Abstract
Nonliving antiviral vaccines traditionally target proteins expressed at the surface of the virion with the hope of inducing neutralizing antibodies. Orthopoxviruses (OPVs), such as the human smallpox virus and the mouse-equivalent ectromelia virus (ECTV; an agent of mousepox), encode immune response modifiers (IRMs) that can increase virulence by decreasing the host immune response. We show that one of these IRMs, the type I interferon (IFN) binding protein (bp) of ECTV, is essential for ECTV virulence and is a natural target of the antibody response. More strikingly, we demonstrate that immunization with recombinant type I IFN bp protects mice from lethal mousepox. Collectively, our experiments have important implications for our understanding of the role of IRMs in OPV virulence and of type I IFNs in OPV infections. Furthermore, our work provides proof of concept that effective antiviral vaccines can be made to prevent disease by targeting virulence factors as an alternative to the traditional approach that attempts to prevent infection by virus neutralization.
The genus Orthopoxvirus (OPV) comprises a large number of morphologically identical viruses, including the agent of human smallpox variola virus (VARV), the agent of mousepox ectromelia virus (ECTV), the vaccine species vaccinia virus (VACV), and monkeypox virus, a zoonotic virus with its natural reservoir in African rodents.
Smallpox, a grave systemic disease characterized by high mortality and responsible for the death of billions over at least three millennia (1), was eradicated worldwide in the late 1970s through vaccination with VACV. However, there is fear that VARV could be used as a weapon. This would be devastating, because massive vaccination against smallpox was discontinued in 1978 and most of the human population is presently not immune. In addition, the reduced level of herd immunity against OPV increases the possibility of infection with zoonotic monkeypox virus, as exemplified by its current endemic status in central and west Africa, as well as a recent outbreak in the US Midwest (2–4). Moreover, because there are many other OPVs in nature, there is a threat of emerging OPV zoonoses.
The current smallpox vaccine uses live VACV, which is normally poorly or nonpathogenic to humans but induces cross-protective immunity against other OPVs. However, because of cases of adverse effects, including generalized infection and death, the live VACV vaccine is not safe by current standards (1, 5, 6). On the other hand, killed VACV induces antibodies but is not effective at preventing disease, perhaps because it does not induce a subset of antibodies important for protection (7–12). Therefore, there is a need to replace the current VACV-based vaccine (Dryvax) with a safer vaccine that, ideally, should be noninfectious, such as subunit vaccines based on recombinant (Rec) viral proteins or DNA. However, it will be difficult to evaluate the efficacy of new vaccines in the absence of human smallpox or information regarding the correlates of immunity. Thus, the design and testing of new types of smallpox vaccines will be facilitated by advances in our understanding of the viral pathogenesis and immunology to lethal poxvirus infections.
OPVs and other poxviruses such as the Leporipoxvirus myxoma virus (the agent of myxomatosis in the European rabbit) encode nonstructural proteins that play important roles in host specificity and virulence (13–21). Some of these proteins are secreted immune response modifiers (IRMs) that may or may not bind to the surface of cells. These secreted IRMs can either mimic (virokines) or compete (viroreceptors) with the function of chemokines, cytokines, and growth factors (22–24). Although we still lack a complete understanding of the role of IRMs in poxvirus pathogenesis, it is clear that some play an important role in the virulence of myxoma virus in rabbits and of VACV when inoculated intranasally to mice (25, 26), Because secreted IRMs are exposed to the extracellular milieu, it is very possible that they are targets of the host's antibody response. However, this has not been formally tested. Furthermore, antibodies to secreted IRMs important for virulence could be significant for protective immunity and be used as targets of novel vaccine strategies.
The OPV ECTV has host specificity for the mouse and, in susceptible strains such as BALB/c mice, produces systemic lethal mousepox when inoculated with as little as ∼1 PFU through the footpad and other routes (24). Moreover, mousepox is remarkably similar to human smallpox and can also be prevented by VACV inoculation (27, 28). Of interest, most of the IRMs in VARV are also present in ECTV. Consequently, it is very likely that they play similar roles in pathogenesis in their respective natural hosts. Thus, ECTV is an excellent model to understand the role of IRMs in pathogenesis and as possible candidates for new anti-OPV vaccines.
IFNs are proinflammatory cytokines produced in high quantities and during the early stages of viral infections. By inducing an antiviral state in cells (29) and modulating the immune response (30), IFNs are major mediators of the antiviral defense (31). Type I IFN-α and -β use a common heterodimeric receptor (IFN-α/βR), composed of IFNAR1 and IFNAR2, that is ubiquitously expressed (32). Type I IFNs can be produced by almost any infected cell, but in some viral infections are mostly produced by plasmacytoid dendritic cells (33–35). The type II IFN-γ is mainly the product of NK cells and CD8+ and CD4+ T cells, and binds to IFN-γR at the surface of cells.
IFNs play an important role in resistance to OPV infections. Mice deficient in type I and II IFNR or IFN-β are highly susceptible to VACV infection (36–39). Similarly, type I and II IFNs are essential for natural resistance to mousepox in 129 and C57BL/6 (B6) mice, as demonstrated in experiments of antibody depletion, or with mice deficient in IFN-γ, IFN α/βR, or IFN-γR (40–43).
OPVs encode IRMs that are type I and II IFN binding proteins (bp's) that compete with type I or II IFNR for their ligands, respectively (13, 44–47). In ECTV, the type I IFN bp is encoded by ECTV Moscow open reading frame no. 166 (EVM166) and is 88% identical with both the VACV and the VARV orthologues (13). However, whether the ECTV protein binds mouse IFN with similar or different affinity than the VACV protein is not known.
The role of IFN bp in OPV virulence is still not completely clear. For example, VACV deficient in the IFN-γ bp was not attenuated in mice (48). However, this is not significant because VACV IFN-γ bp does not inhibit the biological activity of mouse IFN-γ (49). On the other hand, deletion of the IFN-γ bp results in moderate attenuation of ECTV (50). In the case of the type I IFN bp, its deletion in VACV (B19R, formerly B18R) results in ∼100-fold attenuation of VACV in mice challenged through the intranasal route. However, whether the type I IFN bp plays any role in ECTV virulence has not been determined. A comparative analysis is of particular interest given the fact that ECTV is much more pathogenic for the mouse than VACV.
Vaccines provide immunity by mimicking some aspects of the natural infection. Immunity to OPV in humans and mice is characterized by the presence of circulating antiviral antibodies and relatively high frequencies of memory T and B lymphocytes specific for the virus (51–55). Experiments of passive immunization showed that either preexisting antibody or memory CD8+ T cells can protect immunocompetent mice from mousepox (28, 56) However, neither antibody nor memory CD8+ T cells transfer reduced virus replication at the site of entry or completely abrogated systemic virus spread (56). These findings are not opposed to the concept of protective immunity. Indeed, it is well established that VACV immunization does not completely prevent ECTV or VACV replication or spread in mice or in humans, respectively (28, 56). Therefore, although vaccines should ideally prevent infection, the fact that immunization with VACV does not prevent virus replication but was very effective at eradicating smallpox indicates that protective humoral immunity may use antibodies that do not neutralize the viral particles but that target secreted virulence factors. In this study, we demonstrate that the type I IFN bp is essential for ECTV virulence, a natural target of the antibody response, and constitutes an effective target for protective immunization.
RESULTS
The type I IFN bp is essential for ECTV virulence
To elucidate the importance of the type I IFN bp in ECTV virulence, we made ECTV with EVM166 deleted (Δ166), where EVM166 was replaced with enhanced GFP (EGFP; Fig. S1 A, available at http://www.jem.org/cgi/content/full/jem.20071854/DC1) (26). In addition, as a control for any defects that Δ166 may have acquired in other loci during cloning, we also generated a revertant virus (Δ166 revertant to WT [Rev166]) in which EVM166 was reintroduced to its original location in Δ166 (Fig. S1 B). Correct replacement/reinsertion of EVM166 in Δ166 and Rev166 was confirmed by amplification and sequencing of DNA fragments using primers lying outside of the homologous recombined sequences (unpublished data).
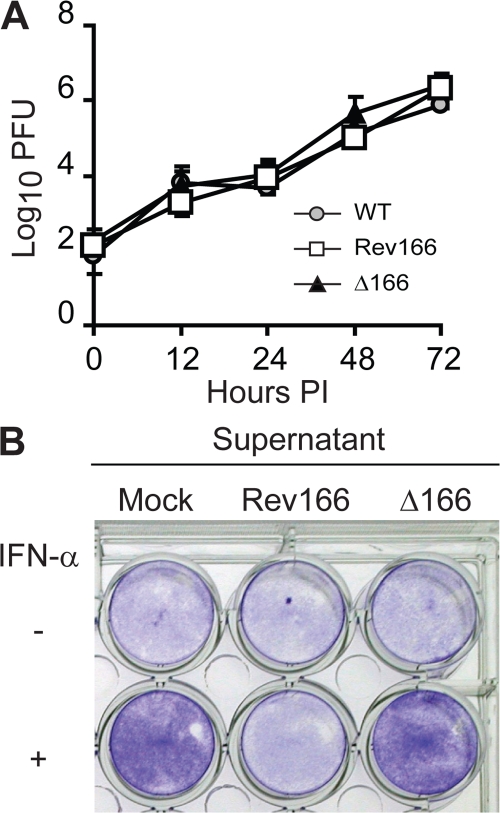
We next characterized the viruses for their ability to replicate and block the biological function of IFN-α in tissue culture. We found that ECTV Δ166, Rev166, and WT produced similar amounts of infectious virus in multistep growth curves, as determined in mouse A9 cells (Fig. 1 A), as well as in monkey BSC-1 cells (not depicted). This indicates that the type I IFN bp is not required for ECTV replication in tissue culture and is consistent with expectations for a nonstructural secreted protein that does not play a role in DNA replication or virus assembly. In addition, supernatants from cells infected with Rev166 but not from Δ166 were able to block the biological function of IFN-α, as determined in vesicular stomatitis virus (VSV) IFN protection assays (Fig. 1 B). This provides a functional confirmation of the correct deletion/reinsertion of EVM166 in Δ166 and Rev166, respectively, and indicates that other than EVM166, ECTV does not encode any secreted protein capable of blocking the function of IFN-α.
Figure 1.
In vitro characterization of Δ166 and Rev166 ECTV. (A) Mouse fibrosarcoma A9 cells were infected with 0.01 PFU/cell of the indicated viruses. Cell-associated and free virus were determined at the indicated times after infection. Data points are means ± SD of three replicate wells. (B) Confluent L929 cells in 24-well plates were treated with 200 μl of supernatants from BSC-1 cells infected as indicated. After 1 h of incubation, 300 μl of media with or without 50 IU IFN-α was added. The cells were incubated for an additional 24 h, followed by infection with 10 PFU/cell VSV. After 24 h of incubation, the cells were washed, fixed, and stained with crystal violet.
The natural route of ECTV entry is through microabrasions of the footpad (57). Via this route, it first multiplies at the site of entry followed by spread to and replication in the local draining lymph node (D-LN). From there, ECTV follows the efferent lymphatic vessels to reach the blood and then visceral organs, such as the liver and the spleen (57). In mousepox-susceptible BALB/c mice, the massive replication of ECTV in the liver results in acute liver necrosis and death within 8–11 d post infection (PI). Any BALB/c mouse that survives this phase develops a typical skin exanthema and experiences weight loss of 25–30% of its initial body weight (28, 56, 58). To determine whether the type I IFN bp is important for efficient ECTV replication and spread in vivo, we infected BALB/c mice in the footpad with 300 PFU of ECTV Δ166, Rev166, or WT. 3, 5, and 7 d PI, we quantified virus loads in the infected footpad to determine local replication, and the spleen and liver to determine spread (Fig. 2 A). The results showed that the type I IFN bp is not very important for ECTV replication at the initial site of infection, because at every time PI, only minor differences in virus loads of the three viruses were found in the infected footpads. However, the virus loads of Δ166 were ∼104- and ∼103-fold lower in spleen and liver, respectively, than those of WT or Rev166, indicating that the type I IFN bp is required for the efficient spread of ECTV to visceral organs. Consistent with these results, infection with Δ166 resulted in decreased anatomical pathology in organs. Macroscopic observation showed that, as expected, the D-LNs (left popliteal) and spleens of mice infected with WT ECTV were reduced in size compared with naive mice, and their spleens and livers were necrotic. Conversely, the D-LNs and spleens of mice infected with Δ166 were enlarged, and their livers had a normal appearance (Fig. 2 B).
Figure 2.
ECTV Δ166 is avirulent in immunocompetent mice but virulent in IFNAR1-deficient mice. (A) BALB/c mice were infected with 300 PFU of the indicated viruses. Virus titers in the infected footpad and spleen were determined at the indicated times. Data points are means ± SD of three mice. (B) BALB/c mice were infected with the indicated viruses, and organs were harvested and photographed 7 d PI. (C) BALB/c mice were infected in the footpad with 300 PFU of the indicated viruses and observed for survival. Data correspond to five mice per group. (D) IFNAR1-deficient or control 129S2/SvPasCrl mice were infected with 300 PFU of the indicated viruses and observed for survival. Data correspond to five mice per group.
To further determine the role of the type I IFN bp in ECTV virulence, we infected BALB/c mice with 300 PFU of ECTV Δ166, Rev166, or WT and determined survival. As an additional control, we also infected a group of mice with ECTV 189898-p7.5-EGFP, which has EGFP inserted in a noncoding region of the ECTV genome (59). Strikingly, all of the mice infected with ECTV Δ166 survived, whereas all of those infected with Rev166, 189898-p7.5-EGFP, or WT ECTV died (Fig. 2 C). Moreover, none of the BALB/c mice infected with Δ166 demonstrated overt symptoms of mousepox or major weight loss (unpublished data). In additional experiments, we found that the dose of ECTV WT, Rev166, or 189898-p7.5-EGFP that killed 50% of BALB/c mice was <5 PFU. On the other hand, no deaths were observed in BALB/c mice infected with up to 5 × 107 PFU Δ166, the maximum dose that our virus stock permitted (Table I). Thus, from these experiments we conclude that Δ166 is attenuated at least 107-fold. Because the LD50s of Rev166, 189898-p7.5-EGFP, and WT ECTV were comparable, we also conclude that the avirulence of Δ166 is caused by the absence of the type I IFN bp and not other factors such as expression of EGFP. Collectively, these results demonstrate that the type I IFN bp is essential for ECTV virulence.
Table I.
Lethality of different ECTV isolates
| Experiment | Virus | Dose (PFU) | Dead/total |
|---|---|---|---|
| 1 | Rev166 | 0.1 | 2/6 |
| 1 | 3/6 | ||
| 300 | 5/5 | ||
| WT | 0.1 | 2/6 | |
| 1 | 4/4 | ||
| 10 | 4/4 | ||
| 300 | 5/5 | ||
| Δ166 | 300 | 0/5 | |
| 106 | 0/5 | ||
| 107 | 0/4 | ||
| 5 × 107 | 0/3 | ||
| 2 | Rev166 | 0.1 | 1/6 |
| 1 | 2/6 | ||
| WT | 1 | 6/6 | |
| Δ166 | 5 × 107 | 0/4 | |
| 3 | Rev166 | 0.2 | 2/6 |
| 2 | 6/6 | ||
| WT | 0.2 | 5/6 | |
| Δ166 | 5 × 107 | 0/4 | |
| 189898-p7.5-EGFP | 0.5 | 2/6 | |
| 5 | 6/6 |
Mice were infected with the indicated viruses serially diluted in PBS from stocks. For experiments 1 and 2, the dose was calculated according to the virus titer determined in the virus stock. For experiment 3, final dilutions were retitered. Based on the results, we estimate LD50 values for the different viruses as follows: Rev166, <2; WT, <0.2; p7.5-EGFP, <5; Δ166, >5 × 107. These experiments are independent of those in Fig. 2 C.
Although the data presented in this section demonstrate that ECTV is avirulent without the type I IFN bp, it is possible that an unknown function of the type I IFN bp (other than type I IFN blockade) was responsible for the phenotype. Similar to B6 mice, 129/Sv mice are naturally resistant to mousepox after infection with WT ECTV. However, 129 mice deficient in IFNAR1 (a component of the IFN-α/βR) rapidly die from WT ECTV infection (43). Importantly, we found that Δ166 regained virulence in IFNAR1-deficient 129/Sv mice (Fig. 2 D). This result strongly suggests that Δ166 ECTV is avirulent in BALB/c mice because it is unable to block the antiviral effects of type I IFN.
The type I IFN bp decreases anti-ECTV innate and adaptive immunity
We and others have shown that T cells are essential for the natural resistance of B6 mice to mousepox (55, 60–62) and that a very strong T cell response can be detected in the D-LN of B6 mice 5 d PI (63). We have also recently shown that, different from B6 mice, the T cell response to WT ECTV in the D-LN of mousepox-susceptible naive BALB/c mice is almost undetectable (56). Interestingly, naive BALB/c mice mounted a very strong CD8+ T cell response to Δ166 in the popliteal D-LN but, as expected (56, 64), not to Rev166 (Fig. 3 A). A similar observation was made for the CD4+ T cell response (unpublished data). In addition, we also found an increase in the D-LN T cell response to Δ166 as compared with Rev166 in mousepox-resistant B6 mice (unpublished data). We next tested whether the type I IFN bp may also affect innate immunity. When RAG-1–deficient mice (which lack adaptive immunity) were infected with Δ166, they died from mousepox, but several days later than mice infected with Rev166 (Fig. 3 B), indicating that in addition to adaptive immunity, the type I IFN bp affects innate immunity. We reasoned that NK cells might be one component of innate immunity affected by the type I IFN bp because we and others have shown that NK cells are essential for resistance to mousepox (59, 62, 65). In addition, recent publications from our laboratory and from Parker et al. (59, 65) showed that on day 2 PI, ECTV recruits NK cells to the D-LN. We found that the proportion of NK cells was four times higher in the D-LN of mice infected with ECTV Δ166 as compared with those infected with Rev166 (Fig. 3 C) or WT (not depicted), indicating that one effect of the type I IFN bp on innate immunity is to reduce the recruitment of NK cells to the D-LN. However, the type I IFN bp must also affect components of innate immunity other than NK cells (e.g., macrophages and/or polymorphonuclear cells), because RAG-1–deficient mice depleted of NK cells with anti-Asialo GM1 antibody and infected with ECTV Δ166 succumbed to mousepox later than those infected with Rev166 (Fig. 3 D).
Figure 3.
ECTV type I IFN bp affects adaptive and innate immunity. (A) Mice were infected with the indicated viruses. 5 d PI, anti-ECTV CD8+ T cell responses were determined in the popliteal D-LN. Plots are gated on CD8+ cells. Data correspond to a pool of three lymph nodes and are representative of three similar experiments. Axes are log10 of fluorescence intensity. Percentages of cells are shown. (B) RAG-1–deficient mice were infected with the indicated viruses and observed for survival. Data correspond to five mice per group. (C) BALB/c mice were infected with the indicated viruses. 3 d PI, cells from the D-LN and contralateral non–DLN (ND-LN) were stained as indicated. Data correspond to a pool of three lymph nodes and are representative of two experiments. Axes are log10 of fluorescence intensity. Percentages of cells are shown. (D) RAG-1–deficient mice depleted of NK cells using anti-Asialo GM1 antibody were infected with the indicated viruses and observed for survival. Data correspond to five mice per group.
The ECTV type I IFN bp binds to mouse IFN-α tightly, blocks its biological function in tissue culture, and complements Δ166 in vivo
To further study the role of the type I IFN bp in ECTV pathogenesis and immunity, we cloned and produced in Escherichia coli a Rec His-tagged ECTV type I IFN bp, which was recovered from inclusion bodies, purified by Ni2+ affinity chromatography, and refolded as previously described for the ECTV EVM135 protein (64). Because the OPV type I IFN bp is predicted to be glycosylated (which potentially could affect its binding to IFN-α), we also produced and purified His-tagged ECTV and VACV type I IFN bp using a baculovirus system, as previously published (66). Irrespective of the viral origin or method of production, all Rec proteins bound to mouse IFN-α with very similar kinetics, as determined by surface plasmon resonance (SPR), and were characterized by a very slow Koff (indicating a very tight binding) and a high affinity (Fig. 4 A). The mean Kd values for two independent experiments for the ECTV protein expressed in E. coli and the VACV protein expressed in the baculovirus system were 25.1 and 17.1, with χ2 values of 0.8 and 0.9, respectively. We were unable to fit the binding data for the ECTV protein expressed in the baculovirus system to the 1:1 Langmuir binding model. This was caused by the very slow off rates, particularly at the lower concentrations of IFN-α (Fig. 4 A, right). However, a partial fit for the Koff and Kon on this interaction was obtained for each of the two highest concentrations of IFN-α. In each case, the calculated Kd was in the nanomolar range or below, indicating that the interaction of the ECTV protein produced in the baculovirus system was similar in its dissociation constant to the interaction of IFN-α with the ECTV protein expressed in E. coli. Importantly, the Kd values that we obtained were consistent with those reported by Symons et al. for the binding of the VACV protein to mouse type I IFNs, which was ∼10-fold lower than the binding of the VACV protein to human type I IFNs (45). Overall, the SPR experiments indicate that the VACV and ECTV type I IFN bp are similar in their ability to bind to mouse IFN-α. Because the protein produced in E. coli bound with similar kinetics as the proteins produced in the baculovirus system, the data also indicate that glycosylation of the type I IFN bp is not required for effective binding to mouse IFN-α. All subsequent experiments in the study were thus performed with the protein expressed in E. coli, which is easier and less expensive to generate in large quantities than the protein produced in the baculovirus system. Of interest, we also found that Rec type I IFN bp inactivated the biological activity of IFN-α in VSV IFN protection assays (Fig. 4 B, left). However, it did not block the biological activity of mouse IFN-β (Fig. 4 B, right), which is consistent with a previous study (44). Interestingly, when BALB/c mice were infected with Δ166 and also inoculated with Rec type I IFN bp, they died from mousepox (Fig. 4 C), but not when inoculated with Rec type I IFN bp alone. This demonstrated that, in addition to binding and blocking the biological function of IFN-α in vitro, the Rec type I IFN bp can complement the defect of Δ166 in vivo.
Figure 4.
ECTV type I IFN bp binds to mouse IFN-α with high affinity, blocks its biological activity in tissue culture, and complements Δ166 in vivo. (A) SPR sensorgrams for the interaction of mouse IFN-α 3 with the indicated Rec type I IFN bp. Values on the y axes are arbitrary response units. Continuous lines correspond to the experimental values after subtracting the control sensorgram; dashed lines correspond to the data fitted to a 1:1 Langmuir binding model. The graph corresponding to the ECTV protein produced in the baculovirus system (right) does not have dashed lines because the data could not be fitted to the 1:1 Langmuir model due to a slow Koff. Data are representative of two identical experiments. (B) MEFs were incubated with the indicated amounts of IFN-α or -β for 24 h in the presence or absence of the indicated amounts of Rec ECTV type I IFN bp, followed by infection with VSV. After an additional 24-h incubation, the cells were fixed and stained with crystal violet. Data are representative of two identical experiments. (C) Mice were inoculated i.v. with 50 μg of Rec ECTV type I IFN bp immediately, and 3 d PI with ECTV Δ166. A group of control-infected mice received PBS instead of Rec ECTV type I IFN bp, whereas a second control group received Rec ECTV type I IFN bp but remained uninfected. Survival was monitored. Data are representative of three experiments.
The type I IFN bp is a natural target of the anti-OPV antibody response
Infection with OPVs are well known to induce a strong antibody response against multiple structural components of the virions (55, 64, 67, 68). Consistent with this, antisera to ECTV WT, Δ166, or Rev166 contained antibodies that recognized purified ECTV virions (Fig. 5 A). However, whether secreted IRMs can also be targets of the antibody response is not known. This question is important because antibodies capable of blocking the function of IRMs essential for virus virulence could contribute to protective immunity. To elucidate whether the type I IFN bp is a natural target of the antibody response, we used Rec type I IFN bp as antigen in ELISA to demonstrate that B6 mice that recovered from infection with Rev166 but not with Δ166 had high titer antibodies to type I IFN bp (Fig. 5 B, compare open squares with closed triangles), indicating that the type I IFN bp is a natural target of anti-ECTV immunity. Sera from BALB/c mice that had been infected with VACV also reacted with Rec type I IFN bp (Fig. 5 B, closed circles), demonstrating that VACV infection induces antibodies to the VACV homologue (B19R) and that antibodies to the two proteins are cross-reactive. Even more important, sera from mice that recovered from Rev166 but not from Δ166 infection had the capacity to block the biological activity of Rec type I IFN bp in tissue culture (Fig. 5 C), suggesting that antibodies to the type I IFN bp may have protective effects in vivo.
Figure 5.
The OPV type I IFN bp is a natural target of the antibody response and can be used as an effective vaccine. (A) Sera from mice infected with the indicated viruses were tested for anti-ECTV antibodies in ELISA using purified virus particles as antigen bound to the plate. (B) As in A, but using Rec ECTV type I IFN bp as antigen. (C) MEFs were treated for 24 h with 20 IU IFN-α in the presence or absence of the indicated amounts of Rec ECTV type I IFN bp and/or sera from ECTV Δ166 or Rev166 immune mice. MEFs were next infected with VSV, incubated for 24 h, and fixed and stained with crystal violet. (D) BALB/c mice were immunized and boosted twice with Rec ECTV type I IFN bp or control Rec human HER2/neu produced in E. coli. As additional controls, BALB/c mice were infected with Δ166, and B6 mice were infected with Rev166. 3 wk after the last boost or infection, sera was collected and anti-ECTV type I IFN bp was determined by ELISA. (E) The immunized mice in D were challenged with 300 PFU of WT ECTV and survival was monitored. Data correspond to five mice per group and are representative of three identical experiments.
Immunization with Rec ECTV type I IFN bp prevents lethal mousepox
Given that the type I IFN bp is essential for ECTV virulence in BALB/c mice and that antibodies can neutralize its biological effect in vitro, we tested whether immunization with Rec type I IFN bp induced a protective immune response. Sera from BALB/c mice immunized three times with Rec type I IFN bp had high antibody titers to ECTV type I IFN bp (Fig. 5 D), and also neutralized the biological activity of Rec type I IFN bp but not ECTV infectivity in tissue culture (not depicted). Strikingly, all BALB/c mice immunized with Rec type I IFN bp and challenged with 300 PFU ECTV WT survived the infection with minimal weight loss (∼10%), whereas all mice immunized with control Rec human Her2/neu died (Fig. 5 E). Thus, anti–type I IFN bp antibodies protect from mousepox, and Rec type I IFN bp can be successfully used as a vaccine to prevent it.
DISCUSSION
Three major conclusions can be drawn from the data described in this paper: (a) the type I IFN bp is essential for ECTV virulence, (b) the type I IFN bp is a natural target of the anti-OPV immune response, and (c) the type I IFN bp is an effective target to immunize against mousepox.
In regard to the role of the type I IFN bp in virulence, it is interesting to note that VACV lacking the type I IFN bp was found to be attenuated ∼100-fold in mice infected through the intranasal route (45). This attenuation, however, was modest when compared with the >107-fold attenuation of ECTV Δ166 inoculated via the footpad, as reported in this paper. This difference is surprising given that the VACV and ECTV proteins bind mouse IFN-α with similar affinities and that both fail to block the biological activity of mouse IFN-β (Fig. 4) (44). Whatever the reason, our demonstration that Δ166 is extremely attenuated further stresses the need to explore the role of virulence factors in animal species that have a natural susceptibility to the virus in question. The finding that deletion of only one protein that is nonessential for virus replication results in a virus that is attenuated >107-fold is very surprising and indicative of the important role of the type I IFN bp in ECTV virulence. A remaining question is whether the type I IFN bp is unique among IRMs and is the only Achilles' heel of ECTV virulence, or whether other IRMs will also have such an essential role. This question can be answered by testing other ECTV IRMs. In this regard, it has recently been shown that ECTV lacking the IFN-γ bp (EVM158) is only mildly attenuated because it spread substantially in vivo, and because it induced mousepox and some deaths in susceptible BALB/c mice even at doses of 500 PFU (50). Another important question is whether the type I IFN bp, which is conserved among most OPVs, is also essential for the virulence of other OPVs in their natural hosts or whether different IRMs will be essential for each virus–host interaction. This question will be more difficult to address, as it requires matching viruses with natural hosts.
IFN-α and -β are known to use the same signaling pathway to induce the antiviral state in cells (29). Because the type I IFN bp inactivates mouse IFN-α but not -β, and because no other IFN-β blocking activity was detected in supernatants of ECTV-infected cells (44), the extreme attenuation of Δ166 is intriguing. Several possibilities could explain this conundrum, all of them enticing. One possibility is that besides IFN-α binding, the ECTV type I IFN bp protein has a second function that is essential for virulence. This second function, however, would also have to be performed in the extracellular environment, because inoculation of the Rec protein rescued virulence of Δ166 and antibodies to the type I IFN bp induced by immunization prevented mousepox after infection with WT ECTV. However, our finding that infection with Δ166 is lethal to IFNAR1-deficient mice strongly argues that it is the IFN-α binding function of the type I IFN bp that is essential for virulence. Another possibility is that IFN-β is not produced or produced in insufficient quantities during ECTV infection. This possibility seems unlikely because IFN-β–deficient mice were found to be very sensitive to intranasal VACV infection (39). Finally it is also possible that the in vivo antiviral functions of IFN-α and -β are nonoverlapping.
The attenuation of Δ166 was accompanied by a major increase in the magnitude of the innate and adaptive immune responses in the D-LN, revealing the important role of the type I IFN bp in modifying the anti-ECTV immune response. This increased immunogenicity may be the result of increased IFN-α signaling to immune cells in the absence of type I IFN bp. Alternatively, the presence of fully active IFN-α during infection with Δ166 may directly affect the level of virus replication, and this may determine the level of activation of the various arms of the immune system.
Because of the risks of emerging OPV infections and OPV weaponization, and because the VACV-based vaccine is not safe by current standards, there is a considerable interest in developing novel anti-OPV vaccines (64, 69–76). In this context, our finding that the type I IFN bp is a natural target of the antibody response indicates that deletion of IRMs from live vaccines may be a double-edged sword. On one hand, it may increase the safety of the vaccine. On the other hand, it is also possible that these deletions will decrease vaccine efficacy because, as we now know, the type I IFN bp of OPVs is a natural target of the antibody response and is cross-reactive. Thus, it is very possible that antibodies to type I IFN bp and other IRMs may substantially contribute to the protective effects induced by live VACV vaccination, and may be one of the reasons why immunization with killed virions is not very effective at inducing protection (77).
Vaccines against pathogen-secreted proteins are in use for bacterial diseases (e.g., tetanus and anthrax). However, the use of secreted virulence factors as vaccines to prevent viral diseases is unheard of. Thus, our work provides the framework for a novel approach to antiviral vaccination using nonstructural virulence factors as targets. Interestingly, over three decades ago it was reported that a soluble early antigen expressed by VACV-infected cells could be used to induce skin resistance to VACV in rabbits (78). Remarkably, this soluble antigen was later found to contain the type I IFN bp (79, 80). Its possible use as a vaccine, however, was never explored further. It could be argued that such an approach may not be useful because it prevents disease but does not provide sterilizing immunity, thus maintaining the possibility of virus spread. This argument can be contested because one of the biggest successes in medical history, VACV immunization, does not provide sterilizing immunity either to humans or mice (1, 56). In the case of subunit vaccines against OPVs, all approaches thus far have focused on structural proteins, with some success (64, 69–71, 73). Given the strong protection induced by Rec type I IFN bp, we now propose that combining essential IRMs and structural proteins in new vaccine formulations may be the best approach for an effective and safe subunit vaccine to OPVs and, possibly, other viruses. In addition, our experiments open the possibility that the type I IFN bp of OPVs could be used as a pharmacological target to prevent disease in individuals exposed or at risk of exposure to pathogenic OPVs.
MATERIALS AND METHODS
Mice.
BALB/c mice were purchased from the National Cancer Institute (NCI). RAG1-deficient mice on a B6 background were originally purchased from the Jackson Laboratory and bred at FCCC. B6 mice were obtained from the FCCC colony. IFNAR1-deficient mice in a 129S2/SvPas background, as previously described (81), were bred at FCCC from breeders obtained from R. Schreiber (Washington University Medical School, St. Louis, MO). Control 129S2/SvPas mice were purchased from Charles River Laboratories. All mice were used in experiments when they were 5–12 wk old. All protocols involving mice were approved by the FCCC Institutional Animal Care and Use Committee.
Media and cells.
Media and cells were as previously described (55, 56, 63, 64), except for mouse embryonic fibroblasts (MEFs) that were prepared according to standard methods (82) from day 12–14 embryos and maintained in DMEM containing 15% FBS and antibiotics.
Viruses.
Stocks of WT VACV Western Reserve strain were produced in tissue culture, as previously described (55, 56, 63, 64). Stocks of ECTV were produced in A9 cells (American Type Culture Collection). When indicated in the figures, ECTV was purified over a 36% sucrose cushion, as previously described (83), but from spleen and liver homogenates from infected BALB/c mice (7 d PI) instead of cell lysates. For ECTV multistep growth curves, BS-C-1 or A9 cells were infected with ECTV at an multiplicity of infection of 0.01 for 1 h. Unabsorbed virus was removed by washing the cells with PBS three times, and the cells were incubated at 37°C/5% CO2 in 3 ml DMEM containing 2.5% FBS. At various times PI, supernatants and cells were harvested separately and virus titers were determined. All growth analyses were performed in triplicate. VACV and ECTV titers in stocks, tissues, and cells were as previously described (55, 56, 63, 64). VSV Indiana strain (provided by J.T. Guo, Drexel Institute for Biotechnology and Virology Research, Doylestown, PA) was expanded in A9 cells, and the viral yield was determined by a plaque assay on Vero cells. ECTV 189898-p7.5-EGFP was previously described (59). To generate ECTV Δ166, we used an adaptation of the method of homologous recombination (model in Fig. S1 A) (26, 59). Briefly, a targeting vector was made with the 5′ 300 base pair of ECTV EVM166 corresponding to nucleotides 187856–188155 of the ECTV Moscow genome (13), the early/late OPV 7.5 promoter, the complete sequence of EGFP, and the 3′ 300 base pair of EVM166 corresponding to nucleotides 188633–188932 of the ECTV Moscow genome, in that order, were inserted into plasmid (Bluescript II SK+; Stratagene). This targeting vector was used to transfect mouse A9 cells using Lipofectamine 2000, according to the manufacturer's instructions (Invitrogen). The transfected cells were infected with 0.3 PFU/cell of WT ECTV Moscow strain in sixwell plates. 2 d later, transfected/infected A9 cells were harvested using a rubber policeman, frozen, and thawed, and different dilutions of cell lysates were used to infect BSC-1 cells in sixwell plates. 2 h after infection, the cells were overlaid with media containing 0.5% agarose. 4 d later, green fluorescent plaques (Fig. S1 C) were picked with a pipette tip and used to infect a new set of cells. The purification procedure was iterated five times until all plaques were fluorescent. The viral DNA from ∼300 base pair downstream and upstream of EVM166 was amplified by PCR, cloned, and sequenced. An isolate with the correct sequence was expanded for subsequent experiments with the same methods as for WT virus. To generate Rev166 (Fig. S1 B), a procedure similar to that for Δ166 was used, but the targeting vector contained the entire coding sequence of EVM166 and the target virus was Δ166. Nonfluorescent plaques were amplified (Fig. S1 C).
Infections and immunizations.
For mouse infections with ECTV, mice were inoculated with 300 PFU of the various ECTV in the left hind footpad in 50 μl PBS. It should be noted that in the past (64), we have used BALB/c mice from the FCCC stock in which 20–40% survival was frequently observed with 3,000 PFU ECTV WT, whereas the LD50 that we recently determined for BALB/c mice from NCI (as well as from the Jackson Laboratory) is <5 PFU, and 5 PFU is 100% lethal. The reason for this difference in susceptibility to lethal mousepox in BALB/c mice from different origins is unknown. Mice were monitored for signs of mousepox and death, and VACV infection was performed as previously described (55, 56, 63, 64). For NK cell depletions, mice were inoculated intraperitoneally with 10 μl of anti-Asialo GM1 antibody (Wako Pure Chemical Industries, Ltd.) 1 d before viral infection. For immunizations, male BALB/c mice were immunized every other week, three times with 20 μg of Rec type I IFN bp or Rec human HER2/neu emulsified in IFA. 3 wk after the last immunization, the mice were bled to determine antibody in serum and were infected with WT ECTV 2 d later. For VACV immunizations, mice were infected IP with 5 × 106 PFU.
Flow cytometry.
Flow cytometry was performed as previously described (55, 56, 59, 63, 64).
Production and use of Rec proteins.
The coding sequence for the ECTV type I IFN bp without the signal peptide (residues 1–31) was amplified by PCR from viral DNA and inserted into vector pET28 (Novagen) with a C-terminal His tag. Expression and refolding of Rec ECTV type I IFN bp was as previously described for EVM135 (64), except that the removal of urea was performed by serial dialysis overnight against 6, 4, 2, 1, and, finally, 0 M urea at room temperature. The full-length, His-tagged ECTV and VACV type I IFN bp's were expressed in a baculovirus system and purified by Ni2+ affinity chromatography, as previously described for other VACV proteins (66).
ELISA.
To determine antibodies to ECTV type I IFN bp in sera from infected or immunized mice, 96-well flat-bottom radio immunoassay/enzyme immunoassay plates (Corning) were coated with 100 ng of Rec ECTV type I IFN bp or 1,000 PFU of sucrose-purified ECTV in 0.1 ml PBS at 4°C overnight. Plates were blocked for 2 h at 37°C with PBS containing 1% BSA. Mice sera were serially diluted in PBS containing 0.5% BSA and 0.05% Tween 20, and 0.1 ml was added to each well. The plates were incubated for 1 h at 37°C and washed three times with PBST (PBS with 0.05% Tween 20). 0.1 ml of peroxidase-conjugated affinity-purified goat anti–mouse IgG (γ chain specific; KPL) was added to each well diluted 1:2,000 in PBST, incubated for 0.5 h at 37°C, and washed five times with PBST. 50 μl of SureBlue TMB 1-Component Microwell Peroxidase Substrate (KPL) was added to each well, and the reaction was stopped by the addition of 0.1 ml of 0.12 M HCl. The OD at 450 nm was determined using a microplate spectrophotometer (μQuant; Bio-Tec).
VSV inhibition assays.
To determine the biological activity of Rec ECTV type I IFN bp in vitro, confluent MEFs or L929 cells in 24-well plates were treated with either 200 μl of supernatants (passed through a 0.1-μM pore filter) from BSC-1 cells infected for 5 d with the indicated ECTV (1 PFU/cell), or with the amounts of Rec ECTV type I IFN bp indicated in the figures. The cells were incubated for 2 h at 37°C in 0.5 ml DMEM with 10% FBS, followed by the addition of the amounts of mouse IFN-α 3 or -β (PBL Interferon Source) indicated in the figures. After overnight incubation, the cells were infected with the indicated PFU of VSV in 0.5 ml DMEM with 2.5% FBS. After 1 h of incubation, the media was replaced with 1 ml of complete DMEM with 10% FBS, and the cells were incubated overnight. The cells were washed twice with PBS, and the monolayer was visualized by fixing with methanol and staining with 1% crystal violet. To determine neutralization of the biological activity of Rec ECTV type I IFN bp with immune sera, the VSV inhibition assay was performed in the presence of the indicated amounts (as shown in the figures) of heat-inactivated (56°C for 30 min) sera from infected (3 wk PI) or Rec ECTV type I IFN bp–immunized mice in 0.5 ml DMEM with 10% FBS 1 h before the addition of Rec ECTV type I IFN bp.
In vivo complementation of Δ166.
To determine the biological activity of Rec ECTV type I IFN bp in vivo, mice were inoculated i.v. with 50 μg of Rec IFN-α bp in 0.5 ml PBS immediately, and 3 d PI with ECTV Δ166. A group of control infected mice received PBS instead of Rec ECTV type I IFN bp, whereas a second control group received Rec ECTV type I IFN bp but remained uninfected. Mousepox and death were determined as previously described (55, 56, 63, 64).
SPR.
Experiments were performed on an optical biosensor (BIACORE 3000; GE Healthcare) at 25°C. The running buffer for the experiments was HBS-EP (10 mM Hepes, 150 mM NaCl, 3 mM EDTA, 0.005% polysorbate 20). Approximately 2,000 response units of purified Rec proteins were coupled to flow cells (Fc) 2–4 of a CM5 sensor chip via primary amines, according to the manufacturer's specifications. Fc1 was activated and blocked without the addition of protein. To characterize the binding of IFN-α 3 to the proteins, the flow path was set to include all flow cells, the flow rate was 50 μl/min, and the data collection rate was set to high. Protein samples were serially diluted in HBS-EP. Binding of each IFN-α concentration was allowed to occur for 2 min, with the wash delay set for an additional 2 min to allow for a smooth dissociation curve. The chip surface was regenerated by injecting brief pulses of buffer containing 1 M NaCL and 0.2 M sodium carbonate, pH 11.5, until the response signal returned to baseline. SPR data were analyzed with BIAevaluation software (version 3.0; GE Healthcare), which employs global fitting. Sensorgrams were corrected for nonspecific binding by subtracting the control sensorgram (Fc1) from the experimental sensorgrams. Model curve fitting was done with a 1:1 Langmuir binding model. This is the simplest model for the interaction between receptor and ligand, as it follows the equation A + B → AB. The rate of association (Kon) is measured from the forward reaction, whereas Koff is measured from the reverse reaction.
Statistics.
Data were analyzed using Excel software (Analysis Tool Pack; Microsoft).
Online supplemental material.
Fig. S1 shows models for the strategies used to generate Δ166 (A) and Rev166 (B), and examples of fluorescent (Δ166) and nonfluorescent (WT) ECTV plaques (C). Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20071854/DC1.
Supplemental Material
Acknowledgments
We thank the FCCC Laboratory Animal, DNA Sequencing, and Flow Cytometry Facilities. We also wish to thank Drs. D. Wiest and W. Mason for helpful comments; H. Gillin for administrative assistance; Dr. J.T. Guo for the VSV; Dr. M. Fang for preparing purified ECTV stocks; Dr. R. Schreiber for IFNAR1-deficient mice; and Dr. C. Krummenacher for help with the interpretation of single-feature polymorphism data.
L.J. Sigal designed and directed the project; R.-H. Xu and L.J. Sigal designed individual experiments; R.-H. Xu performed most experiments; M. Cohen constructed Δ166; Y. Tang helped produce the Rec ECTV protein in E. coli; J.C. Whitbeck produced the Rec ECTV and VACV proteins in the baculovirus system; E. Lazear performed SPR experiments; G.H. Cohen and R.J. Eisenberg supervised the production of the Rec protein in the baculovirus system and the analysis of the SPR experiments; R.-H. Xu and L.J. Sigal analyzed all the data; and L.J. Sigal wrote the manuscript. All authors discussed the results and commented on the manuscript.
This work was supported by National Institutes of Health grants AI065544 (to L.J. Sigal), CA006927 (to the FCCC), and RCE U54-AI57168 (to G.H. Cohen and R.J. Eisenberg), and by an appropriation from the Commonwealth of Pennsylvania.
The authors declare no competing financial interests.
Abbreviations used: bp, binding protein; D-LN, draining lymph node; Δ166, ECTV with EVM166 deleted; ECTV, ectromelia virus; EGFP, enhanced GFP; EVM166, ECTV Moscow open reading frame no. 166; IRM, immune response modifier; MEF, mouse embryonic fibroblast; OPV, orthopoxvirus; PI, post infection; Rec, recombinant; Rev166, Δ166 revertant to WT; SPR, surface plasmon resonance; VACV, vaccinia virus; VARV, variola virus; VSV, vesicular stomatitis virus.
M. Cohen's present address is Division of Infectious Diseases, Dept. of Medicine, University of Pennsylvania, Philadelphia, PA 19104.
References
- 1.Fenner, F., D.A. Henderson, I. Arita, Z. Jezek, D. Ladnyi, and W.H. Organization. 1988. Smallpox and Its Eradication. World Health Organization, Geneva. 1460 pp.
- 2.Cunha, B.E. 2004. Monkeypox in the United States: an occupational health look at the first cases. AAOHN J. 52:164–168. [PubMed] [Google Scholar]
- 3.DiGiulio, D.B., and P.B. Eckburg. 2004. Monkeypox in the Western hemisphere. N. Engl. J. Med. 350:1790–1791. [PubMed] [Google Scholar]
- 4.Nalca, A., A.W. Rimoin, S. Bavari, and C.A. Whitehouse. 2005. Reemergence of monkeypox: prevalence, diagnostics, and countermeasures. Clin. Infect. Dis. 41:1765–1771. [DOI] [PubMed] [Google Scholar]
- 5.Freed, E.R., R.J. Duma, and M.R. Escobar. 1972. Vaccinia necrosum and its relationship to impaired immunologic responsiveness. Am. J. Med. 52:411–420. [DOI] [PubMed] [Google Scholar]
- 6.Cono, J., C.G. Casey, and D.M. Bell. 2003. Smallpox vaccination and adverse reactions. Guidance for clinicians. MMWR Recomm. Rep. 52:1–28. [PubMed] [Google Scholar]
- 7.Appleyard, G., and C. Andrews. 1974. Neutralizing activities of antisera to poxvirus soluble antigens. J. Gen. Virol. 23:197–200. [DOI] [PubMed] [Google Scholar]
- 8.Boulter, E.A., and G. Appleyard. 1973. Differences between extracellular and intracellular forms of poxvirus and their implications. Prog. Med. Virol. 16:86–108. [PubMed] [Google Scholar]
- 9.Payne, L.G. 1980. Significance of extracellular enveloped virus in the in vitro and in vivo dissemination of vaccinia. J. Gen. Virol. 50:89–100. [DOI] [PubMed] [Google Scholar]
- 10.Turner, G.S., E.J. Squires, and H.G. Murray. 1970. Inactivated smallpox vaccine. A comparison of inactivation methods. J. Hyg. (Lond.). 68:197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner, G.S., and E.J. Squires. 1971. Inactivated smallpox vaccine: immunogenicity of inactivated intracellular and extracellular vaccinia virus. J. Gen. Virol. 13:19–25. [DOI] [PubMed] [Google Scholar]
- 12.Smith, G.L., A. Vanderplasschen, and M. Law. 2002. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 83:2915–2931. [DOI] [PubMed] [Google Scholar]
- 13.Chen, N., M.I. Danila, Z. Feng, R.M. Buller, C. Wang, X. Han, E.J. Lefkowitz, and C. Upton. 2003. The genomic sequence of ectromelia virus, the causative agent of mousepox. Virology. 317:165–186. [DOI] [PubMed] [Google Scholar]
- 14.Moss, B., and J.L. Shisler. 2001. Immunology 101 at poxvirus U: immune evasion genes. Semin. Immunol. 13:59–66. [DOI] [PubMed] [Google Scholar]
- 15.Fenner, F. 1996. Poxviruses. In Fields Virology. B.N. Fields, D.M. Knipe, and P.M. Howley, editors. Third edition. Raven Press, Philadelphia. 2673–2702.
- 16.Chen, W., R. Drillien, D. Spehner, and R.M. Buller. 1992. Restricted replication of ectromelia virus in cell culture correlates with mutations in virus-encoded host range gene. Virology. 187:433–442. [DOI] [PubMed] [Google Scholar]
- 17.Shchelkunov, S.N., A.V. Totmenin, I.V. Babkin, P.F. Safronov, O.I. Ryazankina, N.A. Petrov, V.V. Gutorov, E.A. Uvarova, M.V. Mikheev, J.R. Sisler, et al. 2001. Human monkeypox and smallpox viruses: genomic comparison. FEBS Lett. 509:66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shchelkunov, S.N., A.V. Totmenin, P.F. Safronov, M.V. Mikheev, V.V. Gutorov, O.I. Ryazankina, N.A. Petrov, I.V. Babkin, E.A. Uvarova, L.S. Sandakhchiev, et al. 2002. Analysis of the monkeypox virus genome. Virology. 297:172–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langland, J.O., and B.L. Jacobs. 2002. The role of the PKR-inhibitory genes, E3L and K3L, in determining vaccinia virus host range. Virology. 299:133–141. [DOI] [PubMed] [Google Scholar]
- 20.Perkus, M.E., S.J. Goebel, S.W. Davis, G.P. Johnson, K. Limbach, E.K. Norton, and E. Paoletti. 1990. Vaccinia virus host range genes. Virology. 179:276–286. [DOI] [PubMed] [Google Scholar]
- 21.Spehner, D., S. Gillard, R. Drillien, and A. Kirn. 1988. A cowpox virus gene required for multiplication in Chinese hamster ovary cells. J. Virol. 62:1297–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alcami, A. 2003. Viral mimicry of cytokines, chemokines and their receptors. Nat. Rev. Immunol. 3:36–50. [DOI] [PubMed] [Google Scholar]
- 23.McFadden, G., and P.M. Murphy. 2000. Host-related immunomodulators encoded by poxviruses and herpesviruses. Curr. Opin. Microbiol. 3:371–378. [DOI] [PubMed] [Google Scholar]
- 24.Stanford, M.M., G. McFadden, G. Karupiah, and G. Chaudhri. 2007. Immunopathogenesis of poxvirus infections: forecasting the impending storm. Immunol. Cell Biol. 85:93–102. [DOI] [PubMed] [Google Scholar]
- 25.Stanford, M.M., S.J. Werden, and G. McFadden. 2007. Myxoma virus in the European rabbit: interactions between the virus and its susceptible host. Vet. Res. 38:299–318. [DOI] [PubMed] [Google Scholar]
- 26.Johnston, J.B., and G. McFadden. 2004. Technical knockout: understanding poxvirus pathogenesis by selectively deleting viral immunomodulatory genes. Cell. Microbiol. 6:695–705. [DOI] [PubMed] [Google Scholar]
- 27.Turner, P.C., and R.W. Moyer. 2002. Poxvirus immune modulators: functional insights from animal models. Virus Res. 88:35–53. [DOI] [PubMed] [Google Scholar]
- 28.Fenner, F. 1994. Mousepox (ectromelia). In Virus Infections of Rodents and Lagomorphs. A.D.M.E. Osterhaus, editor. Elsevier Science, Amsterdam. 5–25.
- 29.Sen, G.C. 2001. Viruses and interferons. Annu. Rev. Microbiol. 55:255–281. [DOI] [PubMed] [Google Scholar]
- 30.Stetson, D.B., and R. Medzhitov. 2006. Type I interferons in host defense. Immunity. 25:373–381. [DOI] [PubMed] [Google Scholar]
- 31.Vilcek, J. 2006. Fifty years of interferon research: aiming at a moving target. Immunity. 25:343–348. [DOI] [PubMed] [Google Scholar]
- 32.Theofilopoulos, A.N., R. Baccala, B. Beutler, and D.H. Kono. 2005. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu. Rev. Immunol. 23:307–336. [DOI] [PubMed] [Google Scholar]
- 33.Asselin-Paturel, C., A. Boonstra, M. Dalod, I. Durand, N. Yessaad, C. Dezutter-Dambuyant, A. Vicari, A. O'Garra, C. Biron, F. Briere, and G. Trinchieri. 2001. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2:1144–1150. [DOI] [PubMed] [Google Scholar]
- 34.Siegal, F.P., N. Kadowaki, M. Shodell, P.A. Fitzgerald-Bocarsly, K. Shah, S. Ho, S. Antonenko, and Y.-J. Liu. 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science. 284:1835–1837. [DOI] [PubMed] [Google Scholar]
- 35.Dalod, M., T.P. Salazar-Mather, L. Malmgaard, C. Lewis, C. Asselin-Paturel, F. Briere, G. Trinchieri, and C.A. Biron. 2002. Interferon α/β and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J. Exp. Med. 195:517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van den Broek, M.F., U. Muller, S. Huang, M. Aguet, and R.M. Zinkernagel. 1995. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J. Virol. 69:4792–4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang, S., W. Hendriks, A. Althage, S. Hemmi, H. Bluethmann, R. Kamijo, J. Vilcek, R.M. Zinkernagel, and M. Aguet. 1993. Immune response in mice that lack the interferon-gamma receptor. Science. 259:1742–1745. [DOI] [PubMed] [Google Scholar]
- 38.van den Broek, M.F., U. Muller, S. Huang, R.M. Zinkernagel, and M. Aguet. 1995. Immune defence in mice lacking type I and/or type II interferon receptors. Immunol. Rev. 148:5–18. [DOI] [PubMed] [Google Scholar]
- 39.Deonarain, R., A. Alcami, M. Alexiou, M.J. Dallman, D.R. Gewert, and A.C. Porter. 2000. Impaired antiviral response and alpha/beta interferon induction in mice lacking beta interferon. J. Virol. 74:3404–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karupiah, G., T.N. Fredrickson, K.L. Holmes, L.H. Khairallah, and R.M. Buller. 1993. Importance of interferons in recovery from mousepox. J. Virol. 67:4214–4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramshaw, I.A., A.J. Ramsay, G. Karupiah, M.S. Rolph, S. Mahalingam, and J.C. Ruby. 1997. Cytokines and immunity to viral infections. Immunol. Rev. 159:119–135. [DOI] [PubMed] [Google Scholar]
- 42.Jacoby, R.O., P.N. Bhatt, and D.G. Brownstein. 1989. Evidence that NK cells and interferon are required for genetic resistance to lethal infection with ectromelia virus. Arch. Virol. 108:49–58. [DOI] [PubMed] [Google Scholar]
- 43.Panchanathan, V., G. Chaudhri, and G. Karupiah. 2005. Interferon function is not required for recovery from a secondary poxvirus infection. Proc. Natl. Acad. Sci. USA. 102:12921–12926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith, V.P., and A. Alcami. 2002. Inhibition of interferons by ectromelia virus. J. Virol. 76:1124–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Symons, J.A., A. Alcami, and G.L. Smith. 1995. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell. 81:551–560. [DOI] [PubMed] [Google Scholar]
- 46.Alcami, A., J.A. Symons, and G.L. Smith. 2000. The vaccinia virus soluble alpha/beta interferon (IFN) receptor binds to the cell surface and protects cells from the antiviral effects of IFN. J. Virol. 74:11230–11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colamonici, O.R., P. Domanski, S.M. Sweitzer, A. Larner, and R.M. Buller. 1995. Vaccinia virus B18R gene encodes a type I interferon-binding protein that blocks interferon alpha transmembrane signaling. J. Biol. Chem. 270:15974–15978. [DOI] [PubMed] [Google Scholar]
- 48.Symons, J.A., D.C. Tscharke, N. Price, and G.L. Smith. 2002. A study of the vaccinia virus interferon-gamma receptor and its contribution to virus virulence. J. Gen. Virol. 83:1953–1964. [DOI] [PubMed] [Google Scholar]
- 49.Alcami, A., and G.L. Smith. 1995. Vaccinia, cowpox, and camelpox viruses encode soluble gamma interferon receptors with novel broad species specificity. J. Virol. 69:4633–4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakala, I.G., G. Chaudhri, R.M. Buller, A.A. Nuara, H. Bai, N. Chen, and G. Karupiah. 2007. Poxvirus-encoded gamma interferon binding protein dampens the host immune response to infection. J. Virol. 81:3346–3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hammarlund, E., M.W. Lewis, S.G. Hansen, L.I. Strelow, J.A. Nelson, G.J. Sexton, J.M. Hanifin, and M.K. Slifka. 2003. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 9:1131–1137. [DOI] [PubMed] [Google Scholar]
- 52.Demkowicz, W.E. Jr., R.A. Littaua, J. Wang, and F.A. Ennis. 1996. Human cytotoxic T-cell memory: long-lived responses to vaccinia virus. J. Virol. 70:2627–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crotty, S., P. Felgner, H. Davies, J. Glidewell, L. Villarreal, and R. Ahmed. 2003. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J. Immunol. 171:4969–4973. [DOI] [PubMed] [Google Scholar]
- 54.Gardner, I.D., and R.V. Blanden. 1976. The cell-mediated immune response to ectromelia virus infection. II. Secondary response in vitro and kinetics of memory T cell production in vivo. Cell. Immunol. 22:283–296. [DOI] [PubMed] [Google Scholar]
- 55.Fang, M., and L.J. Sigal. 2005. Antibodies and CD8+ T cells are complementary and essential for natural resistance to a highly lethal cytopathic virus. J. Immunol. 175:6829–6836. [DOI] [PubMed] [Google Scholar]
- 56.Xu, R.-H., M. Fang, A. Klein-Szanto, and L.J. Sigal. 2007. Memory CD8+ T cells are gatekeepers of the lymph node draining the site of viral infection. Proc. Natl. Acad. Sci. USA. 104:10992–10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fenner, F. 1949. Mouse-pox; infectious ectromelia of mice; a review. J. Immunol. 63:341–373. [PubMed] [Google Scholar]
- 58.Esteban, D.J., and R.M. Buller. 2005. Ectromelia virus: the causative agent of mousepox. J. Gen. Virol. 86:2645–2659. [DOI] [PubMed] [Google Scholar]
- 59.Fang, M., L.L. Lanier, and L.J. Sigal. 2008. A role for NKG2D in NK cell-mediated resistance to poxvirus disease. PLoS Pathog. 4:e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blanden, R.V. 1971. Mechanisms of recovery from a generalized viral infection: mousepox. II. Passive transfer of recovery mechanisms with immune lymphoid cells. J. Exp. Med. 133:1074–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buller, R.M., K.L. Holmes, A. Hugin, T.N. Frederickson, and H.C. Morse III. 1987. Induction of cytotoxic T-cell responses in vivo in the absence of CD4 helper cells. Nature. 328:77–79. [DOI] [PubMed] [Google Scholar]
- 62.Karupiah, G., R.M. Buller, N. Van Rooijen, C.J. Duarte, and J. Chen. 1996. Different roles for CD4+ and CD8+ T lymphocytes and macrophage subsets in the control of a generalized virus infection. J. Virol. 70:8301–8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fang, M., and L.J. Sigal. 2006. Direct CD28 costimulation is required for CD8+ T cell-mediated resistance to an acute viral disease in a natural host. J. Immunol. 177:8027–8036. [DOI] [PubMed] [Google Scholar]
- 64.Fang, M., H. Cheng, Z. Dai, Z. Bu, and L.J. Sigal. 2006. Immunization with a single extracellular enveloped virus protein produced in bacteria provides partial protection from a lethal orthopoxvirus infection in a natural host. Virology. 345:231–243. [DOI] [PubMed] [Google Scholar]
- 65.Parker, A.K., S. Parker, W.M. Yokoyama, J.A. Corbett, and R.M. Buller. 2007. Induction of natural killer cell responses by ectromelia virus controls infection. J. Virol. 81:4070–4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xiao, Y., L. Aldaz-Carroll, A.M. Ortiz, J.C. Whitbeck, E. Alexander, H. Lou, H.L. Davis, T.J. Braciale, R.J. Eisenberg, G.H. Cohen, and S.N. Isaacs. 2007. A protein-based smallpox vaccine protects mice from vaccinia and ectromelia virus challenges when given as a prime and single boost. Vaccine. 25:1214–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davies, D.H., D.M. Molina, J. Wrammert, J. Miller, S. Hirst, Y. Mu, J. Pablo, B. Unal, R. Nakajima-Sasaki, X. Liang, et al. 2007. Proteome-wide analysis of the serological response to vaccinia and smallpox. Proteomics. 7:1678–1686. [DOI] [PubMed] [Google Scholar]
- 68.Putz, M.M., C.M. Midgley, M. Law, and G.L. Smith. 2006. Quantification of antibody responses against multiple antigens of the two infectious forms of vaccinia virus provides a benchmark for smallpox vaccination. Nat. Med. 12:1310–1315. [DOI] [PubMed] [Google Scholar]
- 69.Hooper, J.W., D.M. Custer, C.S. Schmaljohn, and A.L. Schmaljohn. 2000. DNA vaccination with vaccinia virus L1R and A33R genes protects mice against a lethal poxvirus challenge. Virology. 266:329–339. [DOI] [PubMed] [Google Scholar]
- 70.Hooper, J.W., D.M. Custer, and E. Thompson. 2003. Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates. Virology. 306:181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hooper, J.W., E. Thompson, C. Wilhelmsen, M. Zimmerman, M.A. Ichou, S.E. Steffen, C.S. Schmaljohn, A.L. Schmaljohn, and P.B. Jahrling. 2004. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J. Virol. 78:4433–4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Snyder, J.T., I.M. Belyakov, A. Dzutsev, F. Lemonnier, and J.A. Berzofsky. 2004. Protection against lethal vaccinia virus challenge in HLA-A2 transgenic mice by immunization with a single CD8+ T-cell peptide epitope of vaccinia and variola viruses. J. Virol. 78:7052–7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fogg, C., S. Lustig, J.C. Whitbeck, R.J. Eisenberg, G.H. Cohen, and B. Moss. 2004. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J. Virol. 78:10230–10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fulginiti, V.A., A. Papier, J.M. Lane, J.M. Neff, and D.A. Henderson. 2003. Smallpox vaccination: a review, part II. Adverse events. Clin. Infect. Dis. 37:251–271. [DOI] [PubMed] [Google Scholar]
- 75.Edghill-Smith, Y., H. Golding, J. Manischewitz, L.R. King, D. Scott, M. Bray, A. Nalca, J.W. Hooper, C.A. Whitehouse, J.E. Schmitz, et al. 2005. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 11:740–747. [DOI] [PubMed] [Google Scholar]
- 76.Earl, P.L., J.L. Americo, L.S. Wyatt, L.A. Eller, J.C. Whitbeck, G.H. Cohen, R.J. Eisenberg, C.J. Hartmann, D.L. Jackson, D.A. Kulesh, et al. 2004. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 428:182–185. [DOI] [PubMed] [Google Scholar]
- 77.Galmiche, M.C., J. Goenaga, R. Wittek, and L. Rindisbacher. 1999. Neutralizing and protective antibodies directed against vaccinia virus envelope antigens. Virology. 254:71–80. [DOI] [PubMed] [Google Scholar]
- 78.Ueda, Y., and I. Tagaya. 1973. Induction of skin resistance to vaccinia virus in rabbits by vaccinia-soluble early antigens. J. Exp. Med. 138:1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ueda, Y., S. Morikawa, and Y. Matsuura. 1990. Identification and nucleotide sequence of the gene encoding a surface antigen induced by vaccinia virus. Virology. 177:588–594. [DOI] [PubMed] [Google Scholar]
- 80.Smith, G.L., and Y.S. Chan. 1991. Two vaccinia virus proteins structurally related to the interleukin-1 receptor and the immunoglobulin superfamily. J. Gen. Virol. 72:511–518. [DOI] [PubMed] [Google Scholar]
- 81.Muller, U., U. Steinhoff, L.F. Reis, S. Hemmi, J. Pavlovic, R.M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science. 264:1918–1921. [DOI] [PubMed] [Google Scholar]
- 82.Todaro, G.J., and H. Green. 1963. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J. Cell Biol. 17:299–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Earl, P., B. Moss, L. Wyatt, and M. Carroll. 1998. Generation of Recombinant Vaccinia Viruses. In Current Protocols in Molecular Biology. F. Ausubel, R. Brent, R. Kingston, J. Moore, J. Seidman, J. Smith, and K. Struhl, editors. John Wiley and Sons, Inc., New York. 16.17.11–16.17.19.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.