Abstract
Regulatory T (T reg) cells are the major obstacle to cancer immunotherapy, and their depletion promptly induces conversion of peripheral precursors into T reg cells. We show that T reg cells can be functionally inactivated by OX40 triggering. In tumors, the vast majority of CD4+ T cells are Foxp3+ and OX40bright. However, intratumor injection of the agonist anti-OX40 monoclonal antibody (mAb) OX86, but not anti-CD25 mAb, induces tumor rejection in 80% of mice, an effect that is abrogated by CD8 depletion. Upon intratumor OX40 triggering, increased numbers of infiltrating dendritic cells (DCs) migrate to draining lymph nodes and generate a new wave of tumor-specific cytotoxic T lymphocytes, as detected by tetramer and CD44 staining of node CD8+ T lymphocytes. Tumor-bearing Rag1-knockout (KO) mice reconstituted with OX40-deficient T reg cells and wild-type (WT) effector T cells, or the reciprocal combination, showed that both T reg and effector T cells must be triggered via OX40 for the tumor to be rejected. Accordingly, WT but not OX40-KO mice receiving intratumor coinjection of OX86 and ovalbumin protein were able to revert tumor-induced tolerization of adoptively transferred OX40-competent OTII T lymphocytes. In conclusion, OX40-mediated inactivation of T reg cell function unleashes nearby DCs, allowing them to induce an adaptive immune response. In addition, the known OX40-dependent delivery of fitness signals to activated T cells is boosted by concurrent T reg cell inhibition. OX40 triggering thus has multiple effects that converge to mediate tumor rejection.
Regulatory T (T reg) cells have a major role in tumor-related immune suppression (1). T reg cells accumulate in both human and mouse tumors, as well as in secondary lymphoid organs being recruited (2) and expanded either by the proliferation of preexisting T reg cells (3) or the conversion of CD25− T cells (4, 5). T reg cells suppress a variety of immune cells, such as CD4 and CD8 T lymphocytes (6), NK cells (7), and B lymphocytes (8). Moreover, direct inhibition of DC function has been demonstrated in vitro (9, 10), whereas in vivo evidence is from intravital microscopy applied to mouse models of autoimmune diseases (11, 12). Tumor-associated T reg cells likely contribute to the suppressive milieu in which DCs cannot maturate and activate properly (13, 14).
OX40 is a co-stimulatory molecule belonging to the TNF receptor family. It is transiently expressed on CD4 lymphocytes upon antigen stimulation and is conducive to up-regulation of the antiapoptotic proteins Bcl-xL and Bcl-2 (15). OX40 regulates the differentiation (16), primary clonal expansion, and memory development (17) of CD4 lymphocytes and activation of CD8 cells (18). Triggering OX40 in vivo has been shown to prevent tolerance induction and to revert lymphocyte hyporesponsiveness in experimental tolerogenic systems (19, 20). OX40 is largely involved in the pathogenesis of autoimmune disease; the blockade of the OX40–OX40L axis ameliorates autoimmune symptoms (21).
Although inhibition of OX40 signaling is thought to be beneficial to treat autoimmunity, its triggering improves immune response to cancer. Indeed, systemic administration of OX40L–Ig fusion protein or of the agonist mAb OX86 induces the rejection of different types of mouse transplantable tumors (22, 23). OX40 triggering can synergize with other immunostimulatory molecules. CT26 colon carcinoma cells transduced to stably express both OX40L and GM-CSF, thus combining GM-CSF–mediated DC recruitment and an OX40L-induced T cell boost, are more immunogenic than single OX40L- or GM-CSF–transduced cells (24). The combination of agonist 4-1BB and OX40 antibodies with intratumor adenoviral delivery of IL-12 is more effective than all single agents in treating hepatic colon metastases (25). OX40 engagement on CD8 lymphocytes has been successfully associated with GM-CSF–producing cell vaccination in treating spontaneous mammary tumors arising in FVB MMTV-neu transgenic mice (26).
Emerging evidence attributes a key role in T reg cell biology to OX40. OX40 is constitutively expressed by mouse T reg cells both in the naive state (27, 28) and in the presence of tumors (4). T reg cells from OX40-deficient mice are less numerous at young ages and less suppressive ex vivo than their OX40-sufficient counterparts (28). Triggering OX40 reverts the suppression exerted by T reg cells over CD4 cells in vitro (27, 28), mainly by directly impairing T reg cell function rather than by enabling responder cells to resist T reg cell suppression (27). Moreover, T reg cells preincubated with the agonist mAb OX86 lose the ability to suppress effector T cells in an in vivo mouse model of graft-versus-host disease (27).
Another member of the TNF receptor family, the glucocorticoid-induced TNF receptor (GITR) (29), shares the capability to revert T reg cell suppression (27, 30) and to co-stimulate effector T cell responses (31) with OX40. GITR stimulation eradicates incipient tumors (32, 33) and enhances immune response to antitumor vaccination (34) by boosting CD8 T cells, apparently in the absence of any effect on T reg cell function, while expanding their number (33). Mild autoimmunity has been associated with GITR triggering (34), which can be avoided by confining the treatment to the tumor mass (32).
Because T reg cells constitute the predominant CD4 subpopulation in tumor-bearing hosts, T reg cell manipulation is a necessary step toward immunotherapy. Among possible strategies, CD25 depletion should be excluded as not discriminating between T reg cells and activated effector T cells. On the other hand, CD4 intratumor depletion, although effective in treating growing tumors as it spares CD8-activated effector cells, shows limited application to late-stage lesions (35). Moreover, both approaches produce a local T reg cell depletion that rapidly drives recruitment of both preexisting and newly converted T reg cells (4). Our study shows that the singular administration of agonist anti-OX40 mAb protects naive mice from subsequent tumor challenge and eradicates already established tumors when injected within the lesions. Tumor-infiltrating T reg cell inhibition generate a favorable context that allows DCs to traffic toward the draining lymph node and to prime a new wave of tumor-specific CD8 lymphocytes responsible for tumor rejection. In addition, agonistic mAb boosts OX40 on activated T cells, which also benefit from concurrent T reg cell inactivation. Therefore, OX40 triggering, by suppressing without renewing the T reg cell pool, emerges as a promising component of future cancer immunotherapy.
RESULTS
OX40-triggering mAb protects from tumor challenge
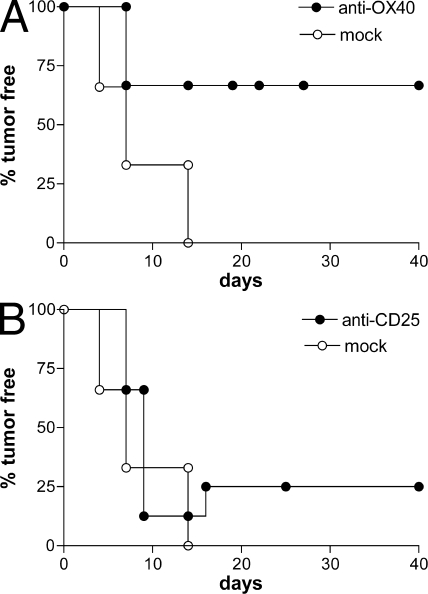
Several studies have demonstrated that T reg cell depletion, by means of anti-CD25 mAb, before tumor injection leads to the rejection of immunogenic tumors (36, 37). We tested whether the functional inhibition of naturally arising T reg cells by OX40 triggering is as effective as T reg cell depletion. Naive BALB/c mice were injected i.p. with 1 mg anti-OX40 (OX86), anti-CD25 (PC61) mAb, or isotype-matched control rat IgG 7 d before the s.c. inoculation of the syngeneic colon carcinoma cell line CT26. All mock-treated animals died of tumor within 2 wk, whereas PC61-treated mice developed tumor that was rejected by 25% of them. In sharp contrast, 70% of OX86-treated mice were completely protected from tumor challenge (Fig. 1). We analyzed the number and distribution of T reg cells at the time of tumor injection. Anti-OX40 mAb did not change T reg cell numbers in the spleen or lymph nodes, whereas anti-CD25 remarkably reduced their numbers in every compartment analyzed (unpublished data). Ex vivo–restimulated splenocytes, recovered from mice rejecting tumors upon either treatment, showed in vitro cytolytic activity against tumor cells and syngeneic blasts pulsed with the MHC class I–restricted tumor-associated immunodominant gp70 peptide AH1 (unpublished data). Mice rejecting CT26 cells after either treatment were resistant to a subsequent challenge with the same cell line but not with tumors lacking the expression of gp70 protein (unpublished data). These results indicate that triggering OX40 in vivo before tumor challenge allows the development of a specific and long-lasting antitumor immune response superior to that induced by T reg cell depletion.
Figure 1.
OX40 triggering protects from tumor challenge. 5 × 104 CT26 colon carcinoma cells were injected s.c. into the right flank of BALB/c mice 7 d after the systemic administration of 1 mg anti-OX40 mAb OX86 (A) or anti-CD25 mAb PC61 (B). As mock treatment, rat IgG was injected. Tumor growth was monitored twice per week. The percentages of tumor-free mice are given at the indicated time points. Six mice were included in each experimental group. Results are from one representative out of three independent experiments.
OX40-mediated protection depends on T reg cell inhibition
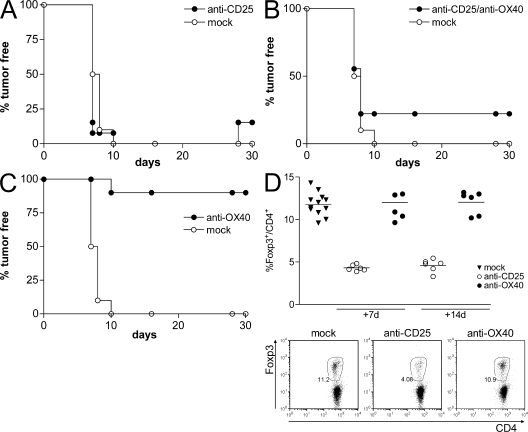
At the naive stage, the only cells constitutively expressing OX40 are the Foxp3+ CD25+ T reg cells (unpublished data) (27); therefore, they are the only possible target of anti-OX40 mAb. To test this hypothesis, we depleted naturally arising T reg cells by injecting anti-CD25 mAb 7 d before OX86 treatment that was 7 d before tumor challenge. As for the previously described experiments, anti-OX40 alone was more effective than anti-CD25, but the effect was lost in mice depleted of natural T reg cells before OX86 treatment (Fig. 2).Fig. 2 D shows the effective reduction of natural T reg cell numbers at the time of anti-OX40 administration that persisted until the time of tumor challenge. From these data, we concluded that OX40 triggering protects from tumor challenge mainly by neutralizing the function of naturally arising T reg cells.
Figure 2.
OX40-induced protection is through T reg cell inhibition. Effect of OX86 mAb in the absence of T reg cells. 1 mg anti-CD25 mAb or anti-OX40 mAb was administered i.p. to BALB/c naive mice before injection of 5 × 104 CT26 cells. As mock treatment, rat IgG was injected. OX86 mAb was given to mice pretreated or not with PC61 mAb, and rat IgG was given as mock treatment. All mice were challenged with 5 × 104 CT26 tumor cells at day 0. (A) PC61 was injected 14 d before tumor challenge. (B) 7 d later, half of the PC61-treated mice received OX86 or rat IgG injection. (C) A mice cohort received OX86 only 7 d before tumor challenge. Tumor growth was monitored twice per week. One representative out of two independent experiments is shown. (D) Effective T reg cell depletion was monitored by Foxp3 staining in lymph nodes 7 and 14 d after PC61 or OX86 injection. Each symbol corresponds to a single lymph node from each mouse analyzed among the experimental groups. The solid line represents the median value. Representative plots of CD4 versus Foxp3 expression (percentages) are shown for the day 7 observation.
However, this does not exclude that the persistence of antibodies in circulation makes possible their effect on primed T cells that gain OX40 and CD25 on the surface. Thus, persisting anti-OX40 mAb could co-stimulate effector T cells and further improve the antitumor response. Conversely, persistent anti-CD25 could result in effector T cell depletion, a process that may counterbalance the favorable consequences of T reg cell depletion. Therefore, these experiments strongly indicate that the superior antitumor effect of OX86 over PC61 is expected to become more evident in a therapeutic setting in which concomitant immunity, although suppressed, should be present.
Tumor-infiltrating T reg cells express functional OX40
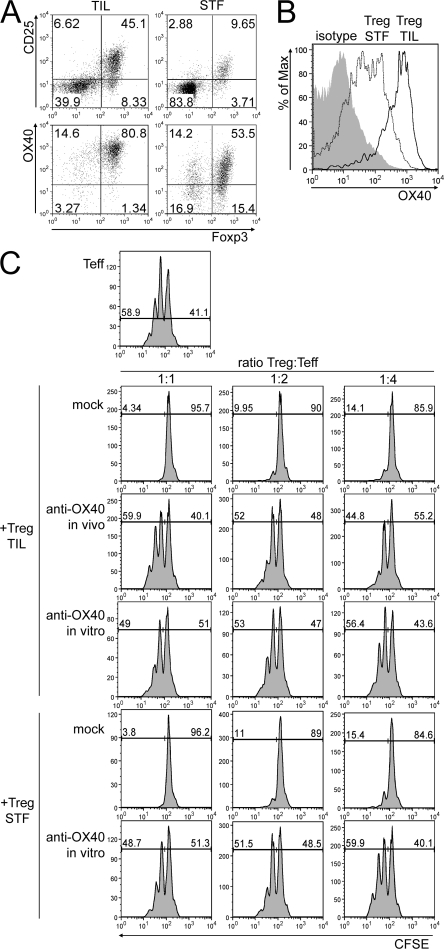
We tested the effects of OX40 triggering in the therapeutic setting and, because of the possible induction of generalized autoimmunity in the event of systemic T reg cell inhibition (32), we decided for intratumor injection of agonistic mAb. To characterize the context in which OX86 mAb should operate, we evaluated the expression of CD25, Foxp3, and OX40 on CD4+ T cells purified from CT26 tumors of ∼3–4 mm in size, in comparison to spleens from tumor-free mice as a baseline. The majority of CD4+ tumor-infiltrating lymphocytes (TILs) were CD25+ and Foxp3+ (Fig. 3 A), and their expression of OX40 was much brighter than in control splenocytes (Fig. 3 B).
Figure 3.
Tumor-infiltrating T reg cells express functional OX40. (A) TILs were purified from a pool of CT26 tumor nodules, and the CD4+ cell population was analyzed by flow cytometry (percentages are shown). As controls, CD4+ T cells were purified from the spleens of tumor-free mice (STF). (top) On gated CD4+ T cells, the expression of Foxp3 versus CD25 was evaluated. (bottom) Among CD4+ CD25+ T cells, Foxp3 versus OX40 expression is shown. (B) OX40 expression was evaluated on gated CD4+ Foxp3+ T cells from TILs of tumor-bearing mice. As controls, isotype staining on Foxp3+ TILs and OX40 staining on Foxp3+ CD4+ splenocytes from tumor-free mice are shown. (C) CD4+ CD25− T cells (Teff), obtained from the spleens of tumor-free mice, were CFSE labeled and seeded either alone or combined at 1:1, 1:2, or 1:4 ratios with unlabeled CD4+ T cells purified from TILs (T reg TIL) of tumor-bearing mice, either untreated or 6 h after intratumor OX86 injection (anti-OX40 in vivo). No major differences in the composition of tumor-infiltrating CD4+ T cells or in Foxp3 expression were evident 6 h after treatment. As controls, CD4+ CD25+ T cells from spleens of tumor-free mice were used (T reg STF). T reg cells were either pretreated with anti-OX40 mAb in vitro or with rat IgG as mock control. After 72 h, effector T cells were tested for CFSE dilution as a marker of proliferation. Representative plots of one out of three independent experiments are shown and indicate percentages of proliferating (CFSElow) versus resting (CFSEhigh) effector T cells.
To test whether OX40 conveys the same inhibitor signal to tumor-infiltrating T reg cells, in comparison to those collected from the spleens of tumor-free mice, T reg cells were incubated for 2 h with OX86 mAb or with control rat IgG before being added to polyclonally activated and CFSE-labeled CD4+ CD25− T cells in a standard in vitro suppression assay. Both tumor- and control spleen–derived T reg cells suppress the proliferation of responder cells in a dose-dependent manner, but not if preincubated with OX86 (Fig. 3 C). More compelling, T reg cells freshly isolated from OX86-treated tumors in vivo lost their suppression when tested ex vivo (Fig. 3 C). These data confirm that CT26-infiltrating T reg cells express OX40 and are efficiently inhibited for the suppressive function by OX40 triggering.
Intratumor OX40 triggering leads to complete tumor rejection
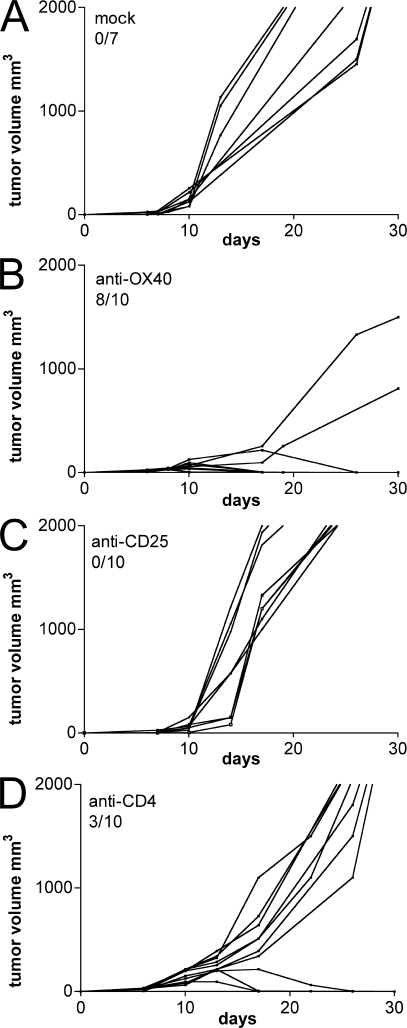
Tumor-infiltrating T reg cells play a major role in establishing and maintaining the state of immune suppression within the tumor microenvironment. Their inhibition could rescue the endogenous immune response from paralysis, thus leading to the rejection of tumors endowed with some immunogenicity. To test this hypothesis, OX86 was injected within the tumor mass when CT26 cells reached ∼3–4 mm in size. Two single injections of 50 μg of mAb were performed within a 2-d interval. As controls, anti-CD25 mAb (PC61) and anti-CD4 (GK1.5)–depleting mAb or rat IgG were used at the same dose and schedule. Although all mock and PC61-treated animals were killed around day 20 because of excessive tumor burden (Fig. 4, A and C), GK1.5-injected mice showed delayed tumor progression, and in 3 out of 10 mice a slow but progressive tumor rejection (Fig. 4 D). In this setting, both PC61 and GK1.5 deplete the CD4+ T reg cell population, but only GK1.5 spares CD8+ effector T lymphocytes.
Figure 4.
Intratumor OX40 triggering induces tumor rejection. 5 × 104 CT26 cells were injected s.c. in the right flank of BALB/c mice. When tumors reached 3–4 mm in size, they received intratumor injection of 100 μg of purified rat IgG (A), anti-OX40 (B), anti-CD25 (C), or anti-CD4 mAb (D). Tumor volume was calculated as longest diameter × (shortest diameter)2 (in cubic millimeters). Lines represent the tumor growth kinetics in each single mouse. The number of tumor-free mice out of the number of treated mice is indicated. One representative of three independent experiments is shown.
Strikingly, OX86 induced complete tumor rejection in 80% of treated mice and markedly reduced the growth of nonrejected tumors (Fig. 4 B). Mice that successfully rejected the primary tumor were protected against a subsequent tumor challenge (unpublished data), and their splenocytes displayed cytotoxic activity against tumor antigens upon ex vivo restimulation (unpublished data). Larger tumors (5–6 mm in diameter) were also rejected in six out of nine mice by anti-OX40 treatment (unpublished data).
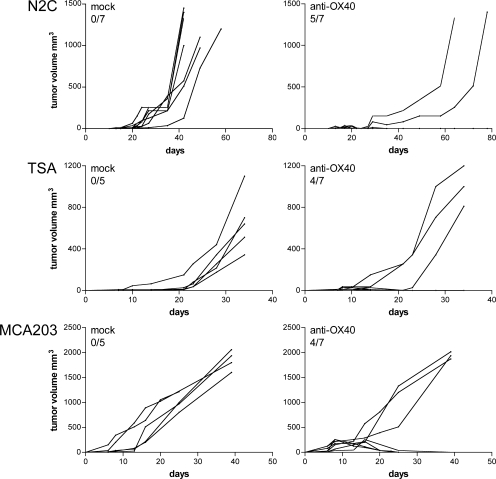
The described therapeutic effect of agonistic anti-OX40 mAb was not restricted to the CT26 colon carcinoma. TSA and N2C mammary tumors of BALB/c mice, as well as MCA203 fibrosarcoma of C57BL/6 mice, were also rejected in ∼70% of treated mice (Fig. 5).
Figure 5.
OX86 intratumor injection eradicates tumors of different hystotypes. N2C and TSA BALB/c mammary carcinomas, and MCA203 C57BL/6 fibrosarcoma of 3–4 mm in size received intratumor injection of 100 μg of purified rat IgG (left) or anti-OX40 (right). Tumor volume was calculated as longest diameter × (shortest diameter)2 (in cubic millimeters). Lines represent the tumor growth kinetics in each single mouse. The number of tumor-free mice out of the total number of treated mice is indicated. One representative of two independent experiments is shown.
Adaptive immune response is required for OX40-induced tumor rejection
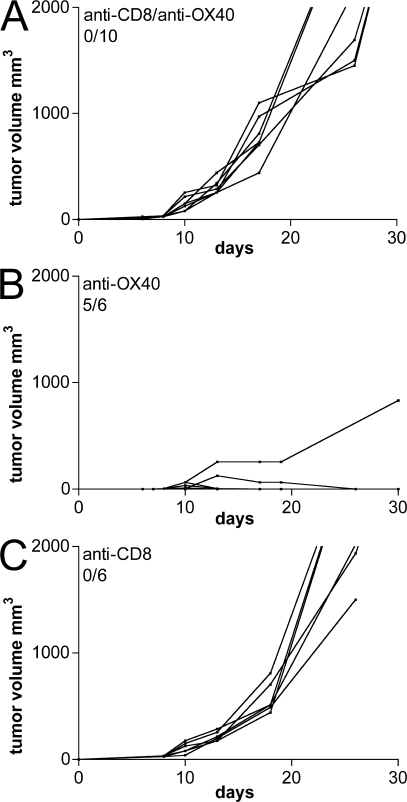
To dissect the immune mechanisms contributing to tumor rejection, mice were systemically injected with anti-CD8 depleting mAb (2.43) the day before and the same day of OX86 intratumor administration. In CD8-depleted mice, OX86 lost effect and tumors grew without difference in comparison to OX86-untreated tumors (Fig. 6).
Figure 6.
CD8+ T cells are required for OX86-mediated rejection. (A) Mice bearing CT26 tumors of 3–4 mm in diameter were depleted of CD8+ T cells immediately before receiving an intratumor injection of 100 μg of purified anti-OX40 mAb. As controls, mice received either anti-OX40 alone (B) or anti-CD8 (C) mAb. Lines represent the tumor growth kinetics in each single mouse within the different experimental groups. The number of tumor-free mice out the total number of treated mice is indicated. One representative of two independent experiments is shown.
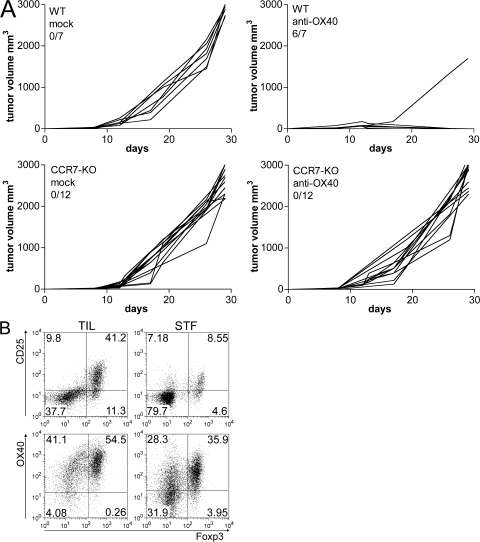
These results raised the question of whether T reg cell inhibition unleashes the activity of preexisting CTLs or favors the generation of new CTLs. Tumor-infiltrating T reg cells could hamper tumor-draining node communication, most likely by inhibiting tumor-infiltrating DC (TIDC) function. To test whether DC migration is necessary for OX40-induced tumor regression, we used CCR7-KO mice, whose DCs cannot enter lymph nodes in response to the chemokine CCL21 and remain in peripheral tissues (38). Tumors grown in WT or CCR7-KO mice were treated with OX86 or with rat IgG as control. 90% of WT but no CCR7-KO mice rejected their tumors upon OX86 treatment (Fig. 7 A). This result suggests that DC migration to draining lymph nodes is required for OX40-mediated tumor rejection, and that newly primed effector T cells are necessary to complete tumor rejection in this experimental setting.
Figure 7.
OX86-induced tumor rejection is lost in CCR7-KO mice. Effect of OX86 treatment in CCR7-KO mice. (A) 5 × 104 CT26 cells were injected s.c. into the right flank of WT (top) or CCR7-KO (bottom) mice. Tumors grew without difference between the two groups. At 3–4 mm in size, mice received intratumor injection of anti-OX40 mAb or rat IgG as control. Each line represents individual tumors. The number of tumor-free out the total number of treated mice is reported. The pooled data of three independent experiments are shown. (B) T cell infiltration in tumors from CCR7-KO mice. CD4+ T cells were purified from a pool of CT26 tumors grown in CCR7-KO mice (TILs). As controls, splenocytes of tumor-free CCR7-KO mice were analyzed (STF). (top) On gated CD4+ T cells, the percentage of CD25+ versus Foxp3+ T cells was evaluated by flow cytometry. (bottom) OX40 and Foxp3 expression were analyzed in the gated CD4+ CD25+ population. Both Foxp3+ and Foxp3− subsets were mostly OX40+, similarly to WT mice (Fig. 3A). However, in contrast to WT mice, TILs from CCR7-KO mice contain a higher percentage of OX40+ Foxp3− T cells, representing activated effector T cells.
It can be argued that T cells, besides TIDCs, have impaired migration to lymph nodes in CCR7-KO mice and that tumor rejection should not be possible at any rate because of an intrinsic defect of T cells rather than DCs. However, this possibility is disputed by the finding of numerous CD25+ Foxp3− T cells within the tumors from CCR7-KO mice, perhaps derived from extranodal priming (Fig. 7 B). Although indirect, these data suggest the hypothesis that T reg cell functional inhibition should restore DC function toward the production of new effector T cells.
Rescue of DC migration results in the priming of tumor-specific effectors at draining lymph nodes
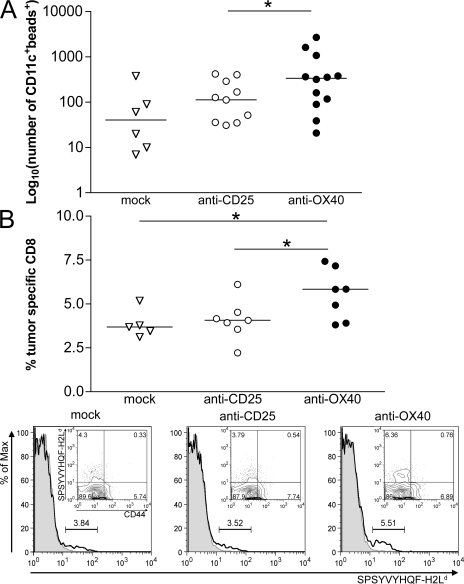
To verify the above hypothesis, we tested whether tumor treatment with OX86 releases TIDCs from suppression and recovers their migration properties. Almost all of the CT26-infiltrating DCs belonged to the myeloid subset, and their surface expression of MHC class II and of co-stimulatory molecules (CD40, CD80, and CD86), which is typically low/intermediate in immature DCs, was not affected 6 or 24 h after OX86 administration (unpublished data). To test whether TIDCs showed increased migratory capacity, we coinjected green fluorescent microbeads together with the OX86 mAb. Beads cannot reach the lymph node unless transported by DCs as engulfed particles. After 24 h, draining lymph nodes were analyzed for the presence of CD11c+ cells emitting green fluorescence because of the engulfed microbeads. As a control, anti-CD25 mAb or rat IgG were coinjected with fluorescent microbeads. The absolute number of green DCs within draining lymph nodes was significantly higher in OX86- versus control-treated mice (Fig. 8 A). This result confirms that tumor-infiltrating T reg cells inhibit nearby DCs and that T reg cell functional inhibition restores their activities.
Figure 8.
T reg cell inhibition by OX40 triggering allows TIDC maturation and priming of new CTLs. (A) Rescue of DC migration after T reg cell inhibition. FITC-conjugated latex particles (2 × 107 beads) of 1 μm in diameter were coinjected with anti-OX40, anti-CD25, or rat IgG (mock) within tumors of 3–4 mm in diameter. After 24 h, axillary and inguinal draining lymph nodes were collected and treated with collagenase D, and the obtained cell suspensions were stained for CD11c. The number of CD11c+ cells showing green fluorescence because of microbead engulfment was evaluated by flow cytometry. Each symbol corresponds to a single draining lymph node; the solid line represents the median value. Results are a pooled representation of three independent experiments. *, P < 0.05. (B) Induction of tumor-specific CD8+ T lymphocytes. (top) Tumor-bearing mice received intratumor injection of anti-CD25, anti-OX40, or rat IgG (mock). 5 d after treatment, axillary and inguinal draining lymph nodes were collected and stained for flow cytometry. On gated CD8+ T cells, the percentage of tumor-specific clones was evaluated by staining with H2Ld tetramers recognizing the SPSYVYHQF peptide of the gp70-env tumor-associated antigen. As a background staining, H2Ld tetramers specific for the unrelated peptide TPHPARIGL (E. coli β-galactosidase 876–884) were used. Percentages of tumor-specific CD8+ T cells were calculated by subtracting the background (β-galactosidase) from the specific (gp70-env) staining. OX86-treated mice showed the highest percentage of tumor-specific CD8+ T cells in the draining lymph nodes. Each symbol represents the draining lymph node of randomly chosen mice from two independent experiments. The solid line represents the median value. *, P < 0.05. (bottom) Representative histograms of tetramer-stained cells gated on CD8+ T cells are shown (indicated as percentages). Gp70-H2Ld tetramer staining (continuous line) was overlaid with β-galactosidase–H2Ld control tetramer staining (shaded line). The subtracted value is indicated for each sample. (insets) Surface expression of the T cell memory marker CD44 on gp70-H2Ld tetramer–positive CD8+ T cells.
The rescue of TIDC function is clearly part of the mechanisms leading to tumor rejection upon OX86 treatment. To confirm such a role, we evaluated the percentage of tetramer-specific CD8+ T cells recognizing AH1, the Ld-restricted gp70-env immunodominant peptide expressed by CT26 carcinoma. 5 d after treatment, the fraction of CD8+ T cells specific for AH1 peptide significantly increased in the draining lymph node of OX86 versus control-treated tumors (Fig. 8 B). Moreover, the CT26-specific CTLs did not express the memory marker CD44 (Fig. 8 B, insets) but instead the early activation marker CD69 (not depicted), suggesting that they are recently activated rather than memory T cells returned from the tumor site. This confirms that the priming of new CTLs actually occurs at the draining lymph node as a consequence of TIDC evasion from T reg cell suppression.
T reg cell inhibition and effector T cell co-stimulation are both required in OX86-induced tumor rejection
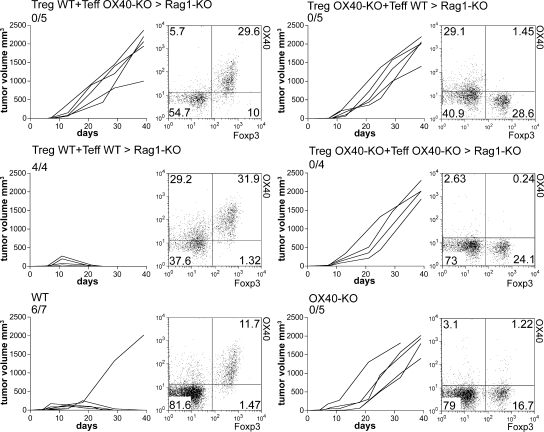
In addition to T reg cells, incipient tumors are infiltrated by some Foxp3− OX40+ activated effector T cells that are readily responsive to the agonistic OX40 mAb. Thus, tumor rejection might result from the dual triggering of both T reg cells and effector T cells. To test this possibility against a distinct, predominant role of T reg cell inhibition versus effector T cell co-stimulation, we generated mice in which T reg cells or effector T cells were alternatively OX40 null. To this end, C57BL/6 Rag1-KO mice were reconstituted with CD4+ CD25− (effector T cells) from WT (CD45.1) mice and CD4+ CD25+ (T reg cells) from OX40-KO (CD45.2) mice, or vice versa, in the presence of CD8 WT T cells. Effector T and T reg cells were mixed at the physiological 10:1 ratio to avoid the conversion of one subpopulation into the other (39). Using the CD45 marker, we verified that CD4+ CD25+ Foxp3− effectors were derived through activation of naive CD4+ CD25− T cells, and that CD4+ CD25+ Foxp3+ cells were from the expansion of CD4+ CD25+ donor T reg cells. 4 wk after reconstitution, mice were injected s.c. with 5 × 105 cells of syngenic MCA203 fibrosarcoma. When the tumor size reached 3–4 mm, mice were treated with intratumor injection of OX86 or control mAb. Fig. 9 shows that control mice receiving both T reg and effector T cells from WT donors rejected the tumor in response to OX86 treatment. Conversely, mice reconstituted with either T reg or effector T cells from OX40-KO donors showed tumor progression despite anti-OX40 treatment. In addition, mice receiving OX40-KO CD4 T cells (both effector T and T reg cells) and OX40-competent CD8 T cells did not reject tumor in response to OX86. The data clearly show that tumor rejection can result from the action of a single agent, the OX86 mAb, capable of double activity: inhibition of T reg cell function and boosting of effector T cells.
Figure 9.
T reg cell inhibition and effector T cell co-stimulation are required for OX86-induced tumor rejection. Rag1-KO C57BL/6 mice were injected i.v. with WT CD8+ T cells in addition to a mixture of CD4+ T cells comprising 1:10 mixed combinations of CD4+ CD25+ (Treg) and CD4+ CD25− (Teff) cells, respectively, purified from spleens of WT CD45.1 or OX40-KO CD45.2 mice. After 4 wk, mice were injected s.c. with 5 × 105 MCA203 tumor cells and treated with intratumor injection of OX86 when tumors reached 3–4 mm in size. Similarly treated WT and OX40-KO mice were used as controls. Tumor growth is reported in cubic millimeters; each line represents a single tumor. The number of mice rejecting tumor out the total number of treated mice is indicated. When mice were killed, draining lymph nodes were collected and stained to check that the donor T cell mixtures were in the right proportions. A representative plot showing Foxp3 versus OX40 expression (percentages) on gated CD4+ T cells is reported for every experimental group. *, P < 0.05.
OX40 triggering renders the tumor microenvironment permissive to immunity against an exogenous antigen
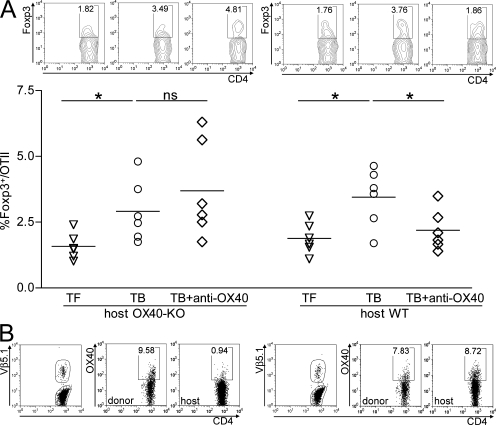
The above activities could also be improved by the newly described effect of OX40 triggering in preventing the conversion of T cells into T reg cells (40, 41), an event that is exacerbated in tumor bearers (4). To test this event in the tumor context, we transferred clonotypic CD4+ CD25− OTII lymphocytes into either OX40-KO or WT mice already bearing MCA203 fibrosarcoma. Tumors of 3–4 mm were injected with 10 μg of OVA protein with or without OX86. 2 d later, draining lymph nodes were collected and OX40 expression by donor OTII cells was interpreted as a function of their activation, which, indeed, occurred without difference between mice treated or not with OX86 and without difference to tumor-free OTII-recipient mice injected s.c. with OVA protein. Although all donor OTII cells were equally expressing OX40 and, therefore, potentially responsive to OX86 mAb, their gaining of Foxp3 in tumor bearers was inhibited by OX86 only if recipient mice were OX40 sufficient (Fig. 10). In OX40-KO recipients, unimpaired conversion of OTII lymphocytes into T reg cells despite OX86 treatment indicates that tumor-infiltrating OX40+, rather than donor, T cells, are the prime targets of intratumoral-injected mAb. Because the absolute counts of Foxp3− clonotypic cells remain constant in all experimental groups, we exclude that preferential expansion of effector cells might overestimate actual conversion rates (unpublished data). Also in TILs, OX86 reduces the conversion rate of clonotypic T cells in WT but not in OX40-KO recipients (unpublished data). These results further support the notion of OX40-mediated local changes that turn the tumor milieu from suppressive into permissive to immune activation.
Figure 10.
OX40-induced T reg cell inhibition prevents the conversion of effector T cells into T reg cells. Effect of intratumor agonistic OX40 mAb on adoptively transferred CD4+ T lymphocytes. To test whether anti-OX40 mAb given intratumorally can directly affect lymphocytes circulating in nearby lymph nodes, 3.5 × 106 CD4+ CD25− OTII cells were injected i.v. in C57BL/6 WT or OX40-KO mice that were either tumor free or bearing MCA203 tumors. After 2 d, OVA protein was injected within the tumor (or s.c. in the flank of tumor-free controls) together with OX86 or control rat IgG. 2 d later, draining lymph nodes were collected and analyzed by flow cytometry. Because tumor bearers can be considered a tolerogenic environment, we tested whether the adoptively transferred OTII cells gain Foxp3 in the course of tolerogenic activation and whether intratumor OX86 can prevent such an event. (A) The percentage of clonotypic OTII cells expressing Foxp3 in all tested conditions (representative dot plots are shown; top). (B) In OX40-KO hosts, intratumor-injected OX86 failed to prevent the gaining of Foxp3 by donor lymphocytes despite their expression of OX40 (percentages are indicated). *, P < 0.05.
DISCUSSION
Growing tumors induce the expansion of natural T reg cells and the conversion of naive T cells into de novo–derived T reg cells. Accumulation of suppressive lymphocytes results in immune paralysis, and inactivation of T reg cell suppression is a major goal for cancer immunotherapy. Indeed, the sole disruption of cell-intrinsic recessive tolerance in tumor bearers may not produce therapeutic effects unless the T reg cell–induced dominant tolerance is effectively and stably broken. In this paper, we show that T reg cell functional inhibition, by means of OX40 triggering, protects from subsequent tumor challenge and induces a complete rejection of already established nodules. We uncover the prominent mechanism allowing the activation of new tumor-specific effectors as a consequence of restored TIDC function by T reg cell inhibition. Effector T cells can then be boosted by an agonistic OX40 mAb, thus concurring with tumor rejection.
In naive mice, naturally arising T reg cells constitutively express OX40 and are virtually the only possible targets of anti-OX40 mAb. In a setting of tumor prevention, the efficacy of anti-OX40 treatment is lost in mice previously depleted of CD25+ T cells. These data demonstrate that the in vivo inhibition of natural T reg cells allows the development of both innate and adaptive immune responses, whose mediators were suppressed by T reg cells. The restored immunity includes DC reactivation and induction of tumor-specific CTLs. Accordingly, mice rejecting the tumor because of T reg cell inhibition established immunological memory and cytotoxic activity that was detected in their splenocytes. At any rate, T reg cell inactivation permits the development of an optimal immune response leading to the clearance of tumor cells depending on their immunogenicity. Although most of the effects initiated by OX40 triggering are caused by T reg cell inactivation, we cannot exclude that the persistent presence of the agonistic mAb will boost effector T cells as soon as they gain OX40 as part of their activation process. This OX40-mediated double effect becomes more evident and testable when agonistic mAb is used in the therapeutic setting (see the following Discussion).
Anti-CD25 depleting mAb is much less efficient than anti-OX40 in tumor prevention. Depletion of CD25+ T cells, checked by means of Foxp3 staining, is incomplete, a finding also reported by others (42–44). The presence of few residual T reg cells may explain its low efficacy in protecting mice from tumor challenge. Another possible explanation is the persistent circulation of anti-CD25 that eliminates the newly generated effector T cells, which gain the CD25 at priming after tumor challenge. Indeed, PC61 persistence in circulation for up to 13 d has been previously reported (45).
The three phases of immunoediting (i.e., elimination, equilibrium, and escape) result from the cross talk between tumor and host immune cells (46). In mice that have had natural T reg cells systemically inactivated by anti-OX40 mAb, the tumor–host interaction stops at the first phase of immunoediting because the development of an optimal endogenous response quickly eliminates tumor cells that are not allowed to establish some equilibrium with the immune cells. This observation suggests that in naive mice natural T reg cells impede the immune activation necessary for primary elimination of tumor cells.
Once tumor cell elimination by host cells has failed, the equilibrium and escape phases soon occur. Growing tumors evade possible immune cell attacks by several strategies, including the increase of T reg cell number and selective recruitment of T reg cells into the tumor bed. The pool of tumor-associated T reg cells derives from the expansion of naturally arising T reg cells and from peripheral conversion of naive precursors into de novo–induced T reg cells, as we and others have recently demonstrated (4, 47). In our tumor model, nodules of 3–4 mm in size are already associated with a significant accumulation of T reg cells in draining lymph nodes, in spleens, and, more importantly, in the tumor infiltrate, which is indicative of an active tumor immune escape. T reg cell inhibition by intratumor injection of agonistic OX40 mAb induces tumor regression in 80% of treated mice, being able to actively revert the previously established state of dominant immune tolerance. Therefore, although natural T reg cells regulate the elimination phase, tumor-associated T reg cells suppress the immune system during the escape phase. In both circumstances, T reg cell inhibition by means of OX40 triggering reverts the tolerant state and allows effective tumor elimination by host immune cells.
The obvious prerequisite for intratumor administration of OX40-agonistic mAb is the expression of OX40 on the surface of target cells. By flow cytometry, we showed that almost all of the CD4+ CD25+ T cells in tumor beds highly express OX40. Among them, the vast majority consists of Foxp3+ T reg cells. Notably, the expression level of OX40 by tumor-infiltrating T reg cells was significantly higher than splenic T reg cells. The reason for this difference is still unknown. Nevertheless, OX40 up-regulation might result from T reg cell activation, behaving similarly to other activation markers up-regulated by tumor-infiltrating T reg cells (4). A similar expression pattern can be suggested for OX40 expression on human T reg cells, which, being OX40 negative in the circulation, acquire OX40 expression at effector sites at inflamed joints in rheumatoid arthritis (48) and likely at the tumor site in cancer patients. The possibility of OX40 up-regulation by tumor-infiltrating human T reg cells implies that anti-OX40 mAb may preferentially trigger tumor-associated T reg cells with less effect on the systemic T reg cell pool that is necessary for self-tolerance control.
OX40L is transiently expressed on the surface of activated B cells and of DCs at late phases of maturation to convey a late co-stimulatory signal to activated T cells. In addition, activated effector T cells can up-regulate OX40L at the very late phases of activation (49, 50). However, those conditions are unlikely in the tumor microenvironment, characterized by profound immunosuppression. Accordingly, we could not detect any OX40L-positive cells in the tumor mass. This observation suggests that in the tumor microenvironment, CD4+ CD25+ T cells cannot receive any triggering signal on OX40 unless the agonist mAb is given.
We compared the efficacy of intratumor administration of OX40-triggering mAb with that of anti-CD25 (PC61) and anti-CD4 (GK1.5) depleting mAb. Intratumor depletion of CD25-positive T cells does not result in any therapeutic effect, whereas few rejections can be obtained by depleting CD4+ T lymphocytes. Most likely, recruitment mechanisms may promptly reestablish T reg cells within the tumor beds after their local depletion. Moreover, anti-CD25 can concurrently deplete CD25+-activated effectors, thus abolishing any potential benefit derived from T reg cell elimination.
Anti-CD4 mAb, although sparing CD8+ T cells, eliminates both T reg and T helper cells and is poorly effective in tumor rejection. In the tumor model that we have extensively examined, the vast majority of tumor-infiltrating T lymphocytes were CD4+ T cells, whereas the few CD8+ T cells expressed low levels of CD25 and OX40 (unpublished data). It is conceivable that in this setting, the few CD8+ T cells are unable to induce tumor rejection and that new CD8+ T cells cannot be generated because of the concurrent elimination of T helper cells by the anti-CD4 mAb. Other more immunogenic tumors, such as the Ag104 model, can be rejected by the sole intratumor CD4 depletion, which rescues the endogenous robust CD8 concomitant immunity otherwise inhibited by T reg cells (35).
The minority of Foxp3− CD4+ CD25+ OX40+ tumor-infiltrating activated T cells can also be triggered by anti-OX40 mAb in vivo. Notably, this stimulation is thought to be beneficial to the overall antitumor immunity by actively boosting effector cells. Indeed, tumor-infiltrating effector cells are supposed to be in a tolerant state that can be actively reversed by OX40 co-stimulation (19). By reconstituting Rag1-KO mice with OX40-competent T reg cells and OX40-deficient effectors, we demonstrated not only that effector co-stimulation occurs upon intratumor OX40 triggering, but also that such a mechanism is necessary to achieve effective tumor therapy. On the other hand, T reg cell inhibition remains a crucial step through which OX40 triggering mediates tumor rejection, because mice bearing OX40-sufficient effector T cells but OX40-deficient T reg cells also fail to reject tumor upon OX86 treatment. We can conclude that although OX40 triggering induces opposite effects on T reg and effector T cells (i.e., inhibition and stimulation), respectively, both effects concur with the same outcome in terms of tumor rejection.
Notably, regardless of the composition of the CD4 mixture, the coinjected OX40-competent CD8+ T cells were not sufficient to achieve tumor rejection. In other words, the sole tolerance reversal of preexisting CD8+ T cells fails to allow immune response unless the CD4+ help is boosted and the T reg cell suppression is inhibited. Similar results have been obtained by Song et al. (51), who demonstrated that the sole CD8 boost through OX40 is ineffective against tumor unless paired to OX40-mediated CD4+ T cell activation.
For T cell priming, TIDCs should reach the draining lymph node. CCR7-null DCs cannot migrate to lymph nodes and CCR7-KO mice have severe structural and functional alterations in lymphoid organs (38). Therefore, in CCR7-KO mice, a cross talk between tumor and lymph node cannot be established. In these mice, the anti-OX40 mAb treatment completely loses any antitumor efficacy. The intrinsic bias of using CCR7-KO mice in these experiments is the impairment of T cell migration to lymph nodes (38). However, this initial finding is challenged by the observation that priming for delayed-type hypersensitivity reactions and OVA-specific proliferation is still possible, although with some delay, in CCR7-KO mice (52). Extranodal priming and compensatory mechanisms could overcome CCR7 deficiency in these systems. Accordingly, we detected CD4+ CD25+ OX40+ Foxp3− T cells among TILs of CCR7-KO tumor bearers. Even if some delayed priming might occur, the rapid tumor growth renders the newly activated T cells useless because tumor burden becomes too large and its fate irreversible. Interestingly, CD4+ TILs of CCR7-KO mice contain a higher proportion of effector T versus T reg cells compared with WT tumors. This is compatible with the accumulation at the tumor site of effector/memory T cells that require CCR7 expression to leave peripheral tissues and enter lymphatics (53, 54).
The possibility that, upon intratumor T reg cell inhibition, DCs are reprogrammed toward an immunogenic rather than tolerogenic pathway finds confirmation by showing their enhanced migration to the draining lymph node. 24 h after the intratumor injection of fluorescent microbeads, the number of CD11c+ cells that have engulfed fluorescent particles at the tumor site and have migrated to the draining lymph node is greatly increased if anti-OX40 mAb was coinjected with the beads. Such improved migration results in a significant increase of tumor-specific CTLs detectable in the draining lymph node 5 d after intratumor OX86 treatment.
Although several papers indicate T reg cell inhibition of T cells priming within the tumor-draining lymph node (55, 56), our data indicate that such inhibition can take place beginning from the tumor site. Rather, TIDCs unleashed from local suppression mature, thus becoming resistant to any further T reg cell suppression at the draining lymph node (57). Alternatively, a small amount of intratumor-injected mAb may reach the draining lymph node via lymphatics, thus restoring the nodal priming activity.
Accordingly, intratumor injection of OVA protein as an exogenous antigen leads to abortive T cell activation unless anti-OX40 mAb is coadministered. A similar pattern of unresponsiveness has been described in A20-HA–bearing mice (58). In both models, transfer of clonotypic CD4+ CD25− T cells shows that tumor induces the tolerogenic activation of transferred T cells and their conversion into Foxp3+ regulatory cells. Injection of OX86 prevents their gaining of Foxp3.
In addition, it has been recently shown that direct co-stimulation through OX40 during the priming impedes the acquisition of a regulatory phenotype (40, 41). Using an adoptive transfer experiment in OX40-KO recipients, we confirm this finding in a tumor setting and provide new evidence that agonistic OX40 mAb given intratumorally mainly acts on environmental, nearby lymphocytes rather than directly on donor clonotypic T cells, which become unable to gain the Foxp3 marker. Therefore, in this model, it is conceivable that preexisting tumor-infiltrating CD4+ T reg and effector T cells, being the prime targets of anti-OX40 mAb, can indirectly modify the tumor microenvironment favoring the immunogenic rather than tolerogenic activation of exogenous OTII cells.
Recent observations have raised a concern about the risk of generalized autoimmune disease in case of excessive co-stimulation (59). Many features of OX40 triggering sustain its safety. Effector cells, only at late phases of activation, express OX40 and can receive co-stimulation (21). Moreover, T reg cells express OX40 constitutively in the periphery (at least in mice) and up-regulate this marker within the tumor; thus, the intratumor route of treatment may preserve systemic tolerance. Mice that rejected the tumor because of OX40 treatment remained perfectly healthy for a 6-mo followup without showing any macroscopic sign of autoimmunity.
Several attempts have been made to exploit T reg cell neutralization as an adjuvant strategy for immunotherapy. Although encouraging results emerge from mouse studies, CD25-directed T reg cell depletion in cancer patients has not provided satisfactory outcomes. The prevalent failure of such an approach may be attributed to the concurrent depletion of activated effectors, the possibility of triggering IL-2 receptor on T reg cells for activation rather than depletion, and the prompt replenishment of the T reg cell pool by peripheral conversion (60). We propose T reg cell inactivation, by means of OX40 triggering, as a suitable alternative in cancer immunotherapy. Indeed, breaking the barrier of dominant tolerance appears to be the sine qua non for effective immunotherapy in cancer patients.
MATERIALS AND METHODS
Mice and treatments.
BALB/c, C57BL/6, Rag1-KO, and OTII mice were purchased from Charles River Laboratories. CCR7-KO and OX40-KO mice were provided by M. Lipp (Max-Delbruck-Center, Berlin, Germany) and N. Killen (University of California, San Francisco, San Francisco, CA), respectively. All mice were maintained under pathogen-free conditions in our animal facility and used at 8 wk of age. CT26 is a carcinogen-induced, undifferentiated colon carcinoma cell line; N2C is a primary mammary carcinoma cell line derived from female BALB-neuT mice; and TSA is a cell line derived from a spontaneous breast adenocarcinoma (all on BALB/c background). MCA203 is a C57BL/6-derived fibrosarcoma induced by 3-methylcholanthrene. Tumor cells were cultured in DMEM (Invitrogen) supplemented with 10% FCS (BioWhittaker). 5 × 104 CT26 cells, 2 × 105 N2C cells, 5 × 104 TSA cells, or 5 × 105 MCA203 cells were inoculated s.c. in the flank of naive mice. Tumor growth was monitored twice per week and recorded as longest diameter × (shortest diameter)2 (in cubic millimeters). In vivo systemic administration of anti-CD25 mAb (clone PC61; hybridoma provided by V. Bronte, University of Padua, Padua, Italy), anti-OX40 mAb (clone OX86; European Collection of Cell Cultures), or anti-CD8 mAb (clone 2.43; American Type Culture Collection) was performed using a daily i.p. injection of 500 μg of ascite for two consecutive days. Purified anti-OX40 mAb, anti-CD25 mAb, and anti-CD4 mAb (clone GK1.5; American Type Culture Collection) were injected within the tumor using two daily injections of 50 μg within a 2-d interval. Animal experiments were authorized by the Fondazione IRCCS Istituto Nazionale dei Tumori Ethical Committee for animal use.
Purification of CD4+ CD25+ and CD4+ CD25− subsets.
Total splenocytes from tumor-free mice were first enriched for T lymphocytes by passing the whole spleen on nylon wool columns (Kisker). CD8+ cells were removed using anti-CD8 microbeads (MACS; Miltenyi Biotec). CD4+ CD25+ cells were then separated from CD4+ CD25− cells using the CD25+ T cell isolation kit (Miltenyi Biotec), according to manufacturer's instruction. Flow cytometry showed that the separate fractions were >90% pure. To obtain tumor-infiltrating T reg cells, nodules of 3–4 mm were pooled and perfused with 400 U/ml of collagenase D solution (Roche) and incubated in collagenase for 30 min at 37°C. After gentle pipetting, the suspension was enriched for viable lymphocytes using a Lympholyte gradient (Cederlane). CD4+ T cells were purified from the obtained TIL population by positive selection with anti-CD4 MACS microbeads. CD4+ TILs were shown to mainly consist of CD25+ Foxp3+ T cells.
Antibodies and flow cytometric analysis.
PE-conjugated anti-CD11c (clone HL3), PE-conjugated anti-Vβ5.1/5.2 (clone MR9-4), FITC-conjugated anti-CD44 (clone IM7), and streptavidin-Cy5 were all purchased from BD Biosciences. Purified, biotin-conjugated, or PE-conjugated anti-OX40 (clone OX86); APC-conjugated anti-Foxp3 (clone FJK-16S); PE-Cy7–conjugated anti-CD4 (clone L3T4); and FITC-conjugated anti-CD25 (clone 7D4) were purchased from e-Bioscience. Alexa Fluor 647–conjugated anti-CD8 (clone 5H10) was purchased from Caltag. PE-conjugated H-2Ld tetramer to peptide SPSYVYHQF (MuLV env gp70 423–431) and PE-conjugated H-2Ld tetramer to peptide TPHPARIGL (Escherichia coli β-galactosidase 876–884) were purchased from Proimmune. Antibodies were used at 5 μg/ml, and surface staining was performed in FACS buffer (10% FCS in PBS) on ice for 45 min. Intracellular staining of Foxp3 (clone FJK-16S) was performed on purified CD4+ CD25+ cells, according to the manufacturer's instructions (e-Bioscience). Flow cytometry data were acquired on a FACSCalibur (Becton Dickinson) and analyzed with FlowJo software (version 8.4.5; Tree Star Inc.).
In vitro suppression assay.
For in vitro assays, purified CD4+ CD25− cells were labeled by incubation with 5 μM CFSE (Invitrogen) in PBS containing 5% FBS for 15 min at 37°C. Cells were washed twice with PBS and set up in a proliferation assay. To test T reg cell suppressive activity, 5 × 104 CD4+ CD25− cells were cultured with 5 × 104 accessory cells (consisting in the whole spleen irradiated with 30 Gy) with or without T reg cells for 72 h in complete medium containing RPMI 1640 (Sigma-Aldrich) supplemented with 5% FCS, 2 mM l-glutamine, 200 U penicillin, and 200 mg/ml streptomycin (Sigma-Aldrich). For stimulation, 1 μg/ml of purified anti-CD3 (e-Bioscience) was added. Where indicated in the figures, T reg cells were preincubated at a concentration of 6 × 106/ml with 30 μg/ml of purified anti-OX40 mAb (clone OX86; e-Bioscience). Every condition was assayed in triplicate and analyzed after 72 h by flow cytometry.
In vivo DC migration.
Mice bearing CT26 tumors were intratumorally injected with the indicated treatment plus 2 × 107 FITC-conjugated latex particles of 1 μm in diameter (Polysciences). After 24 h, mice were killed and draining lymph nodes were collected, teased, and incubated for 30 min in 400 U/ml collagenase D solution. Cells were stained for CD11c expression, and 106 events per lymph node were acquired by flow cytometry. The total number of double-positive PE-CD11c/FITC bead events was calculated for each lymph node.
Reconstitution of Rag1-KO mice.
CD4+ CD25− and CD4+ CD25+ T cells were purified from the spleens of either WT CD45.1 or OX40-KO CD45.2 mice on a C57BL/6 background. CD8+ T cells were obtained from WT mice. Purity of the obtained populations was >90%, as assessed by flow cytometry. Cells were mixed at different combinations and injected i.v. into Rag1-KO mice at 4 wk of age. Every mouse received the following amount of cells: 10 × 106 CD4+ CD25− cells, 106 CD4+ CD25+ cells, and 7 × 106 CD8+ cells, to reconstitute a physiological-like T cell compartment into recipients. After 4 wk, mice were injected s.c. with 5 × 105 MCA203 syngenic tumor cells. At the size of 3–4 mm, intratumor injection with 100 μg anti-OX40 was performed in two doses within a 2-d interval. Tumor growth was monitored every 3 d. When mice were killed, lymph nodes were collected and stained with PE-Cy7 anti-CD4, PE–anti-OX40, allophycocyanin–anti-Foxp3, and FITC–anti-CD8 or FITC–anti-CD45.2 to check for correct reconstitution.
Adoptive transfer of OTII clonotypic T cells.
CD4+ CD25− T cells were purified from the spleens of OTII TCR transgenic mice. The purity of the obtained population was >90%, as assessed by flow cytometry. C57BL/6 WT or OX40-KO mice, tumor free or bearing tumors of 3–4 mm in diameter, received 3.5 × 106 OTII TCR-transgenic cells by tail vein injection. After 2 d, mice were immunized with 10 μg of OVA protein (Sigma-Aldrich) without any adjuvant. The antigen was injected within the tumor in association to the anti-OX40 mAb or the control rat IgG. Tumor-free mice were immunized s.c. in the flank. Mice were killed 2 d later, and lymph nodes draining the site of injection were isolated and stained with PE-Cy7–conjugated anti-CD4, PE-conjugated anti-Vβ5.1/5.2, and allophycocyanin-conjugated anti-Foxp3 to detect conversion, or with biotin-conjugated anti-OX40 followed by streptavidin Cy5 to evaluate OX40 expression on donor versus host cells.
Statistical analysis.
Results are expressed as the means ± SEM. Data were analyzed using a two-sided Student's t test. All analyses were performed using Prism software (GraphPad Software, Inc.). Differences were considered significant at P < 0.05.
Acknowledgments
We thank Mr. Ivano Arioli for technical assistance.
This work was supported by the Associazione Italiana Ricerca sul Cancro and the Italian Ministry of Health. S. Piconese is supported by a fellowship from the Fondazione Italiana Ricerca sul Cancro.
The authors have no conflicting financial interests.
Abbreviations used: GITR, glucocorticoid-induced TNF receptor; TIDC, tumor-infiltrating DC; TIL, tumor-infiltrating lymphocyte.
References
- 1.Zitvogel, L., A. Tesniere, and G. Kroemer. 2006. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat. Rev. Immunol. 6:715–727. [DOI] [PubMed] [Google Scholar]
- 2.Curiel, T.J., G. Coukos, L. Zou, X. Alvarez, P. Cheng, P. Mottram, M. Evdemon-Hogan, J.R. Conejo-Garcia, L. Zhang, M. Burow, et al. 2004. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 10:942–949. [DOI] [PubMed] [Google Scholar]
- 3.Ghiringhelli, F., P.E. Puig, S. Roux, A. Parcellier, E. Schmitt, E. Solary, G. Kroemer, F. Martin, B. Chauffert, and L. Zitvogel. 2005. Tumor cells convert immature myeloid dendritic cells into TGF-β–secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J. Exp. Med. 202:919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valzasina, B., S. Piconese, C. Guiducci, and M.P. Colombo. 2006. Tumor-induced expansion of regulatory T cells by conversion of CD4+CD25− lymphocytes is thymus and proliferation independent. Cancer Res. 66:4488–4495. [DOI] [PubMed] [Google Scholar]
- 5.Zou, W. 2006. Regulatory T cells, tumour immunity and immunotherapy. Nat. Rev. Immunol. 6:295–307. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi, T., Y. Kuniyasu, M. Toda, N. Sakaguchi, M. Itoh, M. Iwata, J. Shimizu, and S. Sakaguchi. 1998. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 10:1969–1980. [DOI] [PubMed] [Google Scholar]
- 7.Ghiringhelli, F., C. Menard, M. Terme, C. Flament, J. Taieb, N. Chaput, P.E. Puig, S. Novault, B. Escudier, E. Vivier, et al. 2005. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor–β–dependent manner. J. Exp. Med. 202:1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao, D.M., A.M. Thornton, R.J. DiPaolo, and E.M. Shevach. 2006. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood. 107:3925–3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veldhoen, M., H. Moncrieffe, R.J. Hocking, C.J. Atkins, and B. Stockinger. 2006. Modulation of dendritic cell function by naive and regulatory CD4+ T cells. J. Immunol. 176:6202–6210. [DOI] [PubMed] [Google Scholar]
- 10.Tang, Q., and M.F. Krummel. 2006. Imaging the function of regulatory T cells in vivo. Curr. Opin. Immunol. 18:496–502. [DOI] [PubMed] [Google Scholar]
- 11.Tang, Q., J.Y. Adams, A.J. Tooley, M. Bi, B.T. Fife, P. Serra, P. Santamaria, R.M. Locksley, M.F. Krummel, and J.A. Bluestone. 2006. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat. Immunol. 7:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tadokoro, C.E., G. Shakhar, S. Shen, Y. Ding, A.C. Lino, A. Maraver, J.J. Lafaille, and M.L. Dustin. 2006. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J. Exp. Med. 203:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vicari, A.P., C. Chiodoni, C. Vaure, S. Ait-Yahia, C. Dercamp, F. Matsos, O. Reynard, C. Taverne, P. Merle, M.P. Colombo, et al. 2002. Reversal of tumor-induced dendritic cell paralysis by CpG immunostimulatory oligonucleotide and anti–interleukin 10 receptor antibody. J. Exp. Med. 196:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dercamp, C., K. Chemin, C. Caux, G. Trinchieri, and A.P. Vicari. 2005. Distinct and overlapping roles of interleukin-10 and CD25+ regulatory T cells in the inhibition of antitumor CD8 T-cell responses. Cancer Res. 65:8479–8486. [DOI] [PubMed] [Google Scholar]
- 15.Rogers, P.R., J. Song, I. Gramaglia, N. Killeen, and M. Croft. 2001. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity. 15:445–455. [DOI] [PubMed] [Google Scholar]
- 16.Huddleston, C.A., A.D. Weinberg, and D.C. Parker. 2006. OX40 (CD134) engagement drives differentiation of CD4+ T cells to effector cells. Eur. J. Immunol. 36:1093–1103. [DOI] [PubMed] [Google Scholar]
- 17.Gramaglia, I., A. Jember, S.D. Pippig, A.D. Weinberg, N. Killeen, and M. Croft. 2000. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J. Immunol. 165:3043–3050. [DOI] [PubMed] [Google Scholar]
- 18.Bansal-Pakala, P., B.S. Halteman, M.H. Cheng, and M. Croft. 2004. Costimulation of CD8 T cell responses by OX40. J. Immunol. 172:4821–4825. [DOI] [PubMed] [Google Scholar]
- 19.Bansal-Pakala, P., A.G. Jember, and M. Croft. 2001. Signaling through OX40 (CD134) breaks peripheral T-cell tolerance. Nat. Med. 7:907–912. [DOI] [PubMed] [Google Scholar]
- 20.Lathrop, S.K., C.A. Huddleston, P.A. Dullforce, M.J. Montfort, A.D. Weinberg, and D.C. Parker. 2004. A signal through OX40 (CD134) allows anergic, autoreactive T cells to acquire effector cell functions. J. Immunol. 172:6735–6743. [DOI] [PubMed] [Google Scholar]
- 21.Sugamura, K., N. Ishii, and A.D. Weinberg. 2004. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nat. Rev. Immunol. 4:420–431. [DOI] [PubMed] [Google Scholar]
- 22.Weinberg, A.D., M.M. Rivera, R. Prell, A. Morris, T. Ramstad, J.T. Vetto, W.J. Urba, G. Alvord, C. Bunce, and J. Shields. 2000. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J. Immunol. 164:2160–2169. [DOI] [PubMed] [Google Scholar]
- 23.Kjaergaard, J., J. Tanaka, J.A. Kim, K. Rothchild, A. Weinberg, and S. Shu. 2000. Therapeutic efficacy of OX-40 receptor antibody depends on tumor immunogenicity and anatomic site of tumor growth. Cancer Res. 60:5514–5521. [PubMed] [Google Scholar]
- 24.Gri, G., E. Gallo, E. Di Carlo, P. Musiani, and M.P. Colombo. 2003. OX40 ligand-transduced tumor cell vaccine synergizes with GM-CSF and requires CD40-Apc signaling to boost the host T cell antitumor response. J. Immunol. 170:99–106. [DOI] [PubMed] [Google Scholar]
- 25.Pan, P.Y., Y. Zang, K. Weber, M.L. Meseck, and S.H. Chen. 2002. OX40 ligation enhances primary and memory cytotoxic T lymphocyte responses in an immunotherapy for hepatic colon metastases. Mol. Ther. 6:528–536. [DOI] [PubMed] [Google Scholar]
- 26.Murata, S., B.H. Ladle, P.S. Kim, E.R. Lutz, M.E. Wolpoe, S.E. Ivie, H.M. Smith, T.D. Armstrong, L.A. Emens, E.M. Jaffee, and R.T. Reilly. 2006. OX40 costimulation synergizes with GM-CSF whole-cell vaccination to overcome established CD8+ T cell tolerance to an endogenous tumor antigen. J. Immunol. 176:974–983. [DOI] [PubMed] [Google Scholar]
- 27.Valzasina, B., C. Guiducci, H. Dislich, N. Killeen, A.D. Weinberg, and M.P. Colombo. 2005. Triggering of OX40 (CD134) on CD4(+)CD25(+) T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 105:2845–2851. [DOI] [PubMed] [Google Scholar]
- 28.Takeda, I., S. Ine, N. Killeen, L.C. Ndhlovu, K. Murata, S. Satomi, K. Sugamura, and N. Ishii. 2004. Distinct roles for the OX40-OX40 ligand interaction in regulatory and nonregulatory T cells. J. Immunol. 172:3580–3589. [DOI] [PubMed] [Google Scholar]
- 29.Nocentini, G., A. Bartoli, S. Ronchetti, L. Giunchi, A. Cupelli, D. Delfino, G. Migliorati, and C. Riccardi. 2000. Gene structure and chromosomal assignment of mouse GITR, a member of the tumor necrosis factor/nerve growth factor receptor family. DNA Cell Biol. 19:205–217. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu, J., S. Yamazaki, T. Takahashi, Y. Ishida, and S. Sakaguchi. 2002. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol. 3:135–142. [DOI] [PubMed] [Google Scholar]
- 31.Shevach, E.M., and G.L. Stephens. 2006. The GITR-GITRL interaction: co-stimulation or contrasuppression of regulatory activity? Nat. Rev. Immunol. 6:613–618. [DOI] [PubMed] [Google Scholar]
- 32.Ko, K., S. Yamazaki, K. Nakamura, T. Nishioka, K. Hirota, T. Yamaguchi, J. Shimizu, T. Nomura, T. Chiba, and S. Sakaguchi. 2005. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J. Exp. Med. 202:885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramirez-Montagut, T., A. Chow, D. Hirschhorn-Cymerman, T.H. Terwey, A.A. Kochman, S. Lu, R.C. Miles, S. Sakaguchi, A.N. Houghton, and M.R. van den Brink. 2006. Glucocorticoid-induced TNF receptor family related gene activation overcomes tolerance/ignorance to melanoma differentiation antigens and enhances antitumor immunity. J. Immunol. 176:6434–6442. [DOI] [PubMed] [Google Scholar]
- 34.Cohen, A.D., A. Diab, M.A. Perales, J.D. Wolchok, G. Rizzuto, T. Merghoub, D. Huggins, C. Liu, M.J. Turk, N.P. Restifo, et al. 2006. Agonist anti-GITR antibody enhances vaccine-induced CD8(+) T-cell responses and tumor immunity. Cancer Res. 66:4904–4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu, P., Y. Lee, W. Liu, T. Krausz, A. Chong, H. Schreiber, and Y.X. Fu. 2005. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J. Exp. Med. 201:779–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimizu, J., S. Yamazaki, and S. Sakaguchi. 1999. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J. Immunol. 163:5211–5218. [PubMed] [Google Scholar]
- 37.Golgher, D., E. Jones, F. Powrie, T. Elliott, and A. Gallimore. 2002. Depletion of CD25+ regulatory cells uncovers immune responses to shared murine tumor rejection antigens. Eur. J. Immunol. 32:3267–3275. [DOI] [PubMed] [Google Scholar]
- 38.Forster, R., A. Schubel, D. Breitfeld, E. Kremmer, I. Renner-Muller, E. Wolf, and M. Lipp. 1999. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 99:23–33. [DOI] [PubMed] [Google Scholar]
- 39.Almeida, A.R., B. Zaragoza, and A.A. Freitas. 2006. Competition controls the rate of transition between the peripheral pools of CD4+CD25− and CD4+CD25+ T cells. Int. Immunol. 18:1607–1613. [DOI] [PubMed] [Google Scholar]
- 40.So, T., and M. Croft. 2007. Cutting edge: OX40 inhibits TGF-beta- and antigen-driven conversion of naive CD4 T cells into CD25+Foxp3+ T cells. J. Immunol. 179:1427–1430. [DOI] [PubMed] [Google Scholar]
- 41.Vu, M.D., X. Xiao, W. Gao, N. Degauque, M. Chen, A. Kroemer, N. Killeen, N. Ishii, and X. Chang Li. 2007. OX40 costimulation turns off Foxp3+ Tregs. Blood. 110:2501–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simon, A.K., E. Jones, H. Richards, K. Wright, G. Betts, A. Godkin, G. Screaton, and A. Gallimore. 2007. Regulatory T cells inhibit Fas ligand-induced innate and adaptive tumour immunity. Eur. J. Immunol. 37:758–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stephens, L.A., D. Gray, and S.M. Anderton. 2005. CD4+CD25+ regulatory T cells limit the risk of autoimmune disease arising from T cell receptor crossreactivity. Proc. Natl. Acad. Sci. USA. 102:17418–17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zelenay, S., and J. Demengeot. 2006. Comment on “Cutting edge: anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells”. J. Immunol. 177:2036–2037. [DOI] [PubMed] [Google Scholar]
- 45.Jones, E., M. Dahm-Vicker, A.K. Simon, A. Green, F. Powrie, V. Cerundolo, and A. Gallimore. 2002. Depletion of CD25+ regulatory cells results in suppression of melanoma growth and induction of autoreactivity in mice. Cancer Immun. 2:1. [PubMed] [Google Scholar]
- 46.Dunn, G.P., L.J. Old, and R.D. Schreiber. 2004. The three Es of cancer immunoediting. Annu. Rev. Immunol. 22:329–360. [DOI] [PubMed] [Google Scholar]
- 47.Zhou, G., and H.I. Levitsky. 2007. Natural regulatory T cells and de novo-induced regulatory T cells contribute independently to tumor-specific tolerance. J. Immunol. 178:2155–2162. [DOI] [PubMed] [Google Scholar]
- 48.Mottonen, M., J. Heikkinen, L. Mustonen, P. Isomaki, R. Luukkainen, and O. Lassila. 2005. CD4+ CD25+ T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clin. Exp. Immunol. 140:360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mendel, I., and E.M. Shevach. 2006. Activated T cells express the OX40 ligand: requirements for induction and costimulatory function. Immunology. 117:196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soroosh, P., S. Ine, K. Sugamura, and N. Ishii. 2006. OX40-OX40 ligand interaction through T cell-T cell contact contributes to CD4 T cell longevity. J. Immunol. 176:5975–5987. [DOI] [PubMed] [Google Scholar]
- 51.Song, A., J. Song, X. Tang, and M. Croft. 2007. Cooperation between CD4 and CD8 T cells for anti-tumor activity is enhanced by OX40 signals. Eur. J. Immunol. 37:1224–1232. [DOI] [PubMed] [Google Scholar]
- 52.Schneider, M.A., J.G. Meingassner, M. Lipp, H.D. Moore, and A. Rot. 2007. CCR7 is required for the in vivo function of CD4+ CD25+ regulatory T cells. J. Exp. Med. 204:735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Debes, G.F., C.N. Arnold, A.J. Young, S. Krautwald, M. Lipp, J.B. Hay, and E.C. Butcher. 2005. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat. Immunol. 6:889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bromley, S.K., S.Y. Thomas, and A.D. Luster. 2005. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat. Immunol. 6:895–901. [DOI] [PubMed] [Google Scholar]
- 55.Hiura, T., H. Kagamu, S. Miura, A. Ishida, H. Tanaka, J. Tanaka, F. Gejyo, and H. Yoshizawa. 2005. Both regulatory T cells and antitumor effector T cells are primed in the same draining lymph nodes during tumor progression. J. Immunol. 175:5058–5066. [DOI] [PubMed] [Google Scholar]
- 56.Munn, D.H., and A.L. Mellor. 2006. The tumor-draining lymph node as an immune-privileged site. Immunol. Rev. 213:146–158. [DOI] [PubMed] [Google Scholar]
- 57.Larmonier, N., M. Marron, Y. Zeng, J. Cantrell, A. Romanoski, M. Sepassi, S. Thompson, X. Chen, S. Andreansky, and E. Katsanis. 2007. Tumor-derived CD4(+)CD25(+) regulatory T cell suppression of dendritic cell function involves TGF-beta and IL-10. Cancer Immunol. Immunother. 56:48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou, G., C.G. Drake, and H.I. Levitsky. 2006. Amplification of tumor-specific regulatory T cells following therapeutic cancer vaccines. Blood. 107:628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Melero, I., S. Hervas-Stubbs, M. Glennie, D.M. Pardoll, and L. Chen. 2007. Immunostimulatory monoclonal antibodies for cancer therapy. Nat. Rev. Cancer. 7:95–106. [DOI] [PubMed] [Google Scholar]
- 60.Colombo, M.P., and S. Piconese. 2007. Regulatory-T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat. Rev. Cancer. 7:880–887. [DOI] [PubMed] [Google Scholar]