Abstract
Bovine tuberculosis (TB) remains a disease of economic and public health importance in developing countries. The aim of this study was to evaluate the use of tuberculin skin-testing and segregation of reactors as a potential control method, and initiate molecular typing of Mycobacterium bovis (M. bovis) in Ethiopia. Comparative intradermal tuberculin test (CIDT), pathological examination, bacteriology, molecular typing were applied. The prevalence of bovine TB was 48% (n=500) in the Holeta Farm as disclosed at the first CIDT conducted in 2002, whereupon the Farm was divided into positive and negative herds. Following three consecutive rounds of skin testing and segregation of skin test positive and negative animals, the incidence of bovine TB was dramatically reduced from 14% to 1% in the negative herd in a year. Spoligotyping of 41 isolates from 17 cows had an identical and uniques poligotypepattern, which can be represented as a binary of 1100000101111110111111100010000000000100000 where 1 indicates the presence of a spacer and 0 represents loss. This spoligotype pattern was not reported previously from elsewhere to the M. bovis database (www.mbovis.org). The new spoligotype pattern was therefore designated as EMbs1, Ethiopian M. bovis strain 1. The VNTR profile of the strain was 5254*33.1 and differed from the VNTR profile of strains reported from United Kingdom. The result of this has shown the potential application of test and segregation for the control of bovine TB particularly in developing countries.
Keywords: Bovine tuberculosis, Molecular typing, Mycobacterium bovis, Test and segregation
INTRODUCTION
Bovine TB is a threat to animal and human health in several countries. Human TB of animal origin, particularly of M. bovis is becoming increasingly important in developing countries while in developed world because of the control bovine TB in farm animals, human infection with M. bovis is minimized although a potential risk remains (Anonymous 1994). Several factors account for the failure of developing countries to control and eradicate bovine TB. Among these are the high cost of a sustainable testing program, social unrest due to political instability and ethnic war, displacement of large numbers of human and animal populations, lack of veterinary expertise and communication networks, insufficient collaboration with bordering countries and hence lack of quarantine, and smuggling of live animals across state boundaries (Ayele and others 2004).
Factors such as lack of quarantine and smuggling of live animals across boundaries, which is very common among east African countries, promote the transmission of M. bovis from one country to another. This underlines the necessity for the investigation of the molecular epidemiology of M. bovis within and across countries so that strains circulating in the cattle, human and wildlife populations are identified. The most common molecular typing method applied to M. bovis in developed countries is spoligotyping (Durr and others 2000) while restriction fragment length polymorphism (RFLP) using insertion sequence (IS) 6110 is the preferred typing method of M. tuberculosis (Van Soolingen and others, 1993). Spoligotyping is a polymerase chain reaction (PCR) based method that exploits polymorphisms within the direct repeat region (DR) of the chromosome (Groenen and others 1993; Kamerbeek and others 1997; Caimi and others 2001), and has been used for the investigation of molecular epidemiology of M. bovis and that of M. tuberculosis with low-copy-number of IS6110 (Bauer and others 1999; Goyal and others 1997; de La Salmoniere and others 1997; Lutze-Wallance and others 2005). Similar to spoligotyping, variable number tandem repeat (VNTR) is a PCR-based technique to determine the number of tandem repeats of the species of M. tuberculosis complex. The VNTR system originally described by Frothingham and Meeker-O'Connell included 6 exact tandem repeats (Frothingham and Meeker-O'Connell 1998). These loci vary in the length of internal repeat units, giving alleles that vary in size. Strains are named on the basis on the number of repeats at each allele, i.e. 7-5-5-4-3-3 would have 7 copies of allele A, 5 of B, etc. Investigation of the spoligotype patterns and VNTR profile of M. bovis strains would be useful for mapping epidemiology of bovine TB and control of its further spread.
This study was undertaken to investigate an outbreak of bovine TB on Farm government-owned at Holeta (Holeta Farm) and evaluate the effect of test and segregation on the incidence of bovine TB on this Farm. Furthermore, this study aimed at initiating molecular typing surveillance of M. bovis in the country.
MATERIALS AND METHODS
History of the Farm
The Holeta Farm was first established in 1955 and started with 120 in-calf Holstein heifers, which had been imported from the United States. In addition, some pure Holstein heifers were introduced from Kenya in 1959. After about 20 years, an additional 120 in-calf Holstein heifers were donated by the Government of Cuba in 1980 and formed the nucleus of the present Farm. The Farm is located 43km west of Addis Ababa at Holetta Town on the main road to western Ethiopia. There are various grades of dairy cattle adjacent to this farm that are kept by smallholders and few small scale commercial dairy farms. Hay, green feed and concentrate are the major feed items fed to animals on the farm. Water for drinking is obtained from a borehole, feeding of calves on un-pasteurized milk has been a common and frequent practice on the Farm as well. Because of the occurrence of clinical signs suggestive of bovine TB ((e.g. respiratory distress, coughing, weight loss, emacication) and post mortem lesions in animals that had died, the herd was tuberculin skin tested in 2001 for the first time. However, due to a technical problem with this skin test the real picture of the disease was not revealed and only in 10 animals tested positive out of over 500 animals (Personal Comm. Farm Manager). Otherwise, neither the animals in the Farm nor animals in the vicinity had been skin-tested previously.
Comparative intradermal tuberculin test
All animals in the Farm except newly born calves were tested during the skin test conducted in 2002. Two sites on the right side of the skin of the mid-neck of the animal, 12cm apart, were shaved and the skin thickness was measured with calipers. Aliqouts of 0.1ml of 2500 IU/ml bovine purified protein derivative (PPD) (Veterinary Laboratories Agency (VLA), Addlestone, Surrey KT15 3NB, U.K.), and 0.1ml of 2500 IU/ml avian PPD (VLA, Addlestone, Surrey KT15 3NB, U.K.) were injected into the dermis at these sites. After 72 h, the thicknesses of the skin at the injection sites were measured using a caliper. Results were interpreted in line with the recommendations of the OIE (2000).
Necropsy and histopathology
Thirty (30) comparative interadermal tuberculin test positive cows were slaughtered and subjected to post mortem examination. Each lobe of the lung was sliced into about 2cm-thick slices so as to facilitate the detection of lesions. Similarly, five lymph nodes namely, mandibular (right and left), medial retropharyngeal, bronchial (left and right), mediastinal (caudal and cranial), and mesenteric from each of the cows were sliced into thin sections and inspected for the presence of visible lesions. Samples from suspicious lesions were collected into 10% buffered formalin and processed for histopathology following OIE (2000) protocol.
The severity of the gross lesions was scored by applying the semi-quantitative procedure developed by Vordermeier and others (2002), with minor modifications to facilitate performance under field conditions. Briefly, lesions in the lobes of the lungs were scored separately as follows: 0 = no visible lesions; 1 = no gross lesions but lesions apparent on slicing of the lobe; 2 = <5 gross lesions; 3= > 5 gross lesions; 4 = gross coalescing lesions. The scores of the individual lobes were added up to calculate the lung score. Similarly, the severity of gross lesions in individual lymph nodes was scored as follows: 0 = no gross lesion; 1 = small lesion at one focus (just starting); 2= small lesions at more than one focus; 3= extensive necrosis. Individual lymph node scores were added up to calculate the lymph node score. Finally, both lymph node and lung pathology scores were added up to determine the total pathology score per animal.
Bacteriology
Suspicious tissue specimens from the 30 slaughtered animals were collected in 5ml of 0.9% saline in universal bottles, and cultured on Lowenstein-Jensen medium according to Aranaz and others (2004). Cultures were incubated aerobically at 37°C for about 5-8 weeks with weekly observation for growth of colonies. Colonies were scrapped and heat-killed at 80°C for 1h, and transported to the Veterinary Laboratories Agency, U.K. for molecular typing.
Molecular typing
Spoligotyping was performed on 41 isolates that were obtained from 17 cows out of the 28 with gross TB lesions. Mycobacterial DNA was extracted from the heat killed cells following the procedure described by Kolk and others (1992), and Kox and others (1994). After DNA extraction, spoligotyping was performed on the isolates as previously described (Kamerbeek and others 1997) with minor modifications (Banu and others 2004). Furthermore, 12 isolates were randomly selected and subjected to VNTR analysis following the protocol described by Frothingham and Meeker-O'Connell (1998), which included 6 exact tandem repeats.
RESULTS
Effect of repeated skin testing and segregation on the incidence of bovine TB
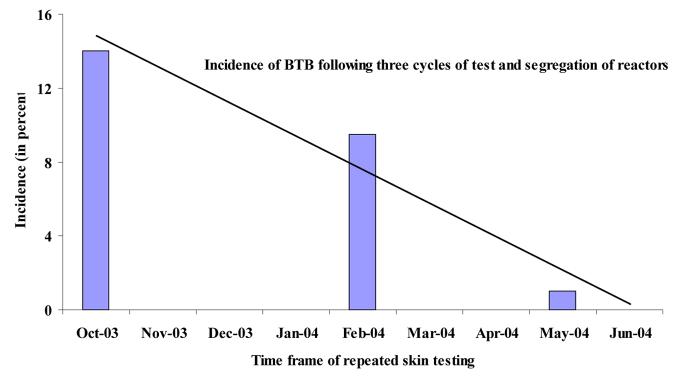
About half (48%) of the herd was CIDT positive by the first test i.e. the prevalence bovine TB was 48% at our first testing in October 2002. On the basis of this result, the Farm was classified into a” positive” and a” negative” herd, and physically separated. This was followed by three consecutive testing rounds on the negative herd and removal of the positive reactors to the positive herd. Table 1 shows the dates of testing and results of the tests. This reduced the incidence of bovine TB from 14% to 1% in a year (Fig 1). The reduction in proportion of positive reactors was significantly associated (r=0.96) with repeated test and segregation with this time frame.
Table 1.
Results of four consecutive test and segregation rounds applied to the Holeta Farm, central Ethiopia
| Frequency of testing | Date of testing | Number of animals tested |
Percent positive |
|---|---|---|---|
| First test | October 2002 | 500 | 48 |
| Second test | October 2003 | 250 | 14 |
| Third test | February 2004 | 200 | 9.5 |
| Fourth test | May 2004 | 200 | 1 |
Figure 1.
Effect of skin testing and segregation on the incidence of bovine tuberculosis in the Holeta Farm, Ethiopia. As can be seen from the Fig 1, the reduction in the incidence prevalence was significantly associated (r=0.96) with repeated test and segregation cycles.
Clincial disease and pathological lesions
With the exception of a few animals that reached the progressive stage of the disease, most of the animals from both the negative and positive herds exhibited similar physical conditions. The average milk production of the two herds was similar during the study period. But sporadic deaths of reactor cows were observed in the positive herd, and post mortems showed TB lesions in different tissues. In additon, there were problems with breeding in the positive herd (CIDT positive animals), in that cows did not come to estrous and also usually failed to conceive.
Post-mortem examinations were conducted on 30 reactors animals. Gross tuberculous lesions were detected in 93.3% of the them. Lung lesions were detected in 30% of the reactors while 90% the reactors had lesions in their lymph nodes. Diaphragmatic lobes of both lungs had greater frequency and intensity of lesions, while lesions were less frequent and intense in the apical lobes of both lungs (Table 2). Mediastinal, bronchial and retropharyngeal lymph nodes were the most frequently and severely affected lymph nodes, while no lesions were found in the mandibular lymph node (Table 3). Lesions were also found in the mesenteric lymph nodes of 11 cows. However, 10 of these cows also had lesions in the lymph nodes of the thoracic cavity. The most commonly observed microscopic lesions were characterized by a central necrosis with mineralization surrounded by granulomatous inflammatory response (data not shown). Macrophages and epitheloid cells were aggregated surrounding the necrotic lesions forming the Langerhan's giant cells (data not shown). Around 29 % (8/27) of the cattle examined by post-mortem presented with disseminated disease (as defined by lesions lung lesions found in >5 lobes and/or lesions in the head and thoracic, or thoracic and mesenteric lymph nodes with total lymph node scores > 10, tables 2 and 3).
Table 2.
Severity and distribution of lesion of bovine TB (pathology score) in the lungs of 30 cows in Holeta Farm
| Tag No | Left apical |
Left cardiac | Left diaphragmatic |
Right apical |
Right cardiac |
Right diaphragmatic |
Right accessory |
Total Severity |
|---|---|---|---|---|---|---|---|---|
| H1-158 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 5 |
| H2-272 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H1-780 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H1-849 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| K-684 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H-729 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H1-851 | 1 | 2 | 1 | 2 | 2 | 3 | 2 | 13 |
| K-882 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H1-514 | 0 | 1 | 0 | 0 | 0 | 3 | 4 | 8 |
| K-577 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H1-842 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| K-801 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H1-760 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 28 |
| S-2057 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H1-210 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H1-193 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H2-434 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 |
| H1-149 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 |
| H1-822 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H1-847 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H1-633 | 4 | 3 | 4 | 1 | 0 | 4 | 3 | 19 |
| H1-814 | 0 | 2 | 2 | 0 | 0 | 2 | 0 | 6 |
| H1-823 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H1-533 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 3 |
| H1-168 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H1-793 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H1-607 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H1-285 | 0 | 0 | 2 | 0 | 0 | 1 | 1 | 4 |
| H1-865 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H1-455 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total animal with TB lesion | 3 | 5 | 6 | 3 | 3 | 9 | 5 | 12 |
Table 3.
Severity and distribution of lesions of bovine TB (pathology score) in the lymph nodes of 30 CIDT positive cows in the Holeta Farm
| Tag No | Mandiblar (left & right) |
Retropharyngeal (lateral &medial) |
Cranial Mediastinal |
Caudal mediastinal |
Left bronchial |
Right bronchial |
Mesenteric | Total Severity |
|---|---|---|---|---|---|---|---|---|
| H1-158 | 0 | 3 | 3 | 3 | 2 | 2 | 0 | 13 |
| H2-272 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| H1-780 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| H1-849 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 |
| K-684 | 0 | 0 | 0 | 2 | 0 | 1 | 2 | 5 |
| H-729 | 0 | 2 | 3 | 0 | 0 | 0 | 0 | 5 |
| H1-851 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 |
| K-882 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 4 |
| H1-514 | 0 | 0 | 1 | 1 | 1 | 2 | 2 | 7 |
| K-577 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H1-842 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 4 |
| K-801 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H1-760 | 0 | 0 | 3 | 3 | 3 | 3 | 3 | 15 |
| S-2057 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| H1-210 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 2 |
| H1-193 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 |
| H2-434 | 0 | 3 | 3 | 3 | 3 | 3 | 3 | 18 |
| H1-149 | 0 | 1 | 2 | 2 | 0 | 0 | 2 | 7 |
| H1-822 | 0 | 2 | 0 | 2 | 2 | 0 | 0 | 6 |
| H1-847 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 3 |
| H1-633 | 0 | 0 | 3 | 3 | 3 | 3 | 3 | 12 |
| H1-814 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 3 |
| H1-823 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 2 |
| H1-533 | 0 | 0 | 3 | 3 | 3 | 3 | 3 | 15 |
| H1-168 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 2 |
| H1-793 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| H1-607 | 0 | 0 | 2 | 3 | 2 | 1 | 1 | 9 |
| H1-285 | 0 | 2 | 2 | 3 | 3 | 2 | 0 | 12 |
| H1-865 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 3 |
| H1-455 | 0 | 2 | 3 | 3 | 3 | 3 | 2 | 16 |
| Total animal with TB lesion | 0 | 10 | 14 | 21 | 14 | 13 | 11 | 28 |
Bacteriology
Growth of mycobacteria was observed in 17 of the 20 animals whose tissues samples were properly processed for culturing. Samples from 10 animals were destroyed because of technical errors (prolonged application of NaOH for decontamination purpose to the first batch of samples) during culturing. A total of 41 isolates were recovered from the 2nd and 3rd batches of samples obtained from the different tissues of the 17 of the 20 animals and, then further subjected to molecular typing.
Spoligotype patterns and VNTR profile
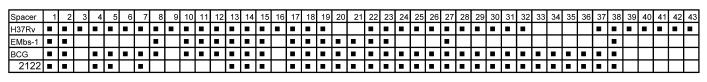
The 41 isolates demonstrated an identical spoligotype pattern, which can be represented as a binary of 1100000101111110111111100010000000000100000 where 1 indicates the presence of a spacer and 0 represents loss. Spacers 3,9,16, and 39-43, which are absent from M. bovis strains were absent from all the isolates, with two gaps as a result of the absence of spacers 3-7 and 28-37 (Fig 2). In addition, spacers 24-26 were absent from the strain. Hence, the Farm appears to be infected with one strain of M. bovis. The new strain was named as EMbs1, which means Ethiopian M. bovis strain 1 (indicating the first spoligotype pattern of M. bovis identified in Ethiopia). Similar to the spoligotype pattern, all the isolates which were analyzed by VNTR had identical i.e. 5254*33.1 VNTR profile.
Figure 2.
Spoligotype pattern of the 41 isolates obtained from 17 skin test positive dairy cows obtained from Holeta Farm, (Ethiopia). All the isolates were found to be identical and thus turned to be the same strain. The new strain did not have spacers 3-7, 24-26, and 27-37. The new strain is unique and no strain with a similar spoligotype pattern has been reported to the M. bovis spoligotype database so far. The strain named 1222 is a sequenced M. bovis strain.
DISCUSSION
The incidence of positive reactors was reduced significantly in the Genetic Farm through the application of consecutive rounds of skin-testing and segregation. After the first test the farm was divided into positive and negative herds. The two herds were separated by a kilometer distance and there was no physical contact between the two herds, and with the negative herd positioned at higher geographical level than the positive herd. Animals in the two herds were fed and watered in a similar way through the same management but with different staff working with the two herds. Three consecutive tests every four months were applied to the negative herd after the first test. The positive reactors were removed and mixed with the positive herd. As the result, the incidence of CIDT positive reactors was reduced from 14% to 1% within a year. Our results therefore support previous findings (reviewed in Proud, 2006) that once reactors and non-reactors are physically separated, cattle-to-cattle transmission is significantly reduced, highlighting the potential effectiveness of this strategy in disease control. Nevertheless, it is necessary to continue the test and segregation policy for some time to maintain a low transmission rate until the disease is fully eradicated. The need for continued vigilance i.e. a continuation of regular skin testing, and also application of other immunological tests alongside skin testing, like the IFN-□ test to be able to remove as many of the infected animals as quickly as possible. This recommendation is based on the observation reported in many studies that the populations recognized by tuberculin skin testing and IFN-□ testing are not fully overlapping and that the optimal use of both tests to detect the maximum number of infected cattle would be the application of both tests in parallel (reviewed by Vordermeier and others, 2006).
With the exception of a few animals that reached the progressive stage of the disease (ie presenting with clinically overt symptoms like respiratory distress or weight loss) most of the animals from both the negative and positive herds exhibited similar physical conditions. It had been reported that prior to the introduction of effective control measures, 90% of the tuberculous cattle had lesions in the lymph nodes (Francis 1958; Collins 1996), and our results confirm these observations (Table 2). In the present study, the diaphragmatic lobes of both lungs had greater frequencies and intensity of lesions. This is in line with previous studies (McIlroy and others 1986), which indicated that 46% of the lung lesions are located in the distal part of the diaphragmatic lobe. Lymph node lesions were predominantly (90%) found in the thoracic lymph nodes although they were also disseminated to the mesenteric lymph node in 30% of the study animals. However, 91% (10/11) of the reactors with mesenteric lesion also had lesions located in the lymph nodes of the thoracic cavity, only 1/11 cows had lesions in the mesenteric lymph nodes only, which is suggestive that alimentary tract infection only is a rare event and that aerogenic infection is the predominant infection route in intensive farms such as the Holeta farm (Menzies and Neill, 200; Ameni and others, 2006).
The spoligotype patterns of all the isolates were identical. Similarly, the VNTR profiles of randomly selected isolates were also identical, which suggests that all the animals in the herd were infected with one strain of M. bovis which has a different spoligotype pattern from previously reported patterns either from Africa (Cadmas and others 2004; Njanpop-Lafourrcade and others 2001), or from other parts of the world (Aranaz and others 1996; Goyal and others 1997; de La Salmoniere and others 1997; Lutze-Wallance and others 2005). Furthermore, the VNTR profile of the strain was different from those reported at least from Great Britain by Smith and others (2003). The predominance of a single M. bovis strain in a local area has been noted before; indeed, in the UK there is a distinct geographical localization of M. bovis molecular types (Smith and others 2003). As it was understood from the history of the Genetic Farm, once the Farm was established there was no subsequent introduction of new animals to the Farm. Hence it is difficult to establish the source of the infection, but it is conceivable that it was either via the introduction of an infected animal when the Farm was established, a wildlife reservoir in the area, or grazing on land or hay contaminated with excretions of an infected herd of the nearby farmers. To investigate this further, additional studies will be needed to survey the molecular types of M. bovis from local wildlife and tuberculous cattle from surrounding farms.
Until the development of an effective vaccine is realized, testing herds using CIDT and segregating the positive reactors from the negatives could help in minimizing the prevalence of the disease. This strategy must, of course, be complemented with milk pasteurization. In addition, molecular typing of M. bovis from the different regions of Ethiopia would be useful for the mapping of the molecular epidemiology of M. bovis in the country.
ACKNOWLEDGEMENTS
This project was supported by the Wellcome Trust under its “Animal Health in the Developing World” initiative. Surane Gemeda, Mellisa Okker and Karon were acknowledged for their expert technical support.
References
- AMENI G, ASEFFA A, ENGERS H, YOUNG D, HEWINSON G, VORDERMEIER M. Cattle husbandry in Ethiopia is a predominant factor affecting the pathology of bovine tuberculosis and IFN-γ responses to mycobacterial antigens. Clinical and Vaccine Immunology. 2006;13:1030–1036. doi: 10.1128/CVI.00134-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANONYMOUS Zoonotic tuberculosis (Mycobacterium bovis): a memorandum from World Health Organization (WHO) with participation of Food and Agricultural Organization (FAO) Bulletin of WHO. 1994;72:851–857. [PMC free article] [PubMed] [Google Scholar]
- ARANAZ A, LIEBENA E, MATEOS A, DOMINGUEZ L, VIDAL D, DOMINGO M, GONZOLEZ O, RODRIGUEZ-FERRI EF, BUNSCHOTEN AE, VAN EMBDEN JDA, COUSINS D. Spacer oligonucleotide typing of Mycobacterium bovis strains from cattle and other animals: a tool for studying epidemiology of tuberculosis. Journal of Clinical Microbiology. 1996;34:2734–2740. doi: 10.1128/jcm.34.11.2734-2740.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARANAZ A, DE JUAN L, MONTERO N, SANVHEZ C, GALKA M, DELSO C, ALVAREZ J, ROMERO B, BEZOS J, VELA AI, BRIONES V, MATEOS A, DOMINGUEZ L. Bovine tuberculosis (Mycobacterium bovis) in wildlife in Spain. Journal of Clinical Microbiology. 2004;42:2602–2608. doi: 10.1128/JCM.42.6.2602-2608.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AYELE WY, NEILL SD, ZINSSTAG J, WEISS MJ, PAVLIK I. Bovine tuberculosis: an old disease but a new threat to Africa. Tubercle and Lung Disease. 4004;8:924–937. [PubMed] [Google Scholar]
- BAUER J, ANDERSEN AB, KREMER K, MIORNER H. Usefulness of spoligotyping to discriminate IS6110 low-copy-number Mycobacterium tuberculosis complex strains cultured in Denmark. Journal of Clinical Microbiology. 1999;37:2602–2606. doi: 10.1128/jcm.37.8.2602-2606.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CADMUS SIB, ATSANDA NN, ONI SO, AKANG EEU. Bovine tuberculosis in one cattle herd in Ibadan in Nigeria. Czech Veterinary Medicine. 2004;49:406–412. [Google Scholar]
- CAIMI K, ROMANO MI, ALITO A, ZUMARRAGA M, BIGI F, CATALDI A. Sequence analysis of the direct repeat region in Mycobacterium bovis. J; Journal of Clinical Microbiology; 2001; pp. 1067–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLINS JD. Factors relevant to M. bovis eradication. Irish Veterinary Journal. 1996;49:241–243. [Google Scholar]
- DE LA SALMONIERE YG, LI HM, TORREA G, BUNSCHOTEN A, VAN EMBDEN J, GICQUEL B. Evaluation of spoligotyping in a study of the transmission of Mycobacterium tuberculosis. Journal of Clinical Microbiology. 1997;35:2210–2214. doi: 10.1128/jcm.35.9.2210-2214.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DURR PA, HEWINSON RG, CLIFTO-HANDLEY RS. Molecular epidemiology of bovine tuberculosis I. Mycobacterium bovis genotyping. Revue. Scientifique et Technique. Office International des. Epizooties. 2000;19:675–688. doi: 10.20506/rst.19.3.1241. [DOI] [PubMed] [Google Scholar]
- FRANCIS J. Tuberculosis in Animals and Man. London: Cassell and Co.; 1958. p. 357. [Google Scholar]
- FROTHINGHAM R, MEEKER-O'CONNELL WA. Genetic diversity in the Mycobacterium tuberculosis complex based on variable numbers of tandem DNA repeats. Microbiology. 1998;144:1189–1196. doi: 10.1099/00221287-144-5-1189. [DOI] [PubMed] [Google Scholar]
- GOYAL M, SAUNDERS NA, VAN EMBDEN JDA, YOUNG DB, SHAW RJ. Differentiation of Mycobacterium tuberculosis isolates by spoligoyping and IS6110 restriction fragment length polymorphism. Journal of Clinical Microbiology. 1997;35:647–651. doi: 10.1128/jcm.35.3.647-651.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROENEN PMA, BUNSCHOTEN AE, VAN SOOLINGEN D, VAN EMBDEN JDA. Nature of DNA polymorphisms in the direct repeat cluster of Mycobacterium tuberculosis; application of strain differentiation by a novel typing method. Molecular Microbiology. 1993;10:1057–1065. doi: 10.1111/j.1365-2958.1993.tb00976.x. [DOI] [PubMed] [Google Scholar]
- KAMERBEEK J, SCHOULS L, KOLK A, VAN AGTERVELD M, VAN SOOLINGEN D, KUIJPER S, BUNSCHOTEN A, MOLHUIZEN H, SHAW R, GOYAL M, VAN EMBDEN J. Simultaneous detection and strain identification of Mycobacterium tuberculosis for diagnosis and epidemiology. Journal of Clinical Microbiology. 1997;35:907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOLK AH, SCHUITEMA AR, KUIJPER, SVAN LEEUWEN J, HERMANS PW, VAN EMBDEN J, HARTSKEERL RA. Detection of Mycobacterium tuberculosis in clinical samples by using polymerase chain reaction and non-radioactive detection system. Journal of Clinical Microbiology. 1992;30:2567–2575. doi: 10.1128/jcm.30.10.2567-2575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOX LF, RHIENTHONG D, MIRANDA AM, UDOMSANTISUK N, ELLIS K, VAN LEEUWEN J, VAN HEUSDEN S, KUIJPER S, KOLK AH. A more reliable PCR for detection of Mycobacterium tuberculosis in clinical samples. Journal of Clinical Microbiology. 1994;32:672–678. doi: 10.1128/jcm.32.3.672-678.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUTZE-WALLACE C, TURCOTTE C, SABORIN M, BERLIE-SURUJBALLI G, BARBEAU Y, WATCHORN D, BELL J. Canadian Journal of Veterinary Research. 2005;69:143–145. [PMC free article] [PubMed] [Google Scholar]
- MCILORY SG, Neill SD, MCCRACKEN RM. Pulmonary lesions and Mycobacterium bovis excretion from the respiratory tract of tuberculin reacting cattle. Veterinary Record. 1986;118:718–721. doi: 10.1136/vr.118.26.718. [DOI] [PubMed] [Google Scholar]
- MENZIES FD, NEILL SD. Cattle-to-cattle transmission of bovine tuberculosis. The Veterinary Journal. 2000;160:92–106. doi: 10.1053/tvjl.2000.0482. [DOI] [PubMed] [Google Scholar]
- NJANPOP-LAFOURCADE BM, INWALD J, OSTYN A, DURAND B, HUGHES S, THOREL MF, HEWINSON G, HADDAD N. Molecular typing of Mycobacterium bovis isolates from Cameroon. Journal of Clinical Microbiology. 2001;39:222–227. doi: 10.1128/JCM.39.1.222-227.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE . Manual of Standards for Diagnostics and Vaccines. Paris, France: Office International de Epizooties (OIE); 2000. [Google Scholar]
- PROUD AJ. Some lesions from the history of the eradication of bovine tuberculosis in Great Britain. Government Veterinary Journal. 2006;16:11–18. [Google Scholar]
- REED SG, ALDERSON MR, DALEMANS W, LOBET Y, SKEIKY YAW. Prospects for a better vaccine against tuberculosis. Tuberculosis. 2003;83:213–219. doi: 10.1016/s1472-9792(02)00080-x. [DOI] [PubMed] [Google Scholar]
- SMITH NH, DALE J, INWALD J, PALMER S, GORDON SV, HEWINSON RG, SMITH JM. The population structure of Mycobacterium bovis in Great Britain: Clonal expansion. Proceedings of National Academy of Science. 2003;100:15271–5. doi: 10.1073/pnas.2036554100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN SOOINGEN D, DE HAAS PE, HERMANS PW, GROENEN PM, VAN EMBDEN JD. Comparison of various repetitive elements as genetic markers for strain differentiation and epidemiology of Mycobacterium tuberculosis. Journal of Clinical Microbiology. 1993;31:1987–1995. doi: 10.1128/jcm.31.8.1987-1995.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VORDERMEIER HM, CHAMBERS MA, COCKLE PJ, WHELAN AO, SIMMONS J, HEWINSON RG. Correlation of ESAT-6 specific gamma-Interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infection and Immunity. 2002;70:3026–32. doi: 10.1128/IAI.70.6.3026-3032.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VORDERMEIER HM, WHELAN A, EWER K, GOODCHILD T, CLIFTON-HADLEY R, WILLIAMS J, HEWINSON RG. The BOVIGAM assay as ancillary test to the tuberculin test. Government Veterinary Journal. 2006;16:72–80. [Google Scholar]