic field.
BMP Antagonists and DV Patterning
The BMP antagonists Chordin, Noggin, Follistatin, and Cerberus are needed for dorsal development in Xenopus (Oelgeschläger et al., 2003a; Kuroda et al., 2004; Khokha et al., 2005). To show that the ubiquitous neural induction seen in quadruple ADMP/BMP2/4/7 knockdowns was due to the loss of ligands rather than to an increase in their antagonists, we performed epistatic experiments in which eight antagonists and ligands were knocked down concomitantly. Knockdown of Chordin/Noggin/Follistatin/Cerberus greatly decreased anterior CNS tissue (compare Figures 6I and 6K). In a recent study, a more complete loss of neural plate was found in Xenopus tropicalis triple Follistatin/Chordin/Noggin-depleted embryos (Khokha et al., 2005); the difference with our results may arise from incomplete targeting of some cDNA isoforms in Xenopus laevis. When Admp/Bmp2/4/7 morpholinos were coinjected into Chordin/Noggin/Follistatin/Cerberus-depleted embryos, it was found that the BMP ligands were epistatic to the secreted antagonists, so that ubiquitous neural induction was still observed (Figure 6M). Thus, the complete neuralization is caused by a lack of BMP ligands and not by an increase of BMP antagonists. In a gain-of-function situation, Chordin (1 μM) or Noggin-Fc (0.5 μM) protein injections (40 nl) into the blastocoele cavity were able to completely block endogenous BMP ligands, causing ubiquitous neural induction throughout the ectoderm as well (Figure 6N).
A particularly informative experiment was carried out taking advantage of triple BMP2/4/7 knockdowns, in which epidermal differentiation should be mediated solely by ADMP. These embryos had an expanded CNS but retained considerable amounts of epidermis on the ventral side, at a great distance from the dorsal source of ADMP secretion (Figures 6C and 6O). Chordin can inhibit ADMP activity, and this inhibition is reversed by the ventrally produced Xlr protease (Figure 6J). Dominant-negative Xlr mRNA inhibits Chordin cleavage (Dale et al., 2002) and moderately expanded the neural plate in wild-type embryos (Figure 6L). In a BMP2/4/7-depleted background, the same dose of DN-Xlr triggered ubiquitous neural differentiation at the expense of epidermis (Figure 6P). This experiment shows that, in conditions in which DV patterning relies exclusively on ADMP, Xolloid-related activity plays a crucial role in self-regulation by releasing active ADMP from Chordin. This result also implies that, in vivo, there is sufficient Chordin made to block the activity of ADMP.
DV Rescue by BMP4 Protein Injection
We next asked whether DV patterning could be rescued in ADMP/BMP2/4/7-depleted embryos by providing an exogenous source of BMP. Rescue was achieved by microinjecting recombinant BMP4 protein into the blastocoele cavity at the late blastula stage (Figures 7A and 7B). Remarkably, lineage-tracing experiments showed that the exogenous BMP4 protein rescued DV pattern according to the original polarity of the zygote (Figures 7C-7E). This was surprising because it occurred even though the BMP4 protein had been microinjected uniformly, filling the blastocoele cavity. We propose that extracellular regulators of the BMP signaling pathway such as the dorsal molecules Chordin, Noggin, and Follistatin and ventral ones such as Xlr, CV-2, Sizzled, Tsg, and Bambi are still functional in BMP-depleted embryos, providing a means of re-establishing a BMP activity gradient utilizing the microinjected BMP4 protein. The morphogenetic field is generated by the localized activity of BMP antagonists, as well as by the opposite transcriptional regulation of dorsal and ventral BMPs.
Figure 7. Rescue of BMP2/4/7/ADMP-Depleted Embryos by BMP4 Protein and Demonstration that Ventral and Dorsal Centers Serve as BMP Sources In Vivo.

(A) Bmp2/4/7/Admp MOs neuralize the entire ectoderm (n = 25 or more per experimental set).
(B) Epidermal fate and DV pattern are rescued in BMP2/4/7/ADMP-depleted embryos by injection of recombinant BMP4 protein (1.5 ng in 40 nl).
(C) Experimental design of blastomere lineage-tracing experiments.
(D) B4 blastomere descendants are distributed along the AP axis of BMP2/4/7/ADMP-depleted embryos (n = 17).
(E) DV pattern in BMP2/4/7/ADMP-depleted embryos is rescued by BMP4 protein injection (n = 56) according to the initial DV polarity of the fertilized egg, as demonstrated by nuclear LacZ staining in the B4 lineage.
(F) Experimental procedure in which wild-type grafts (marked by nuclear lacZ) were transplanted into ADMP/BMP2/4/7-depleted hosts at early gastrula.
(G-I) Ventral-center grafts (inset) rescue neural plate and epidermal differentiation over many cell diameters (n = 19; for control nongrafted hosts, see [A] and Figure 6F). This experiment demonstrates that the ventral center produces “organizing” signals.
(J-L) Dorsal-center grafts elongate and rescue neural and epidermal patterning over the entire embryo (n = 21). The dorsal graft is the sole source of BMP in these quadruple BMP2/4/7/ADMP-depleted hosts. Note that epidermis is induced only at a distance.
Dorsal or Ventral BMP Sources Can Pattern the Embryo
Our proposal that the embryonic DV morphogenetic field is mediated by reciprocal regulation of ventral and dorsal signaling centers had one major weakness: extensive grafting of the ventral-center region of the gastrula had failed to show any inductive activity on neighboring cells. Instead, grafted cells simply became incorporated into the tissues of the embryonic region into which they were transplanted (Smith and Slack, 1983; unpublished data). The availability of quadruple ADMP/BMP2/4/7 knockdowns that undergo ubiquitous neural differentiation (Figures 7A and 7D) allowed us to demonstrate that the wild-type ventral center indeed has inductive activity (Figures 7F-7I). In a BMP-deficient background, transplantation of a ventral center at gastrula was able to rescue the formation of a neural plate flanked by neural folds and epidermis (Figure 7G). Using lineage-traced (nuclear lacZ) grafts and neural Sox2 and epidermal Cytokeratin markers, it was found that while the transplanted cells remained in the ventral-posterior region, they had inductive effects at very considerable distances, patterning the differentiation of CNS and epidermis at the opposite end of the embryo (Figures 7H and 7I; Figure 7A and Figure 6F show nongrafted host embryos). We conclude that a ventral signaling center can regulate histotypic differentiation over great distances, presumably by releasing BMP signals.
We next tested the ability of the dorsal Spemann organizer, a known source of secreted growth-factor antagonists, to function as a source of BMP activity in the embryo. Strikingly, dorsal grafts restored neural plate and epidermal differentiation when grafted into ADMP/BMP2/4/7-deficient embryos (Figures 7J-7L; compare to nongrafted hosts in Figures 7A and 7D and Figure 6F). The lacZ-marked grafts, which serve as the source of BMP in these embryos, elongated and contributed a thin stripe of floor plate, notochord, and prechordal plate (Figure 7J). Signals emanating from the graft induced epidermal differentiation in the opposite, ventral side of the embryo (Figure 7L). The ventralizing activity of the graft only manifested itself at a distance, for the neural plate—a region of low BMP signaling—formed at the sides of the dorsal graft (Figures 7J and 7K). Presumably, in tissues near the grafted cells, BMP antagonists such as Chordin block ADMP signaling, and this inhibition is reversed by Xlr ventrally.
These embryological experiments demonstrate that a ventral center that can signal over large distances is present in the gastrula. In the opposite side of the embryo, the Spemann organizer also serves as a source of ventralizing signals that can act at considerable distances.
DISCUSSION
One of the long-standing unanswered questions in developmental biology is how a self-differentiating morphogenetic field is established. The Xenopus gastrula embryo contains dorsal and ventral centers (reviewed in De Robertis and Kuroda, 2004) that set a new paradigm for understanding how the embryo can self-regulate. Surprisingly, the ventral center expresses secreted molecules of similar structure and biochemical activities to those of the dorsal center or Spemann organizer. This is particularly true for the BMP signaling pathway, in which similar BMP ligands (ADMP and BMP4/7) and extracellular BMP antagonists (Chordin and Crossveinless-2) are expressed at opposite poles of the embryo. This raises a question: how is a BMP activity gradient generated if each pole of the embryo acts as a source of similar biochemical activities?
Here we propose that this can be answered in part through a self-adjusting “conversation” between cells in the ventral and dorsal poles of the embryo via modulation of BMP signals in the extracellular space. This model stems from four key observations. First, the lowering of BMP levels triggers transcription of Admp, allowing compensation to take place. Second, when dorsal and ventral BMPs are simultaneously abrogated (by quadruple ADMP/BMP2/4/7 depletion), the self-regulating morphogenetic field collapses, and ubiquitous neural induction ensues. Third, the response of the system to changes in BMP levels can be dampened by BMP modulators such as Bambi, Sizzled, Xolloid-related, and Chordin. Fourth, ventral or dorsal grafts are able to restore the entire embryonic morphogenetic field in quadruple BMP-deficient hosts.
Dorsal and Ventral BMPs Are Under Opposite Transcriptional Regulation
Low BMP signaling increased Admp expression, whereas BMP4 microinjection positively regulated Bmp4 transcription. At high levels of BMP4, expression of BMP antagonists—namely Bambi (Onichtchouk et al., 1999) and Sizzled/ogon (Collavin and Kirschner, 2003; Yabe et al., 2003), which serve as negative-feedback regulators—was triggered in the ventral center. This suggests a mechanism for controlling the BMP morphogenetic activity gradient by reciprocal regulation of Admp or Bmp4 and their antagonists at opposite poles of the embryo. In this model, the patterning system constitutes a self-sustaining “BMP seesaw” that patterns the ectoderm (Figure 3L and Figure S7). The developing embryo contains many self-regulating morphogenetic fields discovered by experimental embryology methods, such as the limb, heart, lens, ear, and nose fields (Harrison, 1918; Huxley and de Beer, 1934). It is conceivable that genes sharing similar biochemical activities but under opposite transcriptional regulation may provide a means of self-regulation in other systems as well. In particular, human embryonic stem cells (HESCs) are notoriously difficult to differentiate into homogeneous cell types (Brivanlou et al., 2003) and may also be subject to this type of reciprocal regulation.
Quadruple ADMP/BMP2/4/7 Knockdown Causes Complete Neuralization of Ectoderm
How is the right amount of brain tissue allocated in the embryo? During gastrulation, ectodermal cells must choose between two distinct fates: epidermal or neural (Harland, 2000; Munoz-Sanjuan and Brivanlou, 2002; De Robertis and Kuroda, 2004; Stern, 2005). Epidermal differentiation requires BMP signals, and neural tissue is formed when BMP is inhibited. ADMP is a BMP growth factor paradoxically expressed in the dorsal side of the embryo, which is a region of low BMP signaling (Moos et al., 1995; Joubin and Stern, 1999; Dosch and Niehrs, 2000; Lele et al., 2001). ADMP knockdown expanded CNS formation, but embryos retained DV polarity and epidermal differentiation. We had previously shown that, in embryos lacking Spemann’s organizer, BMP2/4/7 inhibition caused radial brain formation but still retained epidermis in about half of the ectoderm (Reversade et al., 2005). The most remarkable finding made in the course of the present study was that inhibition of endogenous BMP2/4/7 and ADMP signals is sufficient to convert the entire ectoderm into neural tissue in vivo. This complete respecification of the ectoderm causes ubiquitous brain differentiation and takes place at the expense of epidermal fate. This experiment demonstrates that the DV morphogenetic field requires both dorsal and ventral BMP sources.
BMP Antagonists Fine Tune the Pattern
In addition to the reciprocal transcriptional regulation of dorsal and ventral BMP growth factors, BMP antagonists play a critical role in regulating the pattern. Several experiments support this view.
First, when quadruple BMP knockdowns were rescued by unlocalized microinjection of BMP4 protein into the blastocoele cavity, DV patterning was rescued according to the original polarity of the embryo (Figures 7A-7E). Since BMP ligand synthesis was blocked in these quadruple morphants, the morphogenetic activity gradient is likely to be restored by extracellular regulators produced in the dorsal center (such as Chordin, Noggin, Follistatin, and Cerberus) and in the ventral center (such as Bambi, Sizzled, Xlr, Tsg, and CV-2) acting on the microinjected BMP4 protein.
Second, we showed that ADMP can form a ternary complex with Chordin and Tsg and prevents binding of ADMP to its cognate receptor, identified here as ALK-2. Previous work had shown that, in chick, ADMP plays an important function as an inhibitor of ectopic Hensen’s node formation, but the authors were unable to detect inhibition of ADMP activity by Chordin (Joubin and Stern, 1999). In Xenopus, it had been observed that Follistatin inhibits ADMP function (Dosch and Niehrs, 2000). We now show that the antineural effects of ADMP are neutralized by Chordin and that Spemann grafts depleted of ADMP have an increased capacity to induce complete head structures.
Third, the importance of Chordin in regulating ADMP biological activity is illustrated by an experiment using DN-Xlr, a protease-inactive mutant that inhibits Chordin degradation (Dale et al., 2002). In BMP2/4/7-depleted embryos, ADMP is the main BMP left, and, in this background, elevating endogenous Chordin levels by injecting DN-Xlr mRNA caused ubiquitous neural induction (Figure 6P). This indicates that pattern regulation is mediated by Chordin, ADMP, and the Xlr metalloproteinase that releases ADMP in the ventral side.
Finally, BMP antagonists from the ventral center also modify the response of the embryo to ADMP signals from the dorsal side. When Bambi and Sizzled were depleted simultaneously, the embryo became hypersensitive to the ventralizing (antineural) activity of Admp mRNA. This indicates that small changes in ADMP levels can be adjusted for by the morphogenetic system through antagonistic activity of Bambi and Sizzled. We conclude that self-regulation in DV patterning results from dorsal and ventral sources of BMPs and their extracellular modulators (Figure 3L).
Establishment of a Morphogenetic Field from Dorsal and Ventral BMP Sources
Ventral-center grafts at gastrula were able to restore a patterned neural plate and epidermis to ADMP/BMP2/4/7-deficient embryos (Figures 7G-7I). Remarkably, the graft was able to induce cell differentiation—for example, of epidermis—at great distances spanning most of the embryo. Showing that a ventral center with inductive activity exists at gastrula was important because ventral-center genes are transcribed as part of the Bmp4 synexpression group, which undergoes dynamic changes during gastrulation as the blastopore closes (Niehrs and Pollet, 1999). Genes of the ventral center are initially expressed in most of the embryo except for the organizer but subsequently narrow to the ventral sector of the midgastrula embryo (Figure 3H). From these transplantation experiments, we conclude that ventral cells can serve as an organizing center releasing signals that can change histotypic differentiation over considerable distances.
The dorsal side can also, paradoxically, serve as a source of ventralizing activity. When a wild-type organizer was transplanted into the dorsal side of a quadruple BMP-depleted embryo, it was able to restore the entire DV pattern, with epidermal tissue forming at the ventralmost side of the embryo (Figures 7J-7L). Although this experiment is highly suggestive, we note that the transport of ADMP to the ventral side was not proven directly by molecular methods as has been possible in Drosophila, in which the existence of a BMP extracellular transport system has been established (Shimmi et al., 2005; Wang and Ferguson, 2005). We found that ADMP binds a type I receptor (ALK-2) distinct from the canonical BMP transducers ALK-3 and ALK-6. Since ALK-2 is most similar to Drosophila Saxophone, a receptor dedicated to the BMP ligand Screw (Neul and Ferguson, 1998), it is possible that vertebrate ADMP fulfills similar functions to those of Screw in Drosophila.
Self-regulation during development is key to ensure that the process is error tolerant and reproducible time after time (Harrison, 1918; Huxley and de Beer, 1934; Spemann, 1938). In Xenopus, the self-regulative nature of the embryo allows formation of a well-proportioned DV pattern in bisected dorsal half-embryos. The molecular nature of pattern formation in a self-regulating field of cells has been one of the most difficult problems in developmental biology. Mathematical reaction-diffusion models suggest that a stable pattern can be generated by a long-range inhibitor and a short-range activator released from a single source. (Meinhardt, 1992; Niehrs and Meinhardt, 2002; http://www.eb.tuebingen.mpg.de/dept4/meinhardt). Chordin and ADMP could provide such a duo, contributing pattern to a morphogenetic field. However, the situation must be much more complex, considering the multitude of additional molecules secreted by the dorsal and ventral centers (De Robertis and Kuroda, 2004). Despite the complexity of the molecular interactions that pattern the gastrula, there is hope that, with the rise of systems biology (Kirschner, 2005), one day this intricate extracellular machinery will be understood as an integrated molecular circuit. In the meantime, the combination of multiple morpholino knockdowns and embryological transplantations possible in Xenopus provide a powerful assay system for investigating how a self-regulating morphogenetic field is established in a vertebrate.
EXPERIMENTAL PROCEDURES
Morpholino Oligos and RNA Injections
Antisense MO for Xenopus laevis Admp, Bambi, and Follistatin were obtained from Gene Tools LLC and consisted of the following sequences: Admp MO, 5′-GGTCCATCTCATCAAGCTGCAGCTC-3′; Bambi MO, 5′-GAGATTCCACCATCCAGCCCAGCGT-3′; Follistatin MO, 5′-CATTTAACATCCTCAGTGCTGGGAG-3′. Bmp2, Bmp4, and Bmp7 MOs (Reversade et al., 2005); Chordin MO (Oelgeschläger et al., 2003a); Noggin MO and Cerberus MO (Kuroda et al., 2004); and Sizzled MO (Collavin and Kirschner, 2003) were as described. MOs were resuspended in sterile water to a concentration of 1 mM, which was then further diluted to give a working solution of 0.25 mM. Prior to microinjections, MO mixtures were heated at 95°C for 25 s and placed on ice. When multiple MOs were tested, a mixture was prepared and embryos were injected four times radially at the two- to four-cell stage. For mRNA microinjections, 100 pg (25 pg four times in each blastomere at the four-cell stage) of zebrafish or Xenopus Admp and 200 pg of DN-Xlr (Dale et al., 2002) were used.
Embryological Methods
Microinjections and mRNA synthesis were performed as described (Piccolo et al., 1997). RT-PCR conditions and primers, as well as the protocol for whole-mount in situ hybridization, can be found at www.hhmi.ucla.edu/derobertis/index.html. Dorsal and ventral halves were prepared from embryos with strong DV polarity (Klein, 1987). Blastula-stage embryos (stage 9) were manually dechorionated in 0.3× modified Barth’s solution, bisected into two equal halves along the prospective dorsoventral axis using a surgical blade or sharp forceps, and cultured in fresh 0.3× Barth’s solution. Ventral and dorsal grafts were transplanted and cultured in Steinberg’s solution.
Protein Injections and Biochemistry
Blastocoele injections of 40 nl of recombinant mouse Chordin (1 μM), mouse Noggin-Fc (0.5 μM), or human BMP4 (0.3 μM) proteins from R&D Systems were carried out at late blastula stage. Crosslinking and immunoprecipitation experiments were as described (Oelgeschläger et al., 2003b) using proteins harvested from supernatants of 293T cells. Briefly, zebrafish Admp, human Tsg, mouse Chordin, and Xenopus Xlr were transfected independently in HEK293T cells and then combined in cocultures. After 48 hr, secreted proteins were either dialyzed overnight into PBS for crosslinking experiments or directly immunoprecipitated using anti-Myc beads (Covance). Receptor binding assays were carried out as described (Larrain et al., 2001) with Protein A beads (Pierce) using human recombinant chimeric ALK-2-Fc, ALK-3-Fc, ALK-6-Fc, BMPR-II-Fc, mouse Tsg, and Chordin proteins from R&D Systems Inc. Anti-mouse Chordin antibody (R&D Systems, AF758) was diluted 1:500, anti-carboxy-phosphorylated Smad1 antibody (a kind gift from C. Heldin, Ludwig Institute, Uppsala) 1:4000, anti-carboxy-phosphorylated Smad2 antibody (Cell Signaling Technology, #3101) 1:1000, anti-β-catenin antibody (Sigma, C2206) 1:3000, anti-diphosphorylated Erk antibody (Sigma, M8159) 1:1000, and anti-actin antibody (Sigma, A4700) 1:500.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. N. Peyrieras for the zebrafish Myc Admp, C. Heldin for the anti-carboxy-phospho-Smad1 serum, L. Dale for DN-Xlr, members of our laboratory for comments on the manuscript, A. Mays and D. Greenberg for technical assistance, and Ana De Robertis and Geraldine Hebras for continued support and encouragement. B.R. is a predoctoral fellow of the Pasteur Institute under the direction of Dr. Benoît Robert. This work was supported by the NIH (R37 HD21502-19). E.M.D.R. is an Investigator of the Howard Hughes Medical Institute.
REFERENCES
- Bertocchini F, Skromne I, Wolpert L, Stern CD. Determination of embryonic polarity in a regulative system: evidence for endogenous inhibitors acting sequentially during primitive streak formation in the chick embryo. Development. 2004;131:3381–3390. doi: 10.1242/dev.01178. [DOI] [PubMed] [Google Scholar]
- Brivanlou AH, Gage FH, Jaenisch R, Jessell T, Melton D, Rossant J. Stem cells. Setting standards for human embryonic stem cells. Science. 2003;300:913–916. doi: 10.1126/science.1082940. [DOI] [PubMed] [Google Scholar]
- Collavin L, Kirschner MW. The secreted Frizzled-related protein Sizzled functions as a negative feedback regulator of extreme ventral mesoderm. Development. 2003;130:805–816. doi: 10.1242/dev.00306. [DOI] [PubMed] [Google Scholar]
- Dale L, Evans W, Goodman SA. Xolloid-related: a novel BMP1/Tolloid-related metalloprotease is expressed during early Xenopus development. Mech. Dev. 2002;119:177–190. doi: 10.1016/s0925-4773(02)00359-3. [DOI] [PubMed] [Google Scholar]
- De Robertis EM, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu. Rev. Cell Dev. Biol. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosch R, Niehrs C. Requirement for anti-dorsalizing morphogenetic protein in organizer patterning. Mech. Dev. 2000;90:195–203. doi: 10.1016/s0925-4773(99)00245-2. [DOI] [PubMed] [Google Scholar]
- Enders AC. Implantation in the nine-banded armadillo: how does a single blastocyst form four embryos? Placenta. 2002;23:71–85. doi: 10.1053/plac.2001.0753. [DOI] [PubMed] [Google Scholar]
- Harland R. Neural induction. Curr. Opin. Genet. Dev. 2000;10:357–362. doi: 10.1016/s0959-437x(00)00096-4. [DOI] [PubMed] [Google Scholar]
- Harrison RG. Experiments on the development of the fore-limb of Amblystoma, a self-differentiating equipotential system. J. Exp. Zool. 1918;25:413–461. [Google Scholar]
- Huxley JS, de Beer GR. The Elements of Experimental Embryology. Cambridge University Press; Cambridge: 1934. [Google Scholar]
- Joubin K, Stern CD. Molecular interactions continuously define the organizer during the cell movements of gastrulation. Cell. 1999;98:559–571. doi: 10.1016/s0092-8674(00)80044-6. [DOI] [PubMed] [Google Scholar]
- Khokha MK, Yeh J, Grammer TC, Harland RM. Depletion of three BMP antagonists from Spemann’s organizer leads to a catastrophic loss of dorsal structures. Dev. Cell. 2005;8:401–411. doi: 10.1016/j.devcel.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Kirschner MW. The meaning of systems biology. Cell. 2005;121:503–504. doi: 10.1016/j.cell.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Klein SL. The first cleavage furrow demarcates the dorsal-ventral axis in Xenopus embryos. Dev. Biol. 1987;120:299–304. doi: 10.1016/0012-1606(87)90127-8. [DOI] [PubMed] [Google Scholar]
- Kuroda H, Wessely O, De Robertis EM. Neural induction in Xenopus: requirement for ectodermal and endomesodermal signals via Chordin, Noggin, beta-Catenin, and Cerberus. PLoS Biol. 2004;2:e92. doi: 10.1371/journal.pbio.0020092. Published online May 11, 2004. 10.1371/journal.pbio.0020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrain J, Oelgeschlager M, Ketpura NI, Reversade B, Zakin L, De Robertis EM. Proteolytic cleavage of Chordin as a switch for the dual activities of Twisted gastrulation in BMP signaling. Development. 2001;128:4439–4447. doi: 10.1242/dev.128.22.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lele Z, Nowak M, Hammerschmidt M. Zebrafish admp is required to restrict the size of the organizer and to promote posterior and ventral development. Dev. Dyn. 2001;222:681–687. doi: 10.1002/dvdy.1222. [DOI] [PubMed] [Google Scholar]
- Macias-Silva M, Hoodless PA, Tang SJ, Buchwald M, Wrana JL. Specific activation of Smad1 signaling pathways by the BMP7 type I receptor, ALK2. J. Biol. Chem. 1998;273:25628–25636. doi: 10.1074/jbc.273.40.25628. [DOI] [PubMed] [Google Scholar]
- Meinhardt H. Pattern formation in biology: a comparison of models and experiments. Rep. Prog. Phys. 1992;55:797–849. [Google Scholar]
- Moos M, Jr., Wang S, Krinks M. Anti-dorsalizing morphogenetic protein is a novel TGF-beta homolog expressed in the Spemann organizer. Development. 1995;121:4293–4301. doi: 10.1242/dev.121.12.4293. [DOI] [PubMed] [Google Scholar]
- Munoz-Sanjuan I, Brivanlou AH. Neural induction, the default model and embryonic stem cells. Nat. Rev. Neurosci. 2002;3:271–280. doi: 10.1038/nrn786. [DOI] [PubMed] [Google Scholar]
- Neul JL, Ferguson EL. Spatially restricted activation of the SAX receptor by SCW modulates DPP/TKV signaling in Drosophila dorsal-ventral patterning. Cell. 1998;95:483–494. doi: 10.1016/s0092-8674(00)81616-5. [DOI] [PubMed] [Google Scholar]
- Niehrs C, Meinhardt H. Modular feedback. Nature. 2002;417:35–36. doi: 10.1038/417035a. [DOI] [PubMed] [Google Scholar]
- Niehrs C, Pollet N. Synexpression groups in eukaryotes. Nature. 1999;402:483–487. doi: 10.1038/990025. [DOI] [PubMed] [Google Scholar]
- Oelgeschläger M, Kuroda H, Reversade B, De Robertis EM. Chordin is required for the Spemann organizer transplantation phenomenon in Xenopus embryos. Dev. Cell. 2003a;4:219–230. doi: 10.1016/s1534-5807(02)00404-5. [DOI] [PubMed] [Google Scholar]
- Oelgeschläger M, Reversade B, Larrain J, Little S, Mullins MC, De Robertis EM. The pro-BMP activity of Twisted gastrulation is independent of BMP binding. Development. 2003b;130:4047–4056. doi: 10.1242/dev.00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onichtchouk D, Chen YG, Dosch R, Gawantka V, Delius H, Massague J, Niehrs C. Silencing of TGF-beta signalling by the pseudoreceptor BAMBI. Nature. 1999;401:480–485. doi: 10.1038/46794. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Lu B, Goodman S, Dale L, De Robertis EM. Cleavage of Chordin by Xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann organizer activity. Cell. 1997;91:407–416. doi: 10.1016/s0092-8674(00)80424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reversade B, Kuroda H, Lee H, Mays A, De Robertis EM. Depletion of Bmp2, Bmp4, Bmp7 and Spemann organizer signals induces massive brain formation in Xenopus embryos. Development. 2005;132:3381–3392. doi: 10.1242/dev.01901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler TW. Langman’s Medical Embryology. Ninth Edition Lippincott Williams & Wilkins; Philadelphia: 2004. [Google Scholar]
- Schier AF, Talbot WS. Molecular Genetics of Axis Formation in Zebrafish. Annu. Rev. Genet. 2005;39:561–613. doi: 10.1146/annurev.genet.37.110801.143752. Published online August 9, 2005. 10.1146/annurev.genet.37.110801.143752. [DOI] [PubMed] [Google Scholar]
- Shimmi O, Umulis D, Othmer H, O’Connor MB. Facilitated transport of a Dpp/Scw heterodimer by Sog/Tsg leads to robust patterning of the Drosophila blastoderm embryo. Cell. 2005;120:873–886. doi: 10.1016/j.cell.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Slack JM. Dorsalization and neural induction: properties of the organizer in Xenopus laevis. J. Embryol. Exp. Morphol. 1983;78:299–317. [PubMed] [Google Scholar]
- Spemann H. Entwicklungsphysiologische Studien am Tritonei III. Arch. f. Entw. Mech. 1903;16:551–631. [Google Scholar]
- Spemann H. Embryonic Development and Induction. Yale University Press; New Haven, CT: 1938. [Google Scholar]
- Spratt NT, Hass H. Integrative mechanism in development of the early chick blastoderm. I. Regulative potentiality of separate parts. J. Exp. Zool. 1960;145:97–137. [Google Scholar]
- Stern CD. Neural induction: old problem, new findings, yet more questions. Development. 2005;132:2007–2021. doi: 10.1242/dev.01794. [DOI] [PubMed] [Google Scholar]
- Wang YC, Ferguson EL. Spatial bistability of Dpp-receptor interactions during Drosophila dorsal-ventral patterning. Nature. 2005;434:229–234. doi: 10.1038/nature03318. [DOI] [PubMed] [Google Scholar]
- Willot V, Mathieu J, Lu Y, Schmid B, Sidi S, Yan YL, Postlethwait JH, Mullins M, Rosa F, Peyrieras N. Cooperative action of ADMP- and BMP-mediated pathways in regulating cell fates in the zebra-fish gastrula. Dev. Biol. 2002;241:59–78. doi: 10.1006/dbio.2001.0494. [DOI] [PubMed] [Google Scholar]
- Yabe T, Shimizu T, Muraoka O, Bae YK, Hirata T, Nojima H, Kawakami A, Hirano T, Hibi M. Ogon/Secreted Frizzled functions as a negative feedback regulator of Bmp signaling. Development. 2003;130:2705–2716. doi: 10.1242/dev.00506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.