Abstract
FcγRIIb is an inhibitory Fc receptor expressed on B cells and myeloid cells. It is important in controlling responses to infection, and reduced expression or function predisposes to autoimmunity. To determine if increased expression of FcγRIIb can modulate these processes, we created transgenic mice overexpressing FcγRIIb on B cells or macrophages. Overexpression of FcγRIIb on B cells reduced the immunoglobulin G component of T-dependent immune responses, led to early resolution of collagen-induced arthritis (CIA), and reduced spontaneous systemic lupus erythematosus (SLE). In contrast, overexpression on macrophages had no effect on immune responses, CIA, or SLE but increased mortality after Streptococcus pneumoniae infection. These results help define the role of FcγRIIb in immune responses, demonstrate the contrasting roles played by FcγRIIb on B cells and macrophages in the control of infection and autoimmunity, and emphasize the therapeutic potential for modulation of FcγRIIb expression on B cells in inflammatory and autoimmune disease.
Fc receptors for IgG are key players in regulating innate and adaptive immunity (1, 2). FcγRIIb, as the only inhibitory Fc receptor for IgG, plays a unique role in regulating immune responses. It is the only Fc receptor expressed on B cells, where it controls the magnitude and persistence of the response to antigen through effects on both mature and memory B cells and plasma cells (3, 4). On myeloid cells, FcγRIIb counteracts activation through activatory Fc receptors (such as FcγRI, III, and IV in the mouse, and FcγRI, IIa, IIIa, and IIIb in the human). The response of the macrophage or dendritic cell to complexed IgG is determined by the specificity of the different low affinity Fc receptors for the isotypes of the IgG involved, as well as the relative ratio of activatory to inhibitory receptor expression on the cell surface (1, 5).
Mice deficient in FcγRIIb develop enhanced inducible immune diseases, such as collagen-induced arthritis (CIA) (6) and inducible alveolitis (7), and on certain genetic backgrounds FcγRIIb deficiency can also contribute to the development of spontaneous systemic lupus erythematosus (SLE) (8). Naturally occurring polymorphisms in the promoter of FcγRIIb reduce expression of the receptor in the mouse and are likely to contribute to spontaneous SLE in several mouse models (9–12). Similar polymorphisms in FcγRIIb may contribute to human SLE (13–15), and a transmembrane mutation associated with SLE is thought to do so by abolishing inhibitory function (16, 17). In addition to this central role in controlling susceptibility to autoimmunity, FcγRIIb controls survival after bacterial infection, balancing bacterial clearance and the risk of septic shock (18), and plays a key role in defense against malaria (19).
Stringent control of FcγRIIb expression is thought to be critical in determining its effect on the immune system. IL-4 enhances FcγRIIb expression and function on human macrophages (20–22), whereas the same cytokine abolishes FcγRIIb function and reduces expression on B cells (23, 24). Expression of FcγRIIb during dendritic cell maturation is also modulated by cytokines, as shown by an increase in FcγRIIb on mature human dendritic cells after treatment with IL-10 and IL-13 (25). Alteration of FcγRIIb expression on plasma cells correlates with the ability of immune complexes to induce their apoptosis (3), and changes in FcγRIIb expression are thought to be responsible for the therapeutic effect of intravenous Ig (26). Thus, physiological modulation of FcγRIIb, via underlying mechanisms that are incompletely understood, is likely to have important implications for immunity.
It has been shown that restoration of FcγRIIb expression using a lentiviral construct could restore tolerance and prevent autoimmune disease in FcγRIIb-deficient mice as well as in strains prone to autoimmunity (27, 28). These experiments demonstrated that restoration of FcγRIIb expression on only 30% of B cells, together with 20% of immature thymocytes and 10% of macrophages, was enough to prevent disease. This work clearly underlines the potential for restoration of FcγRIIb to normal levels, even in a subset of cells, to prevent autoimmunity. Three significant questions remain. The first is the identity of the cell type in which FcγRIIb restoration is most important. The second is whether or not a modest overexpression of FcγRIIb, rather than restoration of deficient expression, is enough to alter disease progression. This has clear implications for the potential for modulation of FcγRIIb to be therapeutically useful. The third question is the relative effect of such a cell-specific increase in FcγRIIb on infection as opposed to autoimmunity, which again has therapeutic implications.
To answer these questions, we generated several lines of mice expressing modestly increased levels of FcγRIIb on either B cells or macrophages. The relatively small increases in expression that were generated could be demonstrated to enhance the inhibitory function of the receptor on both cell types in vitro. Overexpression on the B cell had little effect on T-independent immune responses or on the early IgM response to T-dependent immunization, but it profoundly reduced the IgG response. Overexpression of FcγRIIb on the B cell had no effect on the frequency or initial course of CIA but resulted in early resolution, a marked reduction in joint destruction, and reduced levels of anti–collagen IgG antibodies. Spontaneous SLE was also inhibited, but responses to bacterial infection were not. In contrast, overexpression of FcγRIIb on macrophages had little effect on autoimmune disease or on immune responses but resulted in reduced bacterial phagocytosis and survival in response to Streptococcus pneumoniae. Thus, increasing expression of FcγRIIb on B cells and macrophages within a physiologically relevant range has a profound effect on immune responses, as well as therapeutic implications for the treatment of autoimmune, inflammatory, and infectious diseases.
RESULTS
Modest increases in the expression of FcγRIIb on B cells enhance its inhibitory effect in vitro
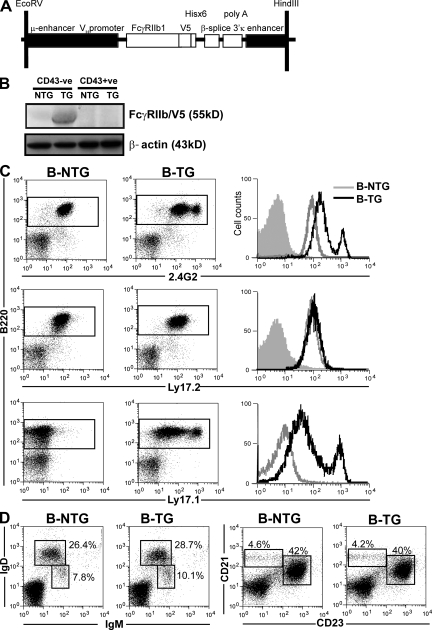
A construct was generated in which FcγRIIb cDNA was placed under the control of the human μ and VH promoter combined with the 3′ κ enhancer to achieve B cell–specific expression (29). Three independent mouse lines were generated on the C57BL/6/CBA background, as identified by screening for a V5 tag incorporated in the construct (Fig. 1, A and B). The FcγRIIb used was of the FcγRIIb1 isoform and of the Ly17.1 allotype, to allow it to be differentiated from Ly17.2, the allotype of the parental strains. Similar expression of the transgene was demonstrated in three independently derived lines, with no effect on expression of the parental FcγRIIb allotype being observed (Fig. 1 C). Expression on a second line is shown in Fig. S1 (available at http://www.jem.org/cgi/content/full/jem.20072565/DC1). The transgene was expressed in all B cell subsets, with highest expression on plasma cells, but was not expressed on other cell types, including follicular dendritic cells (FDCs; Figs. S2 and S3). The major B cell subpopulations in spleen, peripheral blood, and lymph nodes were of normal size in transgenic mice (Fig. 1 D and Table S1). All three founders showed a 2-fold increase in FcγRIIb expression over control levels in 80–90% of the B cells, but interestingly, the remaining 10–20% had an approximately 10-fold increase in expression. This subpopulation was stable throughout the lifetime of the mouse, was composed predominantly of follicular B cells evenly distributed throughout the follicles on histology (Fig. S3), and had no phenotypic or functional features to distinguish them from the larger subpopulation, apart from FcγRIIb expression (see Supplemental discussion, available at http://www.jem.org/cgi/content/full/jem.20072565/DC1).
Figure 1.
Generation of B-TG mice overexpressing FcγRIIb. (A) The construct used to direct B cell–specific expression of transgenic FcγRIIb. (B) Western blot, using a V5-specific antibody, of splenic B cell (CD43−ve) and non–B cell (CD43+ve) populations from B-TG and B-NTG mice. (C) Flow cytometric analysis of transgene expression on B220+ splenic cells (as shown in the gated populations on the dot plots) from B-NTG and B-TG mice. Histograms represent either 2.4G2 (which recognizes all FcγRIIb on B cells), Ly17.2 (endogenous FcγRIIb-specific), or Ly17.1 (transgenic FcγRIIb-specific) expression (shaded histogram = isotype control). No detectable transgene expression was demonstrated on non–B cells (Fig. S2). (D) The proportions of B cell subsets analyzed were normal in B-TG mice. Splenocytes were stained with anti-IgM, -IgD, -CD21, and -CD23 and assessed by flow cytometry (percentages of gated populations are shown; see also Table S1). B–D are representative of at least three independent experiments.
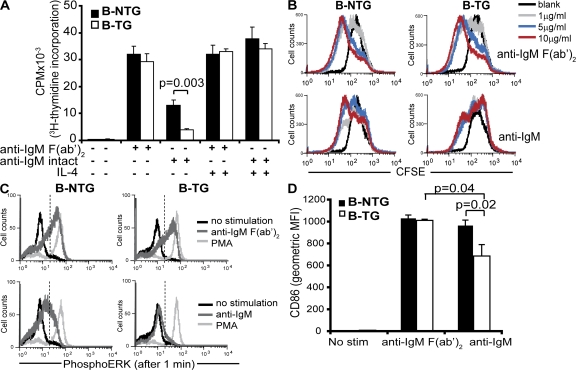
To determine if overexpression of FcγRIIb on B cells resulted in increased inhibition, B cells from B cell–transgenic (B-TG) mice were tested by several established assays of FcγRIIb inhibition. Purified B cells from B-TG mice stimulated with anti-IgM F(ab′)2, which cannot recruit FcγRIIb to the BCR, proliferated equally to controls. As expected, when FcγRIIb was cross-linked using an intact anti-IgM antibody, significant suppression was seen in control mice (30). This suppression was enhanced in the B-TG mice (Fig. 2 A). Addition of IL-4 abolished the inhibitory effect of FcγRIIb (Fig. 2 A), as previously reported (23). Another experiment with an FcγRIIb-deficient control for comparison is shown in Fig. S4 A (available at http://www.jem.org/cgi/content/full/jem.20072565/DC1). An independent measure of proliferation, the reduction of CFSE with increased cell division, was used to demonstrate the increase in activation threshold induced by expression of the transgene on B cells (Fig. 2 B). Finally, increased FcγRIIb expression almost completely abolished the generation of phospho–extracellular signal-regulated kinase (pERK) after BCR cross-linking (Fig. 2 C; quantified in Fig. S4, B and C). The induction of CD86 upon BCR cross-linking was also suppressed in B-TG mice but not in controls (Fig. 2 D). Thus, we generated independent lines of mice that expressed approximately twofold increased FcγRIIb on the majority of B cells, which resulted in an easily discernible increase in inhibitory function.
Figure 2.
Fc-mediated proliferation, ERK phosphorylation, and CD86 expression are suppressed in B-TG B cells. (A) Proliferation of B cells from B-TG and B-NTG mice after 72 h of activation with either intact anti-IgM or anti-IgM F(ab′)2 antibodies, with and without IL-4, assessed by [3H]thymidine incorporation. (B) CFSE staining of B cells from B-NTG and B-TG mice after activation with varying concentrations of either intact anti-IgM or anti-IgM F(ab′)2 antibodies for 120 h before analysis by flow cytometry. (C) Assessment of ERK phosphorylation by intracellular flow cytometry after B cell activation for 1 min with either anti-IgM intact or anti-IgM F(ab′)2 antibodies (PMA = positive control; dashed lines indicate the gate used to quantify the extent of pERK, as shown in Fig. S4). (D) CD86 expression on B-NTG and B-TG B cells after activation with anti-IgM intact or anti-IgM F(ab′)2 molecules for 48 h, analyzed by flow cytometry. Error bars in A represent SD of triplicate wells from three pooled mice; error bars in D represent SD of three mice. p-values were calculated by the Students t test. A is representative of three independent experiments; B, C, and D are representative of two independent experiments.
The predominant effect of FcγRIIb overexpression is to suppress the IgG component of T-dependent immune responses
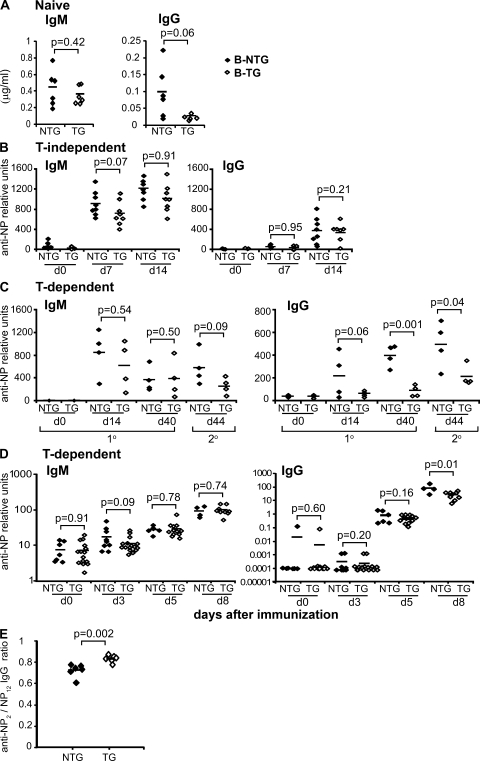
Titers of total serum IgG, but not IgM, were reduced in B-TG mice (Fig. 3 A). To investigate this, we studied the antibody responses to a range of model antigens. FcγRIIb overexpression had surprisingly little effect on T-independent immune responses (Fig. 3 B). Analysis of T-dependent immune responses showed that the IgM response was largely intact in B-TG mice. Anti–4-hydroxyl-3-nitrophenyl acetyl (NP) IgG titers were normal at day 5 of the response, but there was marked suppression of the IgG response from day 8 onwards (Fig. 3, C and D). Consistent with this, ELISPOT analysis showed little difference in the short-lived IgM and IgG plasmablast response (3). In response to secondary immunization, both IgM and IgG levels were reduced in B-TG mice compared with controls (Fig. 3 C), and we have previously described reduced bone marrow plasma cell accumulation and persistence in these mice (3). These results demonstrate that despite the fact that FcγRIIb cross-linking profoundly suppresses proliferation of IgM+ B cells in vitro (Fig. 2, A and B; and Fig. S4 A) (4, 30), IgM production is not influenced in the primary immune response in vivo. Affinity maturation was ascertained by NP2/NP12 ELISA (31) after secondary immunization. There was a significant increase in the ratio of anti-NP2 to anti-NP12 IgG in B-TG mice (Fig. 3 E), consistent with more stringent selection of high affinity B cells when FcγRIIb is increased.
Figure 3.
Overexpression of FcγRIIb on B cells suppresses the T-dependent IgG immune response. (A) Total IgG and IgM titers were measured in serum from naive B-NTG and B-TG mice. Values were calculated from a standard curve of known Ig concentration. Mice were immunized intraperitoneally with NP-haptenated carriers in alum, and antibody responses were assessed by ELISA. B-TG and B-NTG mice were injected with either T-independent (B, NP-ficoll) or T-dependent (C, NP-CGG) antigens and boosted on day 40 with NP-CGG alone or NP-KLH (D). Serum was then assessed for total anti-NP12 IgM and IgG production at various time points after immunization. (E) The affinity of anti-NP IgG was assessed by calculating the ratio of anti-NP2 (high affinity)/anti-NP12 (total) IgG (NP2/NP12) by ELISA from serum obtained from B-NTG and B-TG mice immunized with NP-KLH on day 0, boosted on day 40, and bled on day 49. Each symbol shows data from a single mouse, and the horizontal lines represent the mean. p-values were calculated using the Student's t test. A–C are representative of three independent experiments.
Increased macrophage inhibition by transgenic expression of FcγRIIb
Macrophage-specific expression of FcγRIIb was achieved using a construct in which FcγRIIb2 mRNA was placed under the control of the human CD68 promoter (32). This resulted in macrophage-specific expression in two independent lines, as demonstrated by Western blotting for the V5 tag incorporated in the construct (Fig. 4, A and B). Expression was confirmed on peritoneal macrophages (Fig. 4 C), as well as on macrophages from bone marrow and spleen, and on peritoneal macrophages from an independent strain (Fig. S5, available at http://www.jem.org/cgi/content/full/jem.20072565/DC1). Expression was not seen on dendritic cells, B cells, FDCs, or other cell types (Figs. S5 and S6). Macrophage numbers in all of these organs were normal (Table S2). To determine if the macrophage transgene was functional, Fc receptor–triggered calcium signaling, known to be inhibited by FcγRIIb (33), was assessed in Fura2-loaded peritoneal macrophages. Although there were no differences in peak intracellular calcium signal after Fc receptor aggregation, plateau calcium levels were significantly reduced in cells from transgenic mice (Fig. 4 D). Phosphorylation of ERK was also measured in peritoneal macrophages as a read out of activation after Fc receptor cross-linking. ERK phosphorylation was suppressed by expression of the transgene, in contrast to controls and to the marked increase seen in FcγRIIb-deficient mice (Fig. 4 E). Thus, a relatively modest increase in FcγRIIb expression on macrophages resulted in a significant increase in inhibitory effect in vitro.
Figure 4.
Generation of M-TG mice overexpressing functional FcγRIIb. (A) The construct used to direct macrophage-specific expression of transgenic FcγRIIb. (B) Western blot, using a V5-specific antibody, of peritoneal macrophages from M-TG (lines 1 and 2) and M-NTG mice to detect the presence of the V5-tagged transgene. All further experiments use line 2. (B) Flow cytometric analysis of transgene expression on F4/80+CD11b+ peritoneal macrophages (gated population shown on dot plot) from M-NTG and M-TG mice. Histograms represent either 2.4G2 (recognizes all FcγRIIb, III, and IV), Ly17.2 (endogenous FcγRIIb-specific), or Ly17.1 (transgenic FcγRIIb-specific) expression (shaded histogram = isotype control). No detectable transgene expression was demonstrated on other cell types (Figs. S5 and S6). (D) Intracellular calcium measurements in M-NTG and M-TG peritoneal macrophages after FcγRIIb cross-linking (arrow). Inset shows mean peak and plateau calcium responses. (E) Measurements of ERK phosphorylation in peritoneal macrophages from M-NTG, M-TG, and FcγRIIb−/− mice after FcγRIIb cross-linking. The percent increase in pERK-positive cells after stimulation is shown. Error bars represent the SD of triplicate samples from three or four pooled mice. p-values were calculated using the Student's t test. D and E are representative of two and three independent experiments, respectively.
FcγRIIb overexpression on macrophages does not influence the T-dependent immune response
Macrophage-transgenic (M-TG) mice were immunized with two T-independent antigens (NP-dextran and NP-LPS), and although a possible trend toward a reduction in IgG production was seen at later time points, initial responses to these antigens were not significantly different from control mice (Fig. S7, A and B, available at http://www.jem.org/cgi/content/full/jem.20072565/DC1). Furthermore, the T-dependent response in M-TG mice was indistinguishable from macrophage-nontransgenic (M-NTG) controls (Fig. S7 C).
Increased FcγRIIb expression on B cells, but not macrophages, modifies the course of CIA and spontaneous lupus
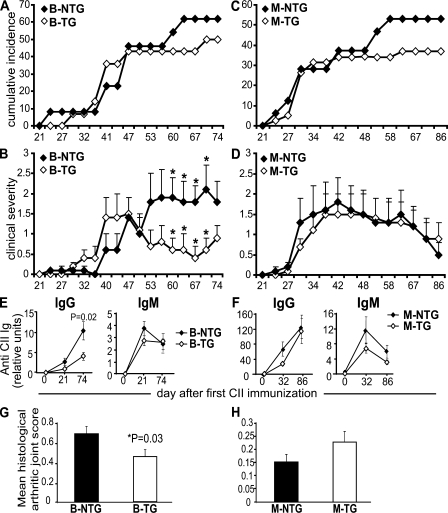
CIA is a widely used model of inflammatory joint disease. It can be induced on the C57BL/6 background using a modified chick type II collagen (CII) immunization protocol (34). B-TG mice showed the same incidence of CIA as controls, and the time of onset and early course of the inflammation were indistinguishable (Fig. 5, A and B). In contrast in the B-TG mice, however, joint inflammation resolved quickly after disease onset, and this resolution was sustained. Consistent with this, there was a reduction in joint damage, as determined by histology at day 74 in B-TG mice compared with controls (Fig. 5 G). IgG anti–collagen antibodies are important in the pathogenesis of CIA (35, 36) and are suppressed in B-TG mice, providing a possible mechanism for the reduction in inflammation (Fig. 5 E). All IgG isotypes were equally suppressed in the B-TG mice (Fig. S8, available at http://www.jem.org/cgi/content/full/jem.20072565/DC1), but IgM was not affected (Fig. 5 E). In contrast, no substantial difference in incidence, disease course, or antibody production was seen in several experiments in the M-TG mice (Fig. 5, C, D, and F), whereas there was a nonsignificant trend toward increased histological joint damage, perhaps because of a failure of macrophage remodeling and/or immune complex clearance (Fig. 5 H).
Figure 5.
Suppressed severity of CIA in B-TG but not M-TG mice. (A) Cumulative arthritis incidence and (B) mean clinical scores (±SEM) for B-TG (n = 14) compared with B-NTG (n = 13) mice over 74 d. (C) Cumulative arthritis incidence and (D) mean clinical scores (±SEM) for M-TG (n = 32) compared with M-NTG (n = 38) mice (data represent three independent experiments combined). p-values for clinical scores were calculated using the unpaired Student's t test. *, P < 0.05; no significant differences were detected in the incidence by χ2 analysis. (E and F) Circulating levels of CII-specific antibody were determined in individual sera by ELISA. Results show mean total IgG and IgM levels (±SEM) as arbitrary units per milliliter (levels of IgG isotypes are shown in Fig. S8). (G and H) At the end of the study, paws were processed for histology and scored blinded for signs of arthritis. Data represent mean ± SEM.
MRL/lpr mice develop a spontaneous autoimmune disease resembling human SLE. The disease is characterized by the development of antinuclear antibodies (ANA), glomerulonephritis, and inflammatory skin lesions (37). Although other cells play their role, the importance of B cells is highlighted by complete abrogation of disease in B cell–deficient MRL/lpr mice (38). B-TG and M-TG mice were crossed onto the MRL/lpr background (Table S3, available at http://www.jem.org/cgi/content/full/jem.20072565/DC1) and assessed for the development of autoimmunity. There was a striking reduction in mortality in the B-TG MRL/lpr mice. All B cell–nontransgenic (B-NTG) MRL/lpr mice were moribund by 34 wk of age, compared with only 14% of B-TG MRL/lpr mice (Fig. 6 A). Consistent with this protection from disease, B-TG mice had significantly lower levels of proteinuria and autoantibodies (Fig. 6, C, E, and F). In contrast, no significant differences in disease progression were observed in the M-TG mice (Fig. 6, B, D, and G).
Figure 6.
Protection from lupus in B-TG mice. B-TG and M-TG mice were crossed with MRL/lpr mice and spontaneous lupus was monitored for up to 40 wk of age. (A and B) Survival rates are shown for groups of B-TG (n = 8), B-NTG (n = 10), M-TG (n = 12), and M-NTG (n = 21) mice. (C and D) Proteinuria levels were recorded weekly in all mice throughout the study. Data are presented as mean ± SEM. p-values were calculated using the unpaired Student's t test. *, P < 0.05. (E) All mice were screened for serum levels of ANA at 20–24 wk of age using Hep-2 slides, and representative results for B-NTG and B-TG mice are shown. (F and G) Serum from each mouse was analyzed for anti-dsDNA IgG. Each symbol represents an individual mouse. Error bars represent SEM. p-values were calculated using the χ2 test (A and B) and the Student's t test (all other values).
FcγRIIb overexpression on macrophages, but not B cells, suppresses responses to bacterial infection
In view of the effect of FcγRIIb on bacterial infection in FcγRIIb-deficient mice (18), we examined the response of the transgenic mice to pneumococcal infection. In the B-TG mice, “natural” IgM antibody titers against pneumococcus were normal, and IgG was only slightly reduced (Fig. S9, available at http://www.jem.org/cgi/content/full/jem.20072565/DC1). In addition, phagocytosis of opsonized bacteria in vitro by peritoneal macrophages was also normal (unpublished data). Consistent with these findings, no effect on mortality was seen in the B-TG mice when they were challenged with pneumococcus (Fig. 7 A). In contrast, although natural antibody levels were normal (Fig. S9), M-TG mice showed a marked reduction in their ability to phagocytose opsonized pneumococci in vitro (Fig. 7 B). In keeping with this, there was a modest but consistent and significant reduction in mortality in M-TG mice after challenge with pneumococcus given either intraperitoneally or intranasally (Fig. 7, C–E).
Figure 7.
M-TG mice are more susceptible to pneumococcal infection. Survival after S. pneumoniae infection. (A) Groups of B-TG (n = 8) and B-NTG (n = 11) mice were inoculated intraperitoneally with 2 × 104 CFU S. pneumoniae type 2 (D39). (B) Peritoneal macrophages were incubated with Alexa Fluor 488–labeled S. pneumoniae either unopsonized or opsonized with heat-inactivated immune serum, followed by flow cytometric analysis. Antibody-dependent phagocytosis is expressed as the percentage of Alexa Fluor 488+ cells relative to the nonopsonized sample. Error bars represent SD of three mice per group and are representative of four independent experiments. (C) Groups of M-TG (n = 8) and M-NTG (n = 9) mice were inoculated intraperitoneally with 102 CFU S. pneumoniae type 2 (D39). A significant reduction in survival was observed for the M-TG mice (P = 0.02). (D) Graphical representation of data pooled from five separate infection experiments (including C) using various doses of S. pneumoniae (3 × 101, 102, 104, 106, and 107). Data represent mean ± SEM. The kinetics differ depending on dose; to normalize for this, data are shown as survival at the time point before 100% mortality of the M-TG mice (indicated by the gray box in C). (E) Intranasal infection of M-NTG (n = 11) and M-TG (n = 10) mice with 2 × 106 CFU S. pneumoniae type 2 (D39). p-values were calculated using the Student's t test (B and D) and the log-rank test (C).
DISCUSSION
Enhancing the expression of FcγRIIb on both B cells and macrophages was shown to increase the inhibitory effect of this receptor on both cell types. The degree of expression difference seen in both transgenic mice was less than the difference that can be induced by cytokine treatment, for example (20–24), and was thus likely to fall within the physiological range of expression of the receptor. Nonetheless there was a profound increase in inhibitory effect, as measured by several independent in vitro assays. This implies that the physiological variation in FcγRIIb expression seen in humans and mice with FcγRIIb promoter polymorphisms, and the changes in expression that can be induced by cytokines, are very likely to have substantial effects on FcγRIIb function.
The effect of FcγRIIb on B cell proliferation has traditionally been measured in vitro by cross-linking surface IgM with or without ligation of FcγRIIb. This has profound effects on the activation threshold of B cells, as shown in several previous studies (4, 30), as well as in Fig. 2. It is therefore interesting that the overexpression of FcγRIIb on B cells has little effect on T-independent responses or on IgM responses to T-dependent antigens. This, together with the fact that IgG was only markedly affected from day 8 after immunization, suggests two possible explanations. The first is that FcγRIIb-mediated suppression of B cell responses requires the production of an adequate titer of antigen-specific IgG that can cross-link FcγRIIb. Such cross-linking may not be achieved in T-independent responses, as they are dominated by IgG3 (39), which has extremely low affinity for FcγRIIb (5). The relative lack of effect on the T-dependent IgM response could reflect the fact that most IgM is made within the first week of immunization, before IgG rises. This model is also consistent with the initial normal production of anti-NP IgG at day 5, with mild differences beginning to be seen at day 8, presumably as anti-NP IgG titers have risen sufficiently to allow FcγRIIb cross-linking to occur. An alternative or additional explanation is that FcγRIIb-mediated suppression in vivo requires substantial cross-linking only achieved on the surface of the FDC. This model would predict that suppression would only occur once the germinal center (GC) reaction had become important, thereby accounting for the lack of effect on T-independent responses, where GCs play at most a minor role. In T-dependent responses, it would explain both the lack of effect on IgM (most early IgM is made by plasmablasts of the extrafollicular foci, and GCs predominantly contain isotype-switched B cells) and on early IgG, made before GC formation has become important (usually estimated to be from 6 d onwards) (40). The low affinity plasmablast response (both IgM and IgG) is controlled independently of FcγRIIb, as we have previously shown using FcγRIIb−/− mice (3). Thus, overexpression of FcγRIIb has helped define the precise stage of the immune response at which FcγRIIb-mediated suppression is important, and suggests the need for further work to elucidate the mechanism underlying this. In addition, we have presented data that suggest increased stringency of affinity maturation in B-TG mice. This suggests that an increased B cell activation threshold controls affinity maturation. Currently, we are testing this at the single-cell level, as described previously (31).
The FcγRIIb-deficient mouse was made using a C57BL/6/129 embryonic stem cell line (4). When other genes in the same region of distal chromosome 1 have been deleted, they have also shown an autoimmune phenotype (e.g., complement receptor 1/2 [41], decay accelerating factor CD55 [42], and serum amyloid P component [43]), much of which has subsequently been found to be caused by the adjacent region of 129 origin rather than to the gene deletion itself (44, 45). The effect of our transgene in suppressing autoimmunity is clearly independent of this 129 region. This confirms that FcγRIIb plays a role in controlling autoimmunity and suggests that the autoimmune phenotype of the FcγRIIb-deficient mouse is not an artifact of 129 contamination.
Antibodies have long been known to be important in the pathogenesis of CIA. Mice deficient in B cells are totally resistant to CIA induction (34), and it is possible to induce arthritis using anti-CII antibodies (36, 46). Despite B-TG mice having decreased anti–collagen antibodies, the incidence and initial course of CIA was unchanged. Rather, the suppression of anti–collagen antibodies by transgenic FcγRIIb results in early disease resolution and an improved long-term outcome. This shows that relatively modest modulation of FcγRIIb expression can have a critical effect on inflammatory arthritis despite not affecting onset. If it was possible to achieve such a change in expression pharmacologically, it would have clear therapeutic implications, particularly in humans, where preemptive therapy is rarely possible and patients present with established disease. The fact that overexpression of FcγRIIb on B cells also markedly reduces spontaneous SLE provides further encouragement that this avenue may be therapeutically useful. The effect of FcγRIIb overexpression on the macrophage, in contrast, had little effect on either CIA or spontaneous SLE. Overall, these results indicate that the B cell, rather than the macrophage, should be the primary target of therapeutic modulation of FcγRIIb in inflammatory disease.
The effect of overexpressing FcγRIIb on autoimmunity will be influenced by the fact that different Fc receptors selectively engage IgG subclasses with varying affinities (1, 47–49). FcγRI binds IgG2a with high affinity and FcγRIV binds IgG2a and IgG2b with intermediate affinity, whereas FcγRIIb and FcγRIII bind IgG2a, IgG2b, and IgG1. Moreover, the affinity with which FcγRIV binds IgG2a or IgG2b is at least twofold higher than FcγRIIb and between 20 and 40 times higher than FcγRIII (50). Thus, IgG2a and IgG2b immune complexes would preferentially bind to FcγRIV, and little effect of FcγRIIb co–cross-linking may be seen. In contrast, immune complexes containing IgG1 would more likely bind FcγRIIb and, thus, induce its inhibitory effect. CIA is predominantly associated with IgG2a anti–collagen antibodies (Fig. S5) (51), and in SLE, autoantibodies of the IgG2a subclass are also dominant (52). Thus, in the M-TG mouse, immune complexes are likely to engage FcγRIV in preference to FcγRIIb, which might explain the lack of effect of FcγRIIb overexpression on macrophages in both CIA and SLE.
Natural antibody levels against pneumococcus were similar to controls in both B-TG and M-TG mice, consistent with the fact that they were also normal in FcγRIIb-deficient mice (18). Given that pneumococcal challenge results in very rapid onset of illness before an IgG response can be initiated, it is thus not surprising that survival was normal in the B-TG mice. In M-TG mice, however, where reduced phagocytosis of pneumococcus could be demonstrated in vitro, there was a significant reduction in survival. Although of a similar magnitude, this effect is the converse of that seen with the FcγRIIb-deficient mouse (18), thus underlining the likelihood that the latter observation was caused by FcγRIIb deficiency on the macrophage. This highlights the importance of FcγRIIb in controlling initial bacterial clearance in infection. It focuses attention on the potential for manipulating expression of the receptor on macrophages to either be useful in therapy, perhaps in the treatment of septic shock (9, 18), or to be responsible for adverse effects if expression is increased in the treatment of inflammatory disease.
Cell-specific overexpression of FcγRIIb on B cells and macrophages highlights the role that therapeutic modulation of receptor expression on B cells might play in inflammatory and autoimmune disease. It has also defined the role of FcγRIIb in suppressing the T-dependent immune response, and has shown that increased expression on macrophages reduces survival after bacterial infection, demonstrating the importance of macrophage FcγRIIb in the control of infection and emphasizing the importance of careful cell-specific targeting in therapeutic modulation.
MATERIALS AND METHODS
Mice
C57BL/6 FcγRIIb−/− mice (4) were provided by J. Ravetch and S. Bolland (The Rockefeller University, New York, NY). MRL/lpr mice were purchased from Harlan. All other mice were obtained from Charles River Laboratories. All experiments were performed according to the regulations of the UK Home Office Scientific Procedures Act (1986). The animal experiments were approved by the UK Home Office.
Generation of B-TG and M-TG mice overexpressing FcγRIIb.
To direct B cell–specific expression of FcγRIIb, we used a construct containing a VH promoter, the IgH intron enhancer, and the Igκ 3′ enhancer, which was a kind gift from T. Tsubata (Tokyo Medical and Dental University, Tokyo, Japan) (29). To direct macrophage-specific expression of FcγRIIb, we used a construct containing the human CD68 (hCD68) promoter, as previously described (32). We introduced the mouse FcγRIIb.1 (Ly17.1 allotype) tagged with both the V5 and Hisx6 epitopes into these constructs. Transgenic mice were established by injecting the DNA fragment containing the tagged FcγRIIb cDNA and the respective promoters, or enhancers in the case of the B cell construct, into CBA-fertilized eggs. The presence of transgene was identified by tail DNA PCR assays (B-TG primers: 5′–3′, 5′-TTCTCGGCTCTGTGAATGACA-3′, and 3′–5′, 5′-CAGCCCTCTCTTGGAAAGGAG-3′; M-TG primers: 5′–3′, 5′-CATAGCTGGAGGAACAAACTACTGAAC-3′, and 3′–5′, 5′-GTAGAATCG AGACCGAGGAGAGG-3′). Transgenic offspring were backcrossed with C57BL/6 mice for at least seven generations. Transgenic mice were crossed to MRL/lpr mice for at least five generations using a marker-selected backcrossing method (Table S3) predicted to result in >99% MRL genetic background by N5 (53).
B cell purification and stimulation
Splenic B cells were negatively selected by magnetic cell purification using anti-CD43 beads (Miltenyi Biotec) to >90% purity. 2 × 106 cells/ml were usually cultured in 24-well flat-bottom plates (Corning Inc.) in RPMI 1640 (Sigma-Aldrich) and 10% FCS (Invitrogen), and were stimulated with either goat anti–mouse IgM μ chain–specific F(ab′)2 or intact IgG at 10 μg/ml, unless otherwise stated in the figures (Jackson ImmunoResearch Laboratories). IL-4 added to these cultures was used at 10 ng/ml (PeproTech). PMA was used at 1 ng/ml.
Peritoneal macrophages, splenic dendritic cells, and bone marrow–derived macrophages (BMDMs) and dendritic cells (BMDCs)
Resident peritoneal macrophages were harvested from mice by peritoneal lavage using 5% BSA in PBS. Splenic dendritic cells were isolated from spleens that were gently disrupted between glass slides and cut up into 5 ml of 2% BSA/PBS containing 1 mg/ml collagenase (Worthington) and 0.1 mg/ml DNase I (Roche). Suspensions were kept at room temperature for 30 min with regular pipetting before RBC lysis using ammonium chloride. Bone marrow cells were flushed with cold RPMI 1640 containing 10% FCS from the dissected tibias and femurs of NTG and TG mice. RBCs were lysed with ammonium chloride, and the remaining cells were washed three times. 2 × 106 bone marrow cells per well were seeded into six-well tissue culture plates. Cells were cultured for 7 d in the presence of 4 ng/ml of recombinant M-CSF (PeproTech) to generate BMDMs, or in the presence of 20 ng/ml of recombinant GM-CSF (PeproTech) to generate BMDCs.
Flow cytometric analysis
Cells were stained with combinations of anti-FcγRII/III–FITC and –biotin (2.4G2), anti-FcγRII (Ly17.1-FITC and Ly17.2-biotin; Cedarlane), CD19-FITC (1D3), CD22-FITC (Cy34.1), B220-allophycocyanin (APC), B220-biotin, B220-PE (RA3-6B2), IgM-FITC (R6-60.2), I-Ab–FITC (25-9-17), IgD-PE (11-26; SouthernBiotech), CD21/CD35-FITC (7G6), CD23-PE (B3B4), CD86-PE (GL1), CD3-PE (17A2), CD4-PerCP (GK1.5), CD8-biotin (RA3-6B2), CD43-biotin (S7), F4/80-RPE (MCA497PE; Serotech), CD11b-APC (M1/70), CD11c-APC, CD11c-PE (HL3), or isotype controls (all antibodies were purchased from BD Biosciences unless otherwise stated). Biotinylated antibodies were cross-linked with streptavidin conjugated to FITC, PerCP, or APC (BD Biosciences). 1% normal rat serum was used to block nonspecific antibody binding. 7-amino-actinomycin D (Invitrogen) was used to exclude dead cells. Cells were analyzed using a flow cytometer (FACSCalibur; BD Biosciences) and FCS Press software (provided by R. Hicks, University of Cambridge, Cambridge, UK).
Immunofluorescence microscopy
Spleens from B-NTG and B-TG mice were sectioned 7 μm thick using a cryostat and left to dry overnight. Sections were fixed in cold acetone for 5 min and blocked for 1 h with 5% BSA/PBS and 5% normal goat or rat serum before staining. Sections were stained in blocking medium at room temperature with 2.4G2-FITC and B220-biotin for 1 h, and then stained with streptavidin–Alexa Fluor 546 for 1 h. For FDC staining, sections were initially stained with rat anti–mouse FDCM-1 (BD Biosciences) and detected with goat anti–rat IgG Alexa Fluor 555 (absorbed against mouse Ig; Invitrogen). Sections were then stained with 2.4G2-FITC, B220-biotin, and goat anti–mouse IgG Alexa Fluor 633 (highly cross absorbed; Invitrogen), followed by streptavidin–Pacific blue (Invitrogen). Alternatively, spleens from M-NTG and M-TG mice were sectioned and stained with FDCM-1, as described, and then stained with 2.4G2-FITC and B220–Alexa Fluor 647 (BioLegend). Vectorshield hardset was used to mount sections before analysis by confocal microscopy.
Intracellular signaling measurements
Purified B cells were stimulated with either goat anti–mouse IgM μ chain–specific F(ab′)2 or intact IgG for between 1 and 30 min. Peritoneal macrophages were stimulated by incubating cells with 2.4G2-biotin before cross-linking with streptavidin for between 1 and 30 min (Dako). To determine the extent of ERK phosphorylation, stimulated cells were fixed in 4% formaldehyde for 10 min at 37°C and permeabilized by adding 100% methanol for 30 min at 4°C. Samples were incubated with phospho-p44/42 Map kinase (Thr202/Tyr204) antibody (Cell Signaling Technology) for 1 h at room temperature, washed, and stained with an Alexa Fluor 488 F(ab′)2 fragment of goat anti–rabbit IgG (H+L; Invitrogen). Cells were analyzed by flow cytometry. To determine intracellular calcium flux, macrophages were loaded with Fura2-AM ester before activation and measured by cuvette fluorimetry, as previously described (54).
Proliferation assays
2 × 106 purified B cells/ml were stimulated with anti-IgM μ chain–specific F(ab′)2 or intact antibody for 72 h. 0.5 μCi tritiated [3H]thymidine (GE Healthcare) was added for the last 8 h of culture, and incorporation was determined using a scintillation counter (PerkinElmer). For CFSE labeling, 5 × 107 purified B cells/ml were loaded with 5 μM CFSE (Invitrogen) before culturing 2 × 106 cells/ml with anti-IgM and anti-IgM F(ab′)2 molecules. The extent of CFSE labeling was analyzed by flow cytometry.
Western blot analysis
Standard Western blot analysis was performed using anti-V5–horseradish peroxidase (HRP) conjugate (Invitrogen) and chemiluminescent detection. Blots were stripped and reprobed with a β-actin–specific antibody (Abcam) as a loading control.
T-independent and T-dependent immunization
Samples of preimmune sera were taken before the initial immunization. Groups of mice were immunized intraperitoneally with 100 μg NP-ficoll, NP-dextran, NP-LPS, NP–chicken γ globulin (CGG), or NP-KLH (Biosearch Technologies) emulsified in alum (Alu-Gel-S; Serva Electrophoresis). Samples of blood from tail veins were taken at various time points after immunization. For secondary responses, mice received a further 100 μg NP-CGG or -KLH/alum on day 28 or 40. Tail bleeds were taken on day 44.
ELISA
For analysis of serum anti-NP antibodies, plates (Nunc Maxisorp; Thermo Fisher Scientific) were coated with 5 μg/ml NP12- or NP2-BSA (Biosearch Technologies) overnight (the NP2/NP12 ratio was used to assess affinity maturation). Plates were blocked by the addition of PBS/1% BSA for 1 h. Serial dilutions of sera were added. To detect specific antibody isotypes, HRP-conjugated secondary antibodies were used (SouthernBiotech). EC50 values were calculated by nonlinear regression analysis using Prism software (Microsoft). Standard curves were constructed from the pooled sera of NP-hyperimmunized mice.
Serum IgM, IgG, IgG2a, IgG2b, IgG1, and IgG3 were assayed using paired capture and HRP-conjugated antibodies (SouthernBiotech), and a standard curve of known concentration of the same mouse Ig isotype.
Anti-dsDNA antibody levels were measured by ELISA, as previously described (55). In brief, 96-well ELISA plates were coated with 5 μg/ml of calf thymus dsDNA (Sigma-Aldrich) in sodium salt citrate buffer at 37°C overnight. Plates were washed with PBS/0.05% Tween 20 and blocked with 1% BSA/PBS, and sera were added in serial dilutions starting at 1:100 and incubated for at least 1 h at room temperature. After a further wash, HRP-conjugated goat anti–mouse IgG was added. Pooled MRL/lpr serum was used as a standard curve.
Levels of antiphosphorylcholine (anti-PC) antibodies were measured by ELISA, as previously described (18). In brief, 96-well Maxisorp microtiter plates were coated with 20 μg/ml PC conjugated to BSA (Biosearch Technologies) overnight at 4°C. Plates were blocked with PBS/10% FCS and washed, and dilutions of serum in PBS/0.05% Tween 20 were added for 2 h. A positive control (pooled serum from mice immunization with Pneumovax II; Aventis-Pasteur MSD) was included on each plate. Plates were revealed with HRP-conjugated goat anti–mouse IgM and IgG.
CIA
The protocol followed to induce CIA in C57BL/6 mice was reported by Campbell et al. (34). CFA was prepared by grinding 100 mg heat-killed Mycobacterium tuberculosis (H37Ra; Difco Laboratories) in 20 ml of incomplete Freund's adjuvant (Sigma-Aldrich). An emulsion was formed by dissolving 2 mg/ml of chick CII (Sigma-Aldrich) overnight at 4°C in 10 mM of acetic acid and combining it with an equal volume of CFA. Mice were injected with 100 μl CII/CFA intradermally into the base of the tail. The same injection was repeated at day 21. Animals were assessed for redness and swelling of limbs, and a clinical score was allocated for each mouse two to three times per week for up to 86 d. The maximum score per mouse was 12. At the end of the experiment, the paws of the mice were removed, fixed, decalcified, and paraffin embedded. 5-μm sections were stained with hematoxylin and eosin and examined for histological changes of inflammation, pannus formation, and cartilage and bone damage. ELISA for antibody to CII was performed as previously described (34). HRP-conjugated goat anti–mouse IgM, IgG, IgG1, IgG2a, IgG2b, or IgG3 (SouthernBiotech) detection antibodies were used. Standard curves were constructed from the pooled sera of CII-hyperimmunized DBA/1 mice.
Proteinuria measurements
Urine protein levels were measured with Multistix 10 SG (Bayer) and scored as follows: 0 = negative; 0.5 = trace; 1 = 30 mg/dl; 2 = 100 mg/dl; 3 = 300 mg/dl; and 4 = 2,000 mg/dl or more.
ANA test
ANAs were examined using a commercial indirect fluorescent antibody assay (ANA Hep-2 slide; Cambridge Life Sciences) according to the manufacturer's instructions. Mouse serum, starting at 1:40 in PBS, was titrated across the slide. Slides were incubated for 30 min at room temperature and washed twice with PBS. Alexa Fluor 488–conjugated rabbit anti–mouse IgG antibody was added to the wells at 1:150 in PBS for 30 min at room temperature. After two more washes, slides were mounted on coverslips for blinded fluorescence microscopic analysis.
Phagocytosis and infection studies with S. pneumoniae
For phagocytosis assays, S. pneumoniae type 2 (D39; provided by J. Brown, University College London, London, UK) was cultured to log phase in Todd-Hewitt broth with 0.5% yeast extract (Oxoid Ltd.), heat inactivated at 60°C for 1 h, and labeled with Alexa Fluor 488 (Invitrogen) (18, 56). Alexa Fluor 488–labeled S. pneumoniae were incubated in PBS or heat-inactivated immune serum at 37°C for 1 h before washing. Immune serum for opsonization was obtained from mice immunized with pneumovax (Aventis-Pasteur MSD). Serum-opsonized and nonopsonized Alexa Fluor 488–labeled pneumococci were added to plastic-adhered peritoneal macrophages at 37°C (and at 4°C as a control). Adhered macrophages were washed, harvested, and analyzed by flow cytometry. Peritoneal macrophages were identified by scatter characteristics and CD11b+ F4/80+ staining. The percentage and geometric mean fluorescence of Alexa Fluor 488+ macrophages were used as measures of phagocytosis. For infection studies, groups of 7–14 B-TG or M-TG mice and littermate NTG controls were inoculated intraperitoneally with 200 μl PBS containing between 3 × 101 and 107 CFU S. pneumoniae, or intranasally with 50 μl PBS containing 2 × 106 CFU S. pneumoniae. The development of disease was monitored as previously described (57). During observation, mice were scored by a blinded observer for the presence or absence of physical signs of progressive sepsis. Mice that became moribund were considered to have reached the end point of the experiment and were killed. Survival data were analyzed using Kaplan-Meier graphs and log-rank tests.
Online supplemental material
Fig. S1 shows transgene expression in two independent strains of B-TG mice. Fig. S2 demonstrates no transgene expression on CD11b+, CD11c+, or CD3+ cells in the B-TG line. Fig. S3 shows transgenic FcγRIIb expression in spleens of B-TG mice by immunohistology. Fig. S4 demonstrates decreased Fc-mediated proliferation in B-TG B cells, and quantification of pERK measurements shown in Fig. 2 C. Fig. S5 shows that transgene expression is restricted to macrophages in two strains of M-TG mice. Fig. S6 shows no transgene expression on dendritic cells and FDCs in M-TG mice. Fig. S7 shows T-independent and T-dependent immune responses in M-TG mice. Fig. S8 shows levels of anti-CII IgG isotypes during CIA. Fig. S9 shows levels of natural anti-PC antibody. Table S1 shows absolute cell numbers of various cell populations in B-TG and B-NTG mice. Table S2 shows absolute cell numbers of various cell populations in M-TG and M-NTG mice. Table S3 lists the primers used for the MRL/lpr backcross. Supplemental discussion discusses the transgenic expression profile in B-TG mice. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20072565/DC1.
Supplemental Material
Acknowledgments
We thank J. Brown for providing S. pneumoniae, J. Walker for helpful discussions, and D. Weekly for performing the microinjections.
K.G.C. Smith is supported by the Wellcome Trust Clinician Research Leave Award (06753AIA) and is a Lister Prize Fellow. K.E. Lawlor was funded by a C.J. Martin Postdoctoral Fellowship (356267), and P.A. Lyons was funded by the Medical Research Council.
The authors declare that they have no competing financial interests.
Abbreviations used: ANA, antinuclear antibodies; B-NTG and -TG, B cell nontransgenic and transgenic, respectively; CGG, chicken γ globulin; CIA, collagen-induced arthritis; CII, type II collagen; ERK, extracellular signal-regulated kinase; FDC, follicular dendritic cell; GC, germinal center; M-NTG and -TG, macrophage nontransgenic and transgenic, respectively; NP, 4-hydroxyl-3-nitrophenyl acetyl; PC, phosphorylcholine; SLE, systemic lupus erythematosus.
A.J. Cutler's present address is Histocompatibility and Immunogenetics, NHS Blood and Transplant, Colindale Centre, London NW9 5BG, England, UK.
R.A. Floto's present address is Cystic Fibrosis and Lung Defence Unit, Papworth Hospital NHS Foundation Trust, Cambridge CB23 3RE, England, UK.
References
- 1.Nimmerjahn, F., and J.V. Ravetch. 2006. Fcgamma receptors: old friends and new family members. Immunity. 24:19–28. [DOI] [PubMed] [Google Scholar]
- 2.Ravetch, J.V., and S. Bolland. 2001. IgG Fc receptors. Annu. Rev. Immunol. 19:275–290. [DOI] [PubMed] [Google Scholar]
- 3.Xiang, Z., A.J. Cutler, R.J. Brownlie, K. Fairfax, K.E. Lawlor, E. Severinson, E.U. Walker, R.A. Manz, D.M. Tarlinton, and K.G.C. Smith. 2007. FcgammaRIIb controls bone marrow plasma cell persistence and apoptosis. Nat. Immunol. 8:419–429. [DOI] [PubMed] [Google Scholar]
- 4.Takai, T., M. Ono, M. Hikida, H. Ohmori, and J.V. Ravetch. 1996. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 379:346–349. [DOI] [PubMed] [Google Scholar]
- 5.Takai, T. 2005. Fc receptors and their role in immune regulation and autoimmunity. J. Clin. Immunol. 25:1–18. [DOI] [PubMed] [Google Scholar]
- 6.Yuasa, T., S. Kubo, T. Yoshino, A. Ujike, K. Matsumura, M. Ono, J.V. Ravetch, and T. Takai. 1999. Deletion of Fcγ receptor IIB renders H-2b mice susceptible to collagen-induced arthritis. J. Exp. Med. 189:187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clynes, R., J.S. Maizes, R. Guinamard, M. Ono, T. Takai, and J.V. Ravetch. 1999. Modulation of immune complex–induced inflammation in vivo by the coordinate expression of activation and inhibitory Fc receptors. J. Exp. Med. 189:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolland, S., Y.S. Yim, K. Tus, E.K. Wakeland, and J.V. Ravetch. 2002. Genetic modifiers of systemic lupus erythematosus in FcγRIIB−/− mice. J. Exp. Med. 195:1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pritchard, N.R., A.J. Cutler, S. Uribe, S.J. Chadban, B.J. Morley, and K.G.C. Smith. 2000. Autoimmune-prone mice share a promoter haplotype associated with reduced expression and function of the Fc receptor FcgammaRII. Curr. Biol. 10:227–230. [DOI] [PubMed] [Google Scholar]
- 10.Xiu, Y., K. Nakamura, M. Abe, N. Li, X.S. Wen, Y. Jiang, D. Zhang, H. Tsurui, S. Matsuoka, Y. Hamano, et al. 2002. Transcriptional regulation of Fcgr2b gene by polymorphic promoter region and its contribution to humoral immune responses. J. Immunol. 169:4340–4346. [DOI] [PubMed] [Google Scholar]
- 11.Rahman, Z.S., H. Niu, D. Perry, E. Wakeland, T. Manser, and L. Morel. 2007. Expression of the autoimmune Fcgr2b NZW allele fails to be upregulated in germinal center B cells and is associated with increased IgG production. Genes Immun. 8:604–612. [DOI] [PubMed] [Google Scholar]
- 12.Jiang, Y., S. Hirose, R. Sanokawa-Akakura, M. Abe, X. Mi, N. Li, Y. Miura, J. Shirai, D. Zhang, Y. Hamano, and T. Shirai. 1999. Genetically determined aberrant down-regulation of FcgammaRIIB1 in germinal center B cells associated with hyper-IgG and IgG autoantibodies in murine systemic lupus erythematosus. Int. Immunol. 11:1685–1691. [DOI] [PubMed] [Google Scholar]
- 13.Kyogoku, C., H.M. Dijstelbloem, N. Tsuchiya, Y. Hatta, H. Kato, A. Yamaguchi, T. Fukazawa, M.D. Jansen, H. Hashimoto, J.G. van de Winkel, C.G. Kallenberg, and K. Tokunaga. 2002. Fcgamma receptor gene polymorphisms in Japanese patients with systemic lupus erythematosus: contribution of FCGR2B to genetic susceptibility. Arthritis Rheum. 46:1242–1254. [DOI] [PubMed] [Google Scholar]
- 14.Su, K., H. Yang, X. Li, X. Li, A.W. Gibson, J.M. Cafardi, T. Zhou, J.C. Edberg, and R.P. Kimberly. 2007. Expression profile of FcgammaRIIb on leukocytes and its dysregulation in systemic lupus erythematosus. J. Immunol. 178:3272–3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu, Z.T., N. Tsuchiya, C. Kyogoku, J. Ohashi, Y.P. Qian, S.B. Xu, C.Z. Mao, J.Y. Chu, and K. Tokunaga. 2004. Association of Fcgamma receptor IIb polymorphism with susceptibility to systemic lupus erythematosus in Chinese: a common susceptibility gene in the Asian populations. Tissue Antigens. 63:21–27. [DOI] [PubMed] [Google Scholar]
- 16.Floto, R.A., M.R. Clatworthy, K.R. Heilbronn, D.R. Rosner, P.A. MacAry, A. Rankin, P.J. Lehner, W.H. Ouwehand, J.M. Allen, N.A. Watkins, and K.G.C. Smith. 2005. Loss of function of a lupus-associated FcgammaRIIb polymorphism through exclusion from lipid rafts. Nat. Med. 11:1056–1058. [DOI] [PubMed] [Google Scholar]
- 17.Kono, H., C. Kyogoku, T. Suzuki, N. Tsuchiya, H. Honda, K. Yamamoto, K. Tokunaga, and Z. Honda. 2005. FcgammaRIIB Ile232Thr transmembrane polymorphism associated with human systemic lupus erythematosus decreases affinity to lipid rafts and attenuates inhibitory effects on B cell receptor signaling. Hum. Mol. Genet. 14:2881–2892. [DOI] [PubMed] [Google Scholar]
- 18.Clatworthy, M.R., and K.G.C. Smith. 2004. FcγRIIb balances efficient pathogen clearance and the cytokine-mediated consequences of sepsis. J. Exp. Med. 199:717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clatworthy, M.R., L. Willcocks, B. Urban, J. Langhorne, T.N. Williams, N. Peshu, N.A. Watkins, R.A. Floto, and K.G.C. Smith. 2007. Systemic lupus erythematosus-associated defects in the inhibitory receptor FcgammaRIIb reduce susceptibility to malaria. Proc. Natl. Acad. Sci. USA. 104:7169–7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, Y., E. Masuda, M.C. Blank, K.A. Kirou, X. Gao, M.S. Park, and L. Pricop. 2005. Cytokine-mediated regulation of activating and inhibitory Fc gamma receptors in human monocytes. J. Leukoc. Biol. 77:767–776. [DOI] [PubMed] [Google Scholar]
- 21.Tridandapani, S., K. Siefker, J.L. Teillaud, J.E. Carter, M.D. Wewers, and C.L. Anderson. 2002. Regulated expression and inhibitory function of Fcgamma RIIb in human monocytic cells. J. Biol. Chem. 277:5082–5089. [DOI] [PubMed] [Google Scholar]
- 22.Pricop, L., P. Redecha, J.L. Teillaud, J. Frey, W.H. Fridman, C. Sautes-Fridman, and J.E. Salmon. 2001. Differential modulation of stimulatory and inhibitory Fc gamma receptors on human monocytes by Th1 and Th2 cytokines. J. Immunol. 166:531–537. [DOI] [PubMed] [Google Scholar]
- 23.Rudge, E.U., A.J. Cutler, N.R. Pritchard, and K.G.C. Smith. 2002. Interleukin 4 reduces expression of inhibitory receptors on B cells and abolishes CD22 and FcγRII-mediated B cell suppression. J. Exp. Med. 195:1079–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Garra, A., K.P. Rigley, M. Holman, J.B. McLaughlin, and G.G. Klaus. 1987. B-cell-stimulatory factor 1 reverses Fc receptor-mediated inhibition of B-lymphocyte activation. Proc. Natl. Acad. Sci. USA. 84:6254–6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu, Y., X. Gao, E. Masuda, P.B. Redecha, M.C. Blank, and L. Pricop. 2006. Regulated expression of FcgammaR in human dendritic cells controls cross-presentation of antigen-antibody complexes. J. Immunol. 177:8440–8447. [DOI] [PubMed] [Google Scholar]
- 26.Samuelsson, A., T.L. Towers, and J.V. Ravetch. 2001. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 291:484–486. [DOI] [PubMed] [Google Scholar]
- 27.McGaha, T.L., B. Sorrentino, and J.V. Ravetch. 2005. Restoration of tolerance in lupus by targeted inhibitory receptor expression. Science. 307:590–593. [DOI] [PubMed] [Google Scholar]
- 28.Nimmerjahn, F., and J.V. Ravetch. 2007. The antiinflammatory activity of IgG: the intravenous IgG paradox. J. Exp. Med. 204:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higuchi, T., Y. Aiba, T. Nomura, J. Matsuda, K. Mochida, M. Suzuki, H. Kikutani, T. Honjo, K. Nishioka, and T. Tsubata. 2002. Cutting Edge: Ectopic expression of CD40 ligand on B cells induces lupus-like autoimmune disease. J. Immunol. 168:9–12. [DOI] [PubMed] [Google Scholar]
- 30.Phillips, N.E., and D.C. Parker. 1983. Fc-dependent inhibition of mouse B cell activation by whole anti-mu antibodies. J. Immunol. 130:602–606. [PubMed] [Google Scholar]
- 31.Smith, K.G.C., A. Light, G.J.V. Nossal, and D.M. Tarlinton. 1997. The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. EMBO J. 16:2996–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gough, P.J., S. Gordon, and D.R. Greaves. 2001. The use of human CD68 transcriptional regulatory sequences to direct high-level expression of class A scavenger receptor in macrophages in vitro and in vivo. Immunology. 103:351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choquet, D., M. Partiseti, S. Amigorena, C. Bonnerot, W.H. Fridman, and H. Korn. 1993. Cross-linking of IgG receptors inhibits membrane immunoglobulin-stimulated calcium influx in B lymphocytes. J. Cell Biol. 121:355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campbell, I.K., J.A. Hamilton, and I.P. Wicks. 2000. Collagen-induced arthritis in C57BL/6 (H-2b) mice: new insights into an important disease model of rheumatoid arthritis. Eur. J. Immunol. 30:1568–1575. [DOI] [PubMed] [Google Scholar]
- 35.Svensson, L., J. Jirholt, R. Holmdahl, and L. Jansson. 1998. B cell-deficient mice do not develop type II collagen-induced arthritis (CIA). Clin. Exp. Immunol. 111:521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holmdahl, R., L. Jansson, A. Larsson, and R. Jonsson. 1990. Arthritis in DBA/1 mice induced with passively transferred type II collagen immune serum. Immunohistopathology and serum levels of anti-type II collagen auto-antibodies. Scand. J. Immunol. 31:147–157. [DOI] [PubMed] [Google Scholar]
- 37.Theofilopoulos, A.N., and F.J. Dixon. 1985. Murine models of systemic lupus erythematosus. Adv. Immunol. 37:269–390. [DOI] [PubMed] [Google Scholar]
- 38.Shlomchik, M.J., M.P. Madaio, D. Ni, M. Trounstein, and D. Huszar. 1994. The role of B cells in lpr/lpr-induced autoimmunity. J. Exp. Med. 180:1295–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perlmutter, R.M., D. Hansburg, D.E. Briles, R.A. Nicolotti, and J.M. Davie. 1978. Subclass restriction of murine anti-carbohydrate antibodies. J. Immunol. 121:566–572. [PubMed] [Google Scholar]
- 40.Jacob, J., R. Kassir, and G. Kelsoe. 1991. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J. Exp. Med. 173:1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu, X., N. Jiang, C. Deppong, J. Singh, G. Dolecki, D. Mao, L. Morel, and H.D. Molina. 2002. A role for the Cr2 gene in modifying autoantibody production in systemic lupus erythematosus. J. Immunol. 169:1587–1592. [DOI] [PubMed] [Google Scholar]
- 42.Miwa, T., M.A. Maldonado, L. Zhou, X. Sun, H.Y. Luo, D. Cai, V.P. Werth, M.P. Madaio, R.A. Eisenberg, and W.C. Song. 2002. Deletion of decay-accelerating factor (CD55) exacerbates autoimmune disease development in MRL/lpr mice. Am. J. Pathol. 161:1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bickerstaff, M.C., M. Botto, W.L. Hutchinson, J. Herbert, G.A. Tennent, A. Bybee, D.A. Mitchell, H.T. Cook, P.J. Butler, M.J. Walport, and M.B. Pepys. 1999. Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nat. Med. 5:694–697. [DOI] [PubMed] [Google Scholar]
- 44.Botto, M. 1998. C1q knock-out mice for the study of complement deficiency in autoimmune disease. Exp. Clin. Immunogenet. 15:231–234. [DOI] [PubMed] [Google Scholar]
- 45.Bygrave, A.E., K.L. Rose, J. Cortes-Hernandez, J. Warren, R.J. Rigby, H.T. Cook, M.J. Walport, T.J. Vyse, and M. Botto. 2004. Spontaneous autoimmunity in 129 and C57BL/6 mice–implications for autoimmunity described in gene-targeted mice. PLoS Biol. 2:E243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terato, K., K.A. Hasty, R.A. Reife, M.A. Cremer, A.H. Kang, and J.M. Stuart. 1992. Induction of arthritis with monoclonal antibodies to collagen. J. Immunol. 148:2103–2108. [PubMed] [Google Scholar]
- 47.Nimmerjahn, F., and J.V. Ravetch. 2008. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 8:34–47. [DOI] [PubMed] [Google Scholar]
- 48.Nimmerjahn, F., and J.V. Ravetch. 2005. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 310:1510–1512. [DOI] [PubMed] [Google Scholar]
- 49.Hamaguchi, Y., Y. Xiu, K. Komura, F. Nimmerjahn, and T.F. Tedder. 2006. Antibody isotype-specific engagement of Fcγ receptors regulates B lymphocyte depletion during CD20 immunotherapy. J. Exp. Med. 203:743–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nimmerjahn, F., P. Bruhns, K. Horiuchi, and J.V. Ravetch. 2005. FcgammaRIV: a novel FcR with distinct IgG subclass specificity. Immunity. 23:41–51. [DOI] [PubMed] [Google Scholar]
- 51.Watson, W.C., and A.S. Townes. 1985. Genetic susceptibility to murine collagen II autoimmune arthritis. Proposed relationship to the IgG2 autoantibody subclass response, complement C5, major histocompatibility complex (MHC) and non-MHC loci. J. Exp. Med. 162:1878–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haas, C., B. Ryffel, and M. Le Hir. 1997. IFN-gamma is essential for the development of autoimmune glomerulonephritis in MRL/lpr mice. J. Immunol. 158:5484–5491. [PubMed] [Google Scholar]
- 53.Wakeland, E., L. Morel, K. Achey, M. Yui, and J. Longmate. 1997. Speed congenics: a classic technique in the fast lane (relatively speaking). Immunol. Today. 18:472–477. [DOI] [PubMed] [Google Scholar]
- 54.Melendez, A., R.A. Floto, D.J. Gillooly, M.M. Harnett, and J.M. Allen. 1998. FcgammaRI coupling to phospholipase D initiates sphingosine kinase-mediated calcium mobilization and vesicular trafficking. J. Biol. Chem. 273:9393–9402. [DOI] [PubMed] [Google Scholar]
- 55.Sekine, H., C.M. Reilly, I.D. Molano, G. Garnier, A. Circolo, P. Ruiz, V.M. Holers, S.A. Boackle, and G.S. Gilkeson. 2001. Complement component C3 is not required for full expression of immune complex glomerulonephritis in MRL/lpr mice. J. Immunol. 166:6444–6451. [DOI] [PubMed] [Google Scholar]
- 56.Martinez, J.E., S. Romero-Steiner, T. Pilishvili, S. Barnard, J. Schinsky, D. Goldblatt, and G.M. Carlone. 1999. A flow cytometric opsonophagocytic assay for measurement of functional antibodies elicited after vaccination with the 23-valent pneumococcal polysaccharide vaccine. Clin. Diagn. Lab. Immunol. 6:581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown, J.S., A.D. Ogunniyi, M.C. Woodrow, D.W. Holden, and J.C. Paton. 2001. Immunization with components of two iron uptake ABC transporters protects mice against systemic Streptococcus pneumoniae infection. Infect. Immun. 69:6702–6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.