Abstract
Experimental autoimmune uveitis (EAU) represents autoimmune uveitis in humans. We examined the role of the interleukin (IL)-23–IL-17 and IL-12–T helper cell (Th)1 pathways in the pathogenesis of EAU. IL–23 but not IL-12 was necessary to elicit disease by immunization with the retinal antigen (Ag) interphotoreceptor retinoid-binding protein (IRBP) in complete Freund's adjuvant. IL-17 played a dominant role in this model; its neutralization prevented or reversed disease, and Th17 effector cells induced EAU in the absence of interferon (IFN)-γ. In a transfer model, however, a polarized Th1 line could induce severe EAU independently of host IL-17. Furthermore, induction of EAU with IRBP-pulsed mature dendritic cells required generation of an IFN-γ–producing effector response, and an IL-17 response by itself was insufficient to elicit pathology. Finally, genetic deficiency of IL-17 did not abrogate EAU susceptibility. Thus, autoimmune pathology can develop in the context of either a Th17 or a Th1 effector response depending on the model. The data suggest that the dominant effector phenotype may be determined at least in part by conditions present during initial exposure to Ag, including the quality/quantity of Toll-like receptor stimulation and/or type of Ag-presenting cells. These data also raise the possibility that the nonredundant requirement for IL-23 in EAU may extend beyond its role in promoting the Th17 effector response and help provide a balance in the current Th1 versus Th17 paradigm.
IL-23 is a recently described member of the IL-12 family. Both cytokines share a common p40 subunit, but IL-23 has a unique p19 subunit whereas IL-12 uses a p35 subunit (1). IL-12 and IL-23 promote overlapping as well as distinct cellular immune functions. Whereas IL–12 is well known for promoting the IFN-γ–producing Th1 effector phenotype in the adaptive response, IL-23 is reported to promote IL-17–producing effector T cells that constitute a separate lineage from Th1 and Th2 and have been appropriately dubbed “Th17.” Recent studies have suggested that these cells may have an important role in cell-mediated autoimmune inflammatory diseases.
Experimental autoimmune uveitis (EAU) serves as a model for several human ocular diseases of suspected autoimmune etiology (2–4). EAU is elicited by immunization with retinal antigens (Ags) or their fragments (5), or by adoptive transfer of retinal Ag-specific CD4+ T cells between syngeneic rodents (6, 7). Published data provide evidence that a Th1-dominant response and the Th1 effector cell are critical for EAU development and that endogenous IL-12 is needed for EAU induction and its full expression (8, 9). However, susceptibility to EAU of IFN-γ–deficient (GKO) mice, exacerbation of EAU by neutralization of endogenous IFN-γ, and the protective effects of high systemic IFN-γ in WT mice (10–12) were in apparent paradox with this notion.
The requirement for IL-12–mediated IFN-γ and Th1 responses in autoimmune inflammation has recently been questioned by several studies in other disease models. Mice deficient in IFN-γ, IFN-γR, IL-12Rβ2, and the IL-12p35 chain were highly susceptible to experimental autoimmune encephalomyelitis (EAE) and collagen-induced arthritis (CIA) (13–15). In contrast, IL-23 and the IL-17–producing effector T cell whose differentiation and maintenance are promoted by IL-23 were found to be necessary for induction of these diseases (15, 16). The activity of the IL-17–producing effector T cells (Th17) was associated with induction of proinflammatory cytokines such as TNF-α, IL-1, IL-6, and IL-8, as well as with enhanced proliferation, maturation, and chemotaxis of neutrophils. These results led to the notion that the pathogenic effects previously attributed to the IL-12–IFN-γ pathway are in fact largely if not solely mediated by IL-23 and the IL-23–driven Th17 effector (15, 17).
The present study was conducted to examine the role of the IL-23–IL-17 pathway in interphotoreceptor retinoid-binding protein (IRBP)-induced EAU. Our data indicate that IL-23, rather than IL-12, is necessary for EAU induction and exerts its role early in the response. We demonstrate that IL-17 plays a role in the pathogenesis of EAU induced by immunization in CFA, and that targeting IL-17 even late in the disease process can ameliorate pathology, indicating an effector role for this cytokine in pathogenesis of this type of EAU. Notably, however, severe EAU could be induced by uveitogenic Th1 cells without participation of host IL-17, and pathology of EAU induced with uveitogenic Ag-pulsed DCs required induction of an IFN-γ–producing effector T cell response. Finally, genetically IL-17–deficient mice were able to develop substantial disease. Thus, in some situations Ag-specific IL-17–producing effector T cells appear to be dispensable for pathogenesis. The data put in perspective the role of Th17 versus other effector mechanisms in autoimmune inflammation and suggest that the essential role of IL-23 in ocular autoimmunity may transcend its ability to drive the Ag-specific IL-17 effector response.
RESULTS
IL-23 is essential for induction of EAU and proinflammatory cytokine responses
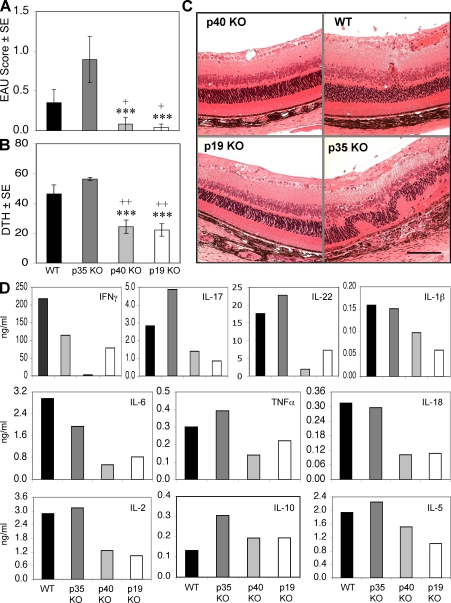
Earlier studies that indicated a necessary role for IL-12 in EAU were based on p40 KO mice and neutralizing anti-p40 antibodies, and did not differentiate between the roles of IL-12 and IL-23. To investigate the effect of IL-23 on EAU development, we immunized WT, p40 KO, p35 KO, and p19 KO strains with a uveitogenic protocol of IRBP in CFA. Determination of EAU score 21 d after immunization revealed a significant reduction in the p19 and p40 KO mice compared with the WT, whereas EAU scores were significantly increased in the p35 KO mice (Fig. 1 A). Evaluation of EAU pathology revealed severe retinal damage in the p35 KO mice, whereas the p19 and p40 KO mice maintained healthy retinas (Fig. 1 C). Interestingly, the composition of the inflammatory infiltrate in the eyes of the p35 KO differed from the WT, with the former dominated by macrophages and the latter by lymphocytes (not depicted), which may be related to differences in the cytokine response profile of these strains (see below).
Figure 1.
IL-23 is essential for EAU induction by up-regulating proinflammatory cytokine. (A) EAU scores were evaluated by histopathology of eyes from WT and the different IL-12 family cytokine KO mice 21 d after immunization. (B) DTH response in mice challenged 48 h before the end of the experiment. These data are representative of three experiments. (C) Representative histopathology of ocular damage in WT and p35 KO mice. Bar, 0.25 mm. (D) LNs were harvested from WT and the different IL-12 family KO mice 21 d after immunization. Cytokine secretion was measured in 48-h IRBP-stimulated (30 μg/ml) culture supernatants. ***, P < 0.001 versus P35 KO; +, P < 0.05; ++, P < 0.01 versus WT. Cytokines were measured in pooled supernatants, so although data represent a group average of five mice, no error bars could be generated.
We next examined the adaptive immune responses 21 d after immunization. Mice challenged with IRBP in their ear pinnae 48 h earlier were assessed for delayed-type hypersensitivity (DTH) responses, and their lymphoid cells were collected for analysis of Ag-specific cytokine production. The results showed that the DTH response to IRBP was well correlated with EAU scores of the respective mice, showing significantly lower response for the p19 and p40 KO mice and significantly higher response for the p35 KO compared with the WT (Fig. 1 B). In keeping with their EAU and DTH responses, the p19 KO and p40 KO mice also revealed reduced amounts of all measured proinflammatory cytokines compared with the WT, without an obvious skewing toward a Th2 cytokine profile (Fig. 1 D). In contrast, p35 KO mice revealed elevated levels of IL-17 and TNF-α compared with WT (Fig. 1 D).
Inductive role of IL-23: systemic neutralization of IL-23 prevents, but does not reverse, EAU
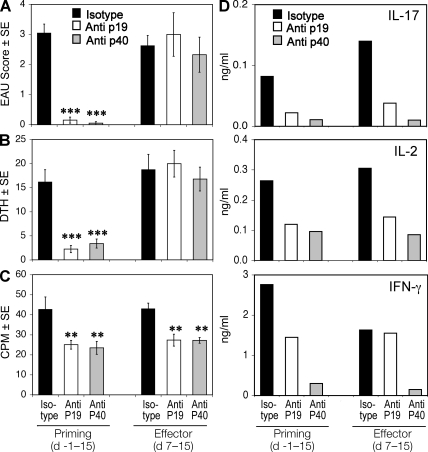
The requirement for IL-23 to develop EAU suggests that this cytokine could serve as a therapeutic target. The most susceptible strain to EAU is the B10.RIII mouse; therefore, we examined whether neutralization of p19 affects EAU development in this strain. Groups of B10.RIII mice were immunized with a uveitogenic protocol of the major pathogenic epitope of IRBP, residues 161–180. Immunized mice were treated with a neutralizing mAb to p19 or p40 or isotype control either starting from the priming phase (prevention) or starting from the effector phase (reversal), as described in Materials and methods. EAU was prevented by early treatment with either anti-p19 or anti-p40 antibodies. However, when treatment was started 7 d after immunization, a time point when uveitogenic effector T cells have already been primed and can be isolated from the LN and spleen, EAU development could not be aborted and the disease scores developed by treated mice were similar to control (Fig. 2 A). This suggests that an inductive requirement for IL-23 occurs at an early stage of EAU pathogenesis.
Figure 2.
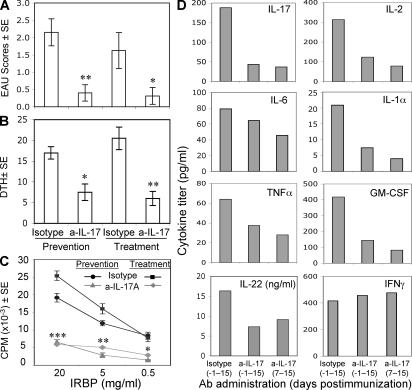
Anti–IL-23 treatment prevents, but does not reverse, EAU. B10RIII mice were immunized with IRBP peptide (161–180) as indicated. Groups of five mice were treated i.p. with antibodies against p19, p40, or with isotype from the priming (starting day −1) or from the effector phase of EAU induction (starting day 7) every other day, as indicated in Materials and methods. (A) EAU score evaluated in eyes by histopathology 17 d after immunization. (B) DTH responses of mice challenged 48 h earlier. (C) LN proliferation to IRBP peptide 161–180. (D) Cytokine secretion from LN cells stimulated with IRBP peptide 161–180 for 48 h. The data are from a representative experiment of two, with five mice per group. **, P < 0.01; ***, P < 0.001 versus the respective isotype control.
We examined the adaptive immune responses of mice that received early or late treatment. DTH was assessed, and lymphoid organs were collected for analysis 17 d after immunization. The effect of the treatment on DTH responses paralleled the effect on EAU scores, showing significantly lower response in mice treated from the priming phase but no reduction in mice treated starting 7 d after immunization (Fig. 2, A and B). In contrast, proliferation (Fig. 2 C) and cytokine responses (Fig. 2 D) tended to be reduced in draining LNs collected from mice treated with either regimen. This apparent discrepancy between responses in vitro (proliferation and cytokines) and in vivo (EAU and DTH) is likely due to the fact that the former reflects newly primed cells present at that point in time in the LN, whereas the latter reflects the sum total of primed effectors that have already exited into the systemic circulation. Thus, although late treatment inhibits further priming of T cells newly arrived in the LN, sufficient effector T cells have already completed priming and exited the LN to mediate DTH and disease.
Th17 cells infiltrate the eye during uveitis; intensity of the IL-17 response is correlated with susceptibility
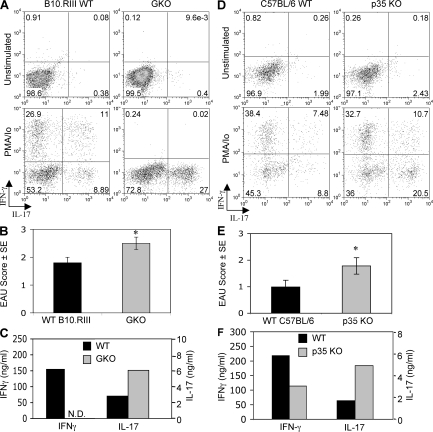
We next wished to examine whether IL-17–producing T cells are present in uveitic eyes. Mice were immunized with a uveitogenic protocol of IRBP. On day 21, when disease was fully developed, eyes were collected for analysis of ocular-infiltrating inflammatory cells by intracellular staining for IFN-γ and IL-17 combined with immunophenotyping, or for histopathological scoring of disease, and LN cells were stimulated in vitro with IRBP for secreted cytokine analysis by ELISA. We also examined eyes and responses of p35 KO and IFN-γ KO mice, both of which characteristically develop more severe inflammation and retinal damage compared with their respective WT counterparts.
Direct ex vivo analysis of eye-infiltrating cells isolated from uveitic WT B10.RIII or C57BL/6 mice for intracellular IFN-γ and IL-17 (after treatment with brefeldin A) revealed few or no cells actively producing these cytokines (Fig. 3, A and D, Unstimulated). However, a brief incubation with PMA plus ionomycin unmasked substantial numbers of CD4+ cells capable of producing IFN-γ and IL-17 within the inflammatory infiltrate (Fig. 3, A and D, PMA/Io). It is difficult to say which condition more accurately reflects the situation in vivo, where infiltrating T cells can come in contact and be stimulated by Ag within ocular microcompartments. Nevertheless, supernatants obtained after mincing uveitic eyes in PBS to release the infiltrating cells and centrifugation can contain detectable levels of inflammatory cytokines despite a considerable dilution factor (see Fig. 7 F).
Figure 3.
Enhanced EAU in IL-12p35 KO and in IFN-γ KO mice is associated with increased systemic and local IL-17 responses. WT and GKO mice on B10.RIII background (A–C) and WT and P35 KO mice on C57BL/6 background (D–F) were immunized with IRBP. On day 21 after immunization, eyes were collected for intracellular cytokine staining of eye-infiltrating inflammatory cells (A and D) or for histopathology (data pooled from two experiments with five mice per group; B and E). *, P < 0.05 versus WT. For FACS analysis, cells isolated from eyes were incubated either with PMA plus ionomycin and brefeldin A (PMA/Io) or with brefeldin A only (unstimulated). Shown are cells gated on CD4. Cytokine secretion from LN cells was measured by ELISA after 48 h of culture with IRBP.
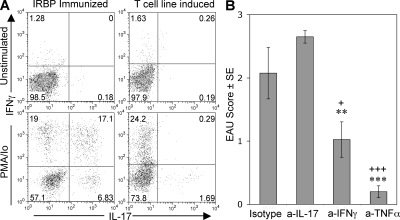
Figure 7.
Host IL-17 does not seem to play a role in pathogenesis of EAU induced with the Th1 cell line. Two million freshly activated Th1 line cells were infused i.v. into naive B10.RIII Thy1.1 homozygous mice. EAU scores were assessed by fundoscopy. (A) Eyes of recipient mice were removed for isolation of infiltrating cells 10 d after cell transfer. Cells isolated from eyes and incubated with PMA plus ionomycin and brefeldin A (PMA/Io) or with brefeldin A only (unstimulated) were stained for intracellular IFN-γ versus IL-17 and were analyzed by FACS. (B) Recipient mice were treated with neutralizing antibodies to IL-17, IFN-γ, or TNF-α, or with isotype control (0.25 mg/mouse/day, starting day 0). 10 d after transfer, EAU was scored by fundoscopy and confirmed by histopathology. Data show histopathology scores of a representative experiment of three. +, P < 0.05; ++, P < 0.01 versus isotype control; **, P < 0.01 and ***, P < 0.001 versus anti–IL-17.
Both p35 KO and GKO mice had significantly increased EAU scores (Fig. 3, B and E) and enhanced IL-17 production (Fig. 3, C and F) compared with their WT counterparts. Furthermore, in both genotypes eye-infiltrating cells isolated from uveitic eyes and analyzed for intracellular cytokine expression contained a higher proportion of cells able to produce IL-17 than their respective WT controls. Although it is not possible to discern whether these were bona fide IRBP-specific MHC class II–restricted Th17 effectors, in all genotypes the vast majority of the intraocular cells capable of producing IL-17 were CD4+ (Fig. 3, B and E).
Effector role of IL-17: IL-17 neutralization prevents and reverses EAU
IL-17 was suggested to be the major pathogenic cytokine in inflammatory autoimmune diseases such as EAE and CIA. We therefore tested whether neutralizing IL-17 in vivo by monoclonal anti–IL-17 antibody can affect EAU development. Neutralization of IL-17 either during the entire course of disease (starting day −1) or during the effector phase only (starting day 7) was highly protective (Fig. 4 A). Immunological responses, including Ag-specific DTH, proliferation, and proinflammatory cytokine production, were strongly reduced in both early and late treatment (Fig. 4, B–D). The inhibitory effect of anti–IL-17 on Ag-specific responses is likely indirect, as T cells lack expression of the IL-17 receptor although it is present on other leukocytes (18).
Figure 4.
Anti–IL-17 treatment prevents and reverses EAU. B10RIII mice were immunized with IRBP as indicated. Groups of five mice were treated i.p. with antibodies against IL-17 or with isotype from the time of priming (starting day −1) or from the effector phase of EAU induction (starting day 7) every other day. (A) EAU score evaluated in eyes by histopathology 17 d after immunization. (B) Ag-specific DTH response. (C and D) LN cells were explanted into culture, and IRBP-specific proliferation and cytokine production were measured by multiplex ELISA or by single-plex ELISA (IL-22). Data show a representative experiment of two. *, P < 0.05; **, P < 0.01; ***, P < 0.001 versus the related isotype control.
Importantly, unlike neutralization of IL-23, neutralization of IL-17 effectively inhibited disease when administered during the effector phase starting from day 7 after immunization, when uveitogenic effectors have already been generated. Thus, unlike IL-23, IL-17 appears to participate in the effector phase of the disease. In the aggregate, the data shown in Figs. 3 and Figs.4 identify the Th17 Ag-specific effector as central to EAU pathogenesis in the mouse EAU model induced by immunization with IRBP in CFA.
Th17 effector cells are able to induce EAU without participation of Th1 effector cells
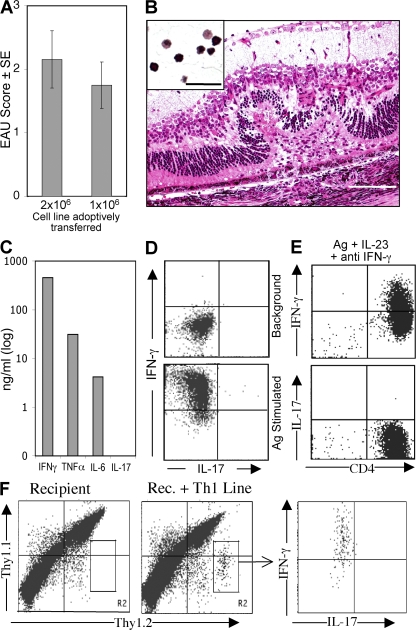
Although the data described thus far indicate that Th17 is an important effector phenotype in EAU, we wished to examine whether this effector phenotype is able to induce EAU in the absence of Th1. To examine this question, we immunized IFN-γ KO mice on the B10.RIII background with IRBP epitope 161–180 and derived a short-term Th17 line by two rounds of stimulation with Ag under Th17-inducing conditions. After the second round of stimulation, ∼50% of the CD4+ cells produced IL-17 by intracellular cytokine staining (Fig. 5 A). In addition, the cells produced very large amounts of IL-6 and considerable amounts of TNF-α, as assayed by ELISA (Fig. 5 B). More than two rounds of stimulation resulted in progressive loss of IL-17 production, suggesting that the Th17 effector phenotype is not stable, at least under the in vitro expansion conditions used here. WT as well as IFN-γ KO recipients infused with 5–10 million cells immediately after the second stimulation reproducibly developed EAU with high incidence and a predominantly neutrophilic infiltrate (Fig. 5, C and D). These data indicate that EAU can be induced by IL-17–producing T effector cells that are unable to produce IFN-γ, and that disease induction by these cells is not dependent on host-produced IFN-γ.
Figure 5.
Th17 effector cells induce EAU in the absence of an IFN-γ response. Short-term Th17 lines were generated from IFN-γ KO mice immunized with p161–180 under Th17 conditions, as described in Materials and methods. (A) Intracellular IL-17 expression after a second stimulation with p161–180. IFN-γ expression is confirmed to be negative. (B) Supernatant from the stimulated cultures was tested for cytokine secretion by ELISA. (C and D) Pathogenicity of Th17 cells. Cells were collected from culture after the second cycle of stimulation, and 5 × 106/mouse were infused into WT or GKO recipients (five mice per group). On day 10 after transfer, eyes were collected for histopathology and disease was scored. Inset shows the inflammatory infiltrate, similar in WT and IFN–γ KO. Bar, 0.25 mm (0.02 mm in inset). A representative experiment of two is shown.
The Th17 effector is dispensable in EAU induced with a Th1 cell line
Previous data in the EAE and arthritis models suggested that IL-17–producing effector T cells have central importance in pathogenesis, whereas the Th1-type IFN-γ–producing effectors have at best a secondary role. Our data shown above appeared to suggest that the same may be true for EAU. We therefore asked the reciprocal question, namely, whether EAU can be induced in the absence of a Th17 response.
Cells from a uvetitogenic CD4+ Th1 cell line specific to IRBP peptide 161–180 were activated in vitro with their peptide Ag. Cytokine production was assayed by ELISA and by intracellular cytokine staining. In parallel, activated cells were infused into naive syngeneic mice for assessment of EAU induction. The T cell line induced severe EAU in recipient mice (Fig. 6, A and B). ELISA analysis of culture supernatants revealed that Th1 cell line in addition to IFN-γ also secretes large amounts of TNF-α and nanogram amounts of IL-6 (Fig. 6 C), but no detectable IL-17. Intracellular staining for IFN-γ and IL-17 revealed that although the line cells expressed abundant IFN-γ, no intracellular IL-17 could be detected (Fig. 6 D). This is not to suggest that IFN-γ is the cytokine directly responsible for pathology, but rather to implicate the downstream mechanisms that it induces. There was also no detectable production of IL–22, a proinflammatory cytokine that was recently associated with the Th17 effector phenotype and with autoimmunity (19–21).
Figure 6.
Severe EAU induced with a T cell line that is stably polarized to the Th1 phenotype. (A and B) After 48 h of stimulation with p161–180, the indicated number of cells was infused into naive B10RIII mice. Shown are average scores (A) and representative histopathology. (B) Inset shows the typical inflammatory infiltrate. Bar, 0.25 mm (0.02 mm in inset). (C) ELISA analysis of cytokines in supernatants of the stimulated cells. (D and E) Intracellular cytokine staining after stimulation with Ag for 24 h in neutral conditions (D) or for 5 d under Th17-inducing conditions (Ag plus IL-23 or Ag plus IL-23 plus anti–IFN-γ; E). (F) “Parking” of Th1 cells in allotype-congenic recipient mice. After stimulation with p161–180 in the presence of irradiated APCs, the T cell line was infused into naive Thy1.1 x Thy1.2 heterozygous recipients (2 × 106/mouse). Note heterozygous Thy1.1/2+ recipient cells versus Thy1.2+ donor cells by FACS analysis. Thy1.2 single-positive cells were sorted out after 90 h, stimulated with IRBP peptide 161–180 for 24 h (with PMA-ionomycin and brefeldin A added during the last 4 h), and stained for intracellular IL-17 and IFN-γ. Representative experiment of two with five mice per group.
To address the possibility that IL-17 production by the Th1 cell line could have delayed kinetics, or could require factor(s) available only in an in vivo environment, we examined the ability of this T cell line to produce IL-17 (a) after a more prolonged stimulation in vitro under Th17-promoting conditions, and (b) several days after adoptive transfer into mice. For the first approach, the T cell line was activated in culture with p161–180 peptide on APCs for 5 d, with rIL-23 plus anti–IFN-γ. In a separate experiment, the activated Th1 cells were adoptively transferred into Thy1–congenic mice (heterozygous Thy1.1/1.2 double-positive recipients), and after 90 h the spleens were analyzed for intracellular IFN-γ versus IL-17 production by the transferred cells (Thy1.2 single-positive). The data showed that (a) even prolonged in vitro stimulation under IL-17–promoting conditions failed to induce IL-17 production in the T cell line (Fig. 6 E), and the cytokine profile remained identical to Fig. 6 C (not depicted); and (b) line cells “parked” in syngeneic hosts, which exhibited ocular signs of EAU, still demonstrated only IFN-γ and no IL-17 production (Fig. 6 F).
Although the Th1 cell line itself cannot produce IL-17, the recipients of these cells are IL-17 competent. Therefore, it was necessary to examine whether host IL-17 was required for pathogenesis of EAU induced by the Th1 line. Flow cytometric analysis of infiltrating cells from uveitic eyes of Th1 cell line recipients revealed few, if any, cells capable of producing IL-17, in sharp contrast to actively immunized mice (Fig. 7 A). More importantly, treatment of the recipient mice with the same dose of neutralizing anti–IL-17 antibody that aborts induction of disease in actively immunized mice had no effect on EAU scores of the Th1 cell line recipients. In contrast, neutralization of IFN-γ or TNF-α reduced or largely prevented disease, indicating that these cytokines, rather than IL-17, have an effector role in the adoptively transferred EAU model (Fig. 7 B).
Th17 without Th1 is insufficient to support pathology in an EAU model induced with in vitro–matured, Ag-pulsed DCs
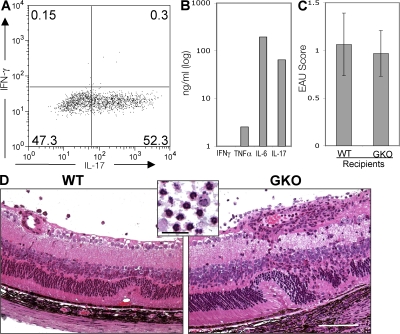
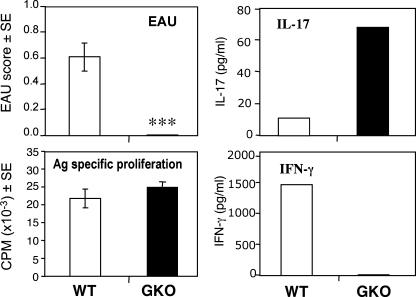
The results above indicated that although IL-17 is clearly dominant in EAU induced by immunization with IRBP in CFA, it is dispensable when EAU is elicited by IRBP-specific Th1 effector cells without use of CFA. Another model of EAU that is not dependent on CFA is a recently developed EAU model induced in B10.RIII mice by injection of IRBP 161–180–pulsed, in vitro–matured splenic DCs (22). In mice with EAU induced by uveitogenic DCs, the Ag-specific IL-17 response is lower and the IFN-γ response is higher than in the traditional EAU model induced by immunization in CFA (22). To examine the need for an IFN-γ–producing effector, we compared the ability of uveitogenic DCs from WT mice to induce disease in WT versus IFN-γ–deficient recipients. DCs isolated from WT mice by immunomagnetic sorting were pulsed with IRBP p161–180 in presence of LPS and anti-CD40 agonistic antibodies and were injected into syngeneic WT or GKO recipients. Only the WT recipients developed ocular inflammation (Fig. 8 A). This was despite efficient Ag priming in both genotypes, as indicated by equivalent Ag-specific proliferation in GKO and WT recipients (Fig. 8 B) and equivalent IL-4 and IL-5 responses (not depicted). Notably, the IL-17 response developed by GKO mice was considerably higher than that of the WT (Fig. 8 C), suggesting that in this model an IL-17 response in the absence of an IFN-γ response is insufficient, and that priming of an IFN-γ–producing effector is needed to elicit disease. These data are consistent with our previous observations and contrast with the enhanced EAU elicited in GKO mice after active immunization in CFA (Fig. 3) (22).
Figure 8.
IFN-γ KO (GKO) mice fail to develop EAU after infusion of uveitogenic DCs despite the presence of a Th17 response. Flt3L-elicited splenic DCs obtained from B10.RIII WT mice were matured and pulsed with IRBP p161–180 in vitro for 4 h and injected into syngeneic WT recipients (n = 8) or GKO recipients (n = 10). EAU (histopathology) and immunological responses were evaluated on day 18 after uveitogenic DC transfer. Data show a representative experiment of three. ***, P < 0.001 versus WT.
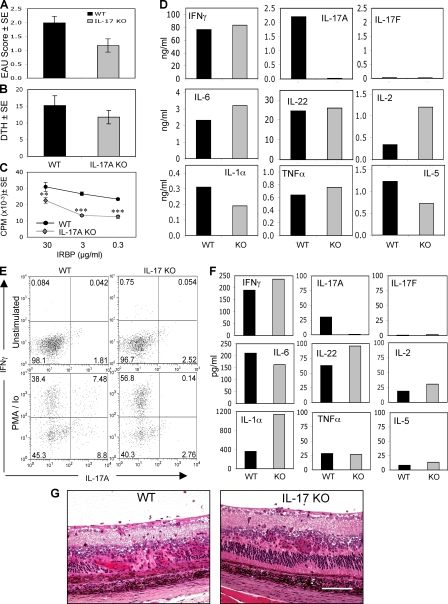
IL-17 KO mice develop EAU and produce proinflammatory cytokines
Inhibition of EAU in actively immunized mice after IL-17 neutralization contrasted with the apparent lack of requirement for IL-17 in the adoptively transferred disease. We therefore examined whether genetic deficiency of IL-17 will prevent induction of EAU similarly to IL-23 deficiency. IL-17 KO mice (23) have a targeted deletion of IL-17A, the same IL-17 family member recognized by the anti–IL-17 antibody clone 1D10 used throughout this study. IL-17 KO mice and WT controls were immunized with a uveitogenic regimen of IRBP. Inhibition of EAU by genetic IL-17 deficiency was only partial and did not attain statistical significance (Fig. 9 A). Although LN cell proliferation was reduced, DTH responses were close to normal (Fig. 9, B and C). There did not appear to be an obvious difference in the histopathological picture that developed in IL-17 KO as opposed to WT mice, other than severity and number of lesions (Fig. 9 G).
Figure 9.
IL-17 KO mice develop EAU and maintain production of proinflammatory cytokines. IL–17 KO mice were immunized with a uveitogenic regimen of IRBP. (A and B) On day 21 after immunization, eyes were collected for EAU evaluation by histopathology (A) and specific DTH responses were recorded as the difference between IRBP- and PBS-injected ears (B). (C and D) LN cells were explanted into culture, and IRBP-specific proliferation (C) and cytokine production (D) were evaluated. The data show averaged responses from 17 animals in three experiments. (E) On day 21 after immunization, eyes were collected and eye-infiltrating cells were extracted and stained for intracellular IFN–γ and IL-17. (F) Ocular extracts prepared as described in Materials and methods were assayed for cytokine levels. (G) Histopathology of EAU in IL-17 KO and WT mice. Bar, 0.25 mm. Data show a representative experiment of three. **, P < 0.01; ***, P < 0.001 versus WT.
These data show that EAU can occur in the complete absence of IL-17A and indicate that other systemic and local mechanisms compensate for genetic deficiency of IL-17A. Multiplex ELISA analysis of Ag-stimulated LN cell supernatants revealed that proinflammatory cytokine responses, with the expected exception of IL-17A, were overall not very severely affected (Fig. 9 D). Flow cytometric analysis of eye-infiltrating cells for intracellular cytokines revealed an increased proportion of CD4+ cells capable of producing IFN-γ in IL-17 KO eyes compared with WT (Fig. 9 E). Notably, supernatants of the minced eye tissue and vitreous fluid that remained after isolation of the eye-infiltrating leukocytes shown in Fig. 9 E (see Materials and methods) analyzed by ELISA revealed proinflammatory cytokines such as IL-22, IFN-γ, IL-6, and IL-1α in amounts similar or even higher than WT mice (Fig. 9 F). IL-17F, another IL-17 family member believed to be proinflammatory, was not detected either in the WT or in the IL-17A KO, suggesting that it was not involved in compensatory mechanisms induced by lack of IL-17A. Positive controls of supernatant samples “spiked” with recombinant IL-17F confirmed that there was no interference with detection of this cytokine under the assay conditions used (not depicted). IL-17E was not examined, as that isoform has already been shown in a previous study to have an inhibitory and not a proinflammatory function (24). Thus, mechanisms other than IL-17A, including but possibly not limited to the Th1 effector response, are sufficient to cause EAU pathology.
DISCUSSION
In this study we demonstrate that autoimmunity to retina can be either Th17 or Th1 driven. The IL-23–IL-17 pathway plays an important role in EAU, and intensity of IL-17 response systemically and locally correlates with disease severity in mice immunized with IRBP/CFA. However, the role of the Th17 effector is redundant with Th1, and each effector phenotype by itself is sufficient to induce pathology in the absence of the reciprocal hallmark cytokine. In contrast, the role of IL-23 in disease pathogenesis is essential and nonredundant, raising the possibility that the requirement for IL-23 in EAU transcends its role in promoting the Th17 effector response. The conditions that drive to a Th17-dominated, or a Th1-dominated, pathogenic effector response appear to include the context in which the first encounter with auto-Ag occurs.
Until recently, the role of IL-12 in promoting the generation of IFN-γ–producing Th1 effector cells was considered the main pathogenic pathway in autoimmune diseases such as EAU and EAE (8, 25, 26). However, the import of the IL-23–IL-17 pathway had put in question the need for “classical” Th1 effector cells in development of these autoimmune diseases (16, 27). IL-23 was found to have a significant role in the induction of EAE (15) and CIA (16). In contrast, IL-12 was found not to be required for EAE and CIA (28). The role of IL-23 in autoimmunity was then proposed to be due to its ability to promote differentiation of T cells to a distinct effector subtype producing mainly IL-17 but not IFN-γ (15, 17, 29). Although more recent data uncovered that the initial signal for commitment to the Th17 lineage is mediated by TGF-β and IL-6, IL-23 is nevertheless required in vivo for expansion and maintenance of the Th17 phenotype (30, 31), such that IL-23–deficient mice—whether genetically or by antibody neutralization—mount a much reduced Th17 response (17 and present data).
Our data are in partial agreement with findings in other autoimmune diseases by showing a requirement for IL-23 rather than IL-12 for disease induction by active immunization with auto-Ag in CFA, and by supporting an important role for the Th17 effector response in pathogenesis of the resultant disease. Of note is the enhanced systemic and local Th17 response in IL-12– and IFN–γ–deficient mice, which typically exhibit more severe disease than their WT counterparts. This suggests that the Th17 effector may become more prominent in pathogenesis when the IL–12–IFN-γ pathway is reduced or eliminated. IFN-γ has been reported to inhibit commitment to the Th17 phenotype in vitro (32). Our present data, as well as the finding that IRBP-immunized mice treated with a neutralizing antibody to IFN–γ develop an elevated IL–17 response (unpublished data), support the notion that this might be the case also in vivo. Studies that preceded the “Th17 era” had already established that under some circumstances the IL-12–IFN-γ pathway (paradoxically) has an inhibitory, rather than a pathogenic, role in autoimmunity and implicated IFN-γ–induced mechanisms such as induction of nitric oxide and apoptosis of T effector cells in this phenomenon (12, 33). IFN-γ–driven activation of IDO in DCs, which causes apoptosis of effector T cells due to tryptophan deprivation (34), and the Tim-3–galectin-9 pathway, which terminates effector T cell responses (35), have also not been excluded. The present results suggest that IFN-γ–driven inhibition of Th17 effector generation might be an additional mechanism that contributes to the increased EAU scores observed in these situations.
However, in apparent contrast to studies in EAE and arthritis, our data in EAU suggest that, under some conditions, Ag-specific Th17 cells may be dispensable. First, EAU can develop in the complete absence of IL–17A, as indicated by substantial ocular pathology in IL-17A KO mice. Susceptibility of IL-17 KO mice contrasts with the protective effect of IL-17 neutralization in WT mice, a phenomenon not uncommon in gene-deficient mice and known as phenotypic compensation (36). It is apparent that congenital lack of IL-17 allows emergence of compensatory mechanisms involving Th1 and other proinflammatory cytokines including IL-22, which is typically produced in large amounts by Th17 cells (19, 20). Although the nature of these compensatory mechanisms requires further investigation, the enhanced Th1 response in LN and in the eyes of IL-17 KO mice raises the possibility that IL-17 may have an antagonistic effect on the development of Th1 effectors, just as IFN-γ inhibits development of Th17 effectors. It should also be noted that although in EAE CIA genetic deficiency of IL-17A was reported to have a strong dampening effect (23, 37, 38), it did not completely abrogate disease, pointing to pathogenic effects of cytokines other than IL-17. Second, severe EAU can be induced by infusion of stably polarized Th1 effector cells that appear to cause pathology without an apparent involvement of host IL-17, acting at least in part through TNF-α and IFN-γ. Finally, uveitogenic DCs are unable to induce EAU in GKO mice despite the presence of a strong Th17 response, suggesting that an IFN-γ–producing effector is needed to induce pathology.
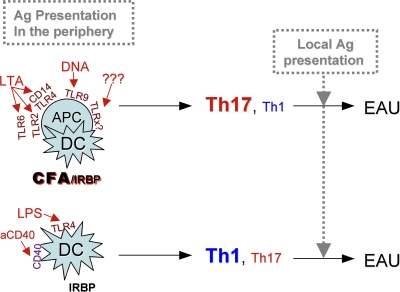
This raises the question of whether the Th17 effector response might be dominant particularly in situations where induction of disease occurs in the context of strong Toll-like receptor (TLR) signals, whereas in other conditions a Th1 response may predominate. It is notable that models in which IL-17 was reported to be highly dominant, EAE and CIA (15, 16) are both induced by immunization with Ag in CFA, as is the classical model of EAU. A necessary role for Th17 in experimental colitis, which is induced in the absence of CFA but occurs in the context of strong innate signaling delivered by gut bacteria, is in line with this notion (39, 40). Thus, factors that may in large measure drive the dominant effector phenotype could include quality/quantity of TLR and other innate signals, as well as the identity and diversity of cells functioning as APCs at the site of initial Ag exposure. A synthesis of our data are presented in schematic form in Fig. 10.
Figure 10.
Schematic representation of the patterns of effector T cell dominance in the different EAU models. Conditions of initial exposure to Ag that may determine effector dominance are the quality/quantity of TLR signals and the type/variety of cells participating as APCs.
The antibody-neutralization data, showing that IL-23 is required early whereas IL-17 acts later in the disease process, are compatible with the established paradigm that IL-23 acts upstream of IL-17 to promote the Th17 effector response. However, the evidence that the Th17 effector response may in some situations be dispensable suggests that the early requirement for IL-23 may reflect function(s) other than supporting the IL-17 effector response. We hypothesize that an early nonredundant requirement for IL-23 could involve effects on APCs, possibly in their maturation and/or early stages of effector T cell priming, preceding the commitment to Th17 or Th1 phenotype. This notion is supported by recent findings showing dependence on IL-23 of a completely non–T cell–driven inflammation, inflammatory bowel disease induced in RAG−/− mice with agonistic anti-CD40 mAbs (41). Additionally, a not very well studied function of IL-23 is its role in promoting IFN-γ–producing Th1 cells. Our current data suggest that IL-23 also affects the Th1 response, as its genetic lack or neutralization by antibodies reduced not only the IL-17, but also the IFN-γ response to Ag, whereas IL-17 gene–deleted mice had high levels of IFN-γ. This is in line with data published by others. IL-23 enhanced the secretion of IFN-γ by human memory T cells (1, 42). Similarly, in Helicobacter hepaticus–induced T cell–dependent colitis, IL-23 was needed to drive both IFN-γ and IL-17 responses that synergized for maximal intestinal inflammation (43). Finally, administration of an IL-23 plasmid to IL-12p40–deficient mice up-regulated production of IFN-γ in response to Mycobacterium tuberculosis in mice (44). Therefore, IL-23 could be required to promote Th1 as well as Th17 effector responses. Studies are underway to dissect the disease checkpoints controlled by IL-23 in this model.
Both IL-17 and IL-23 emerge as potential new targets for therapeutic intervention in the uveitic diseases where this pathway might play a role in pathogenesis. The “holy grail” of immunotherapy is to inhibit an ongoing disease process by affecting effector mechanisms. Neutralization of IL-17 seems to accomplish just that, as it was able to protect even when started 7 d after disease induction, a point at which pathogenic effectors have already been primed. Although IL-23 could only prevent but not reverse disease, neutralization of IL-23 still holds clinical promise. Chronic autoimmunity involves ongoing priming and recruitment of new T cells into the effector pool. This continuous priming and recruitment cycle is also believed to underlie the phenomenon of epitope and Ag spreading, which drives the relapsing nature of diseases such as EAE (45). In keeping with this, neutralization of IL-23 prevents relapses in the EAE model (46), supporting the rationale of IL-23 targeting in chronic relapsing-remitting autoimmune diseases. Although EAU in the B10.RIII mouse is monophasic in nature, the phenomenon of epitope spreading has been reported in chronic autoimmune uveitis in horses, equine recurrent uveitis (47). Importantly, human chronic uveitides such as ocular sarcoidosis, Vogt-Koyanagi-Harada disease, and sympathetic ophthalmia can present with multiple recurrent attacks. If indeed continuous recruitment of new effectors underlies these uveitic diseases, anti–IL-23, possibly as a combination therapy with anti–IL-17 to target already primed effectors, might be a viable therapeutic approach for such types of uveitis.
MATERIALS AND METHODS
Animals.
IL-23 KO (p19 KO) was described previously (15). IL-17 KO mice were produced as described previously (23). IL-12p35 KO (P35 KO), IL-12p40 KO (p40 KO), IFN-γ KO (GKO; all on C57BL/6 background), C57BL/6, and B10RIII mice were purchased from The Jackson Laboratory. Animals were kept in a specific pathogen-free facility and given water and standard laboratory chow ad libitum. Animal care and use were in compliance with institutional guidelines and with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmical and Vision Research. The animal study protocol was approved by the Animal Care and Use Committee of the NIH.
Reagents, Ags, and antibodies.
CFA was purchased from Sigma-Aldrich. M. tuberculosis strain H37RA was purchased from Thomas Scientific. Purified Bordetella pertussis toxin was purchased from Sigma-Aldrich. IRBP was isolated from bovine retinas, as described previously (48). Human IRBP-derived peptide 161–180 (SGIPYIISYLHPGNTILHVD) was synthesized by Fmoc chemistry (model 432A peptide synthesizer; Applied Biosystems). Neutralizing antibodies to mouse IL-23 (clone MB379.490.130) (17) and to mouse IL-17A (clone 1D10) (46) were produced at DNAX. The C17.8 (anti–IL-12p40, rat IgG2a) hybridoma was provided by G. Trinchieri (Wistar Institute, Philadelphia, PA), and neutralizing mAb for IL-12p40, IFN-γ, and TNF-α was produced by Harlan Bioproducts for Science. FITC-labeled anti–mouse CD4 (clone L3T4), PE-labeled anti–mouse IL-17 (clone TC11-18H10), APC-labeled anti–IFN-γ (clone XMG1.2), and cytokine secretion blocker (GolgiStop [brefeldin A]) were purchased from Becton Dickinson. PMA and ionomycin were purchased from LC Laboratories.
EAU induction and scoring.
Induction of EAU by active immunization was described previously (49). In brief, immunization included 150 μg IRBP for C57BL/6 mice and 7 μg IRBP peptide 161–180 for B10RIII mice. For C57BL/6 mice, B. pertussis toxin (0.5 μg/mouse) was injected i.p. Alternatively, EAU was induced by adoptive transfer of 1–2 million freshly stimulated cells from a uveitogenic T cell line. Cells stimulated with Ag for 48 h were suspended in medium plus 2% mouse serum and were injected i.p. into naive syngeneic recipients.
A third method of EAU induction consisted of two subcutaneous injections 4 d apart of 1–2 million IRBP peptide-pulsed, in vitro–matured (LPS plus anti-CD40) splenic DCs and injection of pertussis on day 2. The method has been described previously (22). Clinical EAU was evaluated by fundus examination on a scale of 0–4 based on the extent of inflammation, as described in detail elsewhere (5, 50). Eyes harvested 17–21 d after immunization, or 14 d after adoptive transfer, were processed for histopathology and stained with standard hematoxylin and eosin, and the severity of EAU was evaluated in a masked fashion on a scale of 0–4 using previously published criteria based on the number, type, and size of lesions (5, 50).
Determination of immunological responses.
DTH to IRBP was evaluated by the ear swelling (9). For Ag-specific lymphocyte proliferation and cytokine production in primary cultures, spleens and draining LNs were pooled within the group (five mice per group) and cultured as described previously (51) for evaluation of cytokine responses by ELISA and proliferation by [3H]thymidine uptake. Cytokines in 48-h supernatants of Ag-stimulated cells were quantitated using the Pierce Multiplex SearchLight Arrays technology (Thermo Fisher Scientific) (52), except for IL-22 and IL-17F. IL-22 was measured using an ELISA kit from Antigenix America Inc., per the manufacturer's instructions. IL-17F was determined by an electrochemiluminescence sandwich immunoassay performed in Meso-Scale Discovery (MSD) in 96-well streptavidin-coated plates. Wells were blocked overnight at 4°C with 5% BSA in PBS, washed with a Tween20-containing buffer, and reacted with biotinylated monoclonal anti–mouse IL-17F (clone 8G6) as capture antibody. Test samples were added in duplicate (2 h at room temperature with shaking), washed, and reacted with anti–mouse IL-17F pAb (AF2057; R&D Systems), modified by MSD Sulfo-TAG NHS Ester as detection antibody. Recombinant mouse IL-17F was used to generate the standard curve. The electrochemiluminescence signal was measured in the MSD Discovery SECTOR Imager 6000. Cytokines were assayed in pooled supernatants, yielding a grouped average of five, but no error bars. Due to inter-experiment variation in absolute values, repeat experiments could not be combined. Patterns of response were highly reproducible. Figures depict representative experiments.
In vivo IL-23, IL-12p40, IL-17, IFN-γ, and TNF-α neutralization.
B10RIII mice were immunized with IRBP or IRBP uveitogenic peptide (161–180) as indicated. Mice were injected i.p. with 0.5 mg per dose of anti-p19, anti-p40, anti–IL-17, or isotype controls. Treatment was given every other day starting on day −1 through day 15 after immunization, covering both the priming and effector phase (prevention protocol) or starting day 7 through day 15, covering the effector phase only (treatment). In EAU induced by adoptive transfer of the Th1 cell line, treatment with neutralizing antibodies was given from day −1 through day 10 after transfer. Eyes and lymphoid organs were harvested on day 17 after active immunization or on days 10–14 after adoptive transfer (6 or more days after EAU onset).
Isolation of eye-infiltrating cells.
Uveitic eyes were collected from five mice per group. External tissues were trimmed, and the eyes were carefully dissected along the limbus for lens removal. The remaining tissue was transferred into 1 ml RPMI, minced with scissors, dispersed by vigorous pipetting, and centrifuged. The clarified supernatant was collected for cytokine analysis, and the cell pellet was resuspended in 3 ml RPMI, 10% FCS, and 1 mg/ml collagenase D and incubated for 1 h in 37°C. Samples were then dispersed by pipetting several times, washed, filtered, and suspended in 3 ml RPMI plus 10% FCS for counting. Cells were then delivered into a 24-well plate (4 × 106 cells/well), incubated with or without PMA, ionomycin, and brefeldin A and stained for intracellular cytokine analysis and immunophenotyping by FACS, as described below. Non-inflamed eyes from unimmunized donors did not yield sufficient leukocytes for analysis.
Intracellular cytokine staining and FACS analysis.
In all cases where cells were incubated for FACS staining, PMA plus ionomycin (50 and 500 ng/ml, respectively) were added at the last 4 h of incubation, and 10 μg/ml brefeldin A was added at the last 2 h.
For detection of intracellular expression of IL-17 and IFN-γ by FACS analysis, Th1 line cells were stimulated with Ag for 24 h under standard conditions, or for 5 d under IL-23–polarizing conditions (in the presence of 10 ng/ml recombinant mouse IL-23, or IL-23 plus 10 μg anti–mouse IFN-γ). Th17 line cells were stimulated with Ag under Th17 conditions (as indicated) for 48 h. T cells were then separated on Ficoll, washed, and stained for extracellular CD4, followed by intracellular staining for IFN-γ and IL–17.
Freshly isolated eye-infiltrating cells were immediately incubated ex vivo with or without PMA plus ionomycin for 4 h. Thereafter, cells were stained for extracellular markers, followed by staining for intracellular IFN-γ versus IL-17.
For ex vivo detection of intracellular IL-17 versus IFN-γ after parking allotype-marked T cells, Th1 line cells were adoptively transferred i.v. into naive Thy1.1 × Thy1.2 heterozygous recipients. 4 d later, splenocytes were stimulated with IRBP peptide 161–180 for 24 h with the addition of PMA plus ionomycin, after which cells were stained and analyzed for intracellular IL-17 and IFN-γ as above. The line cells (WT origin) were identified by gating on Thy1.2 single-positive cells.
Culture and characterization of T cell lines.
The uveitogenic Th1 cell line specific to a peptide of human IRBP (p16–180) has been described (53). In brief, the line was derived from draining LNs of B10.RIII mice immunized with human IRBP peptide 161–180 and polarized in vitro toward the Th1 phenotype by culture in the presence of Ag, IL-12, and anti–IL-4. The line cells are routinely maintained by alternating cycles of expansion in IL-2 and restimulation with 1 μg/ml p161–180 every 2 to 3 wk in the presence of syngeneic splenocytes, irradiated with 3,000 rads, as APCs.
To generate Th17 cell lines, IFN-γ KO mice were immunized with 20 μg IRBP peptide 161–180 in CFA. At day 7 after immunization, splenocytes and LN cells were combined and CD4 cells were enriched with AutoMacs by negative selection (Miltenyi Biotec) and stimulated for 48 h with p161–180 on irradiated splenocytes under Th17-inducing conditions: 20 ng/ml rIL-23 (R&D Systems), 3 ng/ml TGF-β (PeproTech), 20 ng/ml IL-6 (PeproTech), and 1 μg/ml anti–IL-4 (clone 11B11; BD Biosciences). Cells were then rested for 1 wk and restimulated as above. At that point, cytokine production was evaluated by ELISA or by intracellular cytokine staining, as described above, or the collected cells were adoptively transferred into naive recipients. Eyes were collected histopathology for 10 d after transfer.
Statistical analysis.
Experiments were repeated at least twice, and usually three or more times. Experimental groups are typically composed of five mice. Figures show data compiled from a representative experiment. Statistical analysis of EAU scores was by Snedecor and Cochran's test for linear trend in proportions (nonparametric, frequency-based) (54). Each mouse (average of both eyes) was treated as one statistical event. DTH and proliferation were examined by two-tailed t test. Cytokine responses were assayed on pooled samples (usually five mice per group). Therefore, although the data represent the average of the group, error bars could not be generated.
Acknowledgments
The authors wish to thank the staff of the NEI Histology Core Facility for expert preparation of histological specimens.
This work has been supported by NIH, NEI Intramural funding. SP Biopharma (formerly DNAX Research) is supported by the Schering-Plough Corporation.
R.R. Caspi, D. Cua, Z. Chen, E.P. Bowman, P.B. Silver, and D. Luger are co-inventors in a patent application “Use of IL-23 and IL-17 antagonists to treat autoimmune ocular inflammatory disease.” The authors have no other conflicting financial interests.
Abbreviations used: Ag, antigen; CIA, collagen-induced arthritis; DTH, delayed-type hypersensitivity; EAE, experimental autoimmune encephalomyelitis; EAU, experimental autoimmune uveitis; IRBP, interphotoreceptor retinoid-binding protein; TLR, Toll-like receptor.
References
- 1.Oppmann, B., R. Lesley, B. Blom, J.C. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, et al. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 13:715–725. [DOI] [PubMed] [Google Scholar]
- 2.Gregerson, D.S., W.F. Obritsch, S.P. Fling, and J.D. Cameron. 1986. S-antigen-specific rat T cell lines recognize peptide fragments of S-antigen and mediate experimental autoimmune uveoretinitis and pinealitis. J. Immunol. 136:2875–2882. [PubMed] [Google Scholar]
- 3.Caspi, R.R., F.G. Roberge, C.G. McAllister, M. el-Saied, T. Kuwabara, I. Gery, E. Hanna, and R.B. Nussenblatt. 1986. T cell lines mediating experimental autoimmune uveoretinitis (EAU) in the rat. J. Immunol. 136:928–933. [PubMed] [Google Scholar]
- 4.Mochizuki, M., T. Kuwabara, C. McAllister, R.B. Nussenblatt, and I. Gery. 1985. Adoptive transfer of experimental autoimmune uveoretinitis in rats. Immunopathogenic mechanisms and histologic features. Invest. Ophthalmol. Vis. Sci. 26:1–9. [PubMed] [Google Scholar]
- 5.Agarwal, R.K., and R.R. Caspi. 2004. Rodent models of experimental autoimmune uveitis. Methods Mol. Med. 102:395–419. [DOI] [PubMed] [Google Scholar]
- 6.Sanui, H., T.M. Redmond, S. Kotake, B. Wiggert, L.H. Hu, H. Margalit, J.A. Berzofsky, G.J. Chader, and I. Gery. 1989. Identification of an immunodominant and highly immunopathogenic determinant in the retinal interphotoreceptor retinoid-binding protein (IRBP). J. Exp. Med. 169:1947–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rizzo, L.V., P. Silver, B. Wiggert, F. Hakim, R.T. Gazzinelli, C.C. Chan, and R.R. Caspi. 1996. Establishment and characterization of a murine CD4+ T cell line and clone that induce experimental autoimmune uveoretinitis in B10.A mice. J. Immunol. 156:1654–1660. [PubMed] [Google Scholar]
- 8.Caspi, R.R. 2002. Th1 and Th2 responses in pathogenesis and regulation of experimental autoimmune uveoretinitis. Int. Rev. Immunol. 21:197–208. [DOI] [PubMed] [Google Scholar]
- 9.Tarrant, T.K., P.B. Silver, C.C. Chan, B. Wiggert, and R.R. Caspi. 1998. Endogenous IL-12 is required for induction and expression of experimental autoimmune uveitis. J. Immunol. 161:122–127. [PubMed] [Google Scholar]
- 10.Caspi, R.R., C.C. Chan, B.G. Grubbs, P.B. Silver, B. Wiggert, C.F. Parsa, S. Bahmanyar, A. Billiau, and H. Heremans. 1994. Endogenous systemic IFN-gamma has a protective role against ocular autoimmunity in mice. J. Immunol. 152:890–899. [PubMed] [Google Scholar]
- 11.Jones, L.S., L.V. Rizzo, R.K. Agarwal, T.K. Tarrant, C.C. Chan, B. Wiggert, and R.R. Caspi. 1997. IFN-gamma-deficient mice develop experimental autoimmune uveitis in the context of a deviant effector response. J. Immunol. 158:5997–6005. [PubMed] [Google Scholar]
- 12.Tarrant, T.K., P.B. Silver, J.L. Wahlsten, L.V. Rizzo, C.C. Chan, B. Wiggert, and R.R. Caspi. 1999. Interleukin 12 protects from a T helper type 1–mediated autoimmune disease, experimental autoimmune uveitis, through a mechanism involving interferon γ, nitric oxide, and apoptosis. J. Exp. Med. 189:219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becher, B., B.G. Durell, and R.J. Noelle. 2002. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J. Clin. Invest. 110:493–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gran, B., G.X. Zhang, S. Yu, J. Li, X.H. Chen, E.S. Ventura, M. Kamoun, and A. Rostami. 2002. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J. Immunol. 169:7104–7110. [DOI] [PubMed] [Google Scholar]
- 15.Cua, D.J., J. Sherlock, Y. Chen, C.A. Murphy, B. Joyce, B. Seymour, L. Lucian, W. To, S. Kwan, T. Churakova, et al. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 421:744–748. [DOI] [PubMed] [Google Scholar]
- 16.Murphy, C.A., C.L. Langrish, Y. Chen, W. Blumenschein, T. McClanahan, R.A. Kastelein, J.D. Sedgwick, and D.J. Cua. 2003. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 198:1951–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langrish, C.L., Y. Chen, W.M. Blumenschein, J. Mattson, B. Basham, J.D. Sedgwick, T. McClanahan, R.A. Kastelein, and D.J. Cua. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spriggs, M.K. 1997. Interleukin-17 and its receptor. J. Clin. Immunol. 17:366–369. [DOI] [PubMed] [Google Scholar]
- 19.Liang, S.C., X.Y. Tan, D.P. Luxenberg, R. Karim, K. Dunussi-Joannopoulos, M. Collins, and L.A. Fouser. 2006. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203:2271–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung, Y., X. Yang, S.H. Chang, L. Ma, Q. Tian, and C. Dong. 2006. Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res. 16:902–907. [DOI] [PubMed] [Google Scholar]
- 21.Chang, H., H. Hanawa, H. Liu, T. Yoshida, M. Hayashi, R. Watanabe, S. Abe, K. Toba, K. Yoshida, R. Elnaggar, et al. 2006. Hydrodynamic-based delivery of an interleukin-22-Ig fusion gene ameliorates experimental autoimmune myocarditis in rats. J. Immunol. 177:3635–3643. [DOI] [PubMed] [Google Scholar]
- 22.Tang, J., W. Zhu, P.B. Silver, S.B. Su, C.C. Chan, and R.R. Caspi. 2007. Autoimmune uveitis elicited with antigen-pulsed dendritic cells has a distinct clinical signature and is driven by unique effector mechanisms: initial encounter with autoantigen defines disease phenotype. J. Immunol. 178:5578–5587. [DOI] [PubMed] [Google Scholar]
- 23.Nakae, S., Y. Komiyama, A. Nambu, K. Sudo, M. Iwase, I. Homma, K. Sekikawa, M. Asano, and Y. Iwakura. 2002. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 17:375–387. [DOI] [PubMed] [Google Scholar]
- 24.Kleinschek, M.A., A.M. Owyang, B. Joyce-Shaikh, C.L. Langrish, Y. Chen, D.M. Gorman, W.M. Blumenschein, T. McClanahan, F. Brombacher, S.D. Hurst, et al. 2007. IL-25 regulates Th17 function in autoimmune inflammation. J. Exp. Med. 204:161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caspi, R.R. 1998. IL-12 in autoimmunity. Clin. Immunol. Immunopathol. 88:4–13. [DOI] [PubMed] [Google Scholar]
- 26.Xu, H., L.V. Rizzo, P.B. Silver, and R.R. Caspi. 1997. Uveitogenicity is associated with a Th1-like lymphokine profile: cytokine-dependent modulation of early and committed effector T cells in experimental autoimmune uveitis. Cell. Immunol. 178:69–78. [DOI] [PubMed] [Google Scholar]
- 27.McKenzie, B.S., R.A. Kastelein, and D.J. Cua. 2006. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 27:17–23. [DOI] [PubMed] [Google Scholar]
- 28.Zhang, G.X., B. Gran, S. Yu, J. Li, I. Siglienti, X. Chen, M. Kamoun, and A. Rostami. 2003. Induction of experimental autoimmune encephalomyelitis in IL-12 receptor-beta 2-deficient mice: IL-12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system. J. Immunol. 170:2153–2160. [DOI] [PubMed] [Google Scholar]
- 29.Aggarwal, S., N. Ghilardi, M.H. Xie, F.J. de Sauvage, and A.L. Gurney. 2003. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278:1910–1914. [DOI] [PubMed] [Google Scholar]
- 30.Veldhoen, M., R.J. Hocking, C.J. Atkins, R.M. Locksley, and B. Stockinger. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 24:179–189. [DOI] [PubMed] [Google Scholar]
- 31.Bettelli, E., Y. Carrier, W. Gao, T. Korn, T.B. Strom, M. Oukka, H.L. Weiner, and V.K. Kuchroo. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 441:235–238. [DOI] [PubMed] [Google Scholar]
- 32.Park, H., Z. Li, X.O. Yang, S.H. Chang, R. Nurieva, Y.H. Wang, Y. Wang, L. Hood, Z. Zhu, Q. Tian, and C. Dong. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6:1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gran, B., N. Chu, G.X. Zhang, S. Yu, Y. Li, X.H. Chen, M. Kamoun, and A. Rostami. 2004. Early administration of IL-12 suppresses EAE through induction of interferon-gamma. J. Neuroimmunol. 156:123–131. [DOI] [PubMed] [Google Scholar]
- 34.Mellor, A. 2005. Indoleamine 2,3 dioxygenase and regulation of T cell immunity. Biochem. Biophys. Res. Commun. 338:20–24. [DOI] [PubMed] [Google Scholar]
- 35.Zhu, C., A.C. Anderson, A. Schubart, H. Xiong, J. Imitola, S.J. Khoury, X.X. Zheng, T.B. Strom, and V.K. Kuchroo. 2005. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 6:1245–1252. [DOI] [PubMed] [Google Scholar]
- 36.Barbaric, I., G. Miller, and T.N. Dear. 2007. Appearances can be deceiving: phenotypes of knockout mice. Brief. Funct. Genomics Proteomics. 6:91–103. [DOI] [PubMed] [Google Scholar]
- 37.Nakae, S., S. Saijo, R. Horai, K. Sudo, S. Mori, and Y. Iwakura. 2003. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc. Natl. Acad. Sci. USA. 100:5986–5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Komiyama, Y., S. Nakae, T. Matsuki, A. Nambu, H. Ishigame, S. Kakuta, K. Sudo, and Y. Iwakura. 2006. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 177:566–573. [DOI] [PubMed] [Google Scholar]
- 39.Yen, D., J. Cheung, H. Scheerens, F. Poulet, T. McClanahan, B. McKenzie, M.A. Kleinschek, A. Owyang, J. Mattson, W. Blumenschein, et al. 2006. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Invest. 116:1310–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang, Z., M. Zheng, J. Bindas, P. Schwarzenberger, and J.K. Kolls. 2006. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm. Bowel Dis. 12:382–388. [DOI] [PubMed] [Google Scholar]
- 41.Uhlig, H.H., B.S. McKenzie, S. Hue, C. Thompson, B. Joyce-Shaikh, R. Stepankova, N. Robinson, S. Buonocore, H. Tlaskalova-Hogenova, D.J. Cua, and F. Powrie. 2006. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 25:309–318. [DOI] [PubMed] [Google Scholar]
- 42.Piskin, G., R.M. Sylva-Steenland, J.D. Bos, and M.B. Teunissen. 2006. In vitro and in situ expression of IL-23 by keratinocytes in healthy skin and psoriasis lesions: enhanced expression in psoriatic skin. J. Immunol. 176:1908–1915. [DOI] [PubMed] [Google Scholar]
- 43.Kullberg, M.C., D. Jankovic, C.G. Feng, S. Hue, P.L. Gorelick, B.S. McKenzie, D.J. Cua, F. Powrie, A.W. Cheever, K.J. Maloy, and A. Sher. 2006. IL-23 plays a key role in Helicobacter hepaticus–induced T cell–dependent colitis. J. Exp. Med. 203:2485–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wozniak, T.M., A.A. Ryan, J.A. Triccas, and W.J. Britton. 2006. Plasmid interleukin-23 (IL-23), but not plasmid IL-27, enhances the protective efficacy of a DNA vaccine against Mycobacterium tuberculosis infection. Infect. Immun. 74:557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vanderlugt, C.L., K.L. Neville, K.M. Nikcevich, T.N. Eagar, J.A. Bluestone, and S.D. Miller. 2000. Pathologic role and temporal appearance of newly emerging autoepitopes in relapsing experimental autoimmune encephalomyelitis. J. Immunol. 164:670–678. [DOI] [PubMed] [Google Scholar]
- 46.Chen, Y., C.L. Langrish, B. McKenzie, B. Joyce-Shaikh, J.S. Stumhofer, T. McClanahan, W. Blumenschein, T. Churakovsa, J. Low, L. Presta, et al. 2006. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J. Clin. Invest. 116:1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deeg, C.A., B. Amann, A.J. Raith, and B. Kaspers. 2006. Inter- and intramolecular epitope spreading in equine recurrent uveitis. Invest. Ophthalmol. Vis. Sci. 47:652–656. [DOI] [PubMed] [Google Scholar]
- 48.Pepperberg, D.R., T.L. Okajima, H. Ripps, G.J. Chader, and B. Wiggert. 1991. Functional properties of interphotoreceptor retinoid-binding protein. Photochem. Photobiol. 54:1057–1060. [DOI] [PubMed] [Google Scholar]
- 49.Grajewski, R.S., P.B. Silver, R.K. Agarwal, S.B. Su, C.C. Chan, G.I. Liou, and R.R. Caspi. 2006. Endogenous IRBP can be dispensable for generation of natural CD4+CD25+ regulatory T cells that protect from IRBP-induced retinal autoimmunity. J. Exp. Med. 203:851–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chan, C.C., R.R. Caspi, M. Ni, W.C. Leake, B. Wiggert, G.J. Chader, and R.B. Nussenblatt. 1990. Pathology of experimental autoimmune uveoretinitis in mice. J. Autoimmun. 3:247–255. [DOI] [PubMed] [Google Scholar]
- 51.Avichezer, D., P.B. Silver, C.C. Chan, B. Wiggert, and R.R. Caspi. 2000. Identification of a new epitope of human IRBP that induces autoimmune uveoretinitis in mice of the H-2b haplotype. Invest. Ophthalmol. Vis. Sci. 41:127–131. [PubMed] [Google Scholar]
- 52.Moody, M.D., S.W. Van Arsdell, K.P. Murphy, S.F. Orencole, and C. Burns. 2001. Array-based ELISAs for high-throughput analysis of human cytokines. Biotechniques. 31:186–190. [DOI] [PubMed] [Google Scholar]
- 53.Silver, P.B., L.V. Rizzo, C.C. Chan, L.A. Donoso, B. Wiggert, and R.R. Caspi. 1995. Identification of a major pathogenic epitope in the human IRBP molecule recognized by mice of the H-2r haplotype. Invest. Ophthalmol. Vis. Sci. 36:946–954. [PubMed] [Google Scholar]
- 54.Snedecor, G.W., and W.G. Cochran. 1967. Statistical Methods. Iowa State University Press, Ames, IA. 248 pp.